Abstract
This study explores the fabrication and electrochemical performance of flexible NiCo2O4-based pseudo-capacitor electrodes, inkjet-printed onto flexible Kapton substrates. To circumvent the insulating nature of Kapton, a thin Au interlayer (20 nm) was introduced, significantly enhancing electrical conductivity. The effect of NiCo2O4 mass loading, ranging from 0.1 to 0.5 mg cm−2, was investigated. Optimal performance was achieved at a loading of 0.3 mg cm−2 on Au/Kapton substrates, yielding a specific capacitance of 520 F g−1 at 3.3 A g−1 and 90% capacitance retention after 1000 charge–discharge cycles. These results confirm that inkjet-printed NiCo2O4 electrodes, when combined with a conductive interlayer, exhibit excellent pseudo-capacitive behavior on flexible, non-conductive substrates. This approach demonstrates the feasibility of scalable, low-temperature fabrication techniques for high-performance flexible energy storage devices, suitable for emerging wearable technologies.
1. Introduction
The rapid advancement of portable and wearable electronics has created an urgent demand for flexible energy storage systems capable of delivering high power density, fast charge–discharge rates, and long cycle life. Among the various energy storage technologies, supercapacitors have garnered significant attention due to their ability to meet these requirements either through electrostatic charge storage mechanisms or fast redox reactions in thin films, which entail faster kinetics compared to faradaic processes in batteries [1,2,3]. Despite their superior power density and cycling stability, supercapacitors typically suffer from lower energy density, which restricts their applicability in scenarios requiring both high energy and power output [4,5,6].
To address this limitation, recent research has focused on the development of advanced electrode materials that can enhance the energy density of supercapacitors without compromising their inherent advantages. Transition metal oxides, particularly nickel cobaltite (NiCo2O4), have emerged as promising candidates due to their high theoretical capacitance, excellent electrical conductivity, and robust electrochemical stability [7,8,9,10]. NiCo2O4, a mixed-valence oxide of nickel and cobalt, exhibits pseudo-capacitive behavior, enabling fast and reversible redox reactions that contribute to higher energy storage capacity compared to conventional electric double-layer capacitors (EDLCs) [11,12,13].
The spinel structure of NiCo2O4 provides a large number of electrochemically active sites, which enhances specific capacitance. Furthermore, the synergistic interaction between nickel and cobalt ions improves electrical conductivity and structural integrity, which are critical for maintaining performance over prolonged cycling [14,15,16,17]. However, the electrochemical performance of NiCo2O4 electrodes is highly dependent on factors such as morphology, surface area, and the nature of the substrate. Optimizing these parameters is essential for realizing the full potential of NiCo2O4 in flexible supercapacitor applications [18,19,20,21].
Flexible substrates play a pivotal role in the integration of supercapacitors into wearable and portable electronics, where mechanical durability and conformability are essential. Traditional substrates such as carbon cloth and nickel foam offer high conductivity and surface area but often lack the mechanical flexibility required for dynamic applications [22,23,24]. Kapton, a polyimide film, has emerged as a promising alternative due to its exceptional thermal stability, mechanical flexibility, and chemical resistance [25,26,27]. When coated with a conductive layer such as gold (Au), Kapton provides a stable and conductive platform for the deposition of active electrode materials like NiCo2O4, thereby enabling the fabrication of flexible supercapacitors with enhanced performance and durability [28,29,30]. The selection of Au as an interlayer was based on its excellent chemical inertness, high electrical conductivity, and strong adhesion to Kapton. Unlike Ag, which is prone to oxidation and migration, or carbon paints, which may suffer from poor film uniformity, Au provides a stable and conductive platform for inkjet printing. Although PET/ITO is a common alternative, its brittleness and limited thermal tolerance make it less suitable for flexible and thermally processed devices.
The fabrication of flexible supercapacitors necessitates precise and scalable deposition techniques that are compatible with advanced materials and substrates. Inkjet printing has gained prominence as a versatile and cost-effective method for fabricating supercapacitor electrodes, offering precise control over material deposition and patterning at the microscale [31,32,33,34]. This technique allows for the modulation of electrode thickness and morphology, which are critical parameters influencing electrochemical performance. Moreover, inkjet printing operates at relatively low temperatures, preserving the integrity of flexible substrates such as Kapton [35,36,37]. Compared to conventional methods like electrodeposition, sputtering, and chemical vapor deposition, inkjet printing offers reduced material waste, design flexibility, and scalability for industrial production [38,39,40].
One of the key factors influencing the performance of NiCo2O4 electrodes is the thickness of the catalyst layer. Electrode thickness directly affects specific capacitance, energy density, and mechanical flexibility. Recent studies have demonstrated that optimizing the number of printed layers can significantly improve electrochemical performance [1,8,36].
The main aim of this work is to report the proof-of-concept for an inkjet printing fabrication technique for flexible supercapacitor electrodes. Toward this end, NiCo2O4 was used as a typical electrocatalyst material and Kapton as a typical flexible support. When compared to other flexible substrates such as carbon cloth and nickel foam, a Kapton support offers a distinct advantage in terms of mechanical flexibility and structural integrity. Although carbon cloth and nickel foam coated with NiCo2O4 typically yield high capacitance values (750–1200 F g−1), their limited flexibility constrains their use in wearable applications [41,42,43,44]. In this work, we investigate whether other supports that can be used with inkjet printing techniques, such as the model Au/Kapton system, can result in electrodes with a balanced electrochemical performance and superior mechanical resilience, opening up new avenues for the design of high-performance, flexible energy storage devices tailored for portable and wearable electronics.
2. Materials and Methods
2.1. Materials
Nickel(II) acetate tetrahydrate ((CH3COO)2Ni.4H2O), cobalt(II) acetate tetrahydrate ((CH3COO)2Co4H2O), oxalic acid (HO2CCO2H.H2O), propylene glycol (C3H8O2), and Triton X-100 (C14H22O(C2H4O)n) were purchased from Merck (Darmstadt, Germany) and used directly without purification. Distilled water was applied in all synthesis and ink preparation steps. Printing was carried out on Kapton substrates coated with a conductive gold layer (surface resistivity: 10 Ω/sq).
2.2. Synthesis of NiCo2O4 Nanoparticles
NiCo2O4 (NCO) nanoparticles were prepared via a hydrothermal process were used as the active material for inkjet printing. A solution containing 5 mmol nickel acetate, 10 mmol cobalt acetate, and 10 mmol oxalic acid in 30 mL distilled water was magnetically stirred for 30 min to form a uniform white suspension. The mixture was sealed in an autoclave and heated at 90 °C for 12 h. The product was centrifuged at 4000 rpm for 20 min, washed twice with ethanol, and dried at 80 °C for 4 h in a vacuum oven. Finally, the obtained powder was annealed in air at 350 °C for 2 h with a heating rate of 5 °C min−1, yielding black NiCo2O4 nanoparticles.
2.3. Ink Formulation
The NCO powder was ground by ball milling. In detail, 3 g of NCO was dispersed in 25 mL of distilled water and milled in a zirconia container containing 100 g of 0.5 mm zirconia balls. The milling–centrifugation cycle (700 rpm, 30 min) was repeated four times to obtain a stable black nanoparticle-rich dispersion. This dispersion was subsequently mixed with propylene glycol at a 7:3 volume ratio and supplemented with Triton X-100 (0.5 mg mL−1) to improve nanoparticle stability. To avoid clogging of the inkjet nozzles, the final ink was passed through a 0.45 μm nylon syringe filter to remove large agglomerates.
2.4. Conductive Substrate Preparation and Inkjet Printing Procedure
Kapton films (85 μm thick) were cut into 2 × 1 cm2 pieces and cleaned sequentially with acetone, isopropanol, and deionized water, followed by drying under a nitrogen stream. A 20 nm thick gold layer was deposited onto the Kapton surface using a metal sputter coater ensuring optimal conductivity and adhesion. The Au layer was sputter-deposited on the Kapton substrate at ambient temperature. Although no thermal analysis was performed in this study, Kapton’s thermal stability is well documented in the literature, with decomposition temperatures typically above 500 °C [45].
Inkjet printing was performed using a Dimatix DMP-2850 drop-on-demand printer (Fujifilm Dimatix, Santa Clara, CA, USA), equipped with a 10 pL piezoelectric cartridge (DMC-11610, Fujifilm Dimatix, Santa Clara, CA, USA) and a 3 mL ink reservoir. Printing was conducted under ambient conditions, with the cartridge temperature maintained between 25 and 35 °C and a drop spacing of 40 μm. Following deposition, the printed films were annealed in air at 350 °C for 2 h using a controlled heating rate of 5 °C min−1 to promote crystallinity and film cohesion.
2.5. Multilayer Electrode Assembly and Thermal Consolidation
To systematically evaluate the impact of film thickness on electrochemical performance, a series of NiCo2O4 electrodes was fabricated via inkjet printing with 2, 6, and 10 sequential layers. These configurations corresponded to active material loadings of approximately 0.1, 0.3, and 0.5 mg cm−2, respectively. Following the initial high-temperature annealing step at 350 °C, all printed electrodes were subjected to a secondary thermal treatment at 150 °C for 30 min. This post-deposition annealing was implemented to enhance interparticle cohesion and promote strong interfacial adhesion between the NiCo2O4 film and the underlying Kapton substrate.
The dual-phase thermal protocol—comprising crystallization-driven annealing and low-temperature consolidation—was critical for achieving mechanically stable and electrochemically responsive electrode architectures. Previous studies have demonstrated that annealing temperatures significantly influence the crystallinity, morphology, and electrochemical behavior of NiCo2O4-based electrodes [46,47]. Moreover, variations in electrode thickness and mass loading have been shown to affect specific capacitance and energy density, underscoring the importance of structural optimization in supercapacitor design [48]. This approach ensured optimal film integrity, minimized interfacial resistance, and facilitated efficient charge transport across the printed layers.
2.6. Characterization Techniques
The crystalline structure of the NCO nanoparticles was examined by X-ray diffraction (XRD) using a Rigaku D/MAX-2000H (Rigaku Corporation, Tokyo, Japan) diffractometer with CuKα radiation (λ = 1.54 Å) and a graphite monochromator. Phase identification and structural analysis were performed with X-Pert Highscore Plus software, version 4.9, PANalytical B.V., Almelo, The Netherlands. The surface morphology was investigated by scanning electron microscopy (SEM) on a JEOL JSM-7000F (JEOL Ltd., Tokyo, Japan) operated at 20 kV. Particle size distribution was determined by dynamic light scattering (DLS) using a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK). Atomic force microscopy (AFM) was employed to study the surface topography and film thickness of the electrodes, using a Solver Pro system (NT-MDT, Moscow, Russia).
2.7. Electrochemical Characterization
The electrochemical performance of the inkjet-printed NiCo2O4 thin film electrodes was evaluated using Cyclic Voltammetry (CV), Galvanostatic Charge–Discharge (GCD), and Electrochemical Impedance Spectroscopy (EIS). All measurements were conducted using a PGSTAT302N electrochemical workstation (Autolab, Metrohm, Herisau, Switzerland) with freshly prepared aqueous 0.1 M KOH as the electrolyte.
CV measurements were performed at potential sweep rates ranging from 5 to 20 mV s−1. EIS analyses were conducted over a frequency range of 105 to 0.1 Hz with an AC amplitude of 10 mV. GCD experiments were carried out via chronopotentiometry at constant current densities between 1.0 and 6.0 mA cm−2, with voltage cut-off values defined by the electrochemical window determined from CV profiles. Long-term cycling stability was assessed at a current density of approximately 0.5 mA cm−2 over 1000 consecutive charge–discharge cycles.
All electrochemical tests employed a conventional three-electrode configuration, consisting of a platinum wire as the counter electrode, a Saturated Calomel Electrode (SCE) as the reference, and NiCo2O4/Au/Kapton working electrodes fabricated with 2, 6, and 10 printed layers, corresponding to active material loadings of approximately 0.1, 0.3, and 0.5 mg cm−2, respectively. The active electrode area exposed to the electrolyte was 0.7 cm2. To minimize iR drop effects, a Luggin capillary was positioned 1 mm from the surface of the working electrode, in accordance with established protocols for reducing solution resistance errors [49].
Finally, to assess the mechanical flexibility and electrochemical resilience of the printed electrodes, CV measurements were performed after mechanically bending the electrode to a 90° angle. Comparative voltammograms recorded before and after bending were used to evaluate any degradation in capacitive behavior or redox activity. This test was designed to simulate mechanical strain conditions relevant to flexible and wearable energy storage devices [50,51].
3. Results
3.1. Structural and Morphological Characterization of Inkjet-Printed NiCo2O4 Electrodes
The crystalline structure of the inkjet-printed NiCo2O4 electrode was examined using X-ray Diffraction (XRD), as shown in Figure 1. The diffraction pattern displays prominent peaks indexed to the (111), (220), (311), (222), (400), (331), (420), (422), (511), and (440) planes, consistent with the spinel phase of NiCo2O4 (JCPDS card No. 40-0276) [1,2]. The sharp and well-defined reflections indicate high crystallinity, with the (311) peak being the most intense, suggesting preferential orientation and phase purity. The absence of secondary phases confirms the successful synthesis of NiCo2O4 with no detectable impurities, validating the integrity of the inkjet printing process for crystalline oxide deposition [3]. These structural characteristics are essential for achieving efficient pseudo-capacitive behavior, as they provide a stable framework for fast and reversible redox reactions [4]. TEM images of similarly prepared NCO nano-particles used in our previous work in semi-transparent supercapacitor electrodes revealed 5–9 nm diameter nanoparticles, aggregated in 100–300 nm clusters (see Figure 4 in [1]).
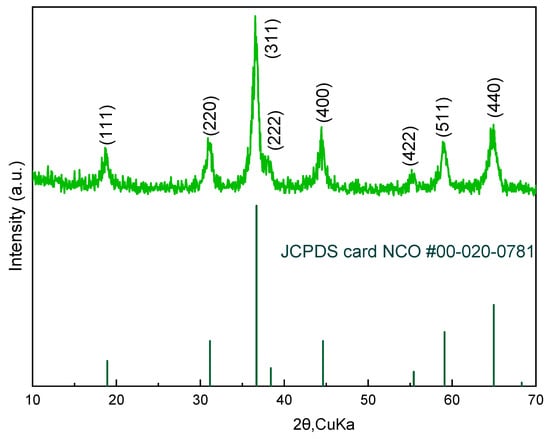
Figure 1.
XRD pattern of inkjet-printed NiCo2O4 showing characteristic spinel peaks.
Figure 2a below shows the top-view photographic image of a NiCo2O4/Au/Kapton electrode prepared by inkjet printing while Figure 2b depicts bending of the flexible electrode.
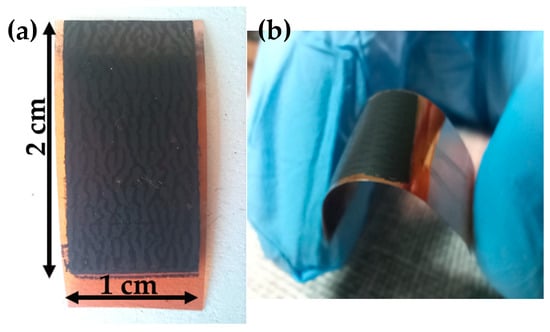
Figure 2.
(a) Top view of inkjet-printed NiCo2O4/Au/Kapton electrode and (b) demonstration of its mechanical flexibility.
Surface morphology of the electrodes was investigated using Scanning Electron Microscopy (SEM), presented in Figure 3. The micrographs (Figure 3a,b) reveal a rough, granular texture composed of interconnected nanoscale particles, forming a porous network across the Au/Kapton substrate. Figure 3b shows a high-magnification SEM image of the NiCo2O4 film, revealing its nanoparticulate structure which is beneficial for ion transport and electrochemical activity. Figure 3c presents the associated particle size analysis, confirming that the particle size in the film (average diameter 60 nm) remains almost the same as in the ball-milled powder. The NCO particles exhibit irregular shapes and a porous morphology, with average diameters ranging from 200 to 800 nm. In some regions, agglomerates reach sizes of 1–2 μm, indicating localized clustering. Following mechanical grinding, the NCO nanoparticles achieve a more uniform size distribution, with diameters reduced to below 200 nm. The average particle size decreases from approximately 450 nm to 60 nm, representing a mean reduction of about 385 nm as shown in Figure 3b (high resolution SEM) and 3c (histogram of particle size analysis), in excellent agreement with similar films prepared on glass substrates and presented in [1]. The average particle size decreases from approximately 450 nm to 65 nm, representing a mean reduction of about 385 nm. This refinement enhances surface homogeneity and increases the electrochemically active area, which is beneficial for ion transport and redox activity [8,27,30]. The uniform distribution of particles and absence of cracks or delamination indicate strong adhesion and consistent film formation, both critical for mechanical stability in flexible devices [9,26]. The observed surface features are characteristic of pseudo-capacitive materials and support efficient electrochemical performance [10,28].
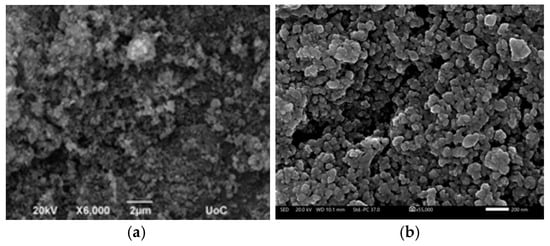
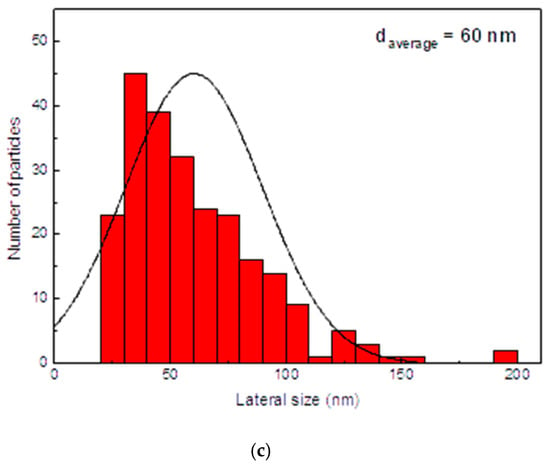
Figure 3.
SEM images of NiCo2O4 films on Au/Kapton substrates, (a) Prepared from NCO particles as synthesized (white scale bar of 2 μm) and (b) after ball-milling (white scale bar of 200 nm); (c) Particle size analysis of the NCO film depicted in (b).
Atomic Force Microscopy (AFM) was employed to assess the topography of the printed NiCo2O4 layer, as shown in Figure 4. The 2D surface profile reveals nanoscale roughness with height variations ranging from 0 to approximately 300 nm. The presence of elevated regions and valleys suggests a textured surface that enhances electrolyte contact and charge storage capacity [30]. The AFM micrograph also reveals a homogeneous and compact film with pronounced nanoroughness, indicating uniform deposition and strong surface cohesion. This topographical complexity complements the SEM findings and confirms the formation of a uniform, high-surface-area film. Together, the XRD, SEM, and AFM analyses demonstrate that inkjet-printed NiCo2O4 on Au/Kapton exhibits the structural and morphological characteristics necessary for high-performance flexible supercapacitor electrodes [8].
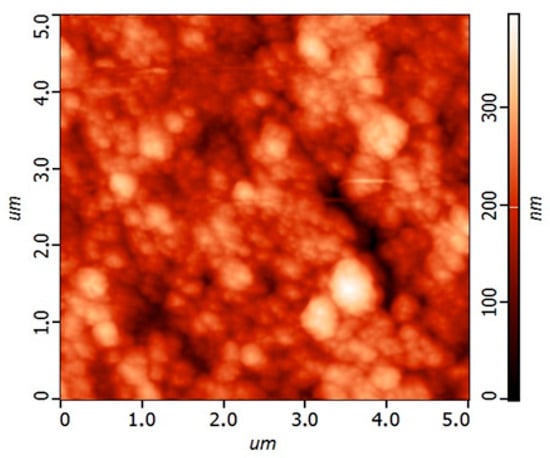
Figure 4.
AFM topography of the printed NiCo2O4 layer showing nanoscale surface roughness.
3.2. Electrochemical Performance
Cyclic voltammetry (CV) was employed to evaluate the capacitive behavior of the inkjet-printed NiCo2O4 electrodes on Au/Kapton substrates as a function of the number of printed catalyst layers. Figure 5 presents the CV responses of the electrode composed of two, six and ten printed layers (corresponding to 0.1, 0.3 and 0.5 mg cm−2 loadings), recorded at scan rates of 5, 10, and 20 mV s−1 within the potential window of 0–0.55 V vs. SCE. The voltammograms exhibit quasi-rectangular shapes with distinguishable redox peaks, indicating the coexistence of electric double-layer capacitive and pseudocapacitive behavior. This hybrid charge storage arises from additional reversible faradaic reactions of Ni2+/Ni3+ and Co2+/Co3+ redox couples. Specifically, the nickel-based redox process follows:
NiO + OH− ⇌ NiOOH + e−
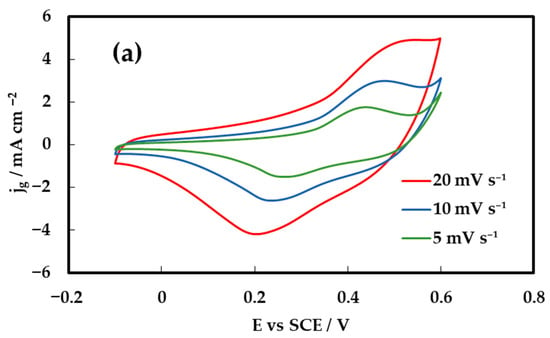
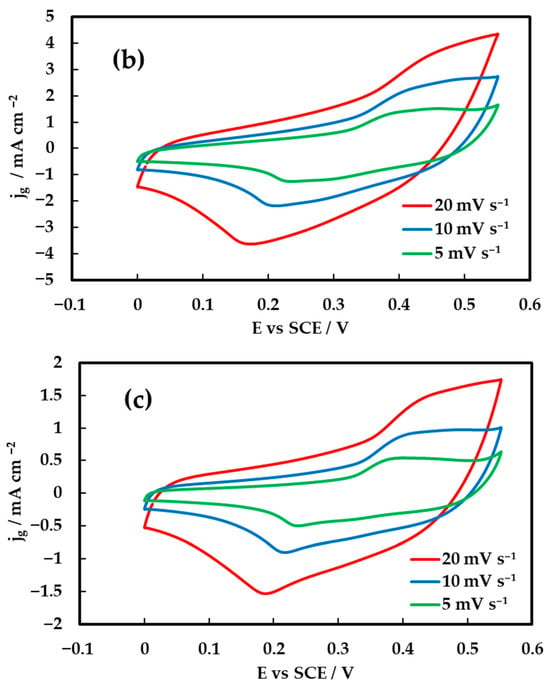
Figure 5.
Cyclic voltammetry (CV) curves of NiCo2O4 electrodes recorded in 1 M KOH within the potential window of 0–0.55 V vs. SCE. (a) for 10-layer electrode, (b) for 6-layer electrode and (c) for 2-layer electrode.
For cobalt, the redox activity proceeds through a two-step mechanism:
Co2O3 + H2O ⇌ 2CoOOH
2CoOOH + 2OH− ⇌ 2CoO2 + 2H2O + 2e−
Alternatively, the overall cobalt reaction can be expressed as:
Co2O3 + 2OH− ⇌ 2CoO2 + H2O + 2e−
These reactions contribute to the broad and overlapping redox peaks observed in the voltammograms, confirming the pseudo-capacitive nature of the inkjet-printed NiCo2O4 electrodes. As the scan rate increases, both anodic and cathodic peak currents increase correspondingly, without significant deformation of the CV shape, suggesting fast surface redox kinetics. The improved current response of 6- and 10-layer electrodes compared to the 2-layer electrode (Figure 5c) confirms the enhancement in electroactive surface area with increasing film thickness. However, the similarity of the current density for the 6- and 10-layer electrodes (Figure 5a,b), indicates that beyond a certain thickness there is a decrease in porosity, limiting the use of the material in the interior of the thick film.
These results suggest that the electrode with six layers (Figure 5b) achieves a balance between sufficient mass loading for effective charge storage and favorable ion diffusion pathways, making it a promising candidate for flexible supercapacitor applications where both performance and mechanical integrity are critical.
EIS measurements (Figure 6a) and subsequent analysis (Figure 6b) were carried out at the open circuit potential of the optimum 6 L electrode (+0.50 V vs. SCE). The recorded arc is associated with a charge transfer resistance (Rct) that may be attributed to Co and Ni redox transformations (change in oxidation state) under kinetic control, followed by a linear tail at low frequencies, indicative of a diffusion-controlled processes (Warburg impedance), most likely corresponding to OH− diffusing through the pores (reactions corresponding to the chemical Equations (1), (3) and (4)).
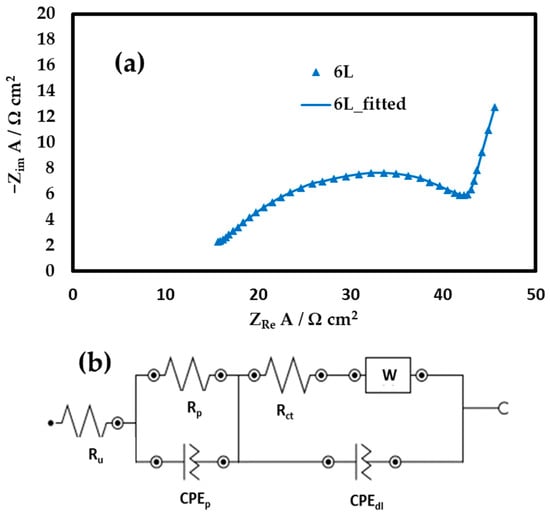
Figure 6.
(a) Nyquist plots of NiCo2O4 electrodes with 6 printed layers measured in 0.1 M KOH at +0.55 V and (b) corresponding fitted electrical circuit.
Fitting an appropriate electrical circuit to the data [Ru(RpCPEp)([RctW]CPEdl)] accounts for the above-mentioned faradaic and diffusion processes second RC circuit in series; ([RctW]CPEdl), as well as for ionic migration inside a porous electrode (first RC circuit in series; Ru(RpCPEp) in agreement with others appearing in the literature [41,42]. In this notation Rp is the ionic pore resistance, Rct the faradaic reaction charge transfer resistance, CPEp and CPEdl the corresponding constant phase elements associated with inhomogeneities and double layer capacitance of the electrode pores and surface and Ru the uncompensated solution resistance up to the outer surface of the electrode.
The values obtained (Table 1) are comparable to those reported for NiCo2O4 electrodes inkjet printed on glass substrates [1], indicating that the Kapton/Au does not compromise electrochemical performance. The values of Ru estimated for all electrodes studied here have been used to correct the potential drop during the discharge part of the GCD presented below.

Table 1.
Component values of the electrical circuit corresponding to the equivalent model illustrated in Figure 6.
Figure 7a presents the galvanostatic charge–discharge (GCD) profiles of inkjet-printed NiCo2O4 electrodes comprising 2, 6, and 10 printed layers, recorded at a current density of 1.5 mA cm−2 in 0.1 M KOH aqueous electrolyte. The voltage–time curves exhibit quasi-linear and symmetric characteristics, with slight deviations from a triangular shape attributed to pseudo-capacitive contributions arising from the reversible faradaic redox reactions involving Ni2+/Ni3+ and Co2+/Co3+ couples. The hybrid charge storage behavior of NiCo2O4 electrodes arises from reversible faradaic reactions involving both nickel and cobalt redox couples. During charging/positive-going potential sweep, Ni2+ ions are oxidized to Ni3+ and Co2+ ions to Co3+, while the reverse reaction occurs during discharging/negative-going potential sweep. These transitions are accompanied by hydroxide ion participation and transport from the alkaline electrolyte into the mixed oxide film, accompanied by fast surface electron transfer. The co-existence of Ni and Co red/ox couples provides multiple oxidation states for charge storage, which contributes to the broad and overlapping redox peaks observed in the cyclic voltammograms. This is the origin of the pseudocapacitive nature of the inkjet-printed NiCo2O4 electrodes and explains their enhanced capacitance and rate capability compared to purely double layer capacitors.
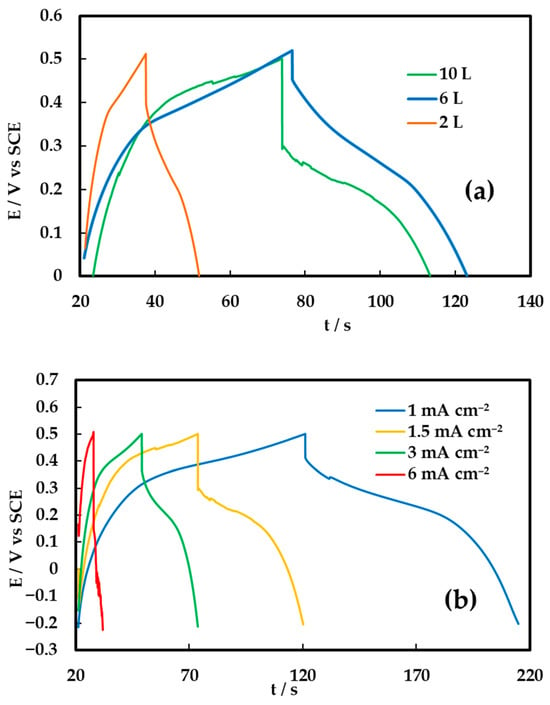
Figure 7.
Galvanostatic charge–discharge curves for (a) NiCo2O4 electrodes with 2, 6, and 10 printed layers at 1.5 mA cm−2 in 0.1 M KOH and (b) the 6 L NiCo2O4 electrode at various current densities.
The shape of the discharge curves suggests that the voltage drop during discharge can be reasonably approximated by a straight line connecting the potential limits. This simplification is justified by the fact that the area under the linear approximation deviates by less than 10% from the actual experimental curve. Therefore, for estimating the specific or areal capacitance (C), one can apply the simplified relation commonly used for electric double-layer (EDL) supercapacitors with potential-independent capacitance:
where is the discharge current density, is the discharge time and is the voltage drop corrected for ohmic losses.
However, for hybrid supercapacitors—where capacitance varies with potential—a more rigorous approach is required. The average areal capacitance () should be calculated using the general expression [5,28,37]:
This formulation accounts for the non-linear voltage profile during discharge and provides a more accurate representation of the hybrid system’s capacitive behavior.
The electrode with 10 printed layers exhibits the longest discharge duration, yielding the highest areal capacitance of 172 mF cm−2, followed by the 6-layer and 2-layer electrodes with values of 156 mF cm−2 and 56 mF cm−2, respectively. The increase in areal capacitance with layer number is ascribed to the greater amount of electroactive material available for charge storage. However, normalization with respect to the active material mass yields a partially reversed trend in mass-specific capacitance, with values of 344 F g−1, 521 F g−1, and 503 F g−1 for the 10-, 6-, and 2-layer electrodes, respectively. The superior gravimetric performance of the 6- and 2-layer electrodes suggests more efficient utilization of the active material, likely due to enhanced ion accessibility and reduced diffusion limitations within thinner films. Figure 7b presents the charge–discharge curves of the optimum 6L electrode at various charge–discharge current densities and the same type of behavior is observed at current densities as high as 6 mA cm−2.
On the other hand, the initial potential drop (IR-drop) observed at the onset of discharge more pronounced as the number of layers decreases, indicating an increase in internal resistance due to a decrease in inter-particle contacts in very thin films. These observations are consistent with the cyclic voltammetry results and highlight the trade-off between areal and mass-specific performance. Optimal electrochemical behavior is thus achieved by balancing active material loading with ionic/electronic transport pathways, which is critical for the development of high-performance flexible supercapacitor devices based on inkjet-printed NiCo2O4 electrodes.
Table 2 presents the specific capacitance values of the NiCo2O4-based electrodes studied in this work. The printed electrodes exhibit specific capacitance values (per substrate geometric area and catalyst mass) in the 50–175 mF cm−2 and 345–520 F g−1 range, depending on electrode configuration and testing conditions. These values are within the range of those reported for flexible NiCo2O4 systems in the literature [17,52,53], underscoring the potential of the inkjet printing approach for the development of high-performance, flexible supercapacitor electrodes.

Table 2.
Capacitance performance of NiCo2O4 electrodes with varying layer thickness.
The long-term electrochemical stability of the NiCo2O4 electrode with 6 printed layers (NCO–Au–Kapton 6 L) was evaluated over 1000 continuous charge–discharge cycles at a fixed current density (1.0 mA cm−2). As shown in Figure 8, the electrode retains approximately 90% of its initial capacitance after 1000 cycles, indicating moderate cycling stability. While the initial performance is satisfactory, the observed fading highlights the need for further optimization of mechanical adhesion, structural durability, or protective coatings to enhance cycling lifespan in flexible supercapacitor applications.
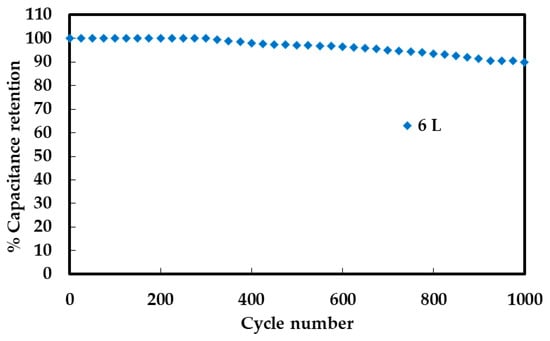
Figure 8.
Capacitance retention of NiCo2O4 (6-layer) electrode on Au/Kapton substrate over 1000 charge–discharge cycles.
3.3. Electrochemical Performance Under Mechanical Stress
To assess the mechanical flexibility and electrochemical stability of the inkjet-printed NiCo2O4 electrodes deposited on Kapton substrates with a gold (Au) interlayer, cyclic voltammetry (CV) measurements were conducted under three mechanical conditions: prior to bending, during bending, and following bending. The CV profiles, recorded at a scan rate of 5 mV s−1 with a mass loading of 0.3 mg cm−2, are presented in Figure 9 (while the inset shows a photograph of the 90°-bent electrode). All three curves—green (before bending), orange (during bending), and blue (after bending)—exhibit quasi-rectangular shapes with discernible redox features, indicative of pseudo-capacitive behavior governed by fast and reversible faradaic reactions [8,10].
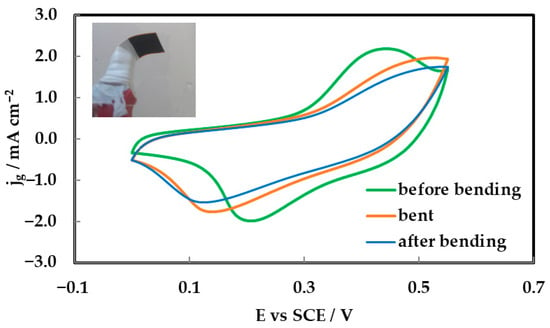
Figure 9.
CV curves of inkjet-printed NiCo2O4 electrodes on Kapton Au (0.3 mg cm−2) in 0.1 M MOH at 5 mV s−1, showing performance before bending (green), during bending (orange), and after bending (blue).
The small deviation between the CV curves obtained before and after bending demonstrates that the electrode retains its electrochemical integrity following mechanical deformation. A modest reduction in current density observed during bending is attributed to transient strain effects, which do not result in permanent degradation. The recovery of current response post-bending confirms the structural robustness and functional resilience of the NiCo2O4/Au/Kapton architecture [4,9].
The bending strain, ε, of the bent 6L NCO/Au/Kapton electrode was estimated using two approaches. The first was an approximate one, simply considering the total electrode thickness, d, of 85 μm Kapton + 20 nm Au + 1.5 μm NCO = 148.52 μm and the radius, r, of the bent structure which is measured as 2.23 cm and the following simple equation [54]:
The bending strain thus estimated was 0.39%.
The second approach, took into consideration an NCO/Kapton bilayer (assuming that the 20 nm Au interlayer was sufficiently thinner than the other two layers, NCO is 1.5 and Kapton 85 μm, to be ignored as an approximation). Taking the Young’s moduli, E, values of NCO and Kapton as 84 and 4 GPa, respectively [54,55], and applying the following equation:
(where η = dNCO/dKapton and χ = ENCO/EKapton), the bending strain thus estimated was 0.29%.
These findings validate the mechanical durability of the inkjet-printed NiCo2O4 electrodes and their ability to maintain stable capacitive performance under dynamic mechanical stress. The integration of a conductive Au interlayer on Kapton not only facilitates efficient charge transport but also supports mechanical compliance, rendering this configuration highly suitable for flexible energy storage systems, particularly in wearable and portable electronic applications where repeated mechanical deformation is anticipated [1,27,28].
4. Discussion
The comprehensive structural and electrochemical characterization of inkjet-printed NiCo2O4 electrodes on Kapton/Au substrates highlights their potential for flexible energy storage applications. Each technique—XRD, SEM, AFM, CV, GCD, EIS, and charge–discharge cycling—offers distinct insights into the material’s behavior, collectively validating its performance and manufacturability.
X-ray diffraction (XRD) confirmed the formation of a crystalline spinel NiCo2O4 phase, with sharp reflections corresponding to the (111), (200), (220), (311), and other characteristic planes (Figure 1). The dominance of the (311) peak and absence of secondary phases indicate high phase purity and preferential orientation. This crystallinity, achieved via low-temperature inkjet printing, rivals that of NiCo2O4 synthesized through hydrothermal or sol–gel methods [5,8], while remaining compatible with flexible substrates.
Scanning Electron Microscopy (SEM) revealed a porous, granular morphology composed of irregularly shaped NiCo2O4 particles (Figure 3). Particle sizes ranged from 200 to 800 nm, with agglomerates reaching up to 2 μm. After mechanical grinding, the particles exhibited a more uniform distribution below 200 nm, with average diameters reduced from ~450 nm to ~60 nm. This refinement enhances surface homogeneity and increases the electrochemically active area, facilitating ion transport and redox reactions. The porous architecture supports electrolyte penetration and is consistent with pseudo-capacitive behavior.
Atomic Force Microscopy (AFM) provided nanoscale insight into the surface topography of the printed films (Figure 4). The 2D profile revealed a compact, homogeneous film with nanoroughness and height variations up to 300 nm. The presence of elevated regions and valleys enhances electrolyte contact and charge storage capacity. This morphology complements the SEM findings and confirms the formation of a high-surface-area electrode layer, critical for both electrochemical performance and mechanical durability.
Cyclic Voltammetry (CV) was used to probe the charge storage mechanism. The CV profiles (Figure 5) display distinct redox peaks corresponding to Ni2+/Ni3+ and Co2+/Co3+ transitions, confirming pseudo-capacitive behavior. The increase in peak current with scan rate and layer number reflects enhanced electrochemical activity due to greater active surface area and improved electron transport. These trends align with prior studies on NiCo2O4 electrodes [5,8], but are here achieved through a scalable inkjet printing process.
Galvanostatic Charge–Discharge (GCD) measurements (Figure 7) quantified the specific capacity. The 10-layer electrode exhibited the highest areal capacitance (345 F g−1), while the 6-layer electrode achieved the highest mass-specific capacitance (520 F g−1), indicating efficient material utilization. This trade-off highlights the challenge of balancing film thickness with ion accessibility, as excessive thickness can lead to underutilization of inner layers due to diffusion limitations.
Electrochemical Impedance Spectroscopy (EIS) (Figure 6) evaluated charge transfer kinetics and internal resistance. Increasing the number of printed layers reduced charge transfer resistance up to an optimal thickness, beyond which transport limitations may arise. This confirms that inkjet-printed NiCo2O4 films can achieve efficient electron and ion conduction when properly optimized.
Charge–discharge cycling behavior, assessed through repeated GCD cycles (Figure 8), revealed good retention over time. The 6-layer electrode maintained approximately 90% of its initial capacitance after 1000 cycles. This decline may result from mechanical fatigue, structural degradation, or loss of active sites during repeated charge–discharge processes. Enhancements such as surface passivation, flexible binders, or hybrid composites may be necessary to improve long-term reliability.
Mechanical flexibility was evaluated via CV measurements before, during, and after bending (Figure 9). The consistent shape and redox features across all conditions confirm that the NiCo2O4/Au/Kapton electrodes maintain electrochemical integrity under mechanical stress. A slight reduction in current during bending was reversible, indicating transient strain effects rather than permanent damage. This resilience supports their application in wearable electronics.
In summary, each characterization technique contributes to a holistic understanding of the material: XRD confirms crystallinity, SEM and AFM reveal surface architecture, CV and GCD quantify charge storage, EIS diagnoses transport efficiency, and charge–discharge cycling tests assess durability. Together, they demonstrate that inkjet-printed NiCo2O4 on Au/Kapton is a viable platform for flexible supercapacitor electrodes. Future research should focus on improving cycling performance, exploring hybrid or solid-state configurations, and refining ink formulation and substrate engineering to further enhance performance and manufacturability.
5. Conclusions
This study demonstrates the successful fabrication and characterization of inkjet-printed NiCo2O4 electrodes on Kapton substrates modified with a thin gold interlayer, offering a scalable and substrate-compatible approach for flexible energy storage devices. Structural analyses confirmed the formation of a crystalline spinel phase with a porous, nanostructured morphology and uniform surface topography—features that directly support efficient charge storage.
Electrochemical characterization revealed pseudo-capacitive behavior driven by reversible Ni2+/Ni3+ and Co2+/Co3+ redox reactions. The six-layer configuration achieved optimal performance, balancing areal and mass-specific capacitance (157 mF cm−2 and 520 F g−1 catalyst), charge–discharge cycling stability, and mechanical flexibility. Impedance analysis and bending tests further validated the robustness of the electrode architecture under dynamic conditions.
Overall, inkjet printing of NiCo2O4 onto Au/Kapton substrates presents a viable pathway toward high-performance, flexible supercapacitor electrodes. Future work should focus on enhancing charge–discharge cycling retention, exploring hybrid material systems, and integrating these electrodes into full-cell configurations for wearable and portable energy storage applications.
Author Contributions
Conceptualization, S.S. and V.B.; methodology, A.B.; investigation, P.P., E.M. and M.C.; writing—original draft preparation, A.B.; writing—review and editing, S.S., A.B. and A.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Banti, A.; Charalampakis, M.; Pardalis, P.; Prochaska, C.; Sotiropoulos, S.; Binas, V. Electrochemical Studies of Inkjet Printed Semi-Transparent NiCo2O4/ITO Supercapacitor Electrodes. Catalysts 2023, 13, 1110. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, C.; Wu, W. Printed flexible supercapacitor: Ink formulation, printable electrode materials and applications. Appl. Phys. Rev. 2021, 8, 021319. [Google Scholar] [CrossRef]
- Hari Narayanan, K.R.; Kannan, S.; Ramadoss, A. Inkjet Printing Fabrication of Supercapacitors. In Functionalized Nanomaterials Based Supercapacitor; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Manjakkal, L.; Dervin, S.; Dahiya, R. Flexible potentiometric pH sensors for wearable systems. RSC Adv. 2020, 10, 8594–8617. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Wang, C.; Yang, F.; Ren, X.; Zhang, X.; Dong, D.; Hu, W. Organic crystalline materials in flexible electronics. Chem. Soc. Rev. 2019, 489, 1492–1530. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Unconventional supercapacitors from nanocarbon-based electrode materials to device configurations. Chem. Soc. Rev. 2016, 45, 4340–4363. [Google Scholar] [CrossRef]
- Goel, P.; Sundriyal, S.; Shrivastav, V.; Mishra, S.; Dubal, D.P.; Kim, K.H.; Deep, A. Perovskite materials as superior and powerful platforms for energy conversion and storage applications. Nano Energy 2021, 80, 105552. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Lin, Y.; Huang, X.; García-Tecedor, M.; Peña O’Shea, V.A.; Murrill, C.; Lazarov, V.K.; Oropeza, F.E.; Zhang, K.H.L. Impact of NiCo2O4/SrTiO3 p–n Heterojunctions on the Interface of Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 28739–28746. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Jia, Y.; Zheng, H. Three-dimensional NiCo2O4@NiWO4 core–shell nanowire arrays for high performance supercapacitors. J. Mater. Chem. A 2017, 5, 1028–1034. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Yang, X.; Adair, K.R.; Wang, C.; Zhao, F.; Sun, X. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 1429–1461. [Google Scholar] [CrossRef]
- Paulsen, B.D.; Wu, R.; Takacs, C.J.; Steinrück, H.-G.; Strzalka, J.; Zhang, Q.; Toney, M.F.; Rivnay, J. Time-Resolved Structural Kinetics of an Organic Mixed Ionic–Electronic Conductor. Adv. Mater. 2020, 32, 2003404. [Google Scholar] [CrossRef]
- Shi, P.; Chen, R.; Hua, L.; Li, L.; Chen, R.; Gong, Y.; Yu, C.; Zhou, J.; Liu, B.; Sun, G.; et al. Highly Concentrated, Ultrathin Nickel Hydroxide Nanosheet Ink for Wearable Energy Storage Devices. Adv. Mater. 2017, 29, 1703455. [Google Scholar] [CrossRef]
- Kim, C.; Marsland, R.; Blick, R.H. The Nanomechanical Bit. Small 2020, 16, 2001580. [Google Scholar] [CrossRef]
- Wang, L.; Fu, X.; He, J.; Shi, X.; Chen, T.; Chen, P.; Wang, B.; Peng, B. Application Challenges in Fiber and Textile Electronics. Adv. Mater. 2019, 32, 1901971. [Google Scholar] [CrossRef]
- Madéo, J.; Man, M.K.L.; Sahoo, C.; Campbell, M.; Pareek, V.; Wong, E.L.; Al-Mahboob, A.; Chan, N.S.; Karmakar, A.; Mariserla, B.M.K.; et al. Directly visualizing the momentum-forbidden dark excitons and their dynamics in atomically thin semiconductors. Science 2020, 370, 1199–1204. [Google Scholar] [CrossRef]
- Bharanitharan, N.T.; Dhinasekaran, D.; Kishore, M.R.A.; Subramanian, B.; Rajendran, A.R. Rational design of NiCo2O4@carbon hollow spheres as a high-performance electrode material for flexible supercapacitors. Nanoscale 2025, 17, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Park, S.J.; Park, S.-J.; Kim, I.; Park, B.; Kim, S.; Jeong, U.; Jin Kon Kim, K.J.; Yang, C. Deformable micro-supercapacitor fabricated via laser ablation patterning of graphene/liquid metal. npj Flex. Electron. 2024, 8, 18. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, W. General Solution Growth of Mesoporous NiCo2O4 Nanosheets on Various Conductive Substrates as High-Performance Electrodes for Supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, E.; He, W.; Deng, X.; Huang, J.; Ding, M.; Wei, X.; Liu, X.; Xu, X. NiCo2O4-Based Supercapacitor Nanomaterials. Nanomaterials 2017, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Li, X.; Huo, P.; Wang, H. NiCo2O4-based nanostructures for high-performance supercapacitors. Dalton Trans. 2021, 50, 1097–1105. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Z.; Xin, N.; Ying, Y.; Shi, W. High-performance supercapacitor based on highly active P-doped one-dimension/two-dimension hierarchical NiCo2O4/NiMoO4 for efficient energy storage. J. Colloid Interface Sci. 2021, 601, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.B. Light scattering measurements to support real-time monitoring and closed-loop control of aerosol jet printing. Addit. Manuf. 2021, 44, 102028. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, D.; Zhang, C.; Meng, F.; Cao, L.; Lin, H. All-solid-state printable supercapacitors based on bimetallic sulfide NiCo2S4 with in-plane interdigital electrode architecture. J. Mater. Sci. 2022, 57, 19381–19395. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Yao, L.; Li, X.; Wang, H.; Pan, H.; Kim, J.; Wang, L. Printed supercapacitors: Materials, printing and applications. Chem. Soc. Rev. 2020, 49, 3229–3264. [Google Scholar] [CrossRef]
- Zub, K.; Winsberg, J.; Schubert, U.S.; Hoeppene, S. Inkjet-printing of supercapacitors. ChemistrySelect 2020, 5, 12345–12352. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.J. Enhanced electrochemical performance of nanoplate NiCo2O4 for supercapacitor applications. RSC Adv. 2020, 10, 1034–1042. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, D.; Li, Q.; Ma, Y.; Yuan, S.; Xie, L.; Chen, C.; Lu, C. Porous NiCo2O4 nanowires supported on carbon cloth for flexible asymmetric supercapacitor with high energy density. J. Energy Chem. 2018, 27, 195–202. [Google Scholar] [CrossRef]
- Peng, X.; Peng, L.; Wu, C.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2020, 49, 3303–3323. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wei, Y.; Chen, X.; Yu, X.; Chen, L. Hierarchical NiCo2O4 nanostructures for supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 10849–10857. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Yu, G.; Hou, X.; Chen, D.; Shen, G. NiCo2O4 nanowires for flexible supercapacitors. Nano Lett. 2020, 20, 1931–1938. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Yang, Z.; Zhang, J.; Hou, S.; Hao, C.; Zhang, J. Recent advances in flexible supercapacitors. J. Solid State Electrochem. 2022, 26, 2627–2658. [Google Scholar] [CrossRef]
- Li, D.; Raza, F.; Wu, Q.; Zhu, X.; Ju, A. NiCo2O4 Nanosheets on Hollow Carbon Nanofibers for Flexible Solid-State Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 14630–14638. [Google Scholar] [CrossRef]
- Priyadarsini, S.S.; Saxena, S.; Ladole, A.H.; Nehru, D.; Dasgupta, S. Inkjet-Printed Transparent Asymmetric Mi-crosupercapacitors with Mesoporous NiCo2O4 and Mn2O3 Electrodes. ACS Appl. Energy Mater. 2024, 7, 715–725. [Google Scholar] [CrossRef]
- Zhanga, J.-N.; Liua, P.; Jina, C.; Jina, L.-N.; Biana, S.-W.; Zhua, Q.; Wang, B. Flexible three-dimensional carbon cloth/carbon fibers/NiCo2O4 composite electrode materials for high-performance all-solid-state electrochemical ca-pacitors. Electrochim. Electrochim. Acta 2017, 256, 90–99. [Google Scholar] [CrossRef]
- Zhao, R.-D.; Cui, D.; Sheng, H.-P.; Gammer, C.; Wu, F.-F.; Xiang, J. Core-shell structured NiCo2O4@Ni(OH)2 nano-materials with high specific capacitance for hybrid capacitors. Ionics 2021, 27, 1369–1376. [Google Scholar] [CrossRef]
- Sun, J.; Wang, w.; Yu, D. NiCo2O4 Nanosheet-Decorated Carbon Nanofiber Electrodes with High Electrochemical Performance for Flexible Supercapacitors. J. Electron. Mater. 2019, 48, 3833–3843. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Rahmanian, E.; Naseri, N. nkjet-Printing Technology for Supercapacitor Application: Current State and Perspectives. ACS Appl. Mater. Interfaces 2020, 12, 34487–34504. [Google Scholar] [CrossRef]
- Chen, J.; Ma, T.; Chen, M.; Peng, Z.; Feng, Z.; Pan, C.; Zou, H.; Yang, W.; Chen, S. Porous NiCo2O4@Ppy core-shell nanowire arrays covered on carbon cloth for flexible all-solid-state hybrid supercapacitors. J. Energy Storage 2020, 32, 101895. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Ni, G.; Li, Q. Facile Synthesis of NiCo2O4 Nanowire Arrays/Few-Layered Ti3C2-MXene Compo-site as Binder-Free Electrode for High-Performance Supercapacitors. Molecules 2022, 27, 6452. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, D.; Liao, L.; Cui, L.; Zheng, R.; Liu, J. Ti3C2Tx MXene based hybrid electrodes for wearable supercapacitors with varied deformation capabilities. Chem. Eng. J. 2022, 429, 132232. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Yang, B.; Liu, J.; Li, R.; Zhang, H.; Liu, L.; Wang, J. Construction of Three-Dimensional Homogeneous NiCo2O4 Core/Shell Nanostructure as High-Performance Electrodes for Supercapacitors. J. Electrochem. Soc. 2015, 162, E319–E324. [Google Scholar] [CrossRef]
- Wang, Q.; Wanga, X.; Xua, J.; Ouyanga, X.; Houa, X.; Chena, D.; Wang, R.; Shen, G. Flexible coaxial-type fiber supercapacitor based on NiCo2O4 nanosheets electrodes. Nano Energy 2014, 8, 44–51. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, H.; Lu, Y.; Qiu, K.; Wang, C.; Zhang, Y.; Liu, X.; Luo, J.; Luo, Y. NiCo2O4 nanostructure materials: Morphol-ogy control and electrochemical energy storage. Dalton Trans. 2014, 43, 15887–15897. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, A.; Li, Y.; Song, D.; Li, Y.; Cheng, L. Thermal decomposition behavior of polyimide containing flame retardant SiO2 and Mg(OH)2. Polymers 2022, 14, 2791. [Google Scholar] [CrossRef] [PubMed]
- Thorat, J.P.; Nikam, R.P.; Lokhande, V.C.; Lokhande, C.D. Porous NiCo2O4 electrodes for high-energy asymmetric supercapacitor: Effect of annealing. J. Mater. Sci. 2023, 58, 9586–9604. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cha, H.-J.; Park, J.; Son, C.-S.; Son, Y.-G.; Hwang, D. Effect of Annealing Temperature on the Structural and Electrochemical Properties of Hydrothermally Synthesized NiCo2O4 Electrodes. Nanomaterials 2024, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Tsay, K.-C.; Zhang, L.; Zhang, J. Effects of electrode layer composition/thickness and electrolyte concentration on both specific capacitance and energy density of supercapacitor. Electrochim. Acta 2012, 60, 428–436. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Xu, Y.; Lin, Z.; Zhong, X.; Huang, X.; Weiss, N.O.; Huang, Y.; Duan, X. Flexible solid-state supercapacitors based on three-dimensional graphene hydrogel films. ACS Nano 2013, 7, 4042–4049. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Flexible supercapacitors based on carbon nanomaterials. J. Mater. Chem. A 2014, 2, 10756–10775. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.-M. 3D Aperiodic Hierarchical Porous Graphitic Carbon Material for High-Rate Electrochemical Capacitive Energy Storage. Angew. Chem. Int. Ed. 2009, 48, 1515–1526. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Xu, J.; Jaber, F.; Musharavati, F.; Zalnezhad, E.; Bae, S.; Hui, K.S.; Hui, K.N.; Liu, J. Synthesis and Characterization of a NiCo2O4@NiCo2O4 Hierarchical Mesoporous Nanoflake Electrode for Supercapacitor Applications. Nanomaterials 2020, 10, 1292. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R.; Fuierer, P. Mechanical integrity of ceramic coatings on Kapton made by a dry aerosol deposition of lunar mare stimulant. Int. J. Appl. Ceram. Technol. 2023, 20, 395–409. [Google Scholar] [CrossRef]
- Farhan, G.K.; Taha, H. A facile method of deriving solar selective nickel-cobalt oxide thin films via spraying process. Semicond. Sci. Technol. 2024, 39, 115017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).