Phase Change Materials for Thermal Management in Lithium-Ion Battery Packs: A Review
Abstract
1. Introduction
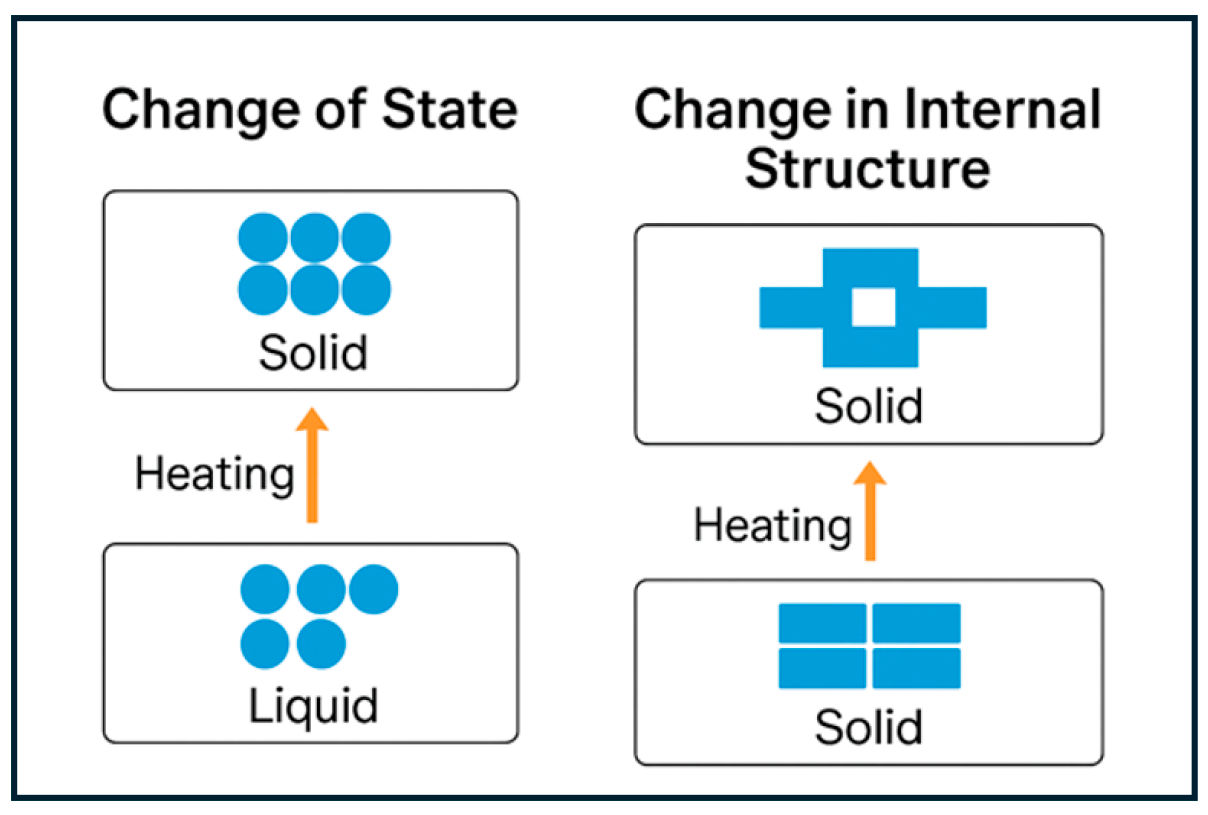
2. Fundamentals of Phase Change Materials
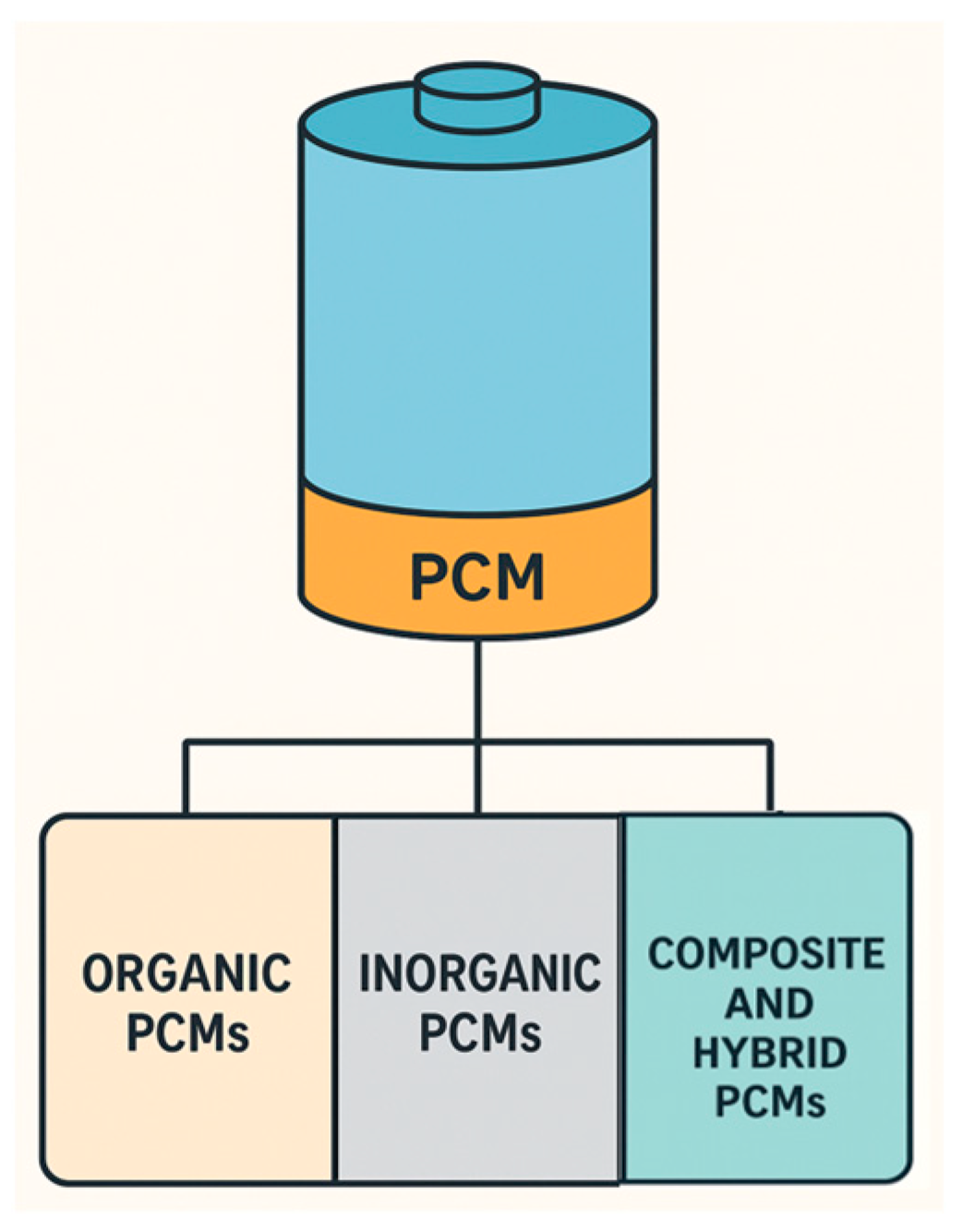
2.1. Basic Principle, Applications, and Types
- 1.
- Organic PCMs—Carbon-based materials that are characterized by their stability in thermal cycling, chemical inactivity, and non-corrosive properties. Organic PCMs have congruently melting and freezing behavior, which means they will melt and solidify without phase segregation [21]. This makes organic PMCs suitable for repeated thermal cycles, as they are very predictable during the two-phase transformations. There are many subtypes of organic PCMs, which can generally be divided into the following: (a) paraffin waxes (e.g., octadecane and hexadecane), which are saturated hydrocarbons and have a wide melting point temperature range depending upon the carbon chain length; and (b) fatty acids (e.g., stearic acid and lauric acid), which are essentially the components from natural fats and oils, and these often have sharp melting points. Organic PCMs are safe, easy to work with, and chemically stable, but they are typically not great for thermal conductivity and are flammable [22].
- 2.
- Inorganic PCMs—Primarily identified as salt hydrates and low-melting metals and alloys. Their notable advantage over organic PCMs is higher thermal conductivity and latent heat storage capacity, and they are generally non-flammable [23]. Types of inorganic PCMs include the following: (a) salt hydrates (calcium chloride hexahydrate and sodium sulfate decahydrate), which are solid and crystalline materials containing water molecules in their structure; and (b) metals and eutectic alloys (gallium and indium-tin), which melt at a specific temperature and provide a rapid thermal response. However, frequent use of these materials can result in phase separation, supercooling, or corrosion [24].
- 3.
- Eutectic PCMs—Prepared by mixing two or more components together as an organic, inorganic, or composite combination, thereby removing erratic melting/freezing points and forming a single, sharp melting/freezing point [20]. This preparation method can lead to phase segregation and compatibility issues over time [25]. These materials are designed to meet the appropriate thermal requirements across a variety of applications. An advantage of this PCM subclass is that you can adjust the phase change temperature to stay within the desired operating range.
2.2. Key Properties for Lithium-Ion Battery Applications
2.3. Advantages of Using PCMs for Li-Ion BTM
2.4. Challenges and Considerations for PCM-Based BTM
3. PCM Materials for Li-Ion Battery Applications
3.1. Organic PCMs
3.2. Inorganic PCMs
3.3. Composite and Hybrid PCMs
3.4. Future Directions in PCM-Based BTM
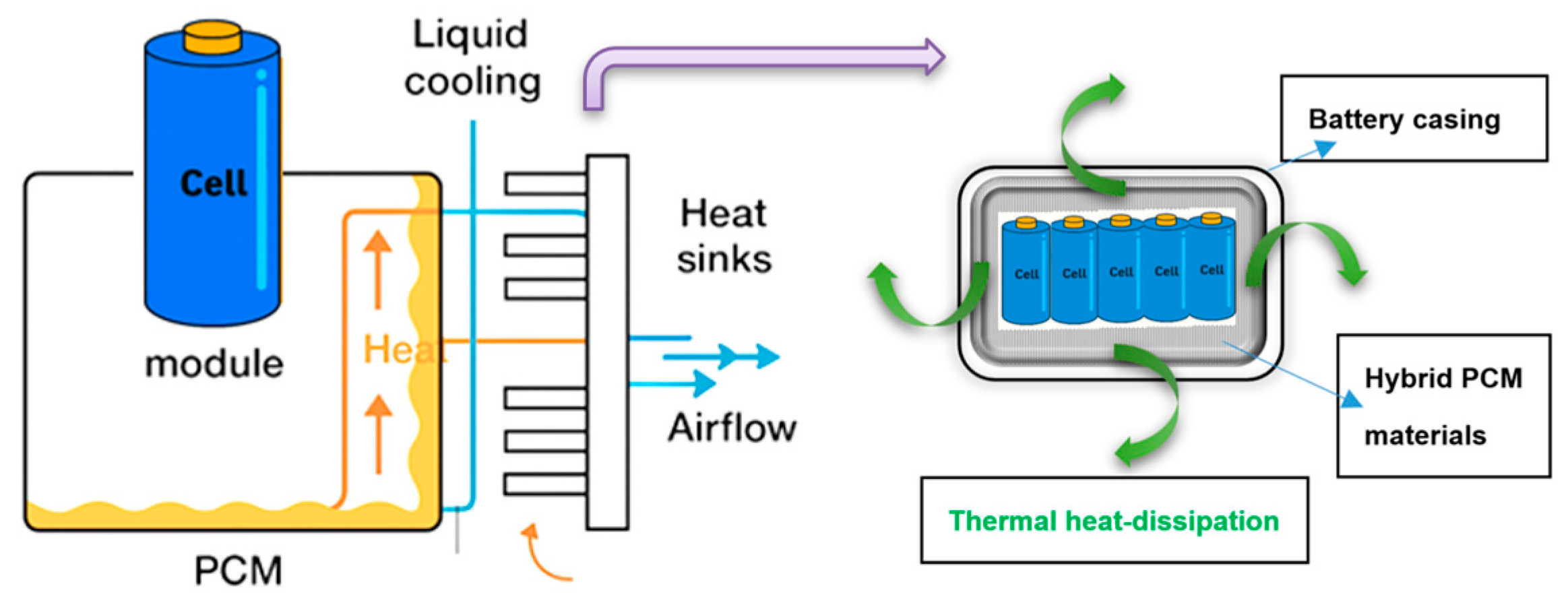
4. Battery–PCM Integration Strategies
4.1. Direct-Contact Integration
4.2. Indirect-Contact Integration
4.3. Hybrid Integration
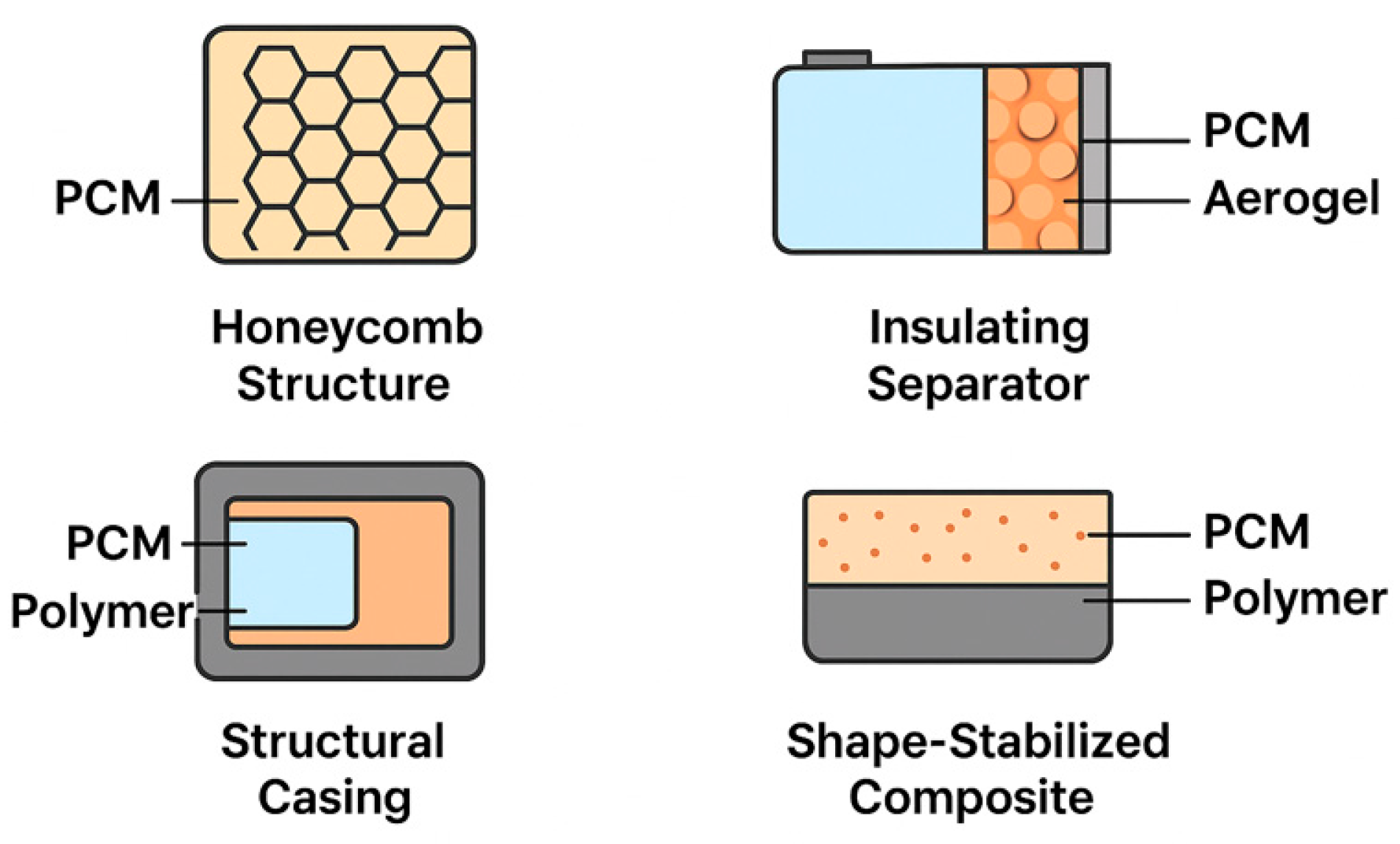
4.4. Structural PCM Integration
4.5. Stability and Safety Enhancements in PCM-Based BTM Systems
5. Performance Metrics and Experimental Evaluation
5.1. Temperature Uniformity: Reduction in Cell-to-Cell Thermal Gradients
5.2. Peak Temperature Suppression: Delays Thermal Runaway Onset
5.3. Thermal Response Time: Speed of Heat Absorption and Dissipation
5.4. Durability and Aging: Long-Term Performance Under Cycling
6. Integration and Application in Battery Systems
7. Challenges and Research Gaps
7.1. Low Thermal Conductivity: Composite Materials Need Optimization
7.2. Material Cost and Scalability: Economic Feasibility for EV-Scale Deployment
7.3. Integration Complexity: Custom Pack Design Required for Efficient Implementation
8. Future Outlook
9. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Afia, S.E.; Cano, A.; Arévalo, P.; Jurado, F. Energy Sources and Battery Thermal Energy Management Technologies for Electrical Vehicles: A Technical Comprehensive Review. Energies 2024, 17, 5634. [Google Scholar] [CrossRef]
- Ahmadian-Elmi, M.; Zhao, P. Review of Thermal Management Strategies for Cylindrical Lithium-Ion Battery Packs. Batteries 2024, 10, 50. [Google Scholar] [CrossRef]
- Khateeb, S.A.; Farid, M.M.; Selman, J.R.; Al-Hallaj, S. Design and Simulation of a Lithium-Ion Battery with a Phase Change Material Thermal Management System for an Electric Scooter. J. Power Sources 2004, 128, 292–307. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent Developments in Phase Change Materials for Energy Storage Applications: A Review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- El Idi, M.M.; Karkri, M.; Kraiem, M. Preparation and effective thermal conductivity of a Paraffin/Metal Foam composite. J. Energy Storage 2021, 33, 102077. [Google Scholar] [CrossRef]
- Joula, M.; Dilibal, S.; Mafratoglu, G.; Danquah, J.O.; Alipour, M. Hybrid Battery Thermal Management System with NiTi SMA and Phase Change Material (PCM) for Li-ion Batteries. Energies 2022, 15, 4403. [Google Scholar] [CrossRef]
- Nistor, C.L.; Gifu, I.C.; Anghel, E.M.; Ianchis, R.; Cirstea, C.-D.; Nicolae, C.A.; Gabor, A.R.; Atkinson, I.; Petcu, C. Novel PEG6000–Silica-MWCNTs Shape-Stabilized Composite Phase-Change Materials (ssCPCMs) for Thermal-Energy Storage. Polymers 2023, 15, 3022. [Google Scholar] [CrossRef]
- Hamad, G.B.; Younsi, Z.; Naji, H.; Salaün, F. A Comprehensive Review of Microencapsulated Phase Change Materials Synthesis for Low-Temperature Energy Storage Applications. Appl. Sci. 2021, 11, 11900. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S. A Review of Power Battery Thermal Energy Management. Renew. Sustain. Energy Rev. 2011, 15, 4554–4571. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, D.; Jiang, L.; Zhang, G.; Wu, H.; Day, R.; Jiang, W. Advanced Thermal Management System Driven by Phase Change Materials for Power Lithium-Ion Batteries: A Review. Renew. Sustain. Energy Rev. 2022, 159, 112207. [Google Scholar] [CrossRef]
- Al-Hallaj, S.; Selman, J.R. Thermal Modeling of Secondary Lithium Batteries for Electric Vehicle/Hybird Electric Vehicle Applications. J. Power Sources 2002, 110, 341–348. [Google Scholar] [CrossRef]
- Ji, C.; Dai, J.; Zhai, C.; Wang, J.; Tian, Y.; Sun, W. A Review on Lithium-Ion Battery Modeling from Mechanism-Based and Data-Driven Perspectives. Processes 2024, 12, 1871. [Google Scholar] [CrossRef]
- Shi, H.; Cheng, M.; Feng, Y.; Qiu, C.; Song, C.; Yuan, N.; Kang, C.; Yang, K.; Yuan, J.; Li, Y. Thermal Management Techniques for Lithium-Ion Batteries Based on Phase Change Materials: A Systematic Review and Prospective Recommendations. Energies 2023, 16, 876. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on Thermal Energy Storage with Phase Change: Materials, Heat Transfer Analysis and Applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Solar Energy Storage Using Phase Change Materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Rashid, F.L.; Al-Obaidi, M.A.; Dulaimi, A.; Mahmood, D.M.N.; Sopian, K. A Review of Recent Improvements, Developments, and Effects of Using Phase-Change Materials in Buildings to Store Thermal Energy. Designs 2023, 7, 90. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Wang, X.; Yang, R.; Lin, K. Influence of Additives on Thermal Conductivity of Shape-Stabilized Phase Change Material. Sol. Energy Mater. Sol. Cells 2006, 90, 1692–1702. [Google Scholar] [CrossRef]
- Sari, A.; Kaygusuz, K. Thermal Energy Storage System Using Stearic Acid as a Phase Change Material. Sol. Energy 2001, 71, 365–376. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in Organic Solid–Liquid Phase Change Materials and Their Applications in Thermal Energy Storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef]
- Abhat, A. Low Temperature Latent Heat Thermal Energy Storage: Heat Storage Materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials Used as PCM in Thermal Energy Storage in Buildings: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Maiti, T.K.; Dixit, P.; Suhag, A.; Bhushan, S.; Yadav, A.; Talapatra, N.; Chattopadhyay, S. Advancements in organic and inorganic shell materials for the preparation of microencapsulated phase change materials for thermal energy storage applications. RSC Sustain. 2023, 1, 665–697. [Google Scholar] [CrossRef]
- Khare, S.; Dell’Amico, M.; Knight, C.; McGarry, S. Selection of Materials for High Temperature Latent Heat Energy Storage. Sol. Energy Mater. Sol. Cells 2012, 107, 20–27. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM Thermal Storage in Buildings: A State of Art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A Review of Materials, Heat Transfer and Phase Change Problem Formulation for Latent Heat Thermal Energy Storage Systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Mondal, S. Phase Change Materials for Smart Textiles—An Overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Baby, R.; Balaji, C. Experimental Investigations on Phase Change Material Based Heat Sinks for Electronic Equipment Cooling. Int. J. Therm. Sci. 2012, 55, 1642–1649. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.J. A Review on Phase Change Materials Integrated in Building Walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal Stability of Phase Change Materials Used in Latent Heat Energy Storage Systems: A Review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Samimi, F.; Babapoor, A.; Azizi, M.; Karimi, G. Thermal management analysis of a Li-ion battery cell using phase change material loaded with carbon fibers. Energy 2016, 96, 355–371. [Google Scholar] [CrossRef]
- Available online: https://thermtest.com/battery-thermal-management-system (accessed on 8 November 2025).
- Ushak, S.; Song, W.; Marín, P.E.; Milian, Y.; Zhao, D.; Grageda, M.; Lin, W.; Chen, M.; Han, Y. A review on phase change materials employed in Li-ion batteries for thermal management systems. Appl. Mater. Today 2024, 37, 102021. [Google Scholar] [CrossRef]
- Nishad, S.; Elmoughni, H.M.; Shakoor, R.A.; Qureshi, Z.A.; Moossa, B.; Krupa, I. A novel design for battery cooling based on highly thermally conductive phase change composites encapsulated by 3D printed polyethylene/boron nitride layer. J. Energy Storage 2025, 112, 115490. [Google Scholar] [CrossRef]
- Bacha, H.B.; Abdullah, A.S.; Essa, F.A.; Omara, Z.M. Energy, Exergy, Economic, and Environmental Prospects of Solar Distiller with Three-Vertical Stages and Thermo-Storing Material. Processes 2023, 11, 3337. [Google Scholar] [CrossRef]
- Rasool, G.; Xinhua, W.; Sun, T.; Hayat, T.; Sheremet, M.; Uddin, A.; Shahzad, H.; Abbas, K.; Razzaq, I.; Yuexin, W. Recent advancements in battery thermal management system (BTMS): A review of performance enhancement techniques with an emphasis on nano-enhanced phase change materials. Heliyon 2024, 10, e36950. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Chen, Y.; Chen, M. Thermal Safety Research of Lithium-Ion Batteries Based on Flame-Retardant Phase Change Materials. Batteries 2025, 11, 50. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Zhang, T.; Jiang, Z.; Ding, J.; Ding, Y. A comprehensive review of composite phase change material based thermal management system for lithium-ion batteries. Renew. Sustain. Energy Rev. 2022, 167, 112667. [Google Scholar] [CrossRef]
- Kizilel, R.; Sabbah, R.; Selman, J.R. Passive Control of Temperature Excursion and Uniformity in High-Energy Li-Ion Battery Packs at High Current and Ambient Temperature. J. Power Sources 2008, 183, 370–375. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, J.; Fang, X.; Zhang, Z.; Xu, T.; Gao, X.; Wang, S. Review on Thermal Management Systems Using Phase Change Materials for Electronic Components, Li-Ion Batteries and Photovoltaic Modules. Renew. Sustain. Energy Rev. 2015, 31, 427–438. [Google Scholar] [CrossRef]
- Wu, W.; Yang, X.; Zhang, G.; Ke, X.; Wang, Z.; Situ, W.; Li, X.; Zhang, J. An experimental study of thermal management system using copper mesh-enhanced composite phase change materials for power battery pack. Energy 2016, 113, 909–916. [Google Scholar] [CrossRef]
- Kisomi, M.K. Thermal Management of Lithium-Ion Batteries: A Comparative Study of Phase Change Materials and Air-Cooling Systems Equipped with Fins. arXiv 2025, arXiv:2503.10244. [Google Scholar] [CrossRef]
- Fu, P.; Zhao, L.; Wang, X.; Sun, J.; Xin, Z. A Review of Cooling Technologies in Lithium-Ion Power Battery Thermal Management Systems for New Energy Vehicles. Processes 2023, 11, 3450. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, Y.; Lv, S.; Ni, H.; Deng, Y.; Yuan, Y. A Review of the Power Battery Thermal Management System with Different Cooling, Heating and Coupling System. Energies 2022, 15, 1963. [Google Scholar] [CrossRef]
- Available online: https://www.kingkatech.com/A-battery-air-cooled-heat-dissipation-id48786547.html (accessed on 12 November 2025).
- Zhao, Y.; Chen, J.; He, W. Design and Performance Evaluation of Liquid-Cooled Heat Dissipation Structure for Lithium Battery Module. Processes 2023, 11, 1769. [Google Scholar] [CrossRef]
- Afzal, A.; Abdul Razak, R.K.; Mohammed Samee, A.D.; Kumar, R.; Ağbulut, Ü.; Park, S.G. A critical review on renewable battery thermal management system using heat pipes. J. Therm. Anal Calorim. 2023, 148, 8403–8442. [Google Scholar] [CrossRef] [PubMed]
- Alsagri, A.S. Approaching a Nearly Zero Energy Building Integrated with PCM by Optimization of Energy Sources. Buildings 2025, 15, 2205. [Google Scholar] [CrossRef]
- Martinez-Albert, M.; Díaz-García, P.; Montava-Seguí, I.; Bou-Belda, E. Experimental Investigation into the Thermal Performance of Personal Cooling Mechanisms. Appl. Sci. 2025, 15, 3296. [Google Scholar] [CrossRef]
- Hyun, S.W.; Kim, J.H.; Shin, D.H. Hybrid PCM–Liquid Cooling System with Optimized Channel Design for Enhanced Thermal Management of Lithium–Ion Batteries. Energies 2025, 18, 4996. [Google Scholar] [CrossRef]
- Rogowski, M.; Fabrykiewicz, M.; Szymański, P.; Andrzejczyk, R. The In-House Method of Manufacturing a Low-Cost Heat Pipe with Specified Thermophysical Properties and Geometry. Appl. Sci. 2023, 13, 8415. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Cen, P.Y.; Chen, Q.; De Cachinho Cordeiro, I.M.; Kong, L.; Lin, P.; Li, A. A Comparative Numerical Study of Lithium-Ion Batteries with Air-Cooling Systems towards Thermal Safety. Fire 2024, 7, 29. [Google Scholar] [CrossRef]
- Sharifi, N.; Shabgard, H.; Millard, C.; Etufugh, U. Hybrid Heat Pipe-PCM-Assisted Thermal Management for Lithium-Ion Batteries. Batteries 2025, 11, 64. [Google Scholar] [CrossRef]
- Ahmad, A.; Navarro, H.; Ghosh, S.; Ding, Y.; Roy, J.N. Evaluation of New PCM/PV Configurations for Electrical Energy Efficiency Improvement through Thermal Management of PV Systems. Energies 2021, 14, 4130. [Google Scholar] [CrossRef]
- Miccoli, F.; Cavargna, A.; Mongibello, L.; Iasiello, M.; Bianco, N. Experimental Characterization and Numerical Simulation of a Low-Scale Personal Cooling System with Integrated PCM. Energies 2024, 17, 1118. [Google Scholar] [CrossRef]
- Rahmani, A.; Dibaj, M.; Akrami, M. Recent Advancements in Battery Thermal Management Systems for Enhanced Performance of Li-Ion Batteries: A Comprehensive Review. Batteries 2024, 10, 265. [Google Scholar] [CrossRef]
- Saber, N.; Richter, C.P.; Unnthorsson, R. Review of Thermal Management Techniques for Prismatic Li-Ion Batteries. Energies 2025, 18, 492. [Google Scholar] [CrossRef]
- Ahmed, Y.E.; Maghami, M.R.; Pasupuleti, J.; Danook, S.H.; Basim Ismail, F. Overview of Recent Solar Photovoltaic Cooling System Approach. Technologies 2024, 12, 171. [Google Scholar] [CrossRef]
- Sun, J.; Dan, D.; Wei, M.; Cai, S.; Zhao, Y.; Wright, E. Pack-Level Modeling and Thermal Analysis of a Battery Thermal Management System with Phase Change Materials and Liquid Cooling. Energies 2023, 16, 5815. [Google Scholar] [CrossRef]
- Hassan, F.; Hussain, A.; Jamil, F.; Arshad, A.; Ali, H.M. Passive Cooling Analysis of an Electronic Chipset Using Nanoparticles and Metal-Foam Composite PCM: An Experimental Study. Energies 2022, 15, 8746. [Google Scholar] [CrossRef]
- Dmitruk, A.; Naplocha, K.; Grzęda, J.; Kaczmar, J.W. Aluminum Inserts for Enhancing Heat Transfer in PCM Accumulator. Materials 2020, 13, 415. [Google Scholar] [CrossRef]
- Dolado, P.; Lazaro, A.; Delgado, M.; Peñalosa, C.; Mazo, J.; Marin, J.M.; Zalba, B. An Approach to the Integrated Design of PCM-Air Heat Exchangers Based on Numerical Simulation: A Solar Cooling Case Study. Resources 2015, 4, 796–818. [Google Scholar] [CrossRef]
- Ahmed, S.E.; Abderrahmane, A.; Alotaibi, S.; Younis, O.; Almasri, R.A.; Hussam, W.K. Enhanced Heat Transfer for NePCM-Melting-Based Thermal Energy of Finned Heat Pipe. Nanomaterials 2022, 12, 129. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q.; Liu, Y.; Lai, B.; Ke, Z.; Wu, W. Investigations of Lithium-Ion Battery Thermal Management System with Hybrid PCM/Liquid Cooling Plate. Processes 2023, 11, 57. [Google Scholar] [CrossRef]
- Górecki, G.; Łęcki, M.; Gutkowski, A.N.; Andrzejewski, D.; Warwas, B.; Kowalczyk, M.; Romaniak, A. Experimental and Numerical Study of Heat Pipe Heat Exchanger with Individually Finned Heat Pipes. Energies 2021, 14, 5317. [Google Scholar] [CrossRef]
- Ren, S.; Han, M.; Fang, J. Personal Cooling Garments: A Review. Polymers 2022, 14, 5522. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Wrobel, L.C.; Hopper, N.; Kolokotroni, M. Numerical Design and Laboratory Testing of Encapsulated PCM Panels for PCM-Air Heat Exchangers. Appl. Sci. 2021, 11, 676. [Google Scholar] [CrossRef]
- Li, K.; Wang, X. Experimental Study on Low-Temperature Thermal Management of Lithium Battery with Pulsating Heat Pipe. World Electr. Veh. J. 2025, 16, 597. [Google Scholar] [CrossRef]
- Karimi, D.; Behi, H.; Akbarzadeh, M.; Van Mierlo, J.; Berecibar, M. A Novel Air-Cooled Thermal Management Approach towards High-Power Lithium-Ion Capacitor Module for Electric Vehicles. Energies 2021, 14, 7150. [Google Scholar] [CrossRef]
- Ganji, M.J.; Agelin-Chaab, M.; Rosen, M.A. Experimental Investigation of Phase Change Material-Based Battery Pack Performance Under Elevated Ambient Temperature. Batteries 2025, 11, 67. [Google Scholar] [CrossRef]
- Diaconu, B.; Cruceru, M.; Anghelescu, L.; Racoceanu, C.; Popescu, C.; Ionescu, M.; Tudorache, A. Latent Heat Storage Systems for Thermal Management of Electric Vehicle Batteries: Thermal Performance Enhancement and Modulation of the Phase Transition Process Dynamics: A Literature Review. Energies 2023, 16, 2745. [Google Scholar] [CrossRef]
- Tahla, M.; Palange, R.; Khan, S.A.; DeBlasio, C. Mitigating thermal runaway in EV batteries using hybrid energy storage and phase change materials. RSC Adv. 2025, 15, 24947–24974. [Google Scholar] [CrossRef]
- Rani, M.G.; Rangasamy, R. Review of phase change material application in thermal management of electric vehicle battery pack. Proc. Inst. Mech. Eng. Part A J. Power Energy 2023, 238, 197–214. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase Change Materials Application in Battery Thermal Management System: A Review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef] [PubMed]
- Katish, M.; Allen, S.; Squires, A.; Ferrándiz-Mas, V. Thermal stability of organic Phase Change Materials (PCMs) by accelerated thermal cycling technique. Thermochim. Acta 2024, 737, 179771. [Google Scholar] [CrossRef]
- Mitra, A.; Kumar, R.; Singh, D.K.; Said, Z. Advances in the improvement of thermal-conductivity of phase change material-based lithium-ion battery thermal management systems: An updated review. J. Energy Storage 2022, 58, 105195. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Gong, Y.; Chen, M. A Novel Paraffin Wax/Expanded Graphite/Bacterial Cellulose Powder Phase Change Materials for the Dependable Battery Safety Management. Batteries 2024, 10, 363. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Lu, Y.; Jiang, B.; Wang, J.; Li, S. An overview of polyethylene glycol composite phase change materials: Preparation, properties and applications. J. Energy Storage 2024, 104 Pt B, 114581. [Google Scholar] [CrossRef]
- Jafaryar, M.; Sheikholeslami, M. Simulation of melting paraffin with graphene nanoparticles within a solar thermal energy storage system. Sci. Rep. 2023, 13, 8604. [Google Scholar] [CrossRef]
- Wang, J.X.; Li, X.; Liu, Y.; Feng, Y.; Xing, Z.; Luo, H.; Yang, J. EG/PCM wrapped around battery pack: A nano-enhanced graphite/PCM composite with high thermal conductivity. Adv. Sci. 2024, 11, 2402190. [Google Scholar] [CrossRef]
- Pilali, E.; Soltani, M.; Hatefi, M.; Shafiei, S.; Salimi, M.; Amidpour, M. Passive thermal management systems with phase change material-based methods for lithium-ion batteries: A state-of-the-art review. J. Power Sources 2025, 632, 236345. [Google Scholar] [CrossRef]
- Balan, A.E.; AL-Sharea, A.; Lavasani, E.J.; Tanasa, E.; Voinea, S.; Dobrica, B.; Stamatin, I. Paraffin-Multilayer Graphene Composite for Thermal Management in Electronics. Materials 2023, 16, 2310. [Google Scholar] [CrossRef]
- Xiao, C.; Zhan, M.; Le, Y. Recent Progress of Phase Change Materials Towards Battery Thermal Management Applications. J. Nucl. Energy Sci. Power Gener. Technol. 2024, 13, 5. [Google Scholar] [CrossRef]
- Budiman, A.C.; Azzopardi, B.; Sudirja; Perdana, M.A.P.; Kaleg, S.; Hadiastuti, F.S.; Hasyim, B.A.; Amin; Ristiana, R.; Muharam, A.; et al. Phase Change Material Composite Battery Module for Thermal Protection of Electric Vehicles: An Experimental Observation. Energies 2023, 16, 3896. [Google Scholar] [CrossRef]
- Huang, J.-B.; Patra, J.; Lin, M.-H.; Ger, M.-D.; Liu, Y.-M.; Pu, N.-W.; Hsieh, C.-T.; Youh, M.-J.; Dong, Q.-F.; Chang, J.-K. A Holey Graphene Additive for Boosting Performance of Electric Double-Layer Supercapacitors. Polymers 2020, 12, 765. [Google Scholar] [CrossRef]
- Murali, S.; Quarles, N.; Zhang, L.L.; Potts, J.R.; Tan, Z.; Lu, Y.; Zhu, Y.; Ruoff, R.S. Volumetric capacitance of compressed activated microwave-expanded graphite oxide (a-MEGO) electrodes. Nano Energy 2013, 2, 764–768. [Google Scholar] [CrossRef]
- Tawiah, B.; Ofori, E.A.; Chen, D.; Ming, Y.; Hou, Y.; Jia, H.; Fei, B. Carbon-Based Thermal Management Solutions and Applications in Li-ion Battery Systems. Batteries 2025, 11, 144. [Google Scholar] [CrossRef]
- Peng, P.; Wang, Y.; Jiang, F. Numerical study of PCM thermal behavior of a novel PCM–heat pipe combined system for Li-ion battery thermal management. Appl. Therm. Eng. 2022, 209, 118293. [Google Scholar] [CrossRef]
- Moaveni, A.; Siavashi, M.; Mousavi, S. Passive and hybrid battery thermal management by cooling flow control, employing nano-PCM, fins, and metal foam. Energy 2024, 288, 129809. [Google Scholar] [CrossRef]
- Wang, T.; Deng, J.; Du, J.; Yang, W.; Zeng, Y.; Wu, T.; Rao, Z.; Li, X. Investigation on the polyethylene glycol based composite phase change materials with coating flame-retardant for battery thermal management. Case Stud. Therm. Eng. 2025, 65, 105616. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, Y.; Zhang, N.; Ju, F.; Yuan, Y. Functionalized Polyethylene Glycol Composite Phase Change Materials: A Review. Adv. Eng. Mater. 2025, 27, 1430. [Google Scholar] [CrossRef]
- Nasiri, M.; Hadim, H. Thermal management of Li-ion batteries using phase change materials: Recent advances and future challenges. J. Energy Storage 2025, 111, 115440. [Google Scholar] [CrossRef]
- Jing, Y.; Zhao, Z.; Cao, X.; Sun, Q.; Yuan, Y.; Li, T. Ultraflexible, cost-effective and scalable polymer-based phase change composites via chemical cross-linking for wearable thermal management. Nat. Commun. 2023, 14, 8060. [Google Scholar] [CrossRef]
- Pereira, J.; Moita, A.; Moreira, A. An Overview of the Nano-Enhanced Phase Change Materials for Energy Harvesting and Conversion. Molecules 2023, 28, 5763. [Google Scholar] [CrossRef]
- Tai, L.D.; Lee, M.-Y. Advances in the Battery Thermal Management Systems of Electric Vehicles for Thermal Runaway Prevention and Suppression. Batteries 2025, 11, 216. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Xu, Y.; Xie, Y.; Hu, T.; Tao, P. Carbon-Enhanced Hydrated Salt Phase Change Materials for Thermal Management Applications. Nanomaterials 2024, 14, 1077. [Google Scholar] [CrossRef]
- Gómez Díaz, K.Y.; De León Aldaco, S.E.; Aguayo Alquicira, J.; Ponce Silva, M.; Portillo Contreras, S.; Sánchez Vargas, O. Thermal Management Systems for Lithium-Ion Batteries for Electric Vehicles: A Review. World Electr. Veh. J. 2025, 16, 346. [Google Scholar] [CrossRef]
- Kong, W.; Dannemand, M.; Johansen, J.B.; Fan, J.; Dragsted, J.; Englmair, G.; Furbo, S. Experimental investigations on heat content of supercooled sodium acetate trihydrate by a simple heat loss method. Sol. Energy 2016, 139, 249–257. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Li, J.; Guo, L.; Fang, M. Review of Encapsulated Salt Hydrate Core-Shell Phase Change Materials. KONA Powder Part. J. 2020, 37, 85–96. [Google Scholar] [CrossRef]
- Hirschey, J.; Goswami, M.; Akamo, D.O.; Kumar, N.; Li, Y.; LaClair, T.J.; Gluesenkamp, K.R.; Graham, S. Effect of expanded graphite on the thermal conductivity of sodium sulfate decahydrate (Na2SO4·10H2O) phase change composites. J. Energy Storage 2022, 52, 104949. [Google Scholar] [CrossRef]
- Yadav, A.; Samykano, M.; Pandey, A.K.; Suraparaju, S.K.; Natarajan, S.K.; Ponnambalam, S.G. Advanced nano-graphite-infused salt-hydrated phase change materials derived from recycled waste for enhancing thermal energy storage with exceptional thermal stability. Therm. Sci. Eng. Prog. 2025, 62, 103621. [Google Scholar] [CrossRef]
- Karimi, D.; Behi, H.; Mierlo, J.V.; Berecibar, M. An Experimental Study on Thermal Performance of Graphite-Based Phase-Change Materials for High-Power Batteries. Energies 2022, 15, 2515. [Google Scholar] [CrossRef]
- Available online: https://batteryuniversity.com/article/bu-210a-why-does-sodium-sulfur-need-to-be-heated#:~:text=Sodium%20batteries%2C%20also%20known%20as,473%E2%80%93662%C2%B0F%29%20temperature (accessed on 8 November 2025).
- Lak, S.N.; Hsieh, C.-M.; Almahbobi, L.; Wang, Y.; Chakraborty, A.; Yu, C.; Pentzer, E.B. Printing Composites with Salt Hydrate Phase Change Materials for Thermal Energy Storage. ACS Appl. Eng. Mater. 2023, 1, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Suchorowiec, K.; Paprota, N.; Pielichowska, K. Aerogels for Phase-Change Materials in Functional and Multifunctional Composites: A Review. Materials 2024, 17, 4405. [Google Scholar] [CrossRef]
- Saudi, M.K.; Emam, M.; Hassan, H.; Sekiguchi, H.; Khalil, A.S.G. Enhancing thermal management of lithium-ion batteries using phase change materials and expanded graphite: An experimental study. J. Energy Storage 2025, 130, 117427. [Google Scholar] [CrossRef]
- Güler, O.; Yazıcı, M.Y. Electrolytic Ni-P and Ni-P-Cu Coatings on PCM-Loaded Expanded Graphite for Enhanced Battery Thermal Management with Mechanical Properties. Materials 2025, 18, 213. [Google Scholar] [CrossRef]
- Yu, X.K.; Tao, Y.B. Improvement of thermal cycle stability of paraffin/expanded graphite composite phase change materials and its application in thermal management. J. Energy Storage 2023, 63, 107019. [Google Scholar] [CrossRef]
- Adnin, R.J.; Lee, H.-S. Advancing Thermal Energy Storage: Synthesis and Thermal Performance of Silica-Encapsulated Paraffin PCMs. Molecules 2025, 30, 1698. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, L.; Hu, X.; Hu, P.; Li, M.; Zhang, X.; Wang, J. Aerogel-Functionalized Phase Change Materials toward Lightweight and Robust Thermal Management. Small Methods 2025, 9, e2500127. [Google Scholar] [CrossRef]
- Talluri, T.; Kim, T.H.; Shin, K.J. Analysis of a Battery Pack with a Phase Change Material for the Extreme Temperature Conditions of an Electrical Vehicle. Energies 2020, 13, 507. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Fan, Y.; Yu, Z.; Pan, W. A Review of Composite Phase Change Materials Used in Battery Thermal Management Systems. J. Energy Storage 2025, 112, 115579. [Google Scholar] [CrossRef]
- Garud, K.S.; Tai, L.D.; Hwang, S.-G.; Nguyen, N.-H.; Lee, M.-Y. A Review of Advanced Cooling Strategies for Battery Thermal Management Systems in Electric Vehicles. Symmetry 2023, 15, 1322. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, S.; Li, T.; Yang, L.; Li, D.; Bai, H.; Wang, X. Review on Thermal Properties with Influence Factors of Solid–Liquid Organic Phase-Change Micro/Nanocapsules. Energies 2024, 17, 604. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.J.; Stamatiou, A.; Worlitschek, J. Synthesis and Investigation of Thermal Properties of Highly Pure Carboxylic Fatty Esters to Be Used as PCM. Appl. Sci. 2018, 8, 1069. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Hamze, S.; Fal, J.; Marcos, M.A.; Estellé, P.; Żyła, G. Thermal and Physical Characterization of PEG Phase Change Materials Enhanced by Carbon-Based Nanoparticles. Nanomaterials 2020, 10, 1168. [Google Scholar] [CrossRef]
- Zbair, M.; Bennici, S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies 2021, 14, 3105. [Google Scholar] [CrossRef]
- Mabrouk, R.; Naji, H.; Dhahri, H. Numerical Investigation of Metal Foam Pore Density Effect on Sensible and Latent Heats Storage through an Enthalpy-Based REV-Scale Lattice Boltzmann Method. Processes 2021, 9, 1165. [Google Scholar] [CrossRef]
- Thalmaier, G.; Cobîrzan, N.; Sechel, N.A.; Vida-Simiti, I. Paraffin Graphite Composite Spheres for Thermal Energy Management. Materials 2025, 18, 1482. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, S.; Liu, Y. The Effect of Expanded Graphite Content on the Thermal Properties of Fatty Acid Composite Materials for Thermal Energy Storage. Molecules 2024, 29, 3146. [Google Scholar] [CrossRef]
- Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5382287 (accessed on 8 November 2025).
- Khlissa, F.; Mhadhbi, M.; Aich, W.; Hussein, A.K.; Alhadri, M.; Selimefendigil, F.; Öztop, H.F.; Kolsi, L. Recent Advances in Nanoencapsulated and Nano-Enhanced Phase-Change Materials for Thermal Energy Storage: A Review. Processes 2023, 11, 3219. [Google Scholar] [CrossRef]
- Mika, Ł.; Radomska, E.; Sztekler, K.; Gołdasz, A.; Zima, W. Review of Selected PCMs and Their Applications in the Industry and Energy Sector. Energies 2025, 18, 1233. [Google Scholar] [CrossRef]
- Yang, G.; Yim, Y.-J.; Lee, J.W.; Heo, Y.-J.; Park, S.-J. Carbon-Filled Organic Phase-Change Materials for Thermal Energy Storage: A Review. Molecules 2019, 24, 2055. [Google Scholar] [CrossRef]
- Radouane, N. A Comprehensive Review of Composite Phase Change Materials (cPCMs) for Thermal Management Applications, Including Manufacturing Processes, Performance, and Applications. Energies 2022, 15, 8271. [Google Scholar] [CrossRef]
- Said, Z.; Pandey, A.K.; Tiwari, A.K.; Kalidasan, B.; Jamil, F.; Thakur, A.K.; Tyagi, V.V.; Sarı, A.; Ali, H.M. Nano-Enhanced Phase Change Materials: Fundamentals and Applications. Prog. Energy Combust. Sci. 2024, 104, 101162. [Google Scholar] [CrossRef]
- Leong, K.Y.; Abdul Rahman, M.R.; Gurunathan, B.A. Nano-Enhanced Phase Change Materials: A Review of Thermo-Physical Properties, Applications and Challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, G.; Li, P.; Xie, Z.; Li, Y.; Luo, T. Cooling Performance of a Nano Phase Change Material Emulsions-Based Liquid Cooling Battery Thermal Management System for High-Capacity Square Lithium-Ion Batteries. Fire 2024, 7, 371. [Google Scholar] [CrossRef]
- Available online: https://ctherm.com/resources/tech-library/thermal-evaluation-of-lithium%E2%80%90ion-battery-modules/ (accessed on 13 November 2025).
- Wen, T.; Zhou, Z.; Zhang, Y.; Xu, X. Advances and Challenges in the Battery Thermal Management Systems of Electric Vehicles. Materials 2025, 18, 4718. [Google Scholar] [CrossRef]
- Paciolla, P.; Papurello, D. Improved Thermal Management of Li-Ion Batteries with Phase-Change Materials and Metal Fins. Batteries 2024, 10, 190. [Google Scholar] [CrossRef]
- Al-Rashed, A.A.A.A. Thermal Management of Lithium-Ion Batteries with Simultaneous Use of Hybrid Nanofluid and Nano-Enhanced Phase Change Material: A Numerical Study. J. Energy Storage 2022, 46, 103730. [Google Scholar] [CrossRef]
- Togun, H.; Basem, A.; Jweeg, M.J.; Anqi, A.E.; Alshamkhani, M.T.; Chattopadhyay, A.; Sharma, B.K.; Niyas, H.; Biswas, N.; Sadeq, A.M.; et al. Revolutionizing Battery Thermal Management: Hybrid Nanofluids and PCM in Cylindrical Pack Cooling. Mater. Renew. Sustain. Energy 2025, 14, 42. [Google Scholar] [CrossRef]
- Saeedipour, S.; Gharehghani, A.; Ahbabi Saray, J.; Andwari, A.M.; Mikulski, M. Proposing a Hybrid Thermal Management System Based on Phase Change Material/Metal Foam for Lithium-Ion Batteries. World Electr. Veh. J. 2023, 14, 240. [Google Scholar] [CrossRef]
- Bozorg, M.V.; Torres, J.F. Multifaceted thermal regulation in electrochemical batteries using cooling channels and foam-embedded phase change materials. arXiv 2024, arXiv:2407.15040. [Google Scholar] [CrossRef]
- Li, Z.; Cao, F.; Zhang, Y.; Zhang, S.; Tang, B. Enhancing Thermal Protection in Lithium Batteries with Power Bank-Inspired Multi-Network Aerogel and Thermally Induced Flexible Composite Phase Change Material. Nano-Micro Lett. 2025, 17, 166. [Google Scholar] [CrossRef]
- Grosu, Y.; Zhao, Y.; Giacomello, A.; Meloni, S.; Dauvergne, J.-L.; Nikulin, A.; Palomo, E.; Ding, Y.; Faik, A. Hierarchical macro-nanoporous metals for leakage-free high-thermal conductivity shape-stabilized phase change materials. arXiv 2020, arXiv:2005.01585. [Google Scholar] [CrossRef]
- Wei, D.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; El Azab, I.H.; Sheng, X.; Huang, M.; El-Bahy, Z.M.; Huang, J. Development of novel biomass hybrid aerogel supported composite phase change materials with improved light-thermal conversion and thermal energy storage capacity. Adv. Compos. Hibrid Mater. 2022, 5, 1910–1921. [Google Scholar] [CrossRef]
- Boonma, K.; Patimaporntap, N.; Mbulu, H.; Trinuruk, P.; Ruangjirakit, K.; Laoonual, Y.; Wongwises, S. A Review of the Parameters Affecting a Heat Pipe Thermal Management System for Lithium-Ion Batteries. Energies 2022, 15, 8534. [Google Scholar] [CrossRef]
- Menale, C.; Mancino, A.N.; Vellucci, F.; Bubbico, R. Solid Foam Insertion to Increase PCM-Based Thermal Energy Storage System Efficiency: Experimental Test and Numerical Simulation of Spherical Macrocapsules. Appl. Sci. 2024, 14, 3326. [Google Scholar] [CrossRef]
- Lyu, Z.; Su, J.; Li, Z.; Li, X.; Yan, H.; Chen, L. A Compact Hybrid Battery Thermal Management System for Enhanced Cooling. arXiv 2024, arXiv:2412.00999. [Google Scholar] [CrossRef]
- Dilbaz, F.; Selimefendigil, F.; Öztop, H.F. Comparisons of Different Cooling Systems for Thermal Management of Lithium-Ion Battery Packs: Phase Change Material, Nano-Enhanced Channel Cooling and Hybrid Method. J. Energy Storage 2024, 90 Pt A, 111865. [Google Scholar] [CrossRef]
- Available online: https://www.fva.rwth-aachen.de/en/2020/01/02/batteriekuehlung (accessed on 8 November 2025).
- Hadded, M.H.; Dardouri, S.; Yüksel, A.; Sghaier, J.; Arıcı, M. Enhancing Energy Efficiency and Thermal Comfort Through Integration of PCMs in Passive Design: An Energetic, Environmental, and Economic (3E) Analysis. Buildings 2025, 15, 3319. [Google Scholar] [CrossRef]
- Wazeer, A.; Das, A.; Abeykoon, C.; Sinha, A.; Karmakar, A. Phase Change Materials for Battery Thermal Management of Electric and Hybrid Vehicles: A Review. Energy Nexus 2022, 7, 100131. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Zhang, J.; Lin, L.; Shi, J. A Review of Composite Phase Change Materials Based on Biomass Materials. Polymers 2022, 14, 4089. [Google Scholar] [CrossRef]
- Xu, X.; Su, Y.; Kong, J.; Chen, X.; Wang, X.; Zhang, H.; Zhou, F. Performance Analysis of Thermal Management Systems for Prismatic Battery Module with Modularized Liquid-Cooling Plate and PCM-Negative Poisson’s Ratio Structural Laminboard. Energy 2024, 286, 129620. [Google Scholar] [CrossRef]
- Li, K.; Yao, X.; Li, Z.; Gao, T.; Zhang, W.; Liao, Z.; Ju, X.; Xu, C. Thermal Management of Li-Ion Batteries with Passive Thermal Regulators Based on Composite PCM Materials. J. Energy Storage 2024, 89, 111661. [Google Scholar] [CrossRef]
- Available online: https://www.evsahihai.com/electric-vehicle-battery-safety (accessed on 8 November 2025).
- Zhi, M.; Fan, R.; Yang, X.; Zheng, L.; Yue, S.; Liu, Q.; He, Y. Recent Research Progress on Phase Change Materials for Thermal Management of Lithium-Ion Batteries. J. Energy Storage 2022, 45, 103694. [Google Scholar] [CrossRef]
- Tang, A.; Pan, J.; Xia, D.; Cai, T.; Zhang, Q.; Tenkolu, G.A.; Jin, Y. Characterization and Experimental Assessment of Hybrid Cooling Strategy for Lithium-Ion Batteries by Integrating Microencapsulated Phase Change Materials. Int. J. Heat Mass Transf. 2024, 224, 125389. [Google Scholar] [CrossRef]
- Ghufran, M.; Huitink, D. Advances in Encapsulated Phase Change Materials for Integration in Thermal Management Applications. Emerg. Mater. 2025, 1–32. [Google Scholar] [CrossRef]
- Sung, N.; Zheng, L.; Wang, P.; Ahmed, F. Cooling-Guide Diffusion Model for Battery Cell Arrangement. arXiv 2024, arXiv:2403.10566. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Li, Y.; Wang, P. Physics-informed Machine Learning for Battery Pack Thermal Management. arXiv 2024, arXiv:2411.09915. [Google Scholar] [CrossRef]
- Subramani, T.; Bartscher, S. Predictive Digital Twins for Thermal Management Using Machine Learning and Reduced-Order Models. arXiv 2025, arXiv:2505.06849. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Song, T.; Zhao, X.; Zhang, Y.; Zhao, S. Optimization of Battery Thermal Management for Real Vehicles via Driving Condition Prediction Using Neural Networks. Batteries 2025, 11, 224. [Google Scholar] [CrossRef]
- Qi, S.; Cheng, Y.; Li, Z.; Wang, J.; Li, H.; Zhang, C. Advanced Deep Learning Techniques for Battery Thermal Management in New Energy Vehicles. Energies 2024, 17, 4132. [Google Scholar] [CrossRef]
- Alawi, A.; Saeed, A.; Sharqawy, M.H.; Al Janaideh, M. A Comprehensive Review of Thermal Management Challenges and Safety Considerations in Lithium-Ion Batteries for Electric Vehicles. Batteries 2025, 11, 275. [Google Scholar] [CrossRef]
- Amiri, M.N.; Håkansson, A.; Burheim, O.S.; Lamb, J.J. Lithium-Ion Battery Digitalization: Combining Physics-Based Models and Machine Learning. Renew. Sustain. Energy Rev. 2024, 200, 114577. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Z.; Li, J.; Cao, X. Performance Analysis and Prediction of Hybrid Battery Thermal Management System Integrating PCM with Air Cooling Based on Machine Learning Algorithm. Appl. Therm. Eng. 2024, 257 Pt C, 124474. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.M.; Irfan, M. Thermal Performance Enhancement of Lithium-Ion Batteries Using Phase Change Material and Fin Geometry Modification. World Electr. Veh. J. 2024, 15, 42. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Zhu, M.; Zhao, L.; Chen, Y.; Chen, M. Experimental Investigation on the Thermal Management for Lithium-Ion Batteries Based on the Novel Flame Retardant Composite Phase Change Materials. Batteries 2023, 9, 378. [Google Scholar] [CrossRef]
- Lokhande, I.K.; Tiwari, N. Experimental and numerical investigation of phase change material filled mini cavity cooling for thermal management of high capacity lithium ion pouch cell. J. Power Sources 2026, 661, 238553. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, D.; Peng, Y.; Li, M.; Wang, B.; Cao, B.; Yang, L. Experimental study on the thermal management performance of phase change material module for the large format prismatic lithium-ion battery. Energy 2022, 238, 122081. [Google Scholar] [CrossRef]
- Pra, F.; Al Koussa, J.; Ludwig, S.; De Servi, C.M. Experimental and Numerical Investigation of the Thermal Performance of a Hybrid Battery Thermal Management System for an Electric Van. Batteries 2021, 7, 27. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, S.; Wang, G.; Weng, J.; Ouyang, D.; Wu, X.; Zhao, L.; Wang, J. Experimental Analysis on the Thermal Management of Lithium-Ion Batteries Based on Phase Change Materials. Appl. Sci. 2020, 10, 7354. [Google Scholar] [CrossRef]
- Grimonia, E.; Andhika, M.R.C.; Aulady, M.F.N.; Rubi, R.V.C.; Hamidah, N.L. Thermal Management System Using Phase Change Material for Lithium-ion Battery. J. Phys. Conf. Ser. 2021, 2117, 012005. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Cheng, W.-L.; Zhao, R. Thermal management of Li-ion battery pack with the application of flexible form-stable composite phase change materials. Energy Convers. Manag. 2019, 182, 9–20. [Google Scholar] [CrossRef]
- Wang, B.; Jiao, C.; Zhang, S. Numerical Improvement of Battery Thermal Management Integrating Phase Change Materials with Fin-Enhanced Liquid Cooling. Energies 2025, 18, 2406. [Google Scholar] [CrossRef]
- Zhu, L.; Li, D.; Wu, Z. Research on Composite Liquid Cooling Technology for the Thermal Management System of Power Batteries. World Electr. Veh. J. 2025, 16, 74. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Zhou, T.; Wang, H.; Li, S.; Yuan, Y.; Ma, Z.; Wei, J.; Zhao, X. Experimental and Numerical Investigations of a Thermal Management System Using Phase-Change Materials and Forced-Air Cooling for High-Power Li-Ion Battery Packs. Batteries 2023, 9, 153. [Google Scholar] [CrossRef]
- Huang, Q.; Zhong, Z.; Li, X.; Zhang, G.; Wei, D.; Yuan, W.; Zhang, J.; Zhou, D. Experimental and Numerical Investigation on an Integrated Thermal Management System for the Li-Ion Battery Module with Phase Change Material. Int. J. Photoenergy 2020, 20204, 695419. [Google Scholar] [CrossRef]
- Gandhi, M.; Kumar, A.; Elangovan, R.; Meena, C.S.; Kulkarni, K.S.; Kumar, A.; Bhanot, G.; Kapoor, N.R. A Review on Shape-Stabilized Phase Change Materials for Latent Energy Storage in Buildings. Sustainability 2020, 12, 9481. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, X.; Wang, C.; Xu, Y. Research on Experimental and Simulated Temperature Control Performance of Power Batteries Based on Composite Phase Change Materials. World Electr. Veh. J. 2024, 15, 302. [Google Scholar] [CrossRef]
- Sabbah, R.; Kizilel, R.; Selman, J.R.; Al-Hallaj, S. Active (air-cooled) vs. passive (phase change material) thermal management of high power lithium-ion packs: Limitation of temperature rise and uniformity of temperature distribution. J. Power Sources 2008, 182, 630–638. [Google Scholar] [CrossRef]
- Joshi, S.; Velumani, D.; Bansal, A. Thermal Management of a Lithium-Ion Battery Pack with Paraffin as PCM. In Proceedings of Fluid Mechanics and Fluid Power (FMFP) 2023, Vol. 5. FMFP 2023; Arun, K.R., Rajesh, G., Arakeri, J.H., Kothadia, H., Eds.; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2025. [Google Scholar] [CrossRef]
- Masood, U.; Haggag, M.; Hassan, A.; Laghari, M. A Review of Phase Change Materials as a Heat Storage Medium for Cooling Applications in the Built Environment. Buildings 2023, 13, 1595. [Google Scholar] [CrossRef]
- Kumar, S.S.; Rao, G.A.P. Recent progress on battery thermal management with composite phase change materials. Energy Storage 2024, 6, e647. [Google Scholar] [CrossRef]
- Kizilel, R.; Sabbah, R.; Selman, J.R.; Al-Hallaj, S. An Alternative Cooling System to Enhance the Safety of Li-Ion Battery Packs. J. Power Sources 2009, 194, 1105–1112. [Google Scholar] [CrossRef]
- Arslan, B.; Ilbas, M. Experimental and Numerical Investigation of Macroencapsulated Phase Change Materials for Thermal Energy Storage. Materials 2024, 17, 2804. [Google Scholar] [CrossRef]
- Singh, R.; Sadeghi, S.; Shabani, B. Thermal Conductivity Enhancement of Phase Change Materials for Low-Temperature Thermal Energy Storage Applications. Energies 2019, 12, 75. [Google Scholar] [CrossRef]
- Xia, Z.; Li, C.; Yu, H.; Wang, Z. Experimental Study of a Passive Thermal Management System Using Expanded Graphite/Polyethylene Glycol Composite for Lithium-Ion Batteries. Energies 2023, 16, 7786. [Google Scholar] [CrossRef]
- Devshette, A.R.; Hole, J.A.; Arakerimath, R.R.; Kumar, A.; Rathore, S.S. Air-cooled and PCM-cooled battery thermal management systems of an electric vehicle: A technical review. Eng. Res. Express 2025, 7, 022502. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Xu, X.; Kong, J.; Song, Z.; Wang, X.; Zhou, F. Thermal performance of a hybrid cooling plate integrated with microchannels and PCM. Appl. Therm. Eng. 2024, 236 Pt D, 121917. [Google Scholar] [CrossRef]
- Ghanbarpour, A.; Hosseini, M.J.; Ranjbar, A.A.; Rahimi, M.; Bahrampoury, R.; Ghanbarpour, M. Evaluation of heat sink performance using PCM and vapor chamber/heat pipe. Renew. Energy 2021, 163, 698–719. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Nada, S.; Hassan, H. Performance study of building cooling system composed of photovoltaic panels, phase change material, and thermoelectric cooler: Impact of its orientation. Int. J. Air-Cond. Refrig. 2025, 33, 3. [Google Scholar] [CrossRef]
- Available online: https://www.nxp.com/company/about-nxp/smarter-world-blog/BL-AUTOMOTIVE-SAFETY-EVOLUTION (accessed on 10 November 2025).
- Elshaer, A.M.; Soliman, A.M.A.; Kassab, M.; Hawwash, A.A. Boosting the Thermal Management Performance of a PCM-Based Module Using Novel Metallic Pin Fin Geometries: Numerical Study. Sci. Rep. 2023, 13, 10955. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Xi, L.; Li, Y.; Gao, J. A Review of Thermal Management and Heat Transfer of Lithium-Ion Batteries. Energies 2024, 17, 3873. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, L.; Li, Z. Research progress on carbon aerogel composite phase-change energy storage materials. Carbon 2025, 244, 120725. [Google Scholar] [CrossRef]
- Duan, J.; Xiong, Y.; Yang, D. Melting Behavior of Phase Change Material in Honeycomb Structures with Different Geometrical Cores. Energies 2019, 12, 2920. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.S. Phase Change Material (PCM) Composite Supported by 3D Cross-Linked Porous Graphene Aerogel. Materials 2022, 15, 4541. [Google Scholar] [CrossRef]
- Pielichowska, K.; Szatkowska, M.; Pielichowski, K. Thermal Energy Storage in Bio-Inspired PCM-Based Systems. Energies 2025, 18, 3548. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, M.; Zhang, S.; Ouyang, D.; Weng, J.; Wei, R.; Chen, Y.; Zhao, L.; Wang, J. Experimental investigation on mitigation of thermal runaway propagation of lithium-ion battery module with flame retardant phase change materials. Appl. Therm. Eng. 2023, 235, 121401. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, M.; Zhao, L.; Chen, Y. Study on Thermal Runaway Propagation Inhibition of Battery Module by Flame-Retardant Phase Change Material Combined with Aerogel Felt. Appl. Energy 2024, 367, 123394. [Google Scholar] [CrossRef]
- Sarcinella, A.; Frigione, M. Selection of PEG-Matrix Combinations to Achieve High Performance Form-Stable Phase Change Materials for Building Applications. Coatings 2024, 14, 250. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. A Summary Review on Experimental Studies for PCM Building Applications: Towards Advanced Modular Prototype. Energies 2022, 15, 1459. [Google Scholar] [CrossRef]
- Chen, P.; Wu, T.; Wu, Z.; Wang, C.; Kong, Z. Biomass Aerogel with Humidity Sensitive for Thermal Runaway Suppression of Battery Modules and Flame-Retardant Application. Energy 2024, 311, 133170. [Google Scholar] [CrossRef]
- Deng, Q.; Liu, Q.; Nian, Y.-L.; Zhao, R.; Cheng, W.-L. A novel flexible composite phase change material with enhanced toughness and shape stability for battery thermal management. J. Energy Storage 2023, 72, 108701. [Google Scholar] [CrossRef]
- Musa, A.A.; Bello, A.; Adams, S.M.; Onwualu, A.P.; Anye, V.C.; Bello, K.A.; Obianyo, I.I. Nano-Enhanced Polymer Composite Materials: A Review of Current Advancements and Challenges. Polymers 2025, 17, 893. [Google Scholar] [CrossRef]
- Rahmani, A.; Dibaj, M.; Akrami, M. Enhancing Battery Pack Cooling Efficiency Through Graphite-Integrated Hybrid-Battery Thermal Management Systems. Batteries 2025, 11, 113. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, C.; Rao, Z. Investigation on the Cooling and Temperature Uniformity of Power Battery Pack Based on Gradient Phase Change Materials Embedded Thin Heat Sinks. Appl. Therm. Eng. 2020, 174, 115304. [Google Scholar] [CrossRef]
- Ren, H.; Yin, L.; Dang, C.; Wu, S.; Jia, L.; Yang, L. Experimental Investigation on Battery Thermal Management Using Phase Change Materials with Different Arrangement Schemes. Appl. Therm. Eng. 2024, 255, 123991. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, B.; Zhu, Y.; Liu, Z.; Liu, Y.; Tao, C.; Gong, L. Enhancement of Heat Transfer for Metallic Honeycomb Cores and Phase Change Materials in Battery Thermal Management Systems. J. Energy Storage 2025, 136, 118354. [Google Scholar] [CrossRef]
- Yang, T.; Su, S.; Xin, Q.; Zeng, J.; Zhang, H.; Zeng, X.; Xiao, J. Thermal Management of Lithium-Ion Batteries Based on Honeycomb-Structured Liquid Cooling and Phase Change Materials. Batteries 2023, 9, 287. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Li, D.; Zuo, X.; Yang, H. Multifunctional composite phase change materials: Preparation, enhanced properties and applications. Compos. Part A Appl. Sci. Manuf. 2024, 185, 108331. [Google Scholar] [CrossRef]
- Mu, B.; Li, M. Fabrication and Thermal Properties of Tetradecanol/Graphene Aerogel Form-Stable Composite Phase Change Materials. Sci. Rep. 2018, 8, 8878. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Deng, Y.; Gao, L.; Bai, J.; Xu, L.; Chen, J.; Yuan, Z. Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid-Expanded Graphite in Spherical Thermal Energy Storage Unit. Energies 2023, 16, 4527. [Google Scholar] [CrossRef]
- Huang, D.; Ma, G.; Yu, Z.; Lv, P.; Zhou, Q.; Liu, Q.; Peng, C.; Xiong, F.; Huang, Y. Highly thermal conductive shape-stabilized composite phase change materials based on boron nitride and expanded graphite for solar thermal applications. RSC Adv. 2023, 13, 13252–13262. [Google Scholar] [CrossRef]
- Xiong, F.; Zhou, J.; Jin, Y.; Zhang, Z.; Qin, M.; Han, H.; Shen, Z.; Han, S.; Geng, X.; Jia, K.; et al. Thermal shock protection with scalable heat-absorbing aerogels. Nat. Commun. 2024, 15, 7125. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, Y.S.; Haghparast, S.N.; Shojaeefard, M.H.; Molaeimanesh, G.R. Numerical Evaluation of a Thermal Management System Consisting of PCM and Porous Metal Foam for Li-Ion Batteries. J. Therm. Anal. Calorim. 2020, 141, 1717–1739. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, M.; Chen, M. Comprehensive Experimental Research on Wrapping Materials Influences on the Thermal Runaway of Lithium-Ion Batteries. Emerg. Manag. Sci. Technol. 2025, 5, e007. [Google Scholar] [CrossRef]
- Kee, S.Y.; Munusamy, Y.; Ong, K.S.; Cornelis Metselaar, H.S.; Chee, S.Y.; Lai, K.C. Thermal Performance Study of Composite Phase Change Material with Polyacrylicand Conformal Coating. Materials 2017, 10, 873. [Google Scholar] [CrossRef]
- Wadee, A.; Walker, P.; McCullen, N.; Ferrandiz-Mas, V. The effect of thermal cycling on the thermal and chemical stability of paraffin phase change materials (PCMs) composites. Mater. Struct. 2025, 58, 25. [Google Scholar] [CrossRef]
- Hussien, S.A.; Ali, A.B.M.; Alkhatib, O.J.; Mahariq, I. Enhanced Passive Thermal Management of Lithium-Ion Batteries with Conical Cylindrical Chamber Incorporating Various Phase Change Materials. Sci. Rep. 2025, 15, 35675. [Google Scholar] [CrossRef] [PubMed]
- Landini, S.; Leworthy, J.; O’Donovan, T.S. A Review of Phase Change Materials for the Thermal Management and Isothermalisation of Lithium-Ion Cells. J. Energy Storage 2019, 25, 1000887. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Ren, Y.; An, Z.; Zhang, Z.; Yang, L.; Cui, W.; Wang, C. Thermal Performance Improvement of Composite Phase-Change Storage Material of Octanoic Acid–Tetradecanol by Modified Expanded Graphite. Energies 2024, 17, 4311. [Google Scholar] [CrossRef]
- Ye, F.; Dong, Y.; Opolot, M.; Zhao, L.; Zhao, C. Assessment of Thermal Management Using a Phase-Change Material Heat Sink under Cyclic Thermal Loads. Energies 2024, 17, 4888. [Google Scholar] [CrossRef]
- He, L.; Wang, H.; Zhu, H.; Gu, Y.; Li, X.; Mao, X. Thermal Properties of PEG/Graphene Nanoplatelets (GNPs) Composite Phase Change Materials with Enhanced Thermal Conductivity and Photo-Thermal Performance. Appl. Sci. 2018, 8, 2613. [Google Scholar] [CrossRef]










| PCM Type | Example Materials | Advantages | Limitations |
|---|---|---|---|
| Organic [21,22] | Paraffin wax, stearic acid | Stable, non-corrosive, non-toxic | Low thermal conductivity, flammable |
| Inorganic [23,24] | Salt hydrates, metallic alloys | High latent heat, better conductivity | Corrosion, supercooling, phase separation |
| Eutectic [25] | Organic–organic or salt mixtures | Precise melting point, customizable profiles | Compatibility and segregation risks |
| Property | Unit | Typical Range | Importance for BTM Systems |
|---|---|---|---|
| Melting Temperature (Tm) [35] | °C | 30–60 | Matches battery operating temperature window |
| Latent Heat of Fusion (ΔH) [35] | kJ/kg | 150–250 | High heat absorption capacity |
| Thermal Conductivity (k) [36] | W/m·K | 0.5–9.3 | Faster heat transfer; improved system response |
| Specific Heat Capacity (Cp) [37] | kJ/kg·K | 1.5–3.0 | Supports pre- and post-melting heat absorption |
| Density (ρ) [38] | kg/m3 | 700–1500 | Affects system weight and energy density |
| Thermal Cycling Stability [38,39] | Cycles | >1000 | Durability and long service life |
| Flammability [39,40] | - | Low or non-flammable | Safety for lithium-ion environments |
| Volume Change [38,40] | - | Minimal | Avoids mechanical stress or damage to the battery casing |
| Compatibility with Battery Materials [40] | - | Chemically inert | Avoids risk of corrosion, leakage, etc. |
| Feature/Criterion | PCM Cooling | Air Cooling | Liquid Cooling | Heat Pipes |
|---|---|---|---|---|
| Temperature Regulation | Excellent peak shaving; maintains narrow range due to latent heat absorption [34,41] | Moderate; depends on airflow and ambient temperature [44] | Very good; can maintain tight temperature control [45] | Good; relies on conduction and evaporation/condensation [46] |
| Hot Spot Reduction | Very good; distributes heat evenly across cells [14] | Poor–moderate; airflow may miss certain areas [47] | Good; uniform coolant distribution if well designed [48] | Good; point-to-point thermal spreading [49] |
| Energy Consumption | Zero during phase change; fully passive [50] | Low–moderate (fans require power) [51] | Moderate–high (pumps, chillers) [52] | Low (capillary action passive but may need fans) [53] |
| Safety Improvement | High; delays/prevents thermal runaway [41,42,43] | Low–moderate [54] | High [52] | High [55] |
| System Complexity | Low; simple encapsulation or embedding [56] | Low; ducting and fans [57] | High; requires pumps, reservoirs, hoses [58] | Moderate; requires sealed tubes and vapor chamber [59] |
| Maintenance Needs | Very low [60] | Low–moderate (fans may fail) [56] | High (fluid leaks, pump wear) [52] | Low [53] |
| Noise Level | Silent [61] | Noticeable (fans) [62] | Noticeable (pump) [63] | Silent [53] |
| Weight Impact | Low–moderate (depends on PCM mass) [64] | Low [65] | Moderate–high (fluid and equipment) [66] | Low [67] |
| Design Flexibility | High; can be molded into any shape [68] | Moderate; ducting constraints [69] | Moderate; plumbing constraints [52] | Limited to linear or planar paths [70] |
| Best Use Case | Peak load shaving, passive backup for active systems [52] | Low-cost, moderate performance needs [71] | High-power, continuous cooling [52] | High heat flux transport in compact layouts [71] |
| Category | Material Examples | Melting Point (°C) | Latent Heat (J/g) | Thermal Conductivity (W/m·K) | Advantages | Drawbacks |
|---|---|---|---|---|---|---|
| Organic PCMs | Paraffins (n-alkanes, C16–C28) [117] | 30–60 | 150–250 | ~0.2 | Abundant, chemically stable, negligible supercooling, tunable melting point | Low conductivity, flammable, leakage |
| Fatty acids (lauric, palmitic, stearic acids) [118] | 30–65 | 150–220 | ~0.2–0.3 | Renewable, higher safety than paraffins, good compatibility | Odor, cost, possible phase separation | |
| Polyethylene glycol (PEG) [119] | 20–65 | 140–200 | ~0.3 | Wide tunable range, stable, flexible use | Hydrophilic (absorbs moisture), leakage without encapsulation | |
| Inorganic PCMs | Salt hydrates (CaCl2·6H2O, Na2SO4·10H2O) [120] | 25–50 | 150–300 | 0.5–1.0 | High latent heat density, higher conductivity than organics | Supercooling, phase segregation, corrosive |
| Molten salts (LiNO3–KNO3–NaNO3) [121] | >100 | 100–200 | 0.5–1.0 | Stable, high-capacity, non-flammable | Too high Tm for EV use, corrosive | |
| Composite/Hybrid PCMs | Paraffin + Expanded Graphite/Graphene [122] | 30–60 | 120–200 | 2–10 | Greatly improved thermal conductivity, leakage prevention | Reduced latent heat, higher cost |
| Fatty acid + Graphite/CNTs [123] | 30–65 | 120–180 | 2–8 | Balance between heat storage and transfer | Additive cost, possible agglomeration | |
| PEG/SiO2 Aerogel, Polymer-encapsulated PCMs [124] | 25–60 | 100–180 | 0.5–5.0 | Shape-stabilized (no leakage), lightweight, tunable | Lower latent heat, fabrication complexity | |
| Nano-encapsulated PCMs (silica, polymer shells) [125] | 25–60 | 100–160 | 0.5–3.0 | Stable, good distribution, scalable for packs | Expensive, lower capacity per weight |
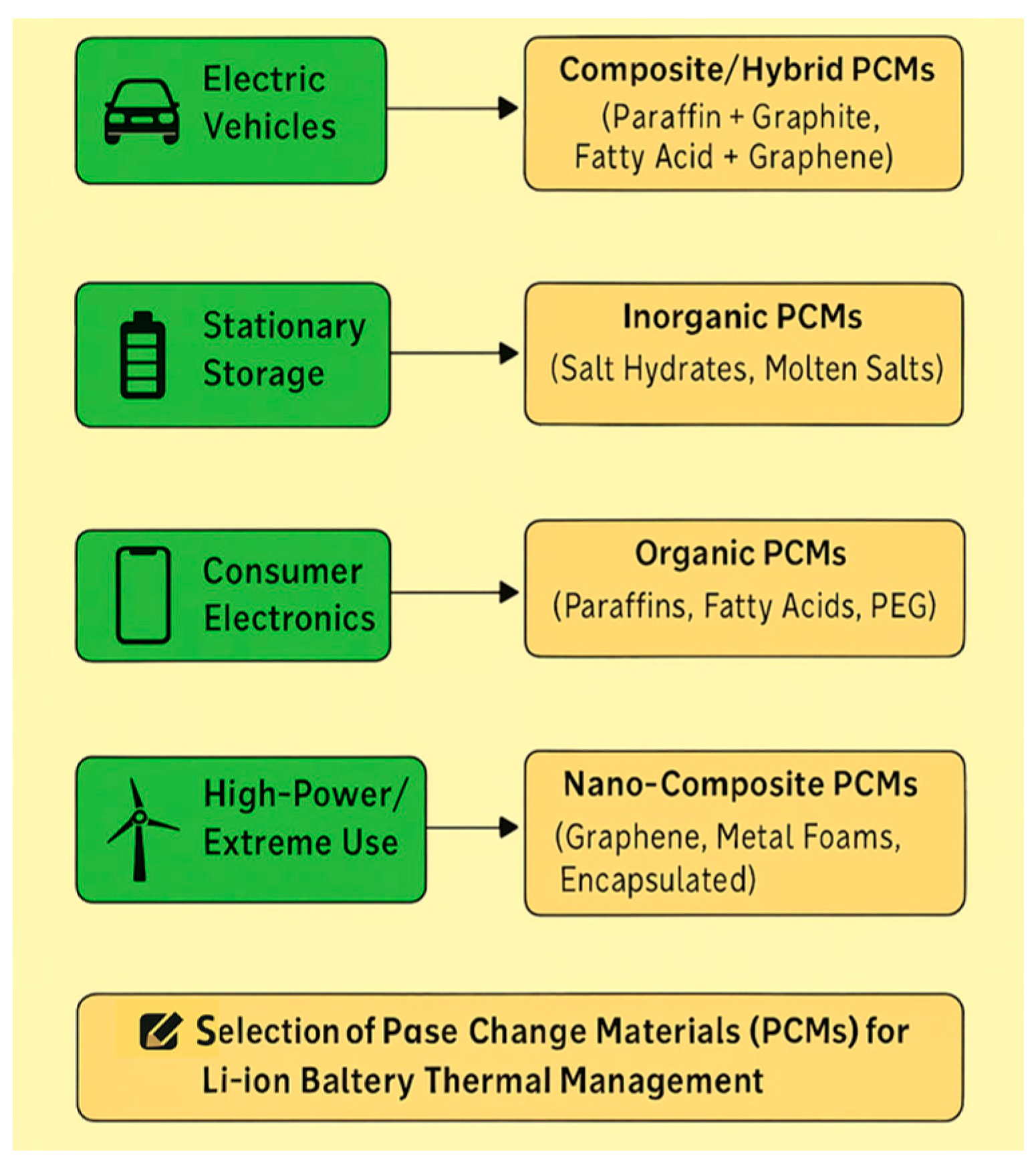
| Application | Best-Fit PCM Type | Examples | Why Suitable | Limitations |
|---|---|---|---|---|
| Electric Vehicles (EVs) | Composite/Hybrid PCMs (organic + fillers) [73] | Paraffin/graphite, fatty acid/graphene, PEG/SiO2 aerogel | -Operating range (30–60 °C) matches Li-ion safety window -High latent heat for heat spikes during fast charging/discharging -Additives improve conductivity (2–10 W/m·K) for rapid heat spreading -Shape-stabilized → no leakage in dynamic environments | Higher cost, reduced latent heat due to additives, fabrication complexity |
| Stationary Energy Storage (grid, renewable integration) | Inorganic PCMs (salt hydrates, molten salts) [126] | CaCl2·6H2O, Na2SO4·10H2O, LiNO3–KNO3 mixtures | -Can tolerate wider temperature ranges -High volumetric energy storage -Low flammability and safer in large installations -Cost-effective for large-scale systems | Supercooling and segregation (salt hydrates) High melting point (molten salts) unsuitable for low-temp ops Corrosion issues |
| Consumer Electronics (laptops, phones, drones, power tools) | Organic PCMs (low-melting paraffins, fatty acids, PEG) [127] | Paraffin (C16–C20), lauric acid, PEG-600 | -Lightweight, compact -Low melting range (30–45 °C) ideal for small Li-ion cells -Easy integration into casings or encapsulated composites -Less complex manufacturing than composites | Low thermal conductivity → may require micro/nano-encapsulation Flammability of paraffins |
| High-power/Extreme Applications (fast-charging EVs, aerospace, defense) | Advanced nano-composite PCMs [128] | Paraffin + graphene nanoplatelets, PCM in metal foams, nano-encapsulated PEG | -Ultra-fast heat dissipation needed -Graphene/metal foam structures boost conductivity >10 W/m·K -Stable and reliable under extreme thermal cycling | Expensive, not yet mass-produced, weight concerns (metal foams) |
| System Type | Nano-Enhanced PCM (nePCM) Integration | Key Advantage |
|---|---|---|
| Emulsion-based liquid cooling [4] | NPCME (octadecane/eicosane) | Lower Tmax and ΔT vs. water cooling |
| Expanded Graphite/PCM/Graphene Composite [131] | Solid nePCM + radiative layer | 26% temperature reduction, passive performance |
| Air-Assisted Hybrid nePCM System with Nano Powder Enhancements [132,133] | nePCM with graphite/nano powder | Maintains safety under real-cycle conditions |
| Hybrid PCM/Liquid Cooling Plate with Graphite Composite [19] | PCM impregnated in graphite matrix | Efficient thermal removal via channels |
| Fin-PCM-Expanded Graphite Composite Heat Sink [134] | Expanded Graphite-enhanced nePCM with fins | Superior under high discharge rates |
| Hybrid Nanofluid + nePCM Cooling for Pouch Cells [135] | Nanofluid + PCM in cold plates | Effective for high C-rate pouch cells |
| Hybrid Nanofluid + nePCM in Cylindrical Packs [136] | Nanofluid + PCM hybrid in cylindrical pack | Enhanced lifespan and thermal uniformity |
| System Type | Matrix Material | Key Outcome and Highlights |
|---|---|---|
| Metal Foam-PCM Composite [137] | Copper/Nickel Foam | Embedding PCM into metal foams significantly improves thermal conductivity and shape stability, achieving lower peak temperatures and more uniform cooling across battery modules |
| Hybrid Metal-Foam and Active Cooling [138] | Metal Foam + Water Channels | A hybrid system embedding PCM in metal foam and integrating water-cooled channels reduces maximum battery temperatures and improves thermal distribution, especially under high discharge rates |
| Aerogel–PCM Composite [139] | Biomass Aerogel Scaffold | A Ge/SA biomass aerogel coupled with a flexible CPCM provides exceptional thermal insulation (~120 °C temperature difference across 1 cm), high latent heat, and robust shape stability—delaying thermal saturation effectively |
| Gelatin/Sodium Alginate Aerogel + CPCM [139] | Biopolymer Aerogel | This multi-network aerogel combined with CPCM performs as a highly insulating, flame-retardant stabilization system, absorbing heat while maintaining structural integrity during thermal events |
| Hierarchical Macro-Nanoporous Metal + PCM [140] | Copper Macro-Nanoporous Matrix | Achieves leakage-free PCM loading (90 vol%) with high energy density, a three-fold thermal conductivity increase, and effective temperature control in simulated battery pack models |
| Ternary MWCNT/Graphene Aerogel-PCM [141] | Carbon Aerogel (MWCNT + Graphene) | This composite PCM achieves up to a 124% increase in thermal conductivity and a 63% reduction in operational temperature in battery applications owing to enhanced heat dispersion and stability |
| System Type | Hybrid Configuration | Key Highlights and Outcomes |
|---|---|---|
| Air Cooling + PCM with Fins [58] | PCM buffer + air convection via biomimetic fins | Reduced power consumption by ~59%; maintained Tmax ≈ 40 °C and ΔT ≈ 3 °C |
| Air Cooling + PCM + Copper Foam [137] | Air flow + PCM in metal foam (prismatic cells) | Hybrid reduced Tmax by 24 °C vs. +13 °C (active) and +11 °C (passive) |
| Liquid Cooling Plate + PCM Composite [66] | PCM impregnated graphite between cells + microchannel liquid plates | Enhances heat transfer via PCM to coolant, improving thermal dissipation |
| Heat Pipe + PCM [142] | PCM reservoir integrated with heat pipes and fin jackets | Maintains lower temperatures at high discharge power, with airflow variation |
| Water Channels + Dual-PCM in Metal Foam [143] | Active water cooling + metal foam with two PCMs | Reduces Tmax by ~2–2.7 °C; improved thermal uniformity by 1.2 °C |
| Liquid Nanofluid + PCM (Nanofluid Cooling) [144] | Nanofluid coolant + PCM in foams and microchannels | Achieves ~3.44 °C lower Tmax at 1C discharge; 6–15% longer cycling; only 5% more power use |
| Liquid + PCM + Nanofluid (Simulated Hybrid BTM systems) [143,145] | Hybrid with PCM, liquid assist, and Al2O3 nanofluid | Hybrid BTM systems improves Tmax by ~28%; only it and PCM achieve ΔT < 5 °C |
| BTM System Strategy | Melting Point Tuning Approach | Key Benefit and Outcome |
|---|---|---|
| Multi-PCM Hybrid Systems [145] | Dual-layer PCM set with distinct melting temperatures embedded in metal foam and water channels | Enhances thermal regulation via staggered heat absorption; reduces Tmax by ~2.7 °C and homogeneity improves by 1.2 °C under 3C discharge |
| Modular PCM in Aviation Packs [138] | Carefully selected PCM with melting point tailored just above ground ambient (42–50 °C) for triggering phase change only during high thermal loads | Prevents premature melting in cool conditions; provides sufficient latent capacity during critical flight phases |
| EV-Friendly PCM (~40 °C) [146] | Use of PCM melting near 40 °C to maintain optimal battery operation; supports life extension and capacity stability | Maintains battery within safe thermal window, particularly in mild-to-warm climates |
| Solar-Climate PCM (75 °C) [147] | PCM with ~75 °C melting point for engine pre-heating in cold climates | Retrieves waste heat effectively, reducing combustion-related fuel use in cold starts (<0 °C) |
| Low-Melting Inorganics (10–20 °C) [148] | Salt hydrates and organics with low melting temps tailored for ambient-sensitive thermal storage | Maintains thermal equilibrium in temperate or cooling-demand sequences |
| System Type | Structural Integration Approach | Key Highlights and Outcomes |
|---|---|---|
| Flexible Dual-Layer FPCM Sleeve [149] | PCM embedded in a flexible composite sleeve with outer conductive and inner insulating layers | Achieves ΔTmax reduction of 14.5 °C at 5C discharge; maintains flexibility and adaptability across climates |
| PCM-Negative Poisson’s Ratio Laminboard [150] | PCM encapsulated in a modular laminboard structure integrated with liquid cooling plates | Reduces Tmax by 3.8 °C and ΔT by 2.5 °C; also decreases mechanical stress and deformation |
| Metal Foam + Dual PCM + Water Channels [143] | Dual PCMs impregnated in metal foam integrated into structural water-cooled channels | Enhances cooling uniformity and reduces Tmax by ~2.7 °C, while providing structural rigidity |
| PCM-Composite Cell Spacers [151] | Shape-stabilized PCM integrated into load-bearing cell spacers | Improves pack compactness and minimized leakage risk, while ensuring both thermal buffering and vibration damping |
| Multifunctional PCM Barriers [152] | PCM-based crash-protection barriers within modules | Provides both thermal runaway mitigation and structural reinforcement under mechanical impact |
| Encapsulation Technique | Description and Outcome |
|---|---|
| Multi-Scale Inorganic PCM Encapsulation [153] | Sodium acetate trihydrate (inorganic PCM) is first micro-encapsulated with expanded graphite to → thermal conductivity ~4.96 W/m·K and prevent leakage; then, it is macro-encapsulated with silicone sealant to ensure long-term chemical stability |
| Microencapsulated PCM Suspensions (MPCMS) [154] | Microcapsules (e.g., paraffin/melamine resin) are dispersed in base liquid to form a latent heat-enhanced coolant; demonstrates ~14.5 °C reduction in module temperature and a ~3.8 °C reduction in temperature variance compared to plain coolant |
| Encapsulated PCM with Enhanced Thermal Conductivity [155] | Use of graphene or CNTs in micro-epoxy or polymer shells; silver-coated nano-PCM improves thermal conductivity from 0.246 to 1.346 W/m·K; CNT-enhanced microcapsules preserve PCM latent heat and improved uniformity |
| Semi-Penetrating Composite Shell Encapsulation [76] | Core–shell PCM structures using CaCO3 or SiO2 shells around octadecane core; increases durability, thermal conductivity, and leakage protection—scalable and cost-effective encapsulation |
| Strategy | Description and Outcomes |
|---|---|
| Cooling-Guided Diffusion Model for Cell Arrangement [156] | A generative AI framework using a diffusion model to optimize battery cell layouts for enhanced thermal diffusion. Achieves superior cooling efficiency—outperforming TabDDPM by 5× and CTGAN by 66× in feasibility and thermal performance |
| Physics-Informed ML Surrogate for Temperature Distribution [157] | Integrates physics laws into ML surrogate modeling (via convolutional neural networks) to predict battery pack temperature. Delivers 15% better accuracy than purely data-driven models using less training data |
| Digital Twin with ROM and ML Integration [158] | Combines reduced-order models from CFD with supervised machine learning (decision trees, SVR, and neural nets) to create a predictive digital twin for thermal dynamics. Offers fast updates and high accuracy |
| Real-World BTM System Optimization via Driving Prediction [159] | Uses neural networks to predict driving profiles for real vehicle BTM systems, enabling adaptive thermal management optimization in real time |
| Review of AI/ML in BTM System Optimization [160] | Highlights the utility of ML models (ANN, LSTM, NSGA-II, and Kriging) for optimizing BTM system performance—achieving an R2 of up to 0.99 and temperature reductions up to 31.7%. Identifies gaps in real-world validation and system variability |
| Hybrid ML-CFD Models for Digital Battery Modeling [161] | Reviews the fusion of physics-based modeling and ML to accelerate battery digitalization—balancing accuracy and computation speed in design and real-time control |
| Surrogate Models for PCM-Liquid Hybrid BTM Systems [162] | Employs surrogate modeling (Adaptive Kriging HDMR) alongside AI (SVR optimized with PSO) to predict and optimize thermal performance, cooling capacity, and system COP |
| AI Prediction for PCM + Air Cooling System Behavior [161,162] | Neural network trained to predict average and peak battery temperatures under varying conditions with <10% error and R ≈ 0.997. Inputs include discharge rate, coolant flow, and inlet temperature |
| PCM Type/Grade | Melting Range (°C) | Form/Example | Recommended Thickness (Cell Contact Layer) | Target Effective Conductivity (W·m−1·K−1) | Notes/Application |
|---|---|---|---|---|---|
| Paraffin (C18–C28) [164,165,166] | 28–55 | Commercial paraffin wax, shape-stabilized with EG or polymer | 3–5 mm (cylindrical); up to 8 mm (prismatic) | ≥1–3 (with EG/graphene) | Widely used; simple; risk of leakage without stabilization |
| PEG (PEG-1000, PEG-1500) [167,168,169] | 30–50 | Solid–solid PEG, PEG/SiO2 or PEG/EG composites | 3–4 mm typical; up to 6 mm for high C-rate | ≥1–5 (with EG, GNPs, BN) | Stable cycling, reduced leakage; effective for cylindrical and pouch cells |
| Salt Hydrates (e.g., CaCl2·6H2O, Na2HPO4·12H2O) [170,171] | 25–40 | Encapsulated or polymer-stabilized | 2–6 mm | ≥1–2 (with graphite/metal foam) | High latent heat but requires encapsulation to avoid leakage and phase separation |
| Shape-stabilized Organic–Inorganic Hybrids [172,173] | 30–45 | Paraffin/PEG + silica, aerogels, polymer matrices | 2–4 mm | 1–3 | Leak-free, mechanical stability, suitable for direct coating or jackets |
| Metallic PCM (low-melting alloys, e.g., Ga–In–Sn) [174,175] | 25–35 | Encapsulated alloy droplets in polymer or graphite foam | 1–3 mm | 10–30 | Very high conductivity, but cost, toxicity, and corrosion issues limit use |
| Microencapsulated PCM (paraffin, PEG in polymer shells) [176,177] | 28–45 | Dispersed microcapsules in polymer resin or adhesive | Thin coating ≤ 1 mm | 0.5–2 | Leak-proof, scalable, good for thin direct-contact layers; lower volumetric enthalpy |
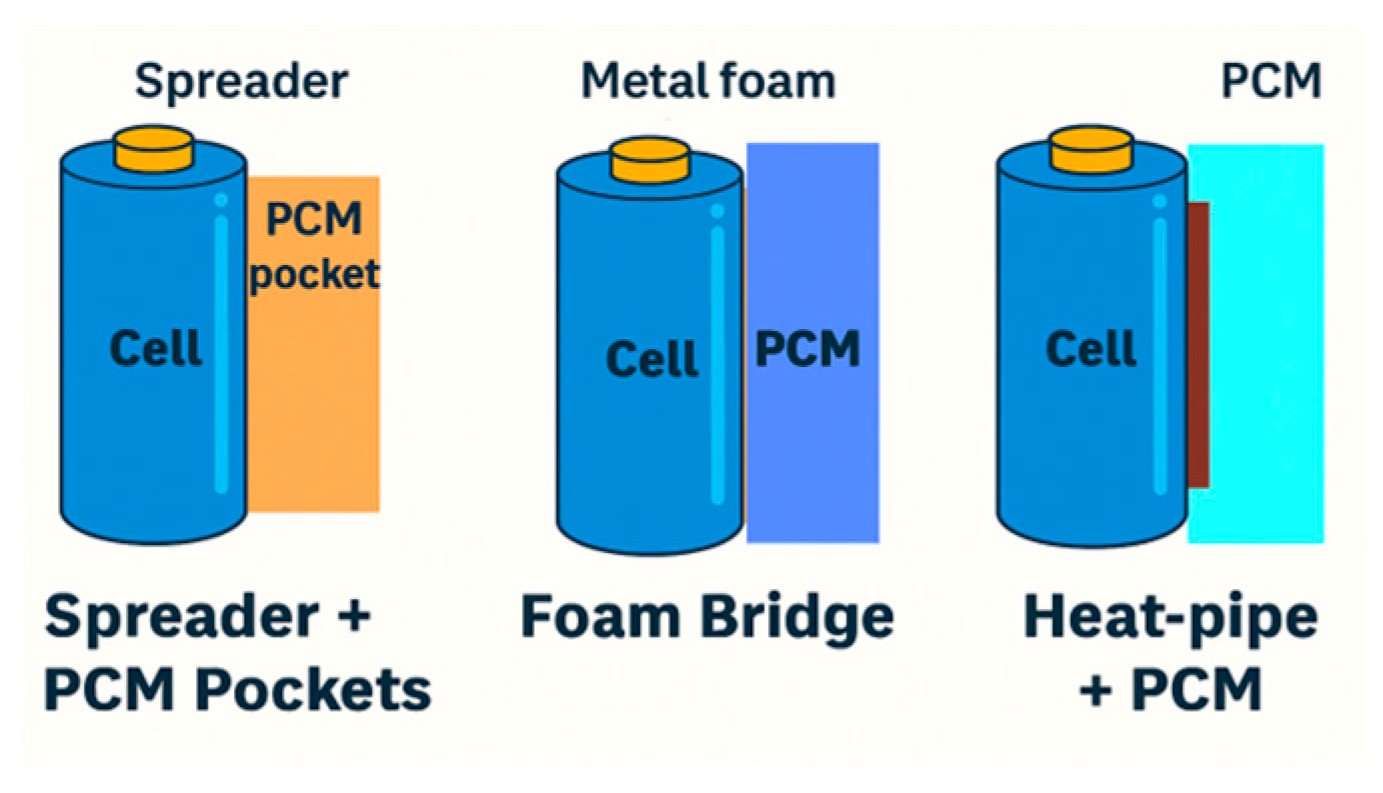
| Criterion | Direct-Contact PCM | Indirect-Contact PCM |
|---|---|---|
| Thermal performance | Very high heat absorption efficiency (low thermal resistance, rapid response) [12,42] | Moderate efficiency (extra thermal resistance via spreaders/foams), but better spatial heat spreading [178] |
| Temperature uniformity | High local absorption, but may cause uneven distribution if PCM placement is non-uniform [10] | Better lateral heat distribution (graphite/metal spreaders reduce hotspots, ΔT typically < 3–5 °C) [5] |
| Recommended PCM grades | Paraffins (C16–C28), PEG (MW 1000–6000), fatty acids; composite PCMs with expanded graphite (EG) or BN for conductivity [140] | Paraffins/paraffin–EG composites, hydrated salts (PCM pockets), metallic foams impregnated with PCM, graphite-enhanced PCMs [6,141] |
| Typical PCM thickness | 2–6 mm jackets or sleeves; 1–3 mm interstitial fills; microcapsules dispersed within 0.1–0.5 mm matrix [179] | PCM pockets/trays: 5–15 mm; foam bridges: 3–10 mm; spreader-linked PCM reservoirs: 10–20 mm [6,180,181] |
| Target effective conductivity | ≥1.0–2.0 W·m−1·K−1 (with fillers) to ensure rapid absorption [182] | ≥3.0–5.0 W·m−1·K−1 for spreaders/foams; PCM pocket conductivity less critical, focus on latent enthalpy [180,181] |
| Weight impact | Lower mass (PCM applied directly, no extra spreader components) [10,179] | Higher due to spreaders, foams, or heat pipes [6] |
| Cost impact | Lower material and manufacturing cost; simpler assembly [179] | Higher due to added conductive materials, TIMs, and assembly steps [6] |
| Safety | Risk of leakage and direct contact with conductive PCMs; electrical insulation required [182] | Safer (PCM isolated in pockets/trays); lower leakage and contamination risks [6] |
| Reliability | Long-term stability depends on PCM encapsulation quality; mechanical stress may damage PCM layer [183] | More robust mechanically; easier to service/replace PCM modules [180,181] |
| Integration complexity | Simple to implement in small packs (e.g., consumer electronics) [179] | More complex, suitable for EV packs and aerospace modules [6] |
| Hybrid PCM Integration | Peak Temp. Reduction (°C) | Max. Temp. Difference ΔT (°C) | Thermal Response Time | Energy Efficiency |
|---|---|---|---|---|
| PCM + Air Cooling [66] | 5–12 | 2–5 | Moderate | Low–Moderate |
| PCM + Liquid Cooling [142] | 15–25 | 2–3 | Fast | High |
| PCM + Heat Pipes [188] | 12–20 | 1–3 | Very Fast | High |
| PCM + Fins/Heat Sinks [191] | 8–15 | 2–4 | Moderate–Fast | Moderate |
| PCM + Hybrid (Air + Liquid) [191] | 20–30 | <2 | Very Fast | Very High |
| Parameter | Conventional PCM Integration | Structural PCM Integration |
|---|---|---|
| Thermal Regulation [39] | High latent heat storage, good peak shaving | Comparable thermal buffering, dependent on composite matrix |
| Mechanical Contribution [197] | None (requires external casing/support) | Provides structural support (e.g., honeycomb, polymer–PCM hybrids) |
| Fire/Impact Protection [198] | Limited (PCM may leak or degrade under fire) | Enhanced (PCM–aerogel composites, flame-retardant matrices) |
| Weight Penalty [199] | Significant (dedicated PCM mass adds to module weight) | Reduced (PCM contributes dual role, lowering parasitic mass) |
| Module Compactness [200] | Lower (PCM requires extra volume for encapsulation) | Higher (PCM integrated in casing/separator reduces packaging volume) |
| Design Complexity [201] | Low–moderate (straightforward encapsulation) | High (requires advanced composites and multifunctional design) |
| TRL (Technology Readiness Level) | Medium (lab to pilot scale, several demos) | Low–medium (emerging research, limited real-world validation) |
| Design Lever | Description | Reported Effect on ΔT |
|---|---|---|
| PCM Placement/Distribution | Gradient thickness, selective positioning near hot spots, or inter-cell gaps instead of uniform blanket | ΔT reduction by 55–77% compared to uniform PCM layouts [205] |
| Thermal Conductivity Enhancers | Addition of expanded graphite, carbon fibers, metallic foams, or graphene sheets to improve lateral spreading | ΔT reduced by 30–50%, faster heat absorption [38] |
| Structural Integration | Embedding PCM into honeycomb cores, metallic skeletons, or casings to combine latent heat buffering with conduction pathways | ΔT reduced to <2–5 °C under 3C cycling [208] |
| Hybridization with Active Cooling | PCM coupled with liquid channels, cooling plates, or forced air to remove stored heat after melting | Maintains ΔT at sub-few °C during high C-rates [38] |
| Design/Method | Test Conditions | Peak Temperature (Tmax) | Suppression vs. Baseline |
|---|---|---|---|
| Baseline (air cooled) [208] | Prismatic cells, 3C discharge, 40 °C ambient | ~60 °C | - |
| PCM-only encapsulation [204] | Cylindrical/prismatic, 2C–3C discharge | 45–52 °C | 8–15 °C lower than baseline |
| Gradient PCM placement [206] | Module with optimized PCM thickness, 3C | Hot-spot reduced by ~18 °C | ≈30–40% reduction |
| Honeycomb + PCM [205] | Prismatic module, 3C, 40 °C ambient | 45.7 °C | ~14 °C lower than baseline |
| Metallic honeycomb PCM core [207] | Module, 3C cycling | Peak reduced by 42% (ΔT < 2 °C) | ~15–20 °C lower |
| Hybrid PCM + liquid cooling plate [66] | 18,650 modules, 3C–4C discharge | Tmax reduction 10–20 °C | Maintained <50 °C |
| PCM Type/ Configuration | Effective Thermal Conductivity (W·m−1·K−1) | Thermal Response Time (s) | Tmax Reduction vs. Baseline |
|---|---|---|---|
| Neat PCM (Paraffin, RT waxes) [205] | ~0.2–0.3 | >70–100 | ~8–10 °C at 2C–3C discharge |
| EG-Enhanced CPCM (5–15 wt%) [38] | 5–15 | 40–60 | ~12–15 °C reduction; ~30–40% faster response |
| Metal-Foam PCM (Al/Cu skeletons) [213] | 20–40 | <30 | ~15–20 °C reduction; ΔT < 2 °C |
| Aerogel–PCM Composites [214] | 1–5 | 35–50 | ~10–14 °C reduction |
| Structural PCM (Honeycomb integration) [215] | 5–10 (distributed pathways) | 30–50 | ~14 °C reduction; improved hot-spot suppression |
| PCM Type | Latent Heat Retention | Cycles Tested | Degradation Mode |
|---|---|---|---|
| Paraffin PCM | ~70–85% after 300 cycles | 100–300 | Leakage, supercooling [38] |
| Salt-hydrate PCM | ~65–70% after 200 cycles | 200 | Phase segregation, incongruent melting [66] |
| EG-enhanced CPCM | >90% after 500 cycles | 300–500 | Minor PCM depletion, stable overall (metallic honeycomb PCM study) [38] |
| Metal-foam PCM [170] | >90% after 500–1000 cycles | 500–1000 | Pore saturation, slight PCM loss [208] |
| Aerogel–PCM composites | ~85–90% after 200–300 cycles | 200–300 | Limited leakage, stable [208] |
| Metric | Evaluation Methods | Benchmark Results (Literature) |
|---|---|---|
| Temperature Uniformity | Thermocouples placed across cells; infrared (IR) thermography; CFD simulations | PCM integration reduces the ΔT between cells by 40–59% compared to air cooling. Hybrid PCM–fin systems achieve ΔT < 3–5 °C at 3C discharge [218] |
| Peak Temperature Suppression | In situ cycling at different C-rates; thermal chamber testing; calorimetry | Maximum cell temperature reduced by 10–20 °C vs. baseline. Critical safety threshold (<60 °C) maintained up to 3–4C discharge [219] |
| Thermal Response Time | Transient heating experiments; step-current load tests; high-resolution IR imaging | Pure PCM: slow response (>100 s). Graphite/foam-enhanced PCM: 30–60% faster heat absorption. Structural PCM near casing reduces lag significantly [220] |
| Durability and Aging | Accelerated thermal cycling (hundreds–thousands of cycles); DSC for latent heat retention; mechanical/vibration tests (EV conditions) | Advanced composites retain >95% latent heat capacity after 500–1000 cycles. Leakage and segregation minimized in encapsulated or polymer-stabilized PCMs [37,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calborean, A.; Máthé, L.; Bruj, O. Phase Change Materials for Thermal Management in Lithium-Ion Battery Packs: A Review. Batteries 2025, 11, 432. https://doi.org/10.3390/batteries11120432
Calborean A, Máthé L, Bruj O. Phase Change Materials for Thermal Management in Lithium-Ion Battery Packs: A Review. Batteries. 2025; 11(12):432. https://doi.org/10.3390/batteries11120432
Chicago/Turabian StyleCalborean, Adrian, Levente Máthé, and Olivia Bruj. 2025. "Phase Change Materials for Thermal Management in Lithium-Ion Battery Packs: A Review" Batteries 11, no. 12: 432. https://doi.org/10.3390/batteries11120432
APA StyleCalborean, A., Máthé, L., & Bruj, O. (2025). Phase Change Materials for Thermal Management in Lithium-Ion Battery Packs: A Review. Batteries, 11(12), 432. https://doi.org/10.3390/batteries11120432







