A MOF-Mediated Strategy for In Situ Niobium Doping and Synthesis of High-Performance Single-Crystal Ni-Rich Cathodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.1.1. Synthesis of MOF-90
2.1.2. Synthesis of SC90
2.1.3. Synthesis of SC90-Nb
2.2. Materials Characterizations
2.3. Electrochemical Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-Nickel Layered Oxide Cathodes for Lithium-Based Automotive Batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, C.; Gong, S.; Sun, Y.; Guan, S.; Gao, Y.; Gao, P.; Zhu, Y.; Lou, S.; Li, X. Electroless Plating Engineering Triggered Shielding Effect Enables Stable Nickel-Rich Cathode for High-Energy-Density Lithium-Ion Batteries. J. Colloid Interface Sci. 2025, 698, 138129. [Google Scholar] [CrossRef]
- Kim, U.-H.; Jun, D.-W.; Park, K.-J.; Zhang, Q.; Kaghazchi, P.; Aurbach, D.; Major, D.T.; Goobes, G.; Dixit, M.; Leifer, N.; et al. Pushing the Limit of Layered Transition Metal Oxide Cathodes for High-Energy Density Rechargeable Li Ion Batteries. Energy Environ. Sci. 2018, 11, 1271–1279. [Google Scholar] [CrossRef]
- Jiang, M.; Danilov, D.L.; Eichel, R.; Notten, P.H.L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Kim, U.-H.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. Quaternary Layered Ni-Rich NCMA Cathode for Lithium-Ion Batteries. ACS Energy Lett. 2019, 4, 576–582. [Google Scholar] [CrossRef]
- Zhou, H.; Xin, F.; Pei, B.; Whittingham, M.S. What Limits the Capacity of Layered Oxide Cathodes in Lithium Batteries? ACS Energy Lett. 2019, 4, 1902–1906. [Google Scholar] [CrossRef]
- Lu, J.; Xu, C.; Dose, W.; Dey, S.; Wang, X.; Wu, Y.; Li, D.; Ci, L. Microstructures of Layered Ni-Rich Cathodes for Lithium-Ion Batteries. Chem. Soc. Rev. 2024, 53, 4707–4740. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-M.; Kim, Y.-S.; Ryu, H.-H.; Sun, Y.-K.; Yoon, C.S. Structural Stability of Single-Crystalline Ni-Rich Layered Cathode upon Delithiation. ACS Energy Lett. 2022, 7, 2919–2926. [Google Scholar] [CrossRef]
- Tang, W.; Shu, Z.; Li, A.; Huang, X.; Li, W. Enhancing Structural Integrity and Long-Term Cycling Stability of High-Voltage Single-Crystalline Ni-Rich Cathodes via Surface/Subsurface Dual-Functional Modification Engineering. Energy Storage Mater. 2025, 77, 104185. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, H.; Sun, J.; Okagaki, J.; Choe, Y.; Yoo, E. Conformational Isomerism Breaks the Electrolyte Solubility Limit and Stabilizes 4.9 V Ni-Rich Layered Cathodes. Nat. Commun. 2024, 15, 9108. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, S.; Di, A.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Challenges and Strategies towards Single-Crystalline Ni-Rich Layered Cathodes. Adv. Energy Mater. 2022, 12, 2201510. [Google Scholar] [CrossRef]
- Zhang, H.; He, X.; Chen, Z.; Yang, Y.; Xu, H.; Wang, L.; He, X. Single-Crystalline Ni-Rich LiNixMnyCo1−x−yO2 Cathode Materials: A Perspective. Adv. Energy Mater. 2022, 12, 2202022. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, X.; Lin, J.; Huang, Y.; Cheng, W.; Liu, Q.; Zhang, H.; Ren, F.; Huang, Y.; Liu, Z.; et al. Restraining Planar Gliding in Single-Crystalline LiNi0.9Co0.05 Mn0.05O2 Cathodes by Combining Bulk and Surface Modification Strategies. Angew. Chem. Int. Ed. 2025, 64, e202419903. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yu, D.; Tang, Y.; Yan, Y.; Qiao, Y.; Bao, J.; Sun, S.-G. The Dilemma of Single-Crystal High-Nickel LiNixCoyMn1-x-yO2 (x ≥ 0.9) Cathodes: Inhomogeneous Delithiation inside and Outside the Particle. Chem. Eng. J. 2025, 504, 158800. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, H.; Gao, J.; Wang, J.; Shao, M.; Zhou, W. Grain-growth Inhibitor with Three-section-sintering for Highly Dispersed Single-crystal NCM90 Cubes. Angew. Chem. 2024, 136, e202314457. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, H.; Liu, X.; Gao, J.; Hao, S.; Yang, Y.; Qiu, J. Perspective on the Preparation Methods of Single Crystalline High Nickel Oxide Cathode Materials. Adv. Energy Mater. 2023, 13, 2300378. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Cheng, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single-Crystal Nickel-Rich Layered-Oxide Battery Cathode Materials: Synthesis, Electrochemistry, and Intra-Granular Fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Van Den Bergh, W.; Karger, L.; Murugan, S.; Janek, J.; Kondrakov, A.; Brezesinski, T. Single Crystal Layered Oxide Cathodes: The Relationship between Particle Size, Rate Capability, and Stability. ChemElectroChem 2023, 10, e202300165. [Google Scholar] [CrossRef]
- Yin, C.; Bao, Z.; Tan, H.; Zhou, H.; Li, J. Metal-Organic Framework-Mediated Synthesis of LiNi0.5Mn1.5O4: Tuning the Mn3+ Content and Electrochemical Performance by Organic Ligands. Chem. Eng. J. 2019, 372, 408–419. [Google Scholar] [CrossRef]
- Yin, C.; Zhou, H.; Yang, Z.; Li, J. Synthesis and Electrochemical Properties of LiNi0.5Mn1.5O4 for Li-Ion Batteries by the Metal–Organic Framework Method. ACS Appl. Mater. Interfaces 2018, 10, 13625–13634. [Google Scholar] [CrossRef]
- Li, T.; Bai, Y.; Wang, Y.; Xu, H.; Jin, H. Advances in Transition-Metal (Zn, Mn, Cu)-Based MOFs and Their Derivatives for Anode of Lithium-Ion Batteries. Coord. Chem. Rev. 2020, 410, 213221. [Google Scholar] [CrossRef]
- Kong, L.; Liu, M.; Huang, H.; Xu, Y.; Bu, X. Metal/Covalent-Organic Framework Based Cathodes for Metal-Ion Batteries. Adv. Energy Mater. 2022, 12, 2100172. [Google Scholar] [CrossRef]
- Shan, R.; Lu, X.; Xu, Y.; Shen, K.; Xia, Y.; Cai, Y.; Yao, J.; Mao, Q.; Wang, Y.; Ji, T. A Ternary MOF Derived Single Crystalline LiNi1/3Mn1/3Co1/3O2 as High-Voltage Cathodes for Lithium-Ion Batteries. Chem. Eng. Sci. 2023, 268, 118416. [Google Scholar] [CrossRef]
- Han, X.; Meng, Q.; Sun, T.; Sun, J. Preparation and Electrochemical Characterization of Single-Crystalline Spherical LiNi1/3Co1/3Mn1/3O2 Powders Cathode Material for Li-Ion Batteries. J. Power Sources 2010, 195, 3047–3052. [Google Scholar] [CrossRef]
- Rawool, C.R.; Karna, S.P.; Srivastava, A.K. Enhancing the Supercapacitive Performance of Nickel Based Metal Organic Framework-Carbon Nanofibers Composite by Changing the Ligands. Electrochim. Acta 2019, 294, 345–356. [Google Scholar] [CrossRef]
- He, F.; Yang, N.; Li, K.; Wang, X.; Cong, S.; Zhang, L.; Xiong, S.; Zhou, A. Hydrothermal Synthesis of Ni-Based Metal Organic Frameworks/Graphene Oxide Composites as Supercapacitor Electrode Materials. J. Mater. Res. 2020, 35, 1439–1450. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, H.; Yu, Z.; Jia, X.; Liang, J.; Li, G.; Li, Y.; Wang, K. Facile Synthesis of 4,4′-Biphenyl Dicarboxylic Acid-Based Nickel Metal Organic Frameworks with a Tunable Pore Size towards High-Performance Supercapacitors. Nanomaterials 2022, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Cao, M.; Zhang, H.; Lv, G.; Zhang, J.; Meng, Y.; Shu, C.; Fan, W.; Zuo, M.; Xiang, W.; et al. Synergistic Effect of Microstructure Engineering and Local Crystal Structure Tuning to Improve the Cycling Stability of Ni-Rich Cathodes. ACS Appl. Mater. Interfaces 2021, 13, 48720–48729. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Yang, X.; Liu, Y.; Wu, Z.; Li, H.; Indris, S.; Ehrenberg, H.; Hua, W. Unravelling the Peculiar Role of Co and Al in Highly Ni-Rich Layered Oxide Cathode Materials. Chem. Eng. J. 2024, 484, 149599. [Google Scholar] [CrossRef]
- Zuo, J.; Wang, J.; Duan, R.; Bai, Y.; Xu, K.; Zhang, K.; Wang, J.; Zhang, K.; Yang, Z.; Yang, Z.; et al. Grain Binding Derived Reinforced Interfacial Mechanical Behavior of Ni-Rich Layered Cathode Materials. Nano Energy 2024, 121, 109214. [Google Scholar] [CrossRef]
- Jiao, T.; Liu, G.; Zou, Y.; Yang, X.; Zhang, X.; Fu, A.; Zheng, J.; Yang, Y. A Novel Trimethylsilyl 2-(Fluorosulfonyl)Difluoroacetate Additive for Stabilizing the Ni-Rich LiNi0.9Co0.05Mn0.05O2/Electrolyte Interface. J. Power Sources 2021, 515, 230618. [Google Scholar] [CrossRef]
- Sheng, H.; Meng, X.; Xiao, D.; Fan, M.; Chen, W.; Wan, J.; Tang, J.; Zou, Y.; Wang, F.; Wen, R.; et al. An Air-Stable High-Nickel Cathode with Reinforced Electrochemical Performance Enabled by Convertible Amorphous Li2CO3 Modification. Adv. Mater. 2022, 34, 2108947. [Google Scholar] [CrossRef]
- Park, G.-T.; Namkoong, B.; Kim, S.-B.; Liu, J.; Yoon, C.S.; Sun, Y.-K. Introducing High-Valence Elements into Cobalt-Free Layered Cathodes for Practical Lithium-Ion Batteries. Nat. Energy 2022, 7, 946–954. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Y.; Zhang, B.; Huang, W.; Cheng, L.; Wang, J.; He, X.; Yu, L.; Xiao, Z.; Wen, J.; et al. Optimized In Situ Doping Strategy Stabling Single-Crystal Ultrahigh-Nickel Layered Cathode Materials. ACS Nano 2024, 18, 8002–8016. [Google Scholar] [CrossRef]
- Qian, R.; Liu, Y.; Cheng, T.; Li, P.; Chen, R.; Lyu, Y.; Guo, B. Enhanced Surface Chemical and Structural Stability of Ni-Rich Cathode Materials by Synchronous Lithium-Ion Conductor Coating for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 13813–13823. [Google Scholar] [CrossRef] [PubMed]

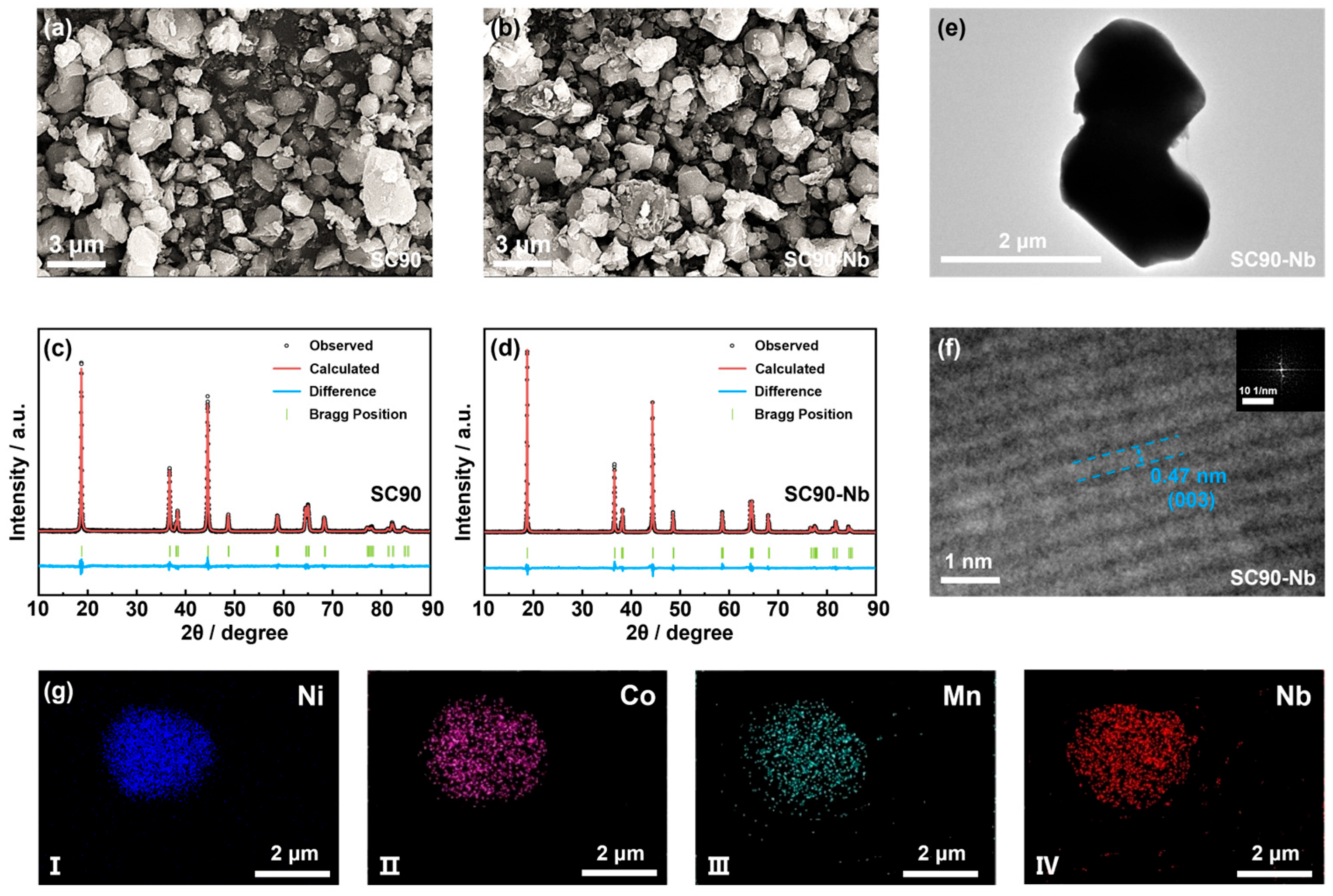
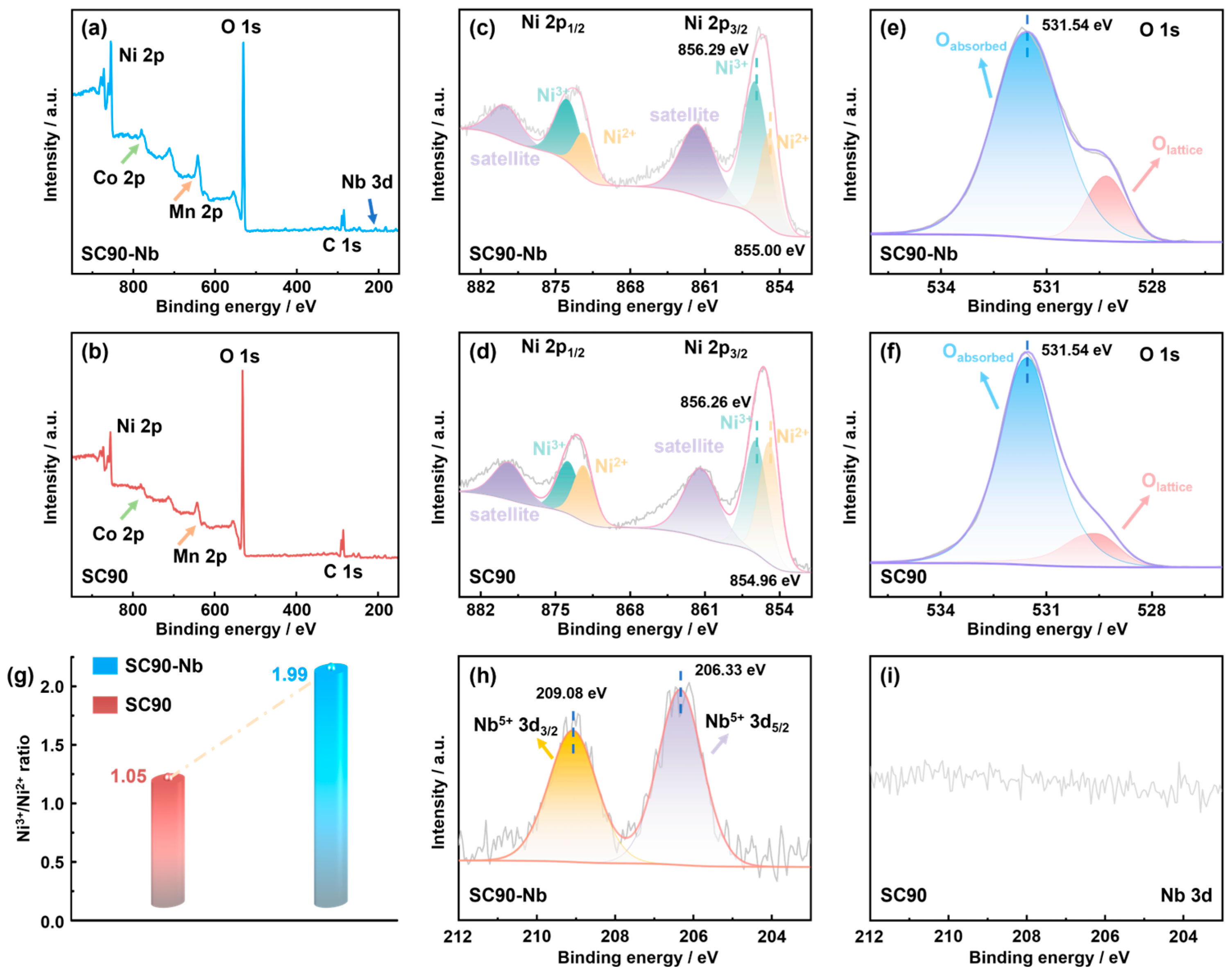


| Sample | Chemical Composition (at. %) | ||||
|---|---|---|---|---|---|
| Li | Ni | Co | Mn | Nb | |
| SC90 | 1.071 | 0.902 | 0.047 | 0.051 | 0 |
| SC90-Nb | 1.066 | 0.892 | 0.049 | 0.050 | 0.009 |
| Sample | a (Å) | c (Å) | V (Å3) | c/a | I003/I104 | Rwp (%) |
|---|---|---|---|---|---|---|
| SC90 | 2.8723 | 14.1961 | 101.43 | 4.9424 | 1.23 | 7.28% |
| SC90-Nb | 2.8726 | 14.1957 | 101.45 | 4.9418 | 1.39 | 6.64% |
| Sample | Three-Cycle Activation | After 100-Cycle | ||
|---|---|---|---|---|
| Rf (Ω) | Rct (Ω) | Rf (Ω) | Rct (Ω) | |
| SC90 | 18.44 | 29.09 | 18.92 | 376.31 |
| SC90-Nb | 21.93 | 30.02 | 22.58 | 64.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhou, H.; Liu, S.; Guan, S.; Liu, M.; Gao, P.; Zhu, Y.; Li, X. A MOF-Mediated Strategy for In Situ Niobium Doping and Synthesis of High-Performance Single-Crystal Ni-Rich Cathodes. Batteries 2025, 11, 368. https://doi.org/10.3390/batteries11100368
Gao Y, Zhou H, Liu S, Guan S, Liu M, Gao P, Zhu Y, Li X. A MOF-Mediated Strategy for In Situ Niobium Doping and Synthesis of High-Performance Single-Crystal Ni-Rich Cathodes. Batteries. 2025; 11(10):368. https://doi.org/10.3390/batteries11100368
Chicago/Turabian StyleGao, Yinkun, Huazhang Zhou, Shumin Liu, Shuyun Guan, Mingyang Liu, Peng Gao, Yongming Zhu, and Xudong Li. 2025. "A MOF-Mediated Strategy for In Situ Niobium Doping and Synthesis of High-Performance Single-Crystal Ni-Rich Cathodes" Batteries 11, no. 10: 368. https://doi.org/10.3390/batteries11100368
APA StyleGao, Y., Zhou, H., Liu, S., Guan, S., Liu, M., Gao, P., Zhu, Y., & Li, X. (2025). A MOF-Mediated Strategy for In Situ Niobium Doping and Synthesis of High-Performance Single-Crystal Ni-Rich Cathodes. Batteries, 11(10), 368. https://doi.org/10.3390/batteries11100368






