MXenes in Solid-State Batteries: Multifunctional Roles from Electrodes to Electrolytes and Interfacial Engineering
Abstract
1. Introduction
2. MXenes: Structure, Synthesis, and Properties
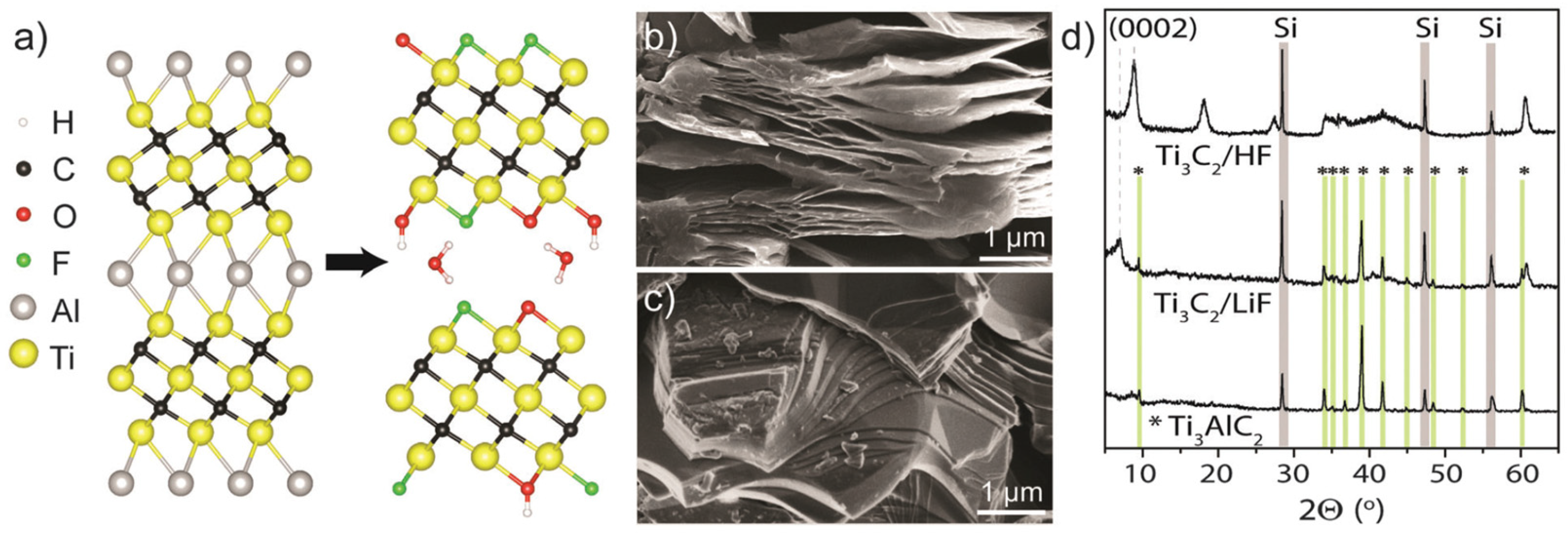
2.1. Crystal Structure and Surface Terminations
2.2. Synthesis Methods
2.2.1. HF Etching
- -
- Toxicity and corrosiveness of HF require stringent safety protocols.
- -
- Environmental hazards due to fluorinated byproducts.
- -
- Limited control over termination chemistry and delamination yield.
2.2.2. In Situ Acid Etching (Fluorine-Free or Reduced-Fluoride Methods)
2.2.3. Molten Salt Synthesis
2.3. Physicochemical Properties Relevant to Solid-State Batteries (SSBs)
2.4. Comparative Advantages over Other 2D Materials (Graphene, MoS2, BN)
3. MXenes as Electrode Materials
3.1. MXenes as Anodes in Solid-State Batteries (SSBs)
3.2. MXenes as Conductive Additives in Cathodes
3.3. MXene-Based Composites for Enhanced Electrode Performance
- -
- Conductive scaffolds that reinforce electron and ion transport paths;
- -
- Structural buffering that accommodates active material expansion and mechanical strain;
- -
- Enhanced redox kinetics by providing accessible electroactive surfaces;
- -
- Improved interface chemistry in solid-state configurations.
4. MXenes for Metal Anode Protection and Dendrite Suppression
4.1. Challenges of Dendrite Growth in Solid-State Batteries (SSBs)
- Interfacial void formation during stripping and plating;
- Current density heterogeneity and local overpotentials;
- Mechanical stress accumulation and interfacial delamination;
- Defect-assisted propagation through grain boundaries and pores;
- Electrochemical instability and formation of resistive interphases;
- Low critical current density thresholds, often below 1 mA cm−2 in practice.
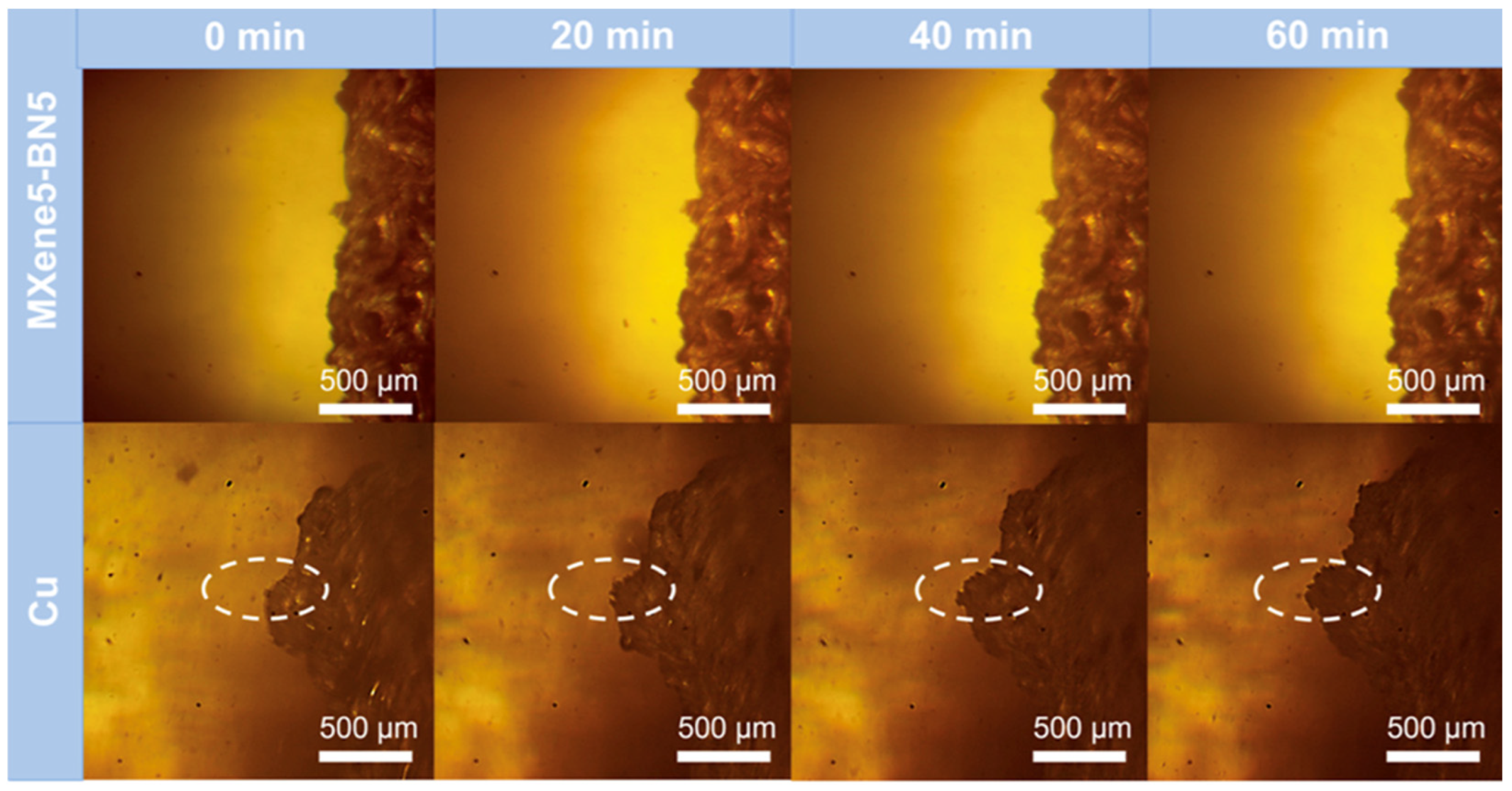
4.2. MXenes as Protective Layers
- -
- Uniform Li+ flux distribution: The polar surface terminations of MXenes (e.g., -O, -OH, -F) act as lithiophilic sites, lowering the nucleation barrier for lithium deposition and facilitating uniform plating beneath the coating.
- -
- Mechanical suppression: Due to their high Young’s modulus (~300–500 GPa), MXene layers can physically resist dendrite protrusion, acting as a mechanical shield without impeding ionic transport.
- -
- Electric field regulation: The excellent electronic conductivity of MXenes helps to redistribute local current densities at the Li–electrolyte interface, thereby mitigating field inhomogeneities that often trigger dendrite nucleation.
- -
- Chemical stability: MXene-based interlayers are relatively inert in contact with both lithium metal and solid electrolytes, reducing interfacial side reactions and preserving long-term interfacial integrity.
4.3. MXenes as Hosts for Metal Anodes
5. MXenes in Solid Electrolytes
5.1. MXenes in Solid Polymer Electrolytes (SPEs)
5.2. MXenes in Inorganic/Polymer Composite Electrolytes
5.3. Impact on Electrochemical Stability Window and Interfacial Compatibility
6. MXene-Based Interfacial Engineering in SSBs
6.1. Interfacial Resistance Issues in SSB Architectures
6.2. MXenes as Interlayers Between Electrodes and Electrolytes
6.3. Chemical Compatibility and Suppression of Interfacial Reactions
6.4. Integration Strategies for Scalable Fabrication
7. Challenges and Limitations
7.1. Material Synthesis Scalability and Environmental Concerns
7.1.1. Limitations of Conventional Wet-Etching Routes
7.1.2. Challenges of Molten-Salt and Fluorine-Free Synthesis
- -
- Energy intensity: High-temperature operation results in elevated energy costs and requires corrosion-resistant reactors.
- -
- Post-processing complexity: Residual salts must be thoroughly removed via extensive washing or vacuum annealing, generating additional wastewater and prolonging processing time.
- -
- Control over stoichiometry: Non-uniform etching or incomplete removal of A-layer elements can produce MXenes with heterogeneous surface chemistry and mixed phases, which impairs reproducibility [131].
7.1.3. Raw Material and Precursor Considerations
7.1.4. Environmental Impact and Sustainability
- -
- Fluoride waste management: Effluents containing LiF, AlF3, and other fluorides require specialized neutralization and disposal.
- -
- Water consumption: Washing and delamination steps consume large quantities of deionized water, with up to 50–100 L of rinse water per gram of MXene reported in some protocols [129].
- -
- Energy footprint: Both MAX phase synthesis and post-etching treatments (e.g., freeze-drying, annealing) are energy-intensive, raising concerns about the net carbon footprint of MXene production.
7.1.5. Pathways Toward Scalable and Sustainable Synthesis
- -
- Continuous-flow microreactors for LiF/HCl etching have been demonstrated to reduce batch time and improve yield consistency, as reported by Kim et al. [133].
- -
- Molten-salt recycling and closed-loop HF recovery systems have been proposed to reduce chemical waste and improve sustainability metrics [134].
- -
- Low-temperature plasma etching and mechanochemical exfoliation are emerging as dry alternatives that bypass liquid etching entirely, although scalability and termination control remain under investigation [135].
- -
- Green chemistry frameworks integrating solvent recovery, fluoride capture, and water reuse are essential to reduce the overall environmental impact and cost.
7.2. Chemical Stability and Oxidation Resistance of MXenes
7.3. Cost and Integration Barriers in SSB Manufacturing
8. Future Perspectives
8.1. Design of MXene Heterostructures and Hybrids for SSBs
8.2. Theoretical Insights and Computational Studies Guiding Material Optimization
- -
- High-throughput compositional discovery, enabling the identification of MXene stoichiometries and terminations with advantageous ion transport, redox, and stability profiles.
- -
- Interface modeling and hybrid architecture optimization, quantifying the roles of termination, heterostructure design, and ion conduction pathways in interfacial layers and solid electrolytes.
- -
- Multiphysics validation, combining DFT with continuum methods (e.g., finite element or phase-field models) to evaluate macroscale stability, throughput potential, and manufacturability.
8.3. Emerging MXene Compositions (e.g., Double Transition Metal MXenes)
8.4. Integration with Emerging Solid Electrolytes (e.g., Sulfide, Halide-Based)
8.5. Roadmap Towards Commercial Implementation
9. Conclusions
9.1. Summary of Key Findings
9.2. Outlook on MXenes as Enablers of Next-Generation SSB Technologies
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALD | Atomic Layer Deposition |
| BC | Carbon Black |
| BN | Boron Nitride |
| CCD | Critical Current Density |
| CNT | Carbon Nanotube |
| COF | Covalent Organic Framework |
| CTAB | Cetyltrimethylammonium Bromide |
| CVD | Chemical Vapor Deposition |
| DE | Delamination Efficiency |
| DFT | Density Functional Theory |
| DMF | Dimethylformamide |
| DMSO | Dimethyl Sulfoxide |
| DTM | Double Transition Metal (MXene subclass) |
| EC | Ethylene Carbonate (also used for the European Commission in a regulatory context) |
| EELS | Electron Energy Loss Spectroscopy |
| EIS | Electrochemical Impedance Spectroscopy |
| EMI | 1-Ethyl-3-methylimidazolium (ionic liquid cation) |
| EPA | United States Environmental Protection Agency |
| ESW | Electrochemical Stability Window |
| FEC | Fluoroethylene Carbonate (common electrolyte additive) |
| GCSE | Garnet-type Ceramic Solid Electrolyte |
| GO | Graphene Oxide |
| GWP | Global Warming Potential |
| LAMS | Lewis Acid Molten Salt (etching route for MXenes) |
| LATP | Lithium Aluminum Titanium Phosphate (Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte) |
| LCA | Life Cycle Assessment |
| LFP | Lithium Iron Phosphate |
| LGPS | Lithium Germanium Phosphorus Sulfide (Li10GeP2S12 solid electrolyte) |
| LLZO | Lithium Lanthanum Zirconium Oxide (Li7La3Zr2O12 garnet-type SSE) |
| LLZTO | Lithium Lanthanum Zirconium Tantalum Oxide (doped garnet-type SSE) |
| MAX | Layered ternary carbides/nitrides (Mn+1AXn phases, where M = early transition metal, A = A-group element, X = C or N) |
| MOF | Metal–Organic Framework |
References
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.-M.; Chen, Z. Review—Practical Challenges Hindering the Development of Solid State Li Ion Batteries. J. Electrochem. Soc. 2017, 164, A1731–A1744. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A Solid Future for Battery Development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Kalnaus, S.; Dudney, N.J.; Westover, A.S.; Herbert, E.; Hackney, S. Solid-State Batteries: The Critical Role of Mechanics. Science 2023, 381, eabg5998. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, Y.; Lotsch, B.; Hu, Y.-S.; Li, H.; Janek, J.; Nazar, L.F.; Nan, C.-W.; Maier, J.; Armand, M.; et al. New Horizons for Inorganic Solid State Ion Conductors. Energy Environ. Sci. 2018, 11, 1945–1976. [Google Scholar] [CrossRef]
- Krauskopf, T.; Hartmann, H.; Zeier, W.G.; Janek, J. Toward a Fundamental Understanding of the Lithium Metal Anode in Solid-State Batteries—An Electrochemo-Mechanical Study on the Garnet-Type Solid Electrolyte Li6.25Al0.25La3Zr2O12. ACS Appl. Mater. Interfaces 2019, 11, 14463–14477. [Google Scholar] [CrossRef]
- Takada, K. Progress and Prospective of Solid-State Lithium Batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-Type Solid-State Fast Li Ion Conductors for Li Batteries: Critical Review. Chem. Soc. Rev. 2014, 43, 4714. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of Inorganic Solid-State Electrolytes for Batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-Based Materials for Electrochemical Energy Storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks. Angew. Chem. Int. Ed. 2017, 56, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Sasaki, T.; Yunoki, S. Electronic Properties and Applications of MXenes: A Theoretical Review. J. Mater. Chem. C 2017, 5, 2488–2503. [Google Scholar] [CrossRef]
- Zhang, C.; Anasori, B.; Seral-Ascaso, A.; Park, S.; McEvoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, Flexible, and Conductive 2D Titanium Carbide (MXene) Films with High Volumetric Capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Er, D.; Li, J.; Naguib, M.; Gogotsi, Y.; Shenoy, V.B. Ti3C2 MXene as a High Capacity Electrode Material for Metal (Li, Na, K, Ca) Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 11173–11179. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P.; et al. Ultra-High-Rate Pseudocapacitive Energy Storage in Two-Dimensional Transition Metal Carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Belay Ibrahim, K.; Ahmed Shifa, T.; Zorzi, S.; Getaye Sendeku, M.; Moretti, E.; Vomiero, A. Emerging 2D Materials beyond Mxenes and TMDs: Transition Metal Carbo-Chalcogenides. Prog. Mater. Sci. 2024, 144, 101287. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Meng, X.; Wang, R. MXene-Engineered Lithium–Sulfur Batteries. J. Mater. Chem. A 2019, 7, 22730–22743. [Google Scholar] [CrossRef]
- Badawi, N.; Bhuyan, M.; Luqman, M.; Alshareef, R.S.; Rafe Hatshan, M.; Al-Warthan, A.; Farooq Adil, S. MXenes the Future of Solid-State Supercapacitors: Status, Challenges, Prospects, and Applications. Arab. J. Chem. 2024, 17, 105866. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene Chemistry, Electrochemistry and Energy Storage Applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- Long, M.Q.; Tang, K.K.; Xiao, J.; Li, J.Y.; Chen, J.; Gao, H.; Chen, W.H.; Liu, C.T.; Liu, H. Recent Advances on MXene Based Materials for Energy Storage Applications. Mater. Today Sustain. 2022, 19, 100163. [Google Scholar] [CrossRef]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides; Wiley-VCH: Hoboken, NJ, USA, 2013. [Google Scholar]
- Borah, A.J.; Natu, V.; Biswas, A.; Srivastava, A. A Review on Recent Progress in Synthesis, Properties, and Applications of MXenes. Oxf. Open Mater. Sci. 2025, 5, itae017. [Google Scholar] [CrossRef]
- Lipatov, A.; Alhabeb, M.; Lu, H.; Zhao, S.; Loes, M.J.; Vorobeva, N.S.; Dall’Agnese, Y.; Gao, Y.; Gruverman, A.; Gogotsi, Y.; et al. Electrical and Elastic Properties of Individual Single-Layer Nb4C3Tx MXene Flakes. Adv. Electron. Mater. 2020, 6, 1901382. [Google Scholar] [CrossRef]
- Hope, M.A.; Forse, A.C.; Griffith, K.J.; Lukatskaya, M.R.; Ghidiu, M.; Gogotsi, Y.; Grey, C.P. NMR Reveals the Surface Functionalisation of Ti3C2 MXene. Phys. Chem. Chem. Phys. 2016, 18, 5099–5102. [Google Scholar] [CrossRef]
- Gouveia, J.D.; Gomes, J.R.B. Effect of Surface Composition on the Stability of Ti- and V-Based Oxycarbide and Oxynitride MXenes. Mater. Today Phys. 2024, 46, 101481. [Google Scholar] [CrossRef]
- Du, W.; Yang, L.; Feng, J.; Zhu, W.; Li, J.; Zhang, P.; Ma, Q. Advancements in Methodologies and Techniques for the Synthesis of Energetic Materials: A Review. Energetic Mater. Front. 2024, 5, 175–190. [Google Scholar] [CrossRef]
- Plaickner, J.; Petit, T.; Bärmann, P.; Schultz, T.; Koch, N.; Esser, N. Surface Termination Effects on Raman Spectra of Ti3C2Tx MXenes: An in Situ UHV Analysis. Phys. Chem. Chem. Phys. 2024, 26, 20883–20890. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes): Synthesis, Properties, and Electrochemical Energy Storage Applications. Energy Environ. Mater. 2020, 3, 29–55. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Shekhirev, M.; Busa, J.; Shuck, C.E.; Torres, A.; Bagheri, S.; Sinitskii, A.; Gogotsi, Y. Ultralarge Flakes of Ti3C2Tx MXene via Soft Delamination. ACS Nano 2022, 16, 13695–13703. [Google Scholar] [CrossRef]
- Shuck, C.E.; Sarycheva, A.; Anayee, M.; Levitt, A.; Zhu, Y.; Uzun, S.; Balitskiy, V.; Zahorodna, V.; Gogotsi, O.; Gogotsi, Y. Scalable Synthesis of Ti3C2Tx MXene. Adv. Eng. Mater. 2020, 22, 1901241. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide ‘Clay’ with High Volumetric Capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Kruger, D.D.; García, H.; Primo, A. Molten Salt Derived MXenes: Synthesis and Applications. Adv. Sci. 2024, 11, 2307106. [Google Scholar] [CrossRef]
- Kareem, S.A.; Ibrahim, M.A.; Anaele, J.U.; Olanrewaju, O.F.; Aikulola, E.O.; Bodunrin, M.O. Recent Advances in Machine Learning Applications for MXene Materials: Design, Synthesis, Characterization, and Commercialization for Energy and Environmental Applications. Next Mater. 2025, 8, 100864. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Anwar, M.T.; Ahmad, R.; Ahmad, M.S.; Usman, M.; Fawy, K.F. MXenes: Transforming Advanced Materials through Electrochemical Reduction Reactions—Opportunities and Challenges. Chem. Eng. J. 2025, 506, 159926. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A Promising Transition Metal Carbide Anode for Lithium-Ion Batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Chen, X.; Zhang, Y.; Zhu, F.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A Hydrothermal Etching Route to Synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced Exfoliation and Improved Adsorption Performance. Ceram. Int. 2018, 44, 18886–18893. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Chy, M.N.U.; Rahman, M.A.; Kim, J.-H.; Barua, N.; Dujana, W.A. MXene as Promising Anode Material for High-Performance Lithium-Ion Batteries: A Comprehensive Review. Nanomaterials 2024, 14, 616. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Guo, F.; Hou, K.; Guan, G.; Lu, L.; Zhang, Y.; Xu, J.; Shang, Y. Highly Flexible All-Solid-State Supercapacitors Based on MXene/CNT Composites. Energy Fuels 2023, 37, 9704–9712. [Google Scholar] [CrossRef]
- Zachariah, S.; Indirajith, R.; Rajalakshmi, M. Recent Advances in Optoelectronic Properties and Applications of Ti3C2Tx MXene. J. Alloys Compd. 2025, 1011, 178296. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase Engineering of Transition Metal Dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Wang, G. MXene-Enabled Interfaces and Architectures for High-Performance Zinc Anodes in Aqueous Zinc-Ion Batteries. Int. J. Electrochem. Sci. 2025, 20, 101023. [Google Scholar] [CrossRef]
- Man, Q.; An, Y.; Shen, H.; Wei, C.; Zhang, X.; Wang, Z.; Xiong, S.; Feng, J. MXenes and Their Derivatives for Advanced Solid-State Energy Storage Devices. Adv. Funct. Mater. 2023, 33, 2303668. [Google Scholar] [CrossRef]
- Protyai, M.I.H.; Bin Rashid, A. A comprehensive overview of recent progress in MXene-based polymer composites: Their fabrication processes, advanced applications, and prospects. Heliyon 2024, 10, e37030. [Google Scholar] [CrossRef]
- Pan, Q.; Zheng, Y.; Kota, S.; Huang, W.; Wang, S.; Qi, H.; Kim, S.; Tu, Y.; Barsoum, M.W.; Li, C.Y. 2D MXene-Containing Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. Nanoscale Adv. 2019, 1, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, N.; Kanwal, N.; Ali, M.; Ali, K.; Hasnain, A.; Ashraf, M.; Ayaz, M.; Ifthikar, J.; Ali, S.; Hendi, A.; et al. Materials Advancements in Solid-State Inorganic Electrolytes for Highly Anticipated All Solid Li-Ion Batteries. Energy Storage Mater. 2024, 71, 103619. [Google Scholar] [CrossRef]
- Yu, R. Recent Advances of Sulfide Electrolytes in All-Solid-State Lithium Batteries. MATEC Web Conf. 2025, 410, 01030. [Google Scholar] [CrossRef]
- Jacob, M.; Moreno Fernández, H.; Haben, A.; Waidha, A.I.; Özel, S.; Hofmann, J.P.; Kautenburger, R.; Clemens, O.; Wissel, K. Direct Recycling of All-Solid-State Batteries with a Halide Solid Electrolyte via Water-Based Separation: Interactions of Electrode Materials in Aqueous Li3 InCl6 Solutions. Batter. Supercaps 2025, 2500189. [Google Scholar] [CrossRef]
- Munir, M.A.; Khalid, S. Focused Review on the Synthesis of Titanium Carbide MXene via Fluorine-Free Methods for Lithium-Ion Batteries. Energy Fuels 2025, 39, 2889–2915. [Google Scholar] [CrossRef]
- Wu, B.; Chen, C.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. All-Solid-State Thin Film Li-Ion Batteries: New Challenges, New Materials, and New Designs. Batteries 2023, 9, 186. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Zhao, F.; Li, J.; Yang, H.; Wang, Y.; He, Y. Multifunction of MXene in Lithium–Sulfur Batteries: A Review. Energy Fuels 2024, 38, 13837–13857. [Google Scholar] [CrossRef]
- Xu, H.; Liu, S.; Li, Z.; Ding, F.; Wang, T.; Liu, T.; Wang, W.; Song, K.; Liu, J.; Hu, L. Ti3C2Tx MXene Enhanced PEO/SN-Based Solid Electrolyte for High-Performance Li Metal Battery. J. Mater. Sci. Technol. 2025, 219, 101–112. [Google Scholar] [CrossRef]
- Zarepour, A.; Ahmadi, S.; Rabiee, N.; Zarrabi, A.; Iravani, S. Self-Healing MXene- and Graphene-Based Composites: Properties and Applications. Nano-Micro Lett. 2023, 15, 100. [Google Scholar] [CrossRef]
- Hu, M.; Chen, L.; Jing, Y.; Zhu, Y.; Dai, J.; Meng, A.; Sun, C.; Jia, J.; Li, Z. Intensifying Electrochemical Activity of Ti3C2Tx MXene via Customized Interlayer Structure and Surface Chemistry. Molecules 2023, 28, 5776. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Rozier, P.; Duployer, B.; Taberna, P.-L.; Anasori, B.; Gogotsi, Y.; Simon, P. Electrochemical and In-Situ X-Ray Diffraction Studies of Ti3C2Tx MXene in Ionic Liquid Electrolyte. Electrochem. Commun. 2016, 72, 50–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Xiao, Y.; Li, C.; Tan, H.H.; Liu, J.; Wu, Y. Theoretical Insights into the Favorable Functionalized Ti2C-Based MXenes for Lithium–Sulfur Batteries. ACS Omega 2020, 5, 29272–29283. [Google Scholar] [CrossRef]
- Kiai, M.S.; Aslfattahi, N.; Karatas, D.; Baydogan, N.; Samylingam, L.; Kadirgama, K.; Kok, C.K. Experimental and DFT Investigations on Multifunctional Ti3C2Tx MXenes/PDAAQ Free Standing Interlayer for Enhancing the Cycle Life and High-Rate Performance of Na–S Batteries. J. Phys. Chem. Solids 2025, 208, 113083. [Google Scholar] [CrossRef]
- Gentile, A.; Pianta, N.; Fracchia, M.; Pollastri, S.; Ferrara, C.; Marchionna, S.; Aquilanti, G.; Tosoni, S.; Ghigna, P.; Ruffo, R. Ti3C2Tx MXenes as Anodes for Sodium-Ion Batteries: The In Situ Comprehension of the Electrode Reaction. ACS Appl. Energy Mater. 2025, 8, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.K.; Askari, M.; Merenda, A.; Shah, D.; Tijing, L. High-Capacity and Selective Lithium-Ion Recovery Via Ti3C2Tx @Sno2 Composite Electrodes Using Hybrid Capacitive Deionization. 2025. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5273042 (accessed on 23 August 2025).
- Shreenag Meda, U.; Madan Raikar, O.; Adaguru Rudregowda, C.; Rangappa, D.; Rani, N.; Ranga, S.S.; Pandey, A. MXenes as Versatile Materials for Hydrogen Technology and Multifunctional Applications. Chem. Asian J. 2025, 20, e202401678. [Google Scholar] [CrossRef] [PubMed]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The World of Two-Dimensional Carbides and Nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef] [PubMed]
- Machín, A.; Morant, C.; Márquez, F. Advancements and Challenges in Solid-State Battery Technology: An In-Depth Review of Solid Electrolytes and Anode Innovations. Batteries 2024, 10, 29. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Han, H.; Mhin, S. MXenes and MXene-Based Composites: Preparation, Characteristics, Theoretical Investigations, and Application in Developing Sulfur Cathodes, Lithium Anodes, and Functional Separators for Lithium–Sulfur Batteries. Batteries 2025, 11, 206. [Google Scholar] [CrossRef]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur Cathodes Based on Conductive MXene Nanosheets for High-Performance Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2015, 54, 3907–3911. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Jiang, Q.; El-Demellawi, J.K.; Kim, H.; Alshareef, H.N. MXene Printing and Patterned Coating for Device Applications. Adv. Mater. 2020, 32, 1908486. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, C.; Li, Q.; Zhang, S.; Wang, C.; Zhang, Z.; Shi, Y.; Yang, L.; Yin, L.; Wang, R. High Performance All-Solid-State Li–Se Battery Based on Selenium Loaded on Ti3C2 MXene Cathode. Green Energy Resour. 2024, 2, 100058. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.; Zhang, Y.; Wei, Y.; Shen, N.; Chen, S.; Xu, B. Burgeoning Silicon/MXene Nanocomposites for Lithium Ion Batteries: A Review. Adv. Funct. Mater. 2024, 34, 2402307. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, C.; Zhou, X.; Hou, J.; Jiang, S.; Meng, L.; Zhang, Y. Recent Progress in Si/Ti3C2Tx MXene Anode Materials for Lithium-Ion Batteries. iScience 2024, 27, 111217. [Google Scholar] [CrossRef] [PubMed]
- Myint, W.; Lolupiman, K.; Yang, C.; Woottapanit, P.; Limphirat, W.; Kidkhunthod, P.; Muzakir, M.; Karnan, M.; Zhang, X.; Qin, J. Exploring the Electrochemical Superiority of V2O5/TiO2@Ti3C2-MXene Hybrid Nanostructures for Enhanced Lithium-Ion Battery Performance. ACS Appl. Mater. Interfaces 2024, 16, 53764–53774. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, L.; Yin, G.; Tao, X.; Yu, L.; Wang, X.; Sun, C.; Sun, Y.; Hong, E.; Zhao, G.; et al. 2D Porous Ti3C2 MXene as Anode Material for Sodium-Ion Batteries with Excellent Reaction Kinetics. Molecules 2025, 30, 1100. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, D.; Li, P.; Guo, X.; Wang, C.; Kang, F.; Li, B.; Wang, G. High-Performance Quasi-Solid-State MXene-Based Li–I Batteries. ACS Cent. Sci. 2019, 5, 365–373. [Google Scholar] [CrossRef]
- Garcı́a, R.E.; Chiang, Y.-M.; Craig Carter, W.; Limthongkul, P.; Bishop, C.M. Microstructural Modeling and Design of Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A255. [Google Scholar] [CrossRef]
- Sharafi, A.; Kazyak, E.; Davis, A.L.; Yu, S.; Thompson, T.; Siegel, D.J.; Dasgupta, N.P.; Sakamoto, J. Surface Chemistry Mechanism of Ultra-Low Interfacial Resistance in the Solid-State Electrolyte Li7La3Zr2O12. Chem. Mater. 2017, 29, 7961–7968. [Google Scholar] [CrossRef]
- Porz, L.; Swamy, T.; Sheldon, B.W.; Rettenwander, D.; Frömling, T.; Thaman, H.L.; Berendts, S.; Uecker, R.; Carter, W.C.; Chiang, Y. Mechanism of Lithium Metal Penetration through Inorganic Solid Electrolytes. Adv. Energy Mater. 2017, 7, 1701003. [Google Scholar] [CrossRef]
- Kazyak, E.; Garcia-Mendez, R.; LePage, W.S.; Sharafi, A.; Davis, A.L.; Sanchez, A.J.; Chen, K.-H.; Haslam, C.; Sakamoto, J.; Dasgupta, N.P. Li Penetration in Ceramic Solid Electrolytes: Operando Microscopy Analysis of Morphology, Propagation, and Reversibility. Matter 2020, 2, 1025–1048. [Google Scholar] [CrossRef]
- Harry, K.J.; Hallinan, D.T.; Parkinson, D.Y.; MacDowell, A.A.; Balsara, N.P. Detection of Subsurface Structures underneath Dendrites Formed on Cycled Lithium Metal Electrodes. Nat. Mater. 2014, 13, 69–73. [Google Scholar] [CrossRef]
- Liu, M.; Song, A.; Zhang, X.; Wang, J.; Fan, Y.; Wang, G.; Tian, H.; Ma, Z.; Shao, G. Interfacial Lithium-Ion Transportation in Solid-State Batteries: Challenges and Prospects. Nano Energy 2025, 136, 110749. [Google Scholar] [CrossRef]
- Lou, S.; Yu, Z.; Liu, Q.; Wang, H.; Chen, M.; Wang, J. Multi-Scale Imaging of Solid-State Battery Interfaces: From Atomic Scale to Macroscopic Scale. Chem 2020, 6, 2199–2218. [Google Scholar] [CrossRef]
- Strauss, F.; Kitsche, D.; Ma, Y.; Teo, J.H.; Goonetilleke, D.; Janek, J.; Bianchini, M.; Brezesinski, T. Operando Characterization Techniques for All-Solid-State Lithium-Ion Batteries. Adv. Energy Sustain. Res. 2021, 2, 2100004. [Google Scholar] [CrossRef]
- Huang, J.; Wu, K.; Xu, G.; Wu, M.; Dou, S.; Wu, C. Recent Progress and Strategic Perspectives of Inorganic Solid Electrolytes: Fundamentals, Modifications, and Applications in Sodium Metal Batteries. Chem. Soc. Rev. 2023, 52, 4933–4995. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Z.; Chen, K.; Jiang, Y.; Yue, M.; Dong, K.; Liu, Y.; Guo, Y.; Wang, Y. MXene-BN-Introduced Artificial SEI to Inhibit Dendrite Growth of Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 56356–56364. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, J.; Chen, W.; Zhang, W.; Sun, C. Recent Advances in Garnet-Based Electrolytes for Solid-State Lithium Metal Batteries: Interfacial Challenges and Engineering Strategies. Mater. Horiz. 2025, 12, 6082–6123. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.; Chen, D.; Ma, F.; Huang, J.; Wang, Y.; Wang, L.; Wu, Y.; Chen, Y. A Dual-Protective MXene/COF Artificial Interface for Dendrite-Free and Stable Lithium Metal Anodes. Adv. Funct. Mater. 2025, 2505390. [Google Scholar] [CrossRef]
- Li, X.L.; Lieu, W.Y.; Wang, L.; Yan, D.; Li, Y.; Ghosh, T.; Li, Y.; Lu, J.; Seh, Z.W.; Yang, H.Y. Silver-Atom Modulation of Ti Vacancies in MXene Enables Uniform Spherical Lithium Deposition. ACS Energy Lett. 2024, 9, 4929–4938. [Google Scholar] [CrossRef]
- Ha, S.; Kim, D.; Lim, H.; Koo, C.M.; Kim, S.J.; Yun, Y.S. Lithiophilic MXene-Guided Lithium Metal Nucleation and Growth Behavior. Adv. Funct. Mater. 2021, 31, 2101261. [Google Scholar] [CrossRef]
- Tao, F.; Xie, D.; Diao, W.-Y.; Liu, C.; Sun, H.-Z.; Li, W.-L.; Zhang, J.-P.; Wu, X.-L. Highly Lithiophilic Ti3C2Tx-Mxene Anchored on a Flexible Carbon Foam Scaffolds as the Basis for a Dendrite-Free Lithium Metal Anode. New Carbon. Mater. 2023, 38, 765–773. [Google Scholar] [CrossRef]
- Yoon, J.; Chae, O.B.; Wu, M.; Jung, H.-T. Dual-Functional Surface of MXene Anodes Boosts Long-Term Cyclability of Lithium-Metal Batteries. J. Mater. Chem. A 2025, 13, 17511–17518. [Google Scholar] [CrossRef]
- Zeng, X.; Mahato, M.; Oh, W.; Yoo, H.; Nguyen, V.H.; Oh, S.; Valurouthu, G.; Jeong, S.; Ahn, C.W.; Gogotsi, Y.; et al. Stoichiometric Ti3C2TxCoating for Inhibiting Dendrite Growth in Anode-Free Lithium Metal Batteries. Energy Environ. Mater. 2024, 7, e12686. [Google Scholar] [CrossRef]
- Wei, C.; Wang, Y.; Zhang, Y.; Tan, L.; Qian, Y.; Tao, Y.; Xiong, S.; Feng, J. Flexible and Stable 3D Lithium Metal Anodes Based on Self-Standing MXene/COF Frameworks for High-Performance Lithium-Sulfur Batteries. Nano Res. 2021, 14, 3576–3584. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, R.; Wang, A.; Guo, W.; Liu, X.; Luo, J. MXene Aerogel Scaffolds for High-Rate Lithium Metal Anodes. Angew. Chem. Int. Ed. 2018, 57, 15028–15033. [Google Scholar] [CrossRef]
- Zhang, B.; Ju, Z.; Xie, Q.; Luo, J.; Du, L.; Zhang, C.; Tao, X. Ti3CNTx MXene/rGO Scaffolds Directing the Formation of a Robust, Layered SEI toward High-Rate and Long-Cycle Lithium Metal Batteries. Energy Storage Mater. 2023, 58, 322–331. [Google Scholar] [CrossRef]
- Wei, C.; Tao, Y.; An, Y.; Tian, Y.; Zhang, Y.; Feng, J.; Qian, Y. Recent Advances of Emerging 2D MXene for Stable and Dendrite-Free Metal Anodes. Adv. Funct. Mater. 2020, 30, 2004613. [Google Scholar] [CrossRef]
- Mao, Y.-Q.; Dong, G.-H.; Zhu, W.-B.; Li, Y.-Q.; Huang, P.; Fu, S.-Y. Novel Sandwich Structured Glass Fiber Cloth/Poly(Ethylene Oxide)-Mxene Composite Electrolyte. Nano Materials Sci. 2024, 6, 60–67. [Google Scholar] [CrossRef]
- Vijayananth, K.; Palaniappan, S.K.; Singh, M.K.; Pudhupalayam Muthukutti, G.; Mavinkere Rangappa, S.; Siengchin, S. Exploring MXene-Polymer Composites for Mechanical, Tribological, and EMI Shielding Applications. Polym. Compos. 2025, 1–24. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Pei, N.; Chen, Z.; Li, R.; Fu, L.; Zhang, P.; Zhao, J. The Critical Role of Fillers in Composite Polymer Electrolytes for Lithium Battery. Nano-Micro Lett. 2023, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Tian, S.; Liu, T.; Yang, M.; Song, B.; Zhang, W. MXene-Coordinated Polymer Electrolytes for High-Performance Solid-State Lithium Metal Batteries. Chem. Eng. J. 2025, 522, 168030. [Google Scholar] [CrossRef]
- Narayanasamy, M.; Zaman, S.; Koo, C.M. 2D MXenes for All-Solid-State Batteries: A Comprehensive Review. Mater. Today Energy 2023, 37, 101405. [Google Scholar] [CrossRef]
- Hadad, S.; Hamrahjoo, M.; Khezraqa, H.; Golshan, M.; Wang, Z.; Salami-Kalajahi, M. Starch Acetate Grafted to MXene Composite Surpasses Room Temperature Liquid Electrolyte Performance for All-Solid-State Lithium-Ion Batteries. Adv. Sci. 2025, 12, e03285. [Google Scholar] [CrossRef]
- Likitaporn, C.; Okhawilai, M.; Kasemsiri, P.; Qin, J.; Potiyaraj, P.; Uyama, H. High Electrolyte Uptake of MXene Integrated Membrane Separators for Zn-Ion Batteries. Sci. Rep. 2022, 12, 19915. [Google Scholar] [CrossRef]
- Lee, A.; Shekhirev, M.; Anayee, M.; Gogotsi, Y. Multi-Year Study of Environmental Stability of Ti3C2Tx MXene Films. Graphene 2D Mater. 2024, 9, 77–85. [Google Scholar] [CrossRef]
- Serajian, S.; Shamsabadi, A.A.; Gnani Peer Mohamed, S.I.; Nejati, S.; Bavarian, M. MXenes in Solid-State Batteries: Current Status and Outlook. J. Power Sources 2024, 610, 234721. [Google Scholar] [CrossRef]
- Sharma, V.; Datta, D. Variation in Interface Strength of Silicon with Surface Engineered Ti3C2 MXenes. arXiv 2020. [Google Scholar] [CrossRef]
- Xu, L.; Wu, T.; Kent, P.R.C.; Jiang, D. Interfacial Charge Transfer and Interaction in the MXene/TiO2 Heterostructures. arXiv 2021. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Bo, S.-H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding Interface Stability in Solid-State Batteries. Nat. Rev. Mater. 2019, 5, 105–126. [Google Scholar] [CrossRef]
- Byeon, Y.-W.; Kim, H. Review on Interface and Interphase Issues in Sulfide Solid-State Electrolytes for All-Solid-State Li-Metal Batteries. Electrochem 2021, 2, 452–471. [Google Scholar] [CrossRef]
- Wang, S.; Fang, R.; Li, Y.; Liu, Y.; Xin, C.; Richter, F.H.; Nan, C.-W. Interfacial Challenges for All-Solid-State Batteries Based on Sulfide Solid Electrolytes. J. Mater. 2021, 7, 209–218. [Google Scholar] [CrossRef]
- Qi, Y.; Swift, M.W.; Fuller, E.J.; Talin, A.A. Interface Potentials inside Solid-State Batteries: Origins and Implications. MRS Bull. 2023, 48, 1239–1246. [Google Scholar] [CrossRef]
- Wen, J.; Huang, L.; Huang, Y.; Luo, W.; Huo, H.; Wang, Z.; Zheng, X.; Wen, Z.; Huang, Y. A Lithium-MXene Composite Anode with High Specific Capacity and Low Interfacial Resistance for Solid-State Batteries. Energy Storage Mater. 2022, 45, 934–940. [Google Scholar] [CrossRef]
- Kawai, K.; Lee, H.; Nomura, Y.; Fujita, M.; Kitaura, H.; Hosono, E.; Nakajima, H.; Tsukasaki, H.; Mori, S.; Sakuda, A.; et al. MXene Electrodes for All Strain-Free Solid-State Batteries. ACS Appl. Mater. Interfaces 2024, 16, 57377–57385. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Shi, X.; Liao, S.; Liu, M.; Xu, Y.; Li, Z.; Liu, J.; Li, X. Structural Design and Investigation of Ti3C2 MXene as a Conductive Interlayer for Improving the Lithium-Storage Performance of PSi@C Anode Material. Electrochim. Acta 2024, 508, 145216. [Google Scholar] [CrossRef]
- Han, X.; Zhou, W.; Chen, M.; Chen, J.; Wang, G.; Liu, B.; Luo, L.; Chen, S.; Zhang, Q.; Shi, S.; et al. Interfacial Nitrogen Engineering of Robust Silicon/MXene Anode toward High Energy Solid-State Lithium-Ion Batteries. J. Energy Chem. 2022, 67, 727–735. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, P.; Xu, Z.; Liu, X.-X.; Feng, S.; Cao, M.; Cao, C.; Wang, X.; Pan, L.; Sun, Z.-M. Ti3C2Tx MXene In-Situ Transformed Li2TiO3 Interface Layer Enabling 4.5 V-LiCoO2/Sulfide All-Solid-State Lithium Batteries with Superior Rate Capability and Cyclability. Chin. Chem. Lett. 2024, 35, 108776. [Google Scholar] [CrossRef]
- Coley, W.; Akhavi, A.-A.; Pena, P.; Shang, R.; Ma, Y.; Moseni, K.; Ozkan, M.; Ozkan, C.S. Charging the Future with Pioneering MXenes: Scalable 2D Materials for Next-Generation Batteries. Nanomaterials 2025, 15, 1089. [Google Scholar] [CrossRef]
- Ren, F.; Liang, Z.; Zhao, W.; Zuo, W.; Lin, M.; Wu, Y.; Yang, X.; Gong, Z.; Yang, Y. The Nature and Suppression Strategies of Interfacial Reactions in All-Solid-State Batteries. Energy Environ. Sci. 2023, 16, 2579–2590. [Google Scholar] [CrossRef]
- Jayan, R.; Islam, M.M. Functionalized MXenes as Effective Polyselenides Immobilizer for Lithium-Selenium Batteries: A Density Functional Theory (DFT) Study. arXiv 2020. [Google Scholar] [CrossRef]
- Ali, I.; Faraz Ud Din, M.; Gu, Z.-G. MXenes Thin Films: From Fabrication to Their Applications. Molecules 2022, 27, 4925. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhou, D.; Deng, S.; Jafarpour, M.; Avaro, J.; Neels, A.; Heier, J.; Zhang, C. Rational Design of Ti3C2Tx MXene Inks for Conductive, Transparent Films. ACS Nano 2023, 17, 3737–3749. [Google Scholar] [CrossRef]
- Yuk, S.; Woo, S.; Kim, S.; Choi, S.; Byun, S.; Song, S.H.; Lee, D. Scalable Liquid-Crystalline MXene Films via Bio-Inspired Surface Bridging Strategy for Enhancing Electrochemical Performance. Adv. Compos. Hybrid. Mater. 2025, 8, 241. [Google Scholar] [CrossRef]
- Awan, H.T.A.; Abdah, M.A.A.M.; Mehar, M.; Walvekar, R.; Chaudhary, V.; Khalid, M.; Khosla, A. MXene-Polymer Hybrid Composites for Advanced Energy Storage: Insights into Supercapacitors and Batteries. J. Energy Storage 2024, 95, 112449. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Heier, J.; Zhang, C. Coating Porous MXene Films with Tunable Porosity for High-Performance Solid-State Supercapacitors. ChemElectroChem 2021, 8, 1911–1917. [Google Scholar] [CrossRef]
- Dadashi Firouzjaei, M.; Nemani, S.K.; Sadrzadeh, M.; Wujcik, E.K.; Elliott, M.; Anasori, B. Life-Cycle Assessment of Ti3C2TxMXene Synthesis. Adv. Mater. 2023, 35, 2300422. [Google Scholar] [CrossRef]
- Zhang, T.; Shevchuk, K.; Wang, R.J.; Kim, H.; Hourani, J.; Gogotsi, Y. Delamination of Chlorine-Terminated MXene Produced Using Molten Salt Etching. Chem. Mater. 2024, 36, 1998–2006. [Google Scholar] [CrossRef]
- Soundiraraju, B.; George, B.K. Two-Dimensional Titanium Nitride (Ti2N) MXene: Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef]
- Ungureanu, A.; Francini, A.; Neri, P.; Girimonte, A.; Giovanardi, R.; Ferrari, A.M.; Rosa, R. Systematic Life Cycle Environmental Impact Comparison of Alternative Synthetic Strategies for Ti3C2Tx MXene. ACS Sustain. Chem. Eng. 2024, 12, 5893–5906. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, H.; Yun, J.; Lee, J.-W.; Cho, J.; Kang, K.; Lim, H.-D. Sustainable and Eco-Friendly Syntheses of Green MXenes for Advanced Battery Applications. Nano Converg. 2025, 12, 39. [Google Scholar] [CrossRef]
- Shuck, C.E.; Ventura-Martinez, K.; Goad, A.; Uzun, S.; Shekhirev, M.; Gogotsi, Y. Safe Synthesis of MAX and MXene: Guidelines to Reduce Risk During Synthesis. ACS Chem. Health Saf. 2021, 28, 326–338. [Google Scholar] [CrossRef]
- Fang, H.; Thakur, A.; Zahmatkeshsaredorahi, A.; Fang, Z.; Rad, V.; Shamsabadi, A.A.; Pereyra, C.; Soroush, M.; Rappe, A.M.; Xu, X.G.; et al. Stabilizing Ti3C2Tx MXene Flakes in Air by Removing Confined Water. Proc. Natl. Acad. Sci. USA 2024, 121, e2400084121. [Google Scholar] [CrossRef] [PubMed]
- Habib, T.; Zhao, X.; Shah, S.A.; Chen, Y.; Sun, W.; An, H.; Lutkenhaus, J.L.; Radovic, M.; Green, M.J. Oxidation Stability of Ti3C2Tx MXene Nanosheets in Solvents and Composite Films. Npj 2D Mater. Appl. 2019, 3, 8. [Google Scholar] [CrossRef]
- Zhao, X.; Vashisth, A.; Prehn, E.; Sun, W.; Shah, S.A.; Habib, T.; Chen, Y.; Tan, Z.; Lutkenhaus, J.L.; Radovic, M.; et al. Antioxidants Unlock Shelf-Stable Ti3C2Tx (MXene) Nanosheet Dispersions. Matter 2019, 1, 513–526. [Google Scholar] [CrossRef]
- Liu, N.; Li, Q.; Wan, H.; Chang, L.; Wang, H.; Fang, J.; Ding, T.; Wen, Q.; Zhou, L.; Xiao, X. High-Temperature Stability in Air of Ti3C2Tx MXene-Based Composite with Extracted Bentonite. Nat. Commun. 2022, 13, 5551. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent Surface Modifications and Superconductivity of Two-Dimensional Metal Carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Björk, J.; Rosen, J. Functionalizing MXenes by Tailoring Surface Terminations in Different Chemical Environments. Chem. Mater. 2021, 33, 9108–9118. [Google Scholar] [CrossRef]
- Adomaviciute-Grabusove, S.; Popov, A.; Ramanavicius, S.; Sablinskas, V.; Shevchuk, K.; Gogotsi, O.; Baginskiy, I.; Gogotsi, Y.; Ramanavicius, A. Monitoring Ti3C2Tx MXene Degradation Pathways Using Raman Spectroscopy. ACS Nano 2024, 18, 13184–13195. [Google Scholar] [CrossRef]
- Choi, E.; Lee, J.; Kim, Y.-J.; Kim, H.; Kim, M.; Hong, J.; Kang, Y.C.; Koo, C.M.; Kim, D.W.; Kim, S.J. Enhanced Stability of Ti3C2Tx MXene Enabled by Continuous ZIF-8 Coating. Carbon 2022, 191, 593–599. [Google Scholar] [CrossRef]
- Iqbal, A.; Hong, J.; Ko, T.Y.; Koo, C.M. Improving Oxidation Stability of 2D MXenes: Synthesis, Storage Media, and Conditions. Nano Converg. 2021, 8, 9. [Google Scholar] [CrossRef]
- Zaed, M.A.; Tan, K.H.; Abdullah, N.; Saidur, R.; Pandey, A.K.; Saleque, A.M. Cost Analysis of MXene for Low-Cost Production, and Pinpointing of Its Economic Footprint. Open Ceram. 2024, 17, 100526. [Google Scholar] [CrossRef]
- Lu, X.; Lian, G.J.; Parker, J.; Ge, R.; Sadan, M.K.; Smith, R.M.; Cumming, D. Effect of Carbon Blacks on Electrical Conduction and Conductive Binder Domain of Next-Generation Lithium-Ion Batteries. J. Power Sources 2024, 592, 233916. [Google Scholar] [CrossRef]
- Jiang, M.; Jiang, D.; Cao, X.; Wang, J.; Sun, Y.; Zhang, M.; Liu, J. Scalable 2D/2D Assembly of Ultrathin MOF/MXene Sheets for Stretchable and Bendable Energy Storage Devices. Adv. Funct. Mater. 2024, 34, 2312692. [Google Scholar] [CrossRef]
- Wan, S.; Li, X.; Chen, Y.; Liu, N.; Du, Y.; Dou, S.; Jiang, L.; Cheng, Q. High-Strength Scalable MXene Films through Bridging-Induced Densification. Science 2021, 374, 96–99. [Google Scholar] [CrossRef]
- Rana, A.S.; Raza, N.; Anwar, M.J.; Nazar, M.F. Advancing MXenes through Green Chemistry for Sustainable Future. Mater. Res. Bull. 2025, 193, 113720. [Google Scholar] [CrossRef]
- ECHA—REACH (EC No. 1907/2006). European Chemicals Agency. Available online: https://osha.europa.eu/en/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council#:~:text=Search-,Regulation%20(EC)%20No%201907/2006%20%2D%20Registration%2C%20Evaluation,some%20on%2Dsite%20isolated%20intermediates (accessed on 23 August 2025).
- Choi, J.; Oh, M.S.; Cho, A.; Ryu, J.; Kim, Y.-J.; Kang, H.; Cho, S.-Y.; Im, S.G.; Kim, S.J.; Jung, H.-T. Simple Approach to Enhance Long-Term Environmental Stability of MXene Using Initiated Chemical Vapor Deposition Surface Coating. ACS Nano 2023, 17, 10898–10905. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, M.; Tang, C.; Quan, K.; Tong, Q.; Cao, H.; Jiang, J.; Yang, H.; Zhang, J. ZIF-8@MXene-Reinforced Flame-Retardant and Highly Conductive Polymer Composite Electrolyte for Dendrite-Free Lithium Metal Batteries. J. Colloid Interface Sci. 2022, 620, 478–485. [Google Scholar] [CrossRef]
- Xu, H.; Liu, S.; Li, Z.; Ding, F.; Liu, J.; Wang, W.; Song, K.; Liu, T.; Hu, L. Synergistic Effect of Ti3C2Tx MXene/PAN Nanofiber and LLZTO Particles on High-Performance PEO-Based Solid Electrolyte for Lithium Metal Battery. J. Colloid Interface Sci. 2024, 668, 634–645. [Google Scholar] [CrossRef]
- Li, N.; Fan, J. Computational Insights into Modulating the Performance of MXene Based Electrode Materials for Rechargeable Batteries. Nanotechnology 2021, 32, 252001. [Google Scholar] [CrossRef]
- Caffrey, N.M. Effect of Mixed Surface Terminations on the Structural and Electrochemical Properties of Two-Dimensional Ti3C2T2 and V2CT2 MXenes Multilayers. Nanoscale 2018, 10, 13520–13530. [Google Scholar] [CrossRef]
- Syamsai, R.; Rodriguez, J.R.; Pol, V.G.; Van Le, Q.; Batoo, K.M.; Adil, S.F.; Pandiaraj, S.; Muthumareeswaran, M.R.; Raslan, E.H.; Grace, A.N. Double Transition Metal MXene (TixTa4−xC3) 2D Materials as Anodes for Li-Ion Batteries. Sci. Rep. 2021, 11, 688. [Google Scholar] [CrossRef]
- Nykiel, K.; Strachan, A. High-Throughput Density Functional Theory Screening of Double Transition Metal MXene Precursors. Sci. Data 2023, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Dihingia, K.D.; Saikia, S.; Yedukondalu, N.; Saha, S.; Sastry, G.N. 2D-Double Transition Metal MXenes for Spintronics Applications: Surface Functionalization Induced Ferromagnetic Half-Metallic Complexes. J. Mater. Chem. C 2022, 10, 17886–17898. [Google Scholar] [CrossRef]
- Guan, Q.; Yan, H.; Cai, Y. Flatten the Li-Ion Activation in Perfectly Lattice-Matched MXene and 1T-MoS2 Heterostructures via Chemical Functionalization. Adv. Mater. Interfaces 2022, 9, 2101838. [Google Scholar] [CrossRef]
- He, X.; Xiang, Y.; Yao, W.; Yan, F.; Zhang, Y.; Gerlach, D.; Pei, Y.; Rudolf, P.; Portale, G. MXene Surface Engineering Enabling High-Performance Solid-State Lithium Metal Batteries. Adv. Funct. Mater. 2025, 35, 2416040. [Google Scholar] [CrossRef]
- Zhou, S.; Guan, Y.; Tan, L.; Li, X.; Zhu, H.; Zhang, Q.; Dong, Z.; Yang, N.; Cong, Y. Recent Advances in Multiple Transition Metal MXenes: Synthesis, Properties, and Applications in Energy Storage. J. Energy Storage 2025, 120, 116419. [Google Scholar] [CrossRef]
- Li, N.; Zeng, Z.; Zhang, Y.; Chen, X.; Kong, Z.; Arramel; Li, Y.; Zhang, P.; Nguyen, B.-S. Double Transition Metal Carbides MXenes (D-MXenes) as Promising Electrocatalysts for Hydrogen Reduction Reaction: Ab Initio Calculations. ACS Omega 2021, 6, 23676–23682. [Google Scholar] [CrossRef]
- Liu, M.; Hong, J.J.; Sebti, E.; Zhou, K.; Wang, S.; Feng, S.; Pennebaker, T.; Hui, Z.; Miao, Q.; Lu, E.; et al. Surface Molecular Engineering to Enable Processing of Sulfide Solid Electrolytes in Humid Ambient Air. Nat. Commun. 2025, 16, 213. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Carbone, M.R.; Komurcuoglu, C.; Shekhawat, J.S.; Sun, K.; Guo, H.; Liu, S.; Chen, K.; Bak, S.-M.; Du, Y.; et al. Atomic Insights into the Oxidative Degradation Mechanisms of Sulfide Solid Electrolytes. arXiv 2023, arXiv:2310.00794. [Google Scholar] [CrossRef]
- Gogotsi, Y. The Future of MXenes. Chem. Mater. 2023, 35, 8767–8770. [Google Scholar] [CrossRef]
- Mim, M.; Habib, K.; Farabi, S.N.; Ali, S.A.; Zaed, M.A.; Younas, M.; Rahman, S. MXene: A Roadmap to Sustainable Energy Management, Synthesis Routes, Stabilization, and Economic Assessment. ACS Omega 2024, 9, 32350–32393. [Google Scholar] [CrossRef]
- Roadmap to Commercialize All-Solid-State Batteries. In ACerS Bulletin/Ceramic Tech Today; Summarizing UCSD Roadmap Including Interface Stability, Process Scale-Up, and Recyclability Priorities. 2020. Available online: https://Ceramics.Org/Ceramic-Tech-Today/Roadmap-to-Commercialize-All-Solid-State-Batteries/?Utm_source=chatgpt.Com (accessed on 23 August 2025).
- Zhao, Z.; Xu, Z.; Wang, Y.; Huang, W.; Cheng, Y.; Wong, W.-Y. Scalable Assembly of Flexible Ultrathin All-in-One MXene-Based Supercapacitors. J. Mater. Chem. A 2025, 13, 13175–13185. [Google Scholar] [CrossRef]
- European Commission. REACH Regulation (Regulation EC No. 1907/2006)—Overview and Legal Scope; Updated 2025; European Chemicals Agency: Helsinki, Finland, 2006; Available online: https://Environment.Ec.Europa.Eu/Topics/Chemicals/REACH-Regulation_en (accessed on 23 August 2025).


| Route | Reagents & Conditions | Phases | Main Terminations (Tx) | Salient Outcomes | Reference |
|---|---|---|---|---|---|
| Direct HF etching | Aqueous HF (≈10–50 wt%) at RT-35 °C; 12–48 h; post-washing & intercalation (DMSO, TBAOH, Li+) for delamination | Ti3C2Tx (from Ti3AlC2), Nb2CTx (from Nb2AlC), etc. | -F, -OH, -O (fluoride-rich; mixed) | Produces multilayer powders; high conductivity; F-rich surfaces can lower polymer compatibility but aid hydrophilicity. | [42] |
| In situ HF (LiF/HCl, “MILD”) | LiF + HCl (e.g., 1 M/6 M) at 35–40 °C; 12–24 h; spontaneous Li+ intercalation; easy delamination | Ti3C2Tx (highly delaminable “clay”) | -OH/-O + reduced -F; Li+ pre-intercalation | Larger interlayer spacing (often > 1.2 nm) and better processability; high volumetric capacitance. | [36,43] |
| Fluorine-free alkaline/hydrothermal | Concentrated NaOH/KOH/LiOH(e.g., 27.5–30 M NaOH) at 180–270 °C (autoclave); 15–48 h; fluorine-free | Ti3C2Tx (T = OH, O) | -OH, -O (no F) | Improves compatibility; greener route; current scope most mature for Ti- and Nb-MAX; careful control required. | [38,44] |
| Lewis-acid molten-salt (LAMS) | ZnCl2, CuCl2, etc., 500–800 °C; A-site replacement + etching; HF-free; often yields Cl-terminatedMXenes; subsequent delamination protocols | Ti3C2Cl2, Ti2CCl2; also Zn-MAX intermediates (Ti3ZnC2,…) | -Cl (and other halides), sometimes mixed; F-free | Halogen-terminated MXenes with good stability; scalable batches; requires salt removal; high-T processing. | [38,39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez, F. MXenes in Solid-State Batteries: Multifunctional Roles from Electrodes to Electrolytes and Interfacial Engineering. Batteries 2025, 11, 364. https://doi.org/10.3390/batteries11100364
Márquez F. MXenes in Solid-State Batteries: Multifunctional Roles from Electrodes to Electrolytes and Interfacial Engineering. Batteries. 2025; 11(10):364. https://doi.org/10.3390/batteries11100364
Chicago/Turabian StyleMárquez, Francisco. 2025. "MXenes in Solid-State Batteries: Multifunctional Roles from Electrodes to Electrolytes and Interfacial Engineering" Batteries 11, no. 10: 364. https://doi.org/10.3390/batteries11100364
APA StyleMárquez, F. (2025). MXenes in Solid-State Batteries: Multifunctional Roles from Electrodes to Electrolytes and Interfacial Engineering. Batteries, 11(10), 364. https://doi.org/10.3390/batteries11100364






