Impacts of Curing-Induced Phase Segregation in Silicon Nanoparticle-Based Electrodes
Abstract
1. Introduction
2. Results
2.1. Pristine Electrodes
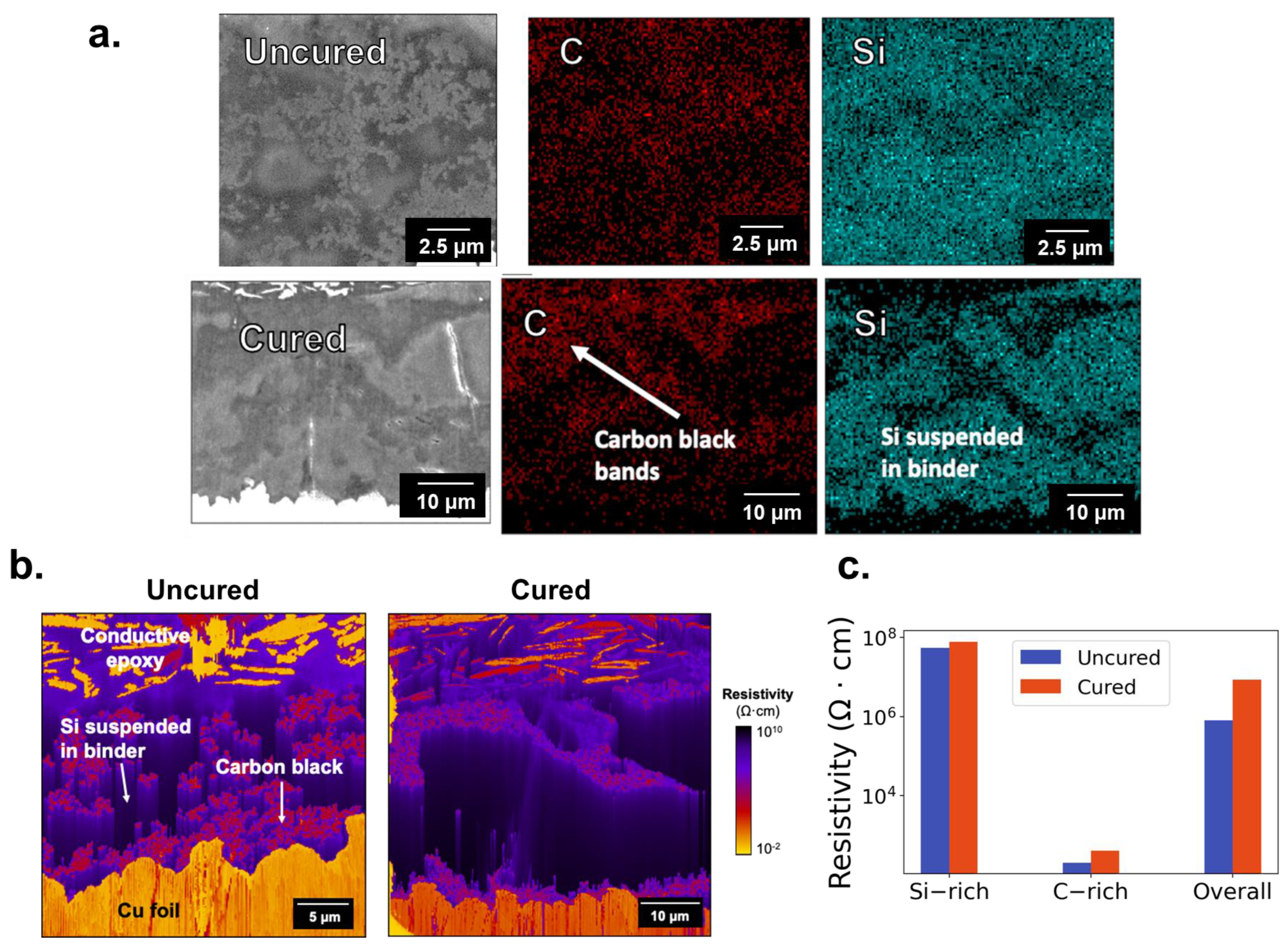
2.1.1. Phase Morphology and Identification
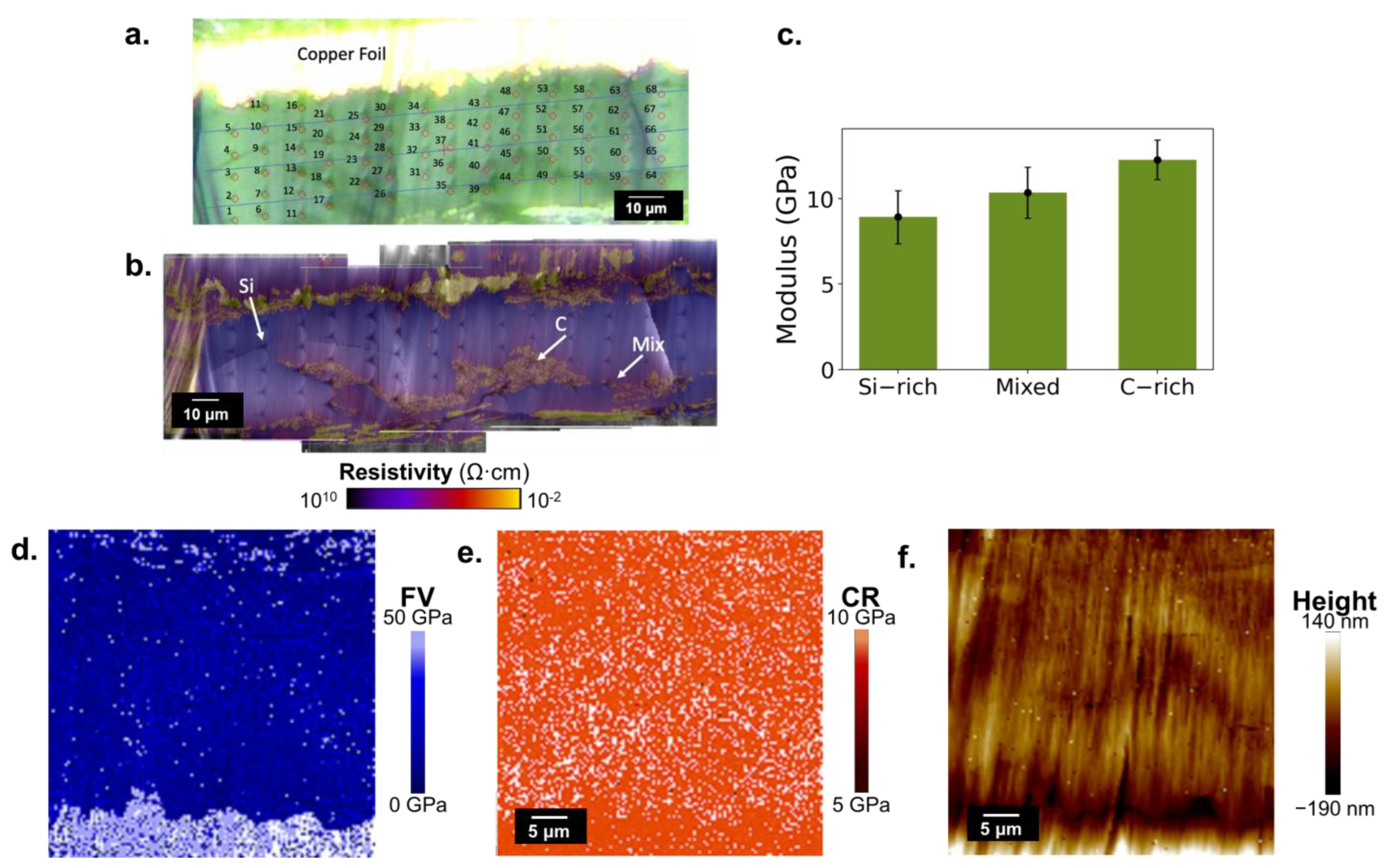
2.1.2. Mechanical Properties
2.2. Cycled Electrodes
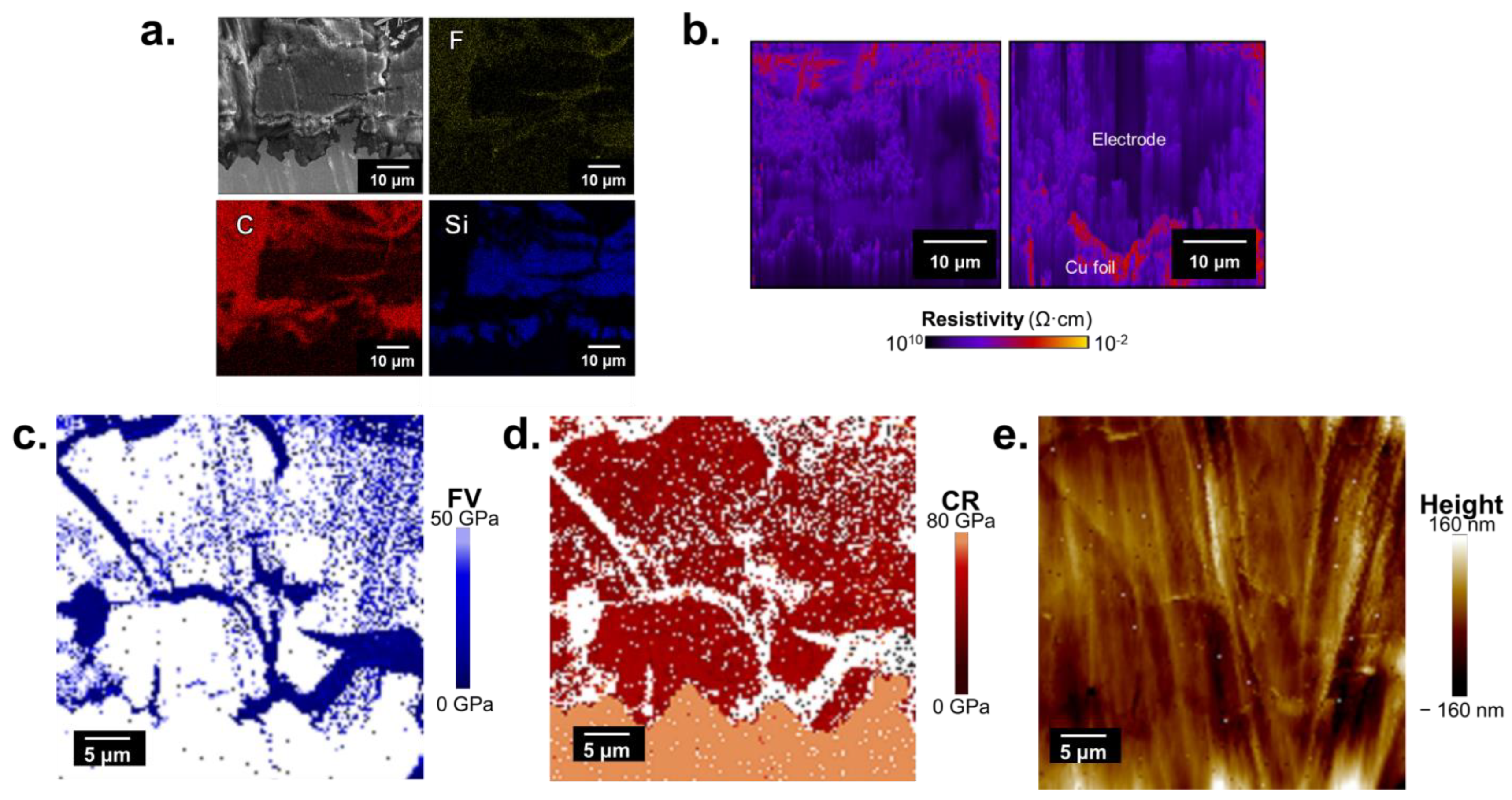
2.2.1. Cycled Uncured Electrodes
2.2.2. Cycled Cured Electrodes
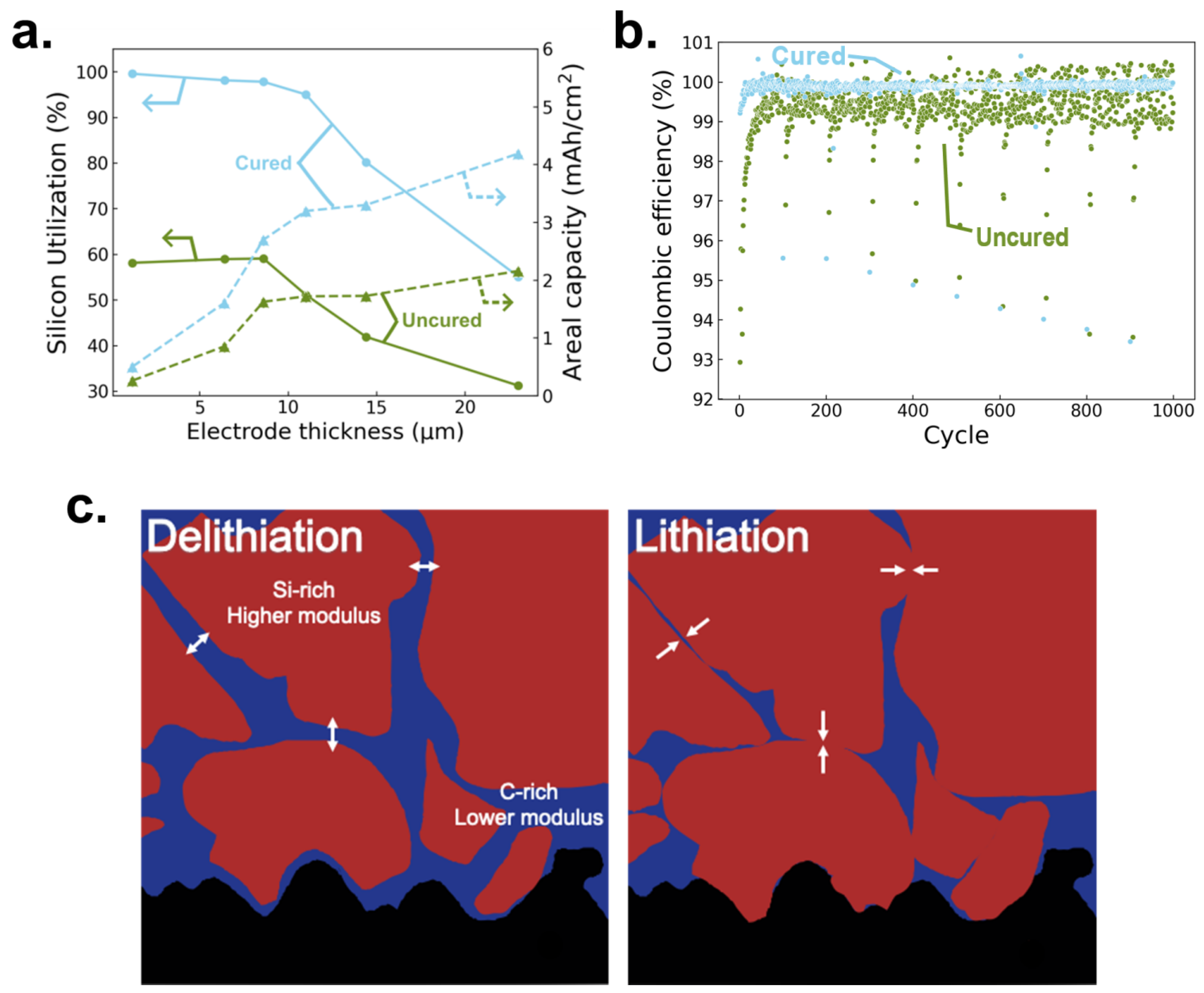
3. Discussion
3.1. Carbon and Silicon Phase Segregation
3.2. Contact Resonance-Force Volume for Composite Electrodes
3.3. Electrochemical Performance
4. Conclusions
5. Experimental Details
5.1. Electrode and Cell Fabrication and Cycling
5.2. SEM-EDS
5.3. FIB-EDS for Air-Free Sample Transfer and Element Mapping
5.4. SSRM
5.5. CR-FV
5.6. Nanoindentation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-Based Anodes for Lithium-Ion Batteries: From Fundamentals to Practical Applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hwang, J.Y.; Sun, Y.K. Nano/Microstructured Silicon-Graphite Composite Anode for High-Energy-Density Li-Ion Battery. ACS Nano 2019, 13, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Steinruck, H.G.; Cao, C.; Veith, G.M.; Toney, M.F. Toward Quantifying Capacity Losses Due to Solid Electrolyte Interphase Evolution in Silicon Thin Film Batteries. J. Chem. Phys. 2020, 152, 084702. [Google Scholar] [CrossRef]
- Nadimpalli, S.P.V.; Sethuraman, V.A.; Dalavi, S.; Lucht, B.; Chon, M.J.; Shenoy, V.B.; Guduru, P.R. Quantifying Capacity Loss Due to Solid-Electrolyte-Interphase Layer Formation on Silicon Negative Electrodes in Lithium-Ion Batteries. J. Power Sources 2012, 215, 145–151. [Google Scholar] [CrossRef]
- Schulze, M.C.; Urias, F.; Dutta, N.S.; Huey, Z.; Coyle, J.; Teeter, G.; Doeren, R.; Tremolet de Villers, B.J.; Han, S.-D.; Neale, N.R.; et al. Control of Nanoparticle Dispersion, SEI Composition, and Electrode Morphology Enables Long Cycle Life in High Silicon Content Nanoparticle-Based Composite Anodes for Lithium-Ion Batteries. J. Mater. Chem. A 2023, 11, 5257–5266. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Mangolini, L.; Thimsen, E.; Kortshagen, U. High-Yield Plasma Synthesis of Luminescent Silicon Nanocrystals. Nano Lett. 2005, 5, 655–659. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Conveying Advanced Li-Ion Battery Materials into Practice The Impact of Electrode Slurry Preparation Skills. Adv. Energy Mater. 2016, 6, 1600655. [Google Scholar] [CrossRef]
- Schulze, M.C.; Carroll, G.M.; Martin, T.R.; Sanchez-Rivera, K.; Urias, F.; Neale, N.R. Hydrophobic versus Hydrophilic Interfacial Coatings on Silicon Nanoparticles Teach Us How to Design the Solid Electrolyte Interphase in Silicon-Based Li-Ion Battery Anodes. ACS Appl. Energy Mater. 2021, 4, 1628–1636. [Google Scholar] [CrossRef]
- Weidong, Z.; Wentao, S.; Ling-Zhi, C.; Deyu, W.; Hong, L.; Flemming, B.; Fuqiang, H.; Cai, S. Beyond Imaging: Applications of Atomic Force Microscopy for the Study of Lithium-Ion Batteries. Ultramicroscopy 2019, 204, 34–48. [Google Scholar] [CrossRef]
- Stetson, C.; Huey, Z.; Downard, A.; Li, Z.; To, B.; Zakutayev, A.; Jiang, C.S.; Al-Jassim, M.M.; Finegan, D.P.; Han, S.D.; et al. Three-Dimensional Mapping of Resistivity and Microstructure of Composite Electrodes for Lithium-Ion Batteries. Nano Lett. 2020, 20, 8081–8088. [Google Scholar] [CrossRef]
- Stetson, C.; Yoon, T.; Coyle, J.; Nemeth, W.; Young, M.; Norman, A.; Pylypenko, S.; Ban, C.; Jiang, C.-S.; Al-Jassim, M.; et al. Three-Dimensional Electronic Resistivity Mapping of Solid Electrolyte Interphase on Si Anode Materials. Nano Energy 2019, 55, 477–485. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Frisco, S.; Pekarek, R.T.; Stetson, C.; Huey, Z.; Harvey, S.; Li, X.; Key, B.; Fang, C.; Liu, G. Examining CO2 as an Additive for Solid Electrolyte Interphase Formation on Silicon Anodes. J. Electrochem. Soc. 2021, 168, 030534. [Google Scholar] [CrossRef]
- Huey, Z.; Ha, Y.; Frisco, S.; Norman, A.; Teeter, G.; Jiang, C.-S.; DeCaluwe, S.C. Multi-Modal Characterization Methods of Solid-Electrolyte Interphase in Silicon-Graphite Composite Electrodes. J. Power Sources 2023, 564, 232804. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S.; Baek, W.J.; Heo, S.; Yun, D.J.; Han, S.; Jung, H. Nanoscale Electrical Degradation of Silicon-Carbon Composite Anode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 24549–24553. [Google Scholar] [CrossRef]
- Yuya, P.A.; Hurley, D.C.; Turner, J.A. Contact-Resonance Atomic Force Microscopy for Viscoelasticity. J. Appl. Phys. 2008, 104, 074916. [Google Scholar] [CrossRef]
- Killgore, J.P.; Yablon, D.G.; Tsou, A.H.; Gannepalli, A.; Yuya, P.A.; Turner, J.A.; Proksch, R.; Hurley, D.C. Viscoelastic Property Mapping with Contact Resonance Force Microscopy. Langmuir 2011, 27, 13983–13987. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. X-rays. In Scanning Electron Microscopy and X-ray Microanalysis; Springer: New York, NY, USA, 2018; pp. 39–63. ISBN 978-1-4939-6674-5. [Google Scholar]
- Heiskanen, S.K.; Kim, J.; Lucht, B.L. Generation and Evolution of the Solid Electrolyte Interphase of Lithium-Ion Batteries. Joule 2019, 3, 2322–2333. [Google Scholar] [CrossRef]
- Burdette-Trofimov, M.K.; Armstrong, B.L.; Heroux, L.; Doucet, M.; Márquez Rossy, A.E.; Hoelzer, D.T.; Kanbargi, N.; Naskar, A.K.; Veith, G.M. Competitive Adsorption within Electrode Slurries and Impact on Cell Fabrication and Performance. J. Power Sources 2022, 520, 230914. [Google Scholar] [CrossRef]
- Dimension Icon User Guide. Available online: https://www.nanophys.kth.se/nanolab/afm/icon/bruker-help/Content/Cover%20page.htm (accessed on 4 March 2024).
- Zhou, X.; Fu, J.; Li, F. Contact Resonance Force Microscopy for Nanomechanical Characterization: Accuracy and Sensitivity. J. Appl. Phys. 2013, 114, 064301. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, K.; Jervis, R.; Shearing, P.R.; Miller, T.S.; Brett, D.J.L. Operando Electrochemical Atomic Force Microscopy of Solid-Electrolyte Interphase Formation on Graphite Anodes: The Evolution of SEI Morphology and Mechanical Properties. ACS Appl. Mater. Interfaces 2020, 12, 35132–35141. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, J.; Wang, S.; Luo, F.; Ben, L.; Li, H. Influence of Fluoroethylene Carbonate on the Solid Electrolyte Interphase of Silicon Anode for Li-Ion Batteries: A Scanning Force Spectroscopy Study. Chin. Phys. B 2020, 29, 048203. [Google Scholar] [CrossRef]
- McBrayer, J.D.; Apblett, C.A.; Harrison, K.L.; Fenton, K.R.; Minteer, S.D. Mechanical Studies of the Solid Electrolyte Interphase on Anodes in Lithium and Lithium Ion Batteries. Nanotechnology 2021, 32, 502005. [Google Scholar] [CrossRef]
- Lin, E.Y.; Frischknecht, A.L.; Riggleman, R.A. Chain and Segmental Dynamics in Polymer–Nanoparticle Composites with High Nanoparticle Loading. Macromolecules 2021, 54, 5335–5343. [Google Scholar] [CrossRef]
- Odegard, G.M.; Clancy, T.C.; Gates, T.S. Modeling of the Mechanical Properties of Nanoparticle/Polymer Composites. Polymer 2005, 46, 553–562. [Google Scholar] [CrossRef]
- Hurley, D.C. Contact Resonance Force Microscopy Techniques for Nanomechanical Measurements. In Applied Scanning Probe Methods; Bhushan, B., Fuchs, H., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 2009; Volume XI, pp. 97–138. [Google Scholar]
- Rabe, U.; Kopycinska, M.; Hirsekorn, S.; Arnold, W. Evaluation of the Contact Resonance Frequencies in Atomic Force Microscopy as a Method for Surface Characterisation (Invited). Ultrasonics 2002, 40, 49–54. [Google Scholar] [CrossRef]
- Stan, G.; Cook, R.F. Mapping the Elastic Properties of Granular Au Films by Contact Resonance Atomic Force Microscopy. Nanotechnology 2008, 19, 235701. [Google Scholar] [CrossRef]
- Polyimide P84NT Technical Brochure. Available online: https://www.pp-evonik.com/assets/img/uploads/Evonik%20Polyimide%20P84NT%20technical%20brochure%20A4.pdf (accessed on 9 February 2024).
- TECAPOWDER PI—Polyimide Powder from Ensinger. Available online: https://www.ensingerplastics.com/en/thermoplastic-materials/pi-polyimide/polyimide-powder (accessed on 9 February 2024).
- Rosenberger, M.R.; Chen, S.; Prater, C.B.; King, W.P. Micromechanical Contact Stiffness Devices and Application for Calibrating Contact Resonance Atomic Force Microscopy. Nanotechnology 2017, 28, 044003. [Google Scholar] [CrossRef]
- Pittenger, B.; Yablon, D. Improving the Accuracy of Nanomechanical Measurements with Force-Curve-Based AFM Techniques. Bruker Appl. Notes 2017, 1, 1–7. [Google Scholar]
- Ma, C.; Wang, W.; Chen, Y.; Arnold, W.; Chu, J. Depth-Sensing Using AFM Contact-Resonance Imaging and Spectroscopy at the Nanoscale. J. Appl. Phys. 2019, 126, 124302. [Google Scholar] [CrossRef]
- Freund, L.B.; Suresh, S. Film Stress and Substrate Curvature. In Thin Film Materials: Stress, Defect Formation, and Surface Evolution; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2003. [Google Scholar]
- Guo, D.; Xie, G.; Luo, J. Mechanical Properties of Nanoparticles: Basics and Applications. J. Phys. D Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef]
- Nam, K.-H.; Hwa Chae, K.; Choi, J.-H.; Jeon, K.-J.; Park, C.-M. Superior Carbon Black: High-Performance Anode and Conducting Additive for Rechargeable Li- and Na-Ion Batteries. Chem. Eng. J. 2021, 417, 129242. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, X.; Liu, D.; Wang, S.; Zhang, L.; Zhao, R.; Kang, F.; Li, B. Understanding the Conductive Carbon Additive on Electrode/Electrolyte Interface Formation in Lithium-Ion Batteries via in Situ Scanning Electrochemical Microscopy. Front. Chem. 2020, 8, 114. [Google Scholar] [CrossRef]
- Madzvamuse, A.; Hamenu, L.; Mohammed, L.; Ko, J.M. Effect of Morphologically Different Conductive Agents on the Performance of Silicon Anode in Lithium-Ion Batteries. ChemistrySelect 2018, 3, 10805–10810. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X.; et al. Hierarchical Porous Silicon Structures with Extraordinary Mechanical Strength as High-Performance Lithium-Ion Battery Anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Magri, M.; Boz, B.; Cabras, L.; Salvadori, A. Quantitative Investigation of the Influence of Electrode Morphology in the Electro-Chemo-Mechanical Response of Li-Ion Batteries. Electrochim. Acta 2022, 405, 139778. [Google Scholar] [CrossRef]
- Kim, J.H.; Huey, Z.; Veith, G.M.; Jiang, C.-S.; Neale, N.R.; Carroll, G.M. Blended 1D Carbon Nanostructures Synergistically Enhance Electron and Ion Transport in Silicon Nanoparticle Electrodes. Cell Rep. Phys. Sci. 2024, 5, 101974. [Google Scholar] [CrossRef]
- Kumar, R.; Woo, J.H.; Xiao, X.; Sheldon, B.W. Internal Microstructural Changes and Stress Evolution in Silicon Nanoparticle Based Composite Electrodes. J. Electrochem. Soc. 2017, 164, A3750–A3765. [Google Scholar] [CrossRef]
- Zhang, W.-J. A Review of the Electrochemical Performance of Alloy Anodes for Lithium-Ion Batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Hu, J.; Lu, B.; Cheng, Y.-T.; Zhang, J. In Situ Measurement of Mechanical Property and Stress Evolution in a Composite Silicon Electrode. J. Power Sources 2017, 366, 80–85. [Google Scholar] [CrossRef]
- Wang, H.; Lu, S.-H.; Wang, X.; Xia, S.; Beng Chew, H. A Review of the Multiscale Mechanics of Silicon Electrodes in High-Capacity Lithium-Ion Batteries. J. Phys. D Appl. Phys. 2021, 55, 063001. [Google Scholar] [CrossRef]
- Eyben, P.; Vandervorst, W.; Alvarez, D.; Xu, M.; Fouchier, M. Probing Semiconductor Technology and Devices with Scanning Spreading Resistance Microscopy. In Scanning Probe Microscopy: Electrical and Electromechanical Phenomena at the Nanoscale; Kalinin, S., Gruverman, A., Eds.; Springer: New York, NY, USA, 2007; pp. 31–87. ISBN 978-0-387-28668-6. [Google Scholar]
- Wagner, R.; Moon, R.J.; Raman, A. Mechanical Properties of Cellulose Nanomaterials Studied by Contact Resonance Atomic Force Microscopy. Cellulose 2016, 23, 1031–1041. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of Contact Deformations on the Adhesion of Particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Cappella, B.; Dietler, G. Force-Distance Curves by Atomic Force Microscopy. Surf. Sci. Rep. 1999, 34, 1–104. [Google Scholar] [CrossRef]


| Sample | Uncycled | Cycled | ||
|---|---|---|---|---|
| FV Modulus (GPa) | CR Modulus (GPa) | FV Modulus (GPa) | CR Modulus (GPa) | |
| Uncured | 7.75 | 7.66 | 73.3 | 41.0 |
| Cured | 11.2 | 8.50 | 6.63 (low) | 10.9 (low) |
| 48.2 (high) | 43.7 (high) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huey, Z.; Carroll, G.M.; Coyle, J.; Walker, P.; Neale, N.R.; DeCaluwe, S.; Jiang, C. Impacts of Curing-Induced Phase Segregation in Silicon Nanoparticle-Based Electrodes. Batteries 2024, 10, 313. https://doi.org/10.3390/batteries10090313
Huey Z, Carroll GM, Coyle J, Walker P, Neale NR, DeCaluwe S, Jiang C. Impacts of Curing-Induced Phase Segregation in Silicon Nanoparticle-Based Electrodes. Batteries. 2024; 10(9):313. https://doi.org/10.3390/batteries10090313
Chicago/Turabian StyleHuey, Zoey, G. Michael Carroll, Jaclyn Coyle, Patrick Walker, Nathan R. Neale, Steven DeCaluwe, and Chunsheng Jiang. 2024. "Impacts of Curing-Induced Phase Segregation in Silicon Nanoparticle-Based Electrodes" Batteries 10, no. 9: 313. https://doi.org/10.3390/batteries10090313
APA StyleHuey, Z., Carroll, G. M., Coyle, J., Walker, P., Neale, N. R., DeCaluwe, S., & Jiang, C. (2024). Impacts of Curing-Induced Phase Segregation in Silicon Nanoparticle-Based Electrodes. Batteries, 10(9), 313. https://doi.org/10.3390/batteries10090313







