Revealing a Correlation between Physical Parameters and Differential Voltage Analysis of a Commercial Li-Ion Battery Based on Fiber Optic Sensors
Abstract
1. Introduction
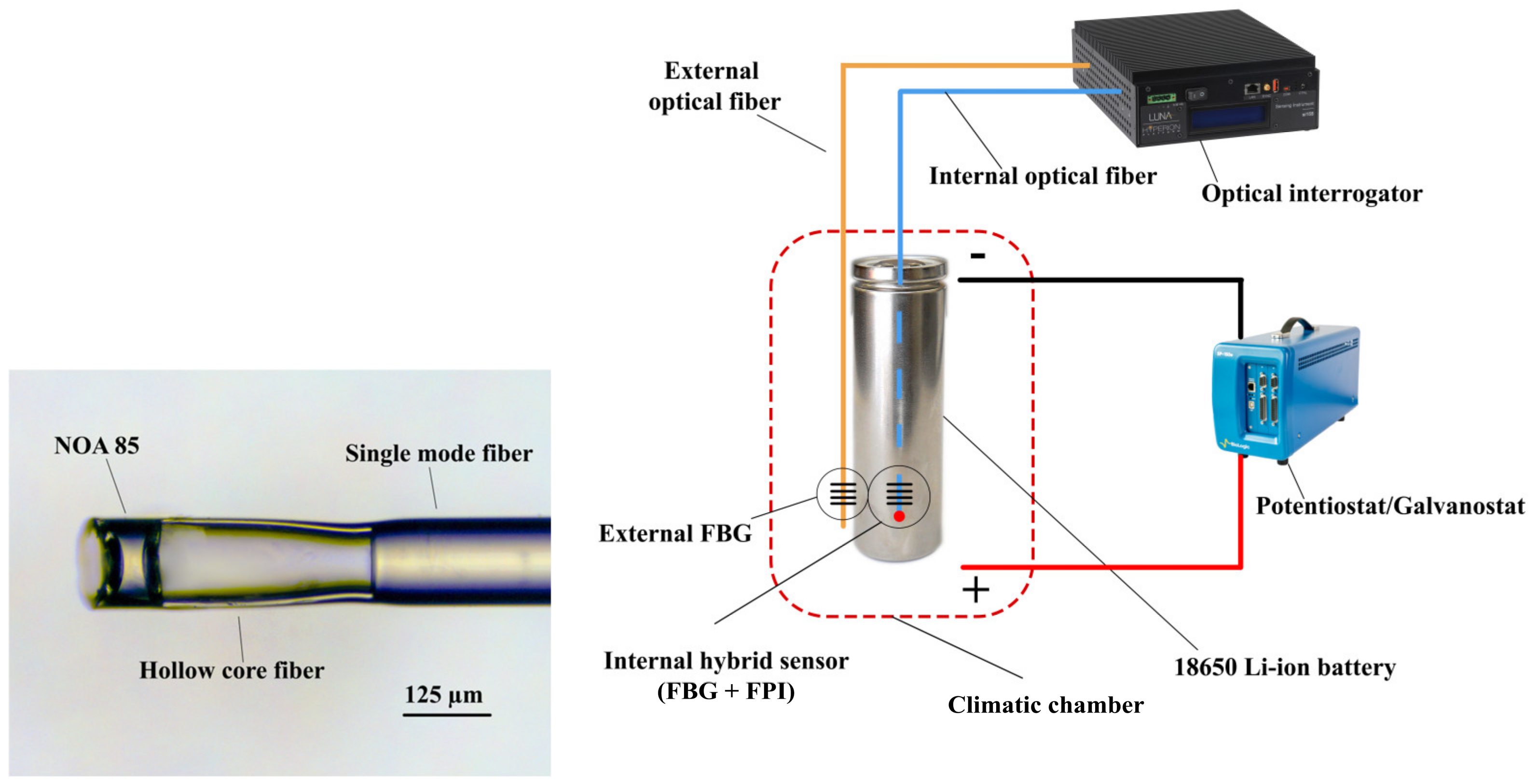
2. Materials and Methods
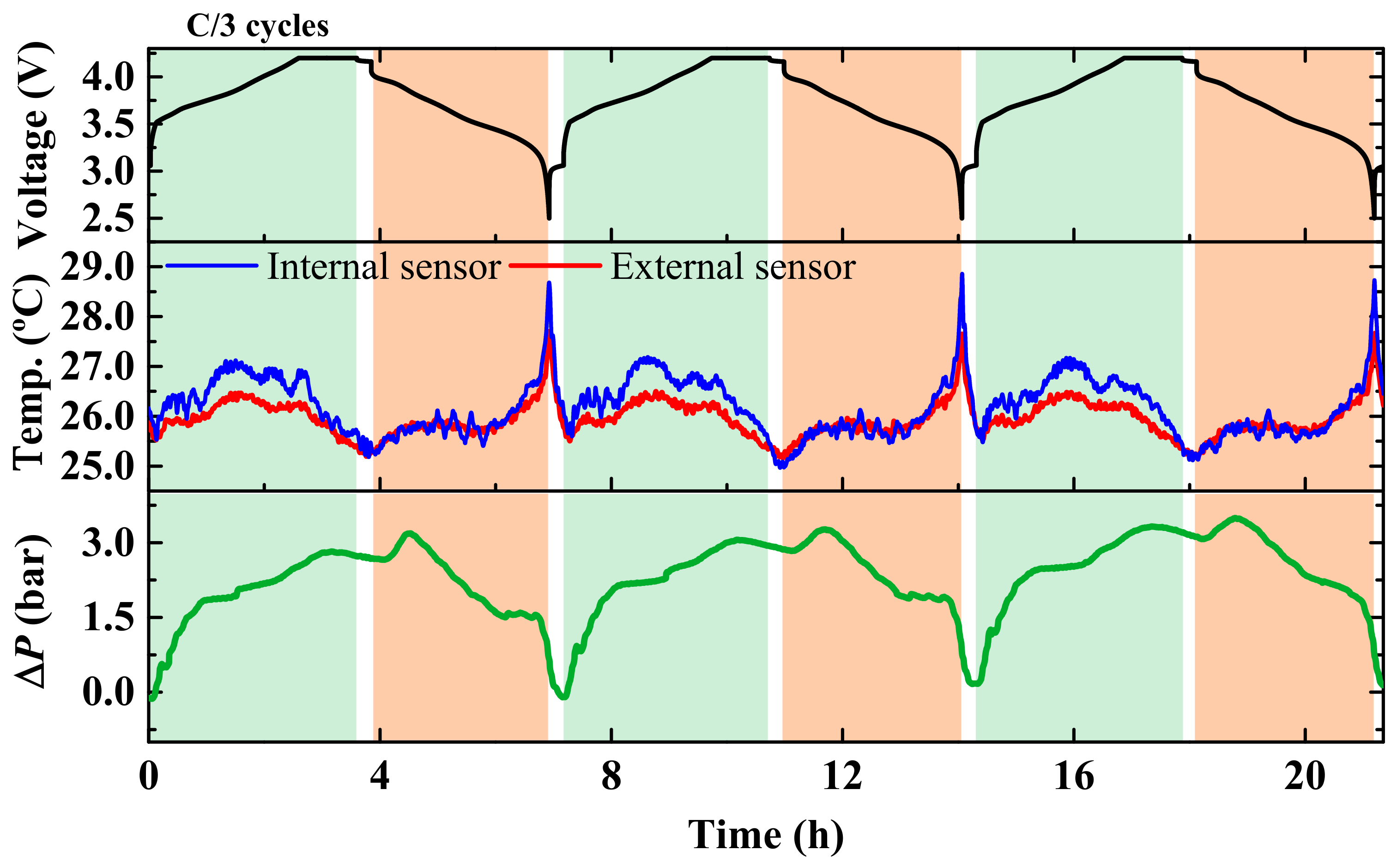
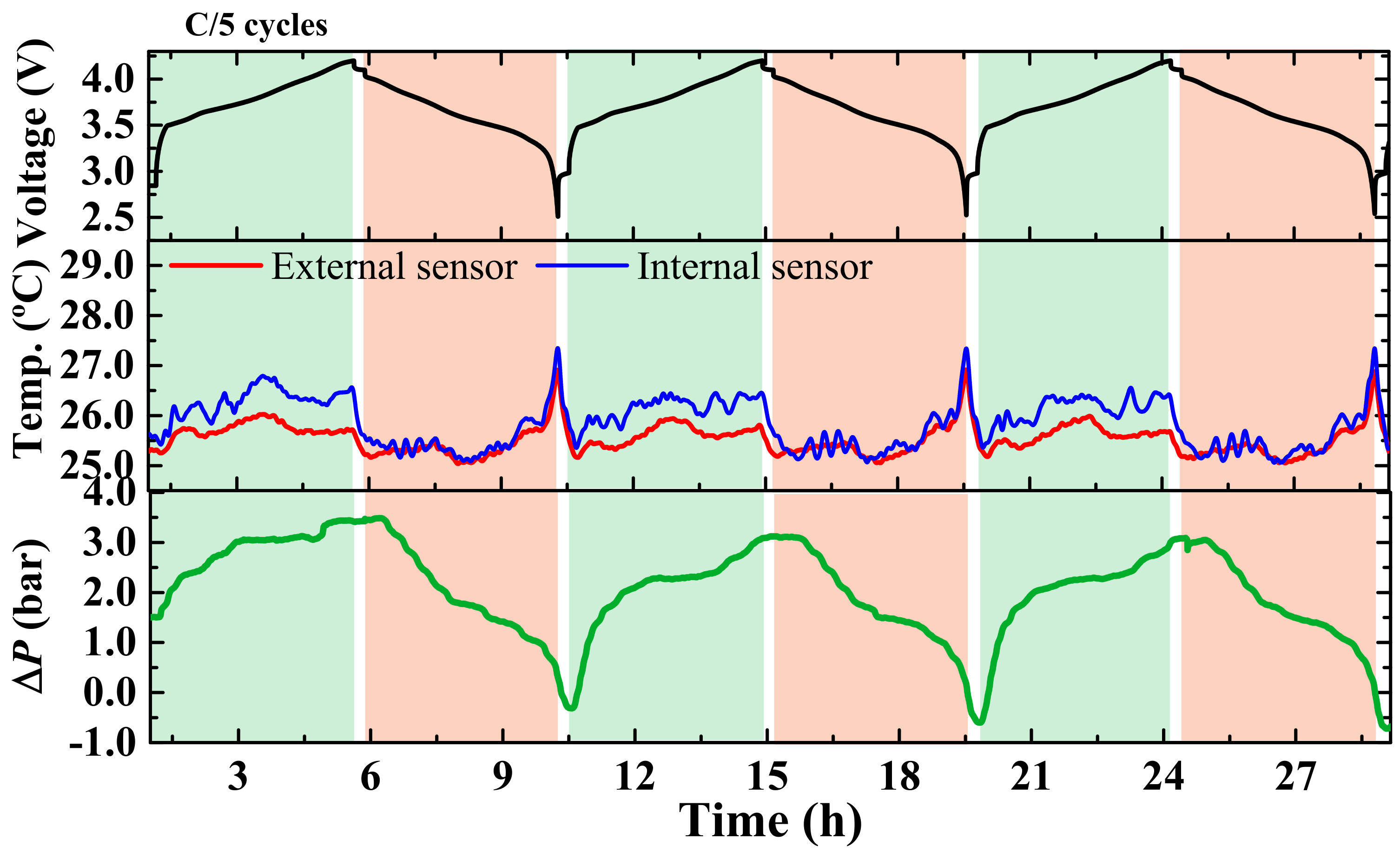
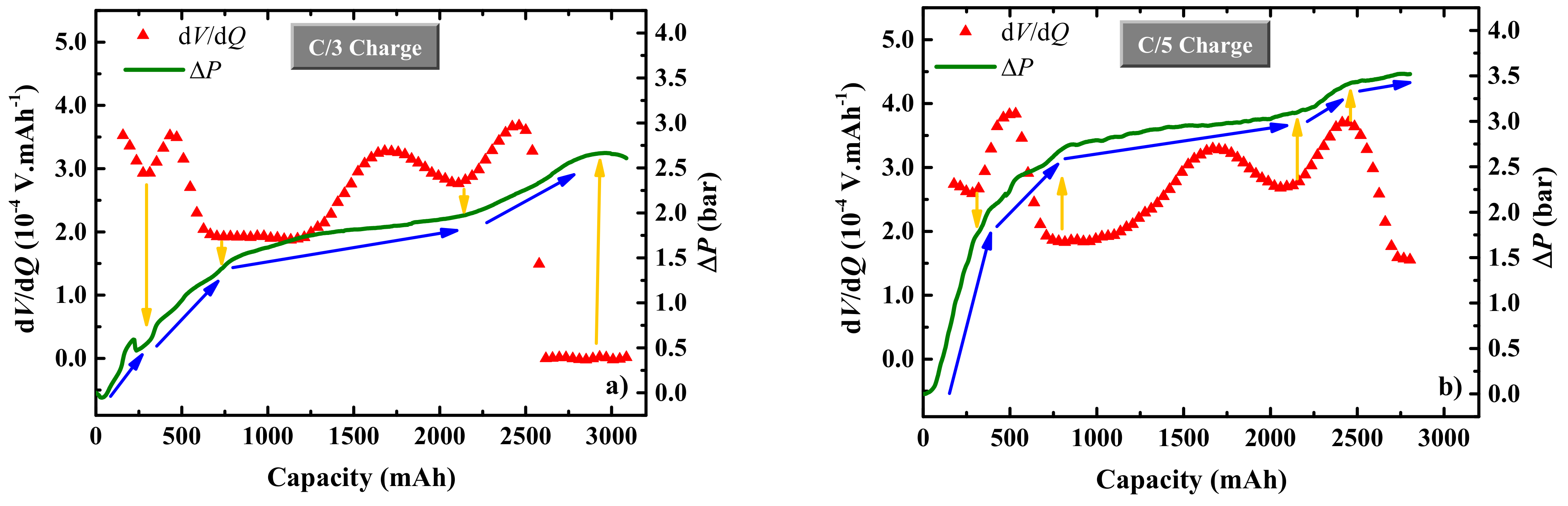
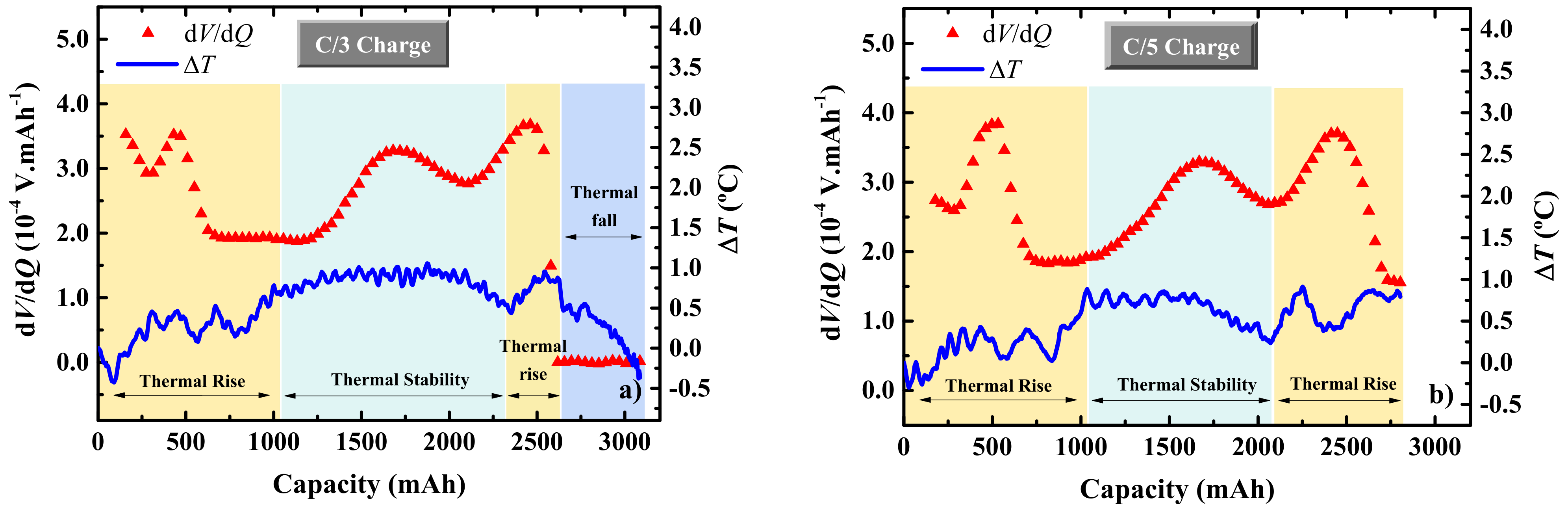
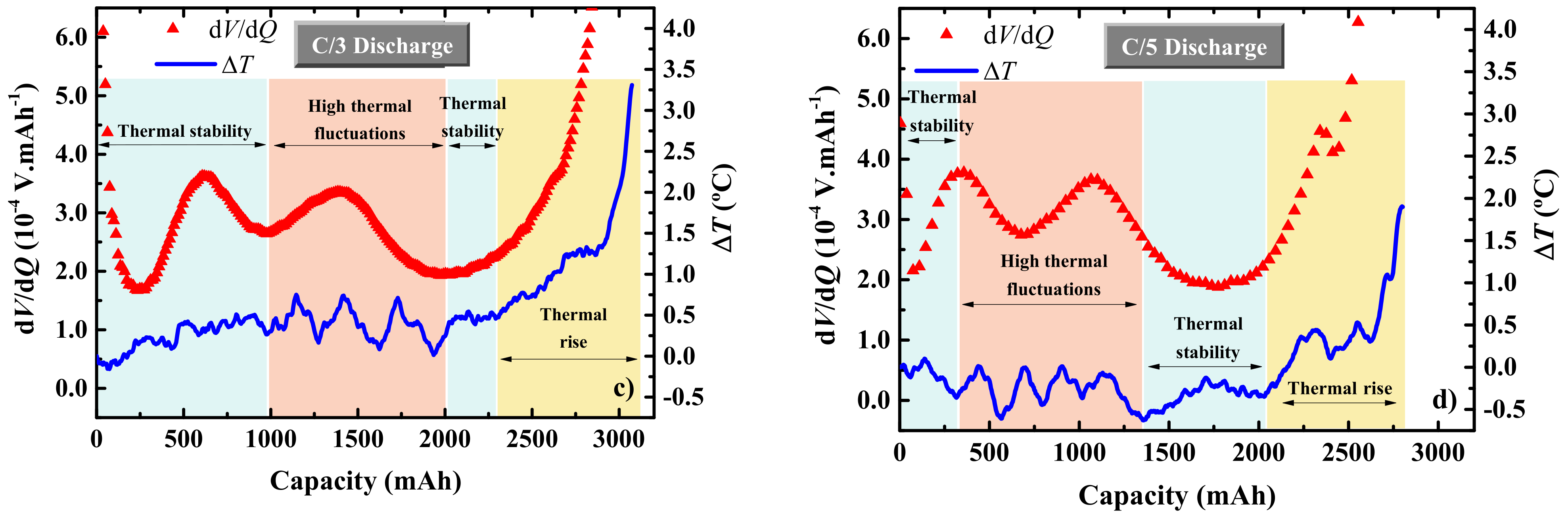
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giarola, S.; Molar-Cruz, A.; Vaillancourt, K.; Bahn, O.; Sarmiento, L.; Hawkes, A.; Brown, M. The Role of Energy Storage in the Uptake of Renewable Energy: A Model Comparison Approach. Energy Policy 2021, 151, 112159. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy Storage Systems: A Review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The Path towards Sustainable Energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chombo, P.V.; Laoonual, Y. A Review of Safety Strategies of a Li-Ion Battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Li, B.; Parekh, M.H.; Adams, R.A.; Adams, T.E.; Love, C.T.; Pol, V.G.; Tomar, V. Lithium-Ion Battery Thermal Safety by Early Internal Detection, Prediction and Prevention. Sci. Rep. 2019, 9, 13255. [Google Scholar] [CrossRef]
- Yang, G.; Leitão, C.; Li, Y.; Pinto, J.; Jiang, X. Real-Time Temperature Measurement with Fiber Bragg Sensors in Lithium Batteries for Safety Usage. Measurement 2013, 46, 3166–3172. [Google Scholar] [CrossRef]
- Nascimento, M.; Ferreira, M.S.; Pinto, J.L. Real Time Thermal Monitoring of Lithium Batteries with Fiber Sensors and Thermocouples: A Comparative Study. Measurement 2017, 111, 260–263. [Google Scholar] [CrossRef]
- Matuck, L.; Pinto, J.L.; Marques, C.; Nascimento, M. Simultaneous Strain and Temperature Discrimination in 18650 Li-Ion Batteries Using Polarization-Maintaining Fiber Bragg Gratings. Batteries 2022, 8, 233. [Google Scholar] [CrossRef]
- Freitas, F.; Matuck, L.; Bierlich, J.; Ferreira, M.; Marques, C.; Nascimento, M. Innovative Hybrid Optical Sensing Design to Simultaneously Discriminate Pressure and Temperature. J. Phys. Conf. Ser. 2022, 2407, 12023. [Google Scholar] [CrossRef]
- Lao, J.; Sun, P.; Liu, F.; Zhang, X.; Zhao, C.; Mai, W.; Guo, T.; Xiao, G.; Albert, J. In Situ Plasmonic Optical Fiber Detection of the State of Charge of Supercapacitors for Renewable Energy Storage. Light Sci. Appl. 2018, 7, 34. [Google Scholar] [CrossRef]
- Bae, C.-J.; Manandhar, A.; Kiesel, P.; Raghavan, A. Monitoring the Strain Evolution of Lithium-Ion Battery Electrodes Using an Optical Fiber Bragg Grating Sensor. Energy Technol. 2016, 4, 851–855. [Google Scholar] [CrossRef]
- Huang, J.; Han, X.; Liu, F.; Gervillié, C.; Blanquer, L.A.; Guo, T.; Tarascon, J.M. Monitoring Battery Electrolyte Chemistry: Via in-Operando Tilted Fiber Bragg Grating Sensors. Energy Environ. Sci. 2021, 14, 6464–6475. [Google Scholar] [CrossRef]
- Fortier, A.; Tsao, M.; Williard, N.D.; Xing, Y.; Pecht, M.G. Preliminary Study on Integration of Fiber Optic Bragg Grating Sensors in Li-Ion Batteries and In Situ Strain and Temperature Monitoring of Battery Cells. Energies 2017, 10, 838. [Google Scholar] [CrossRef]
- Peng, J.; Jia, S.; Jin, Y.; Xu, S.; Xu, Z. Design and Investigation of a Sensitivity-Enhanced Fiber Bragg Grating Sensor for Micro-Strain Measurement. Sens. Actuators A Phys. 2018, 285, 437–447. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Liu, Q.; Liu, F.; Han, X.; Liu, X.; Li, K.; Xiao, G.; Albert, J.; Lu, X.; et al. Operando Monitoring of Ion Activities in Aqueous Batteries with Plasmonic Fiber-Optic Sensors. Nat. Commun. 2022, 13, 547. [Google Scholar] [CrossRef]
- Rente, B.; Fabian, M.; Vidakovic, M.; Liu, X.; Li, X.; Li, K.; Sun, T.; Grattan, K.T.V. Lithium-Ion Battery State-of-Charge Estimator Based on FBG-Based Strain Sensor and Employing Machine Learning. IEEE Sens. J. 2021, 21, 1453–1460. [Google Scholar] [CrossRef]
- Nascimento, M.; Paixão, T.; Ferreira, M.S.; Pinto, J.L. Thermal Mapping of a Lithium Polymer Batteries Pack with FBGs Network. Batteries 2018, 4, 67. [Google Scholar] [CrossRef]
- Peng, J.; Jia, S.; Yu, H.; Kang, X.; Yang, S.; Xu, S. Design and Experiment of FBG Sensors for Temperature Monitoring on External Electrode of Lithium-Ion Batteries. IEEE Sens. J. 2021, 21, 4628–4634. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Sun, Z.; Wang, L.; Xu, R.; Li, M.; Chen, Z. A Comprehensive Review of Battery Modeling and State Estimation Approaches for Advanced Battery Management Systems. Renew. Sustain. Energy Rev. 2020, 131, 110015. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Bloom, I.; Jansen, A.N.; Abraham, D.P.; Knuth, J.; Jones, S.A.; Battaglia, V.S.; Henriksen, G.L. Differential Voltage Analyses of High-Power, Lithium-Ion Cells 1. Technique and Application. J. Power Sources 2005, 139, 295–303. [Google Scholar] [CrossRef]
- Dahn, H.M.; Smith, A.J.; Burns, J.C.; Stevens, D.A.; Dahn, J.R. User-Friendly Differential Voltage Analysis Freeware for the Analysis of Degradation Mechanisms in Li-Ion Batteries. J. Electrochem. Soc. 2012, 159, A1405–A1409. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, J.; Lu, D.D.C.; Wang, G.; He, T. Incremental Capacity Analysis and Differential Voltage Analysis Based State of Charge and Capacity Estimation for Lithium-Ion Batteries. Energy 2018, 150, 759–769. [Google Scholar] [CrossRef]
- Sturm, J.; Frank, A.; Rheinfeld, A.; Erhard, S.V.; Jossen, A. Impact of Electrode and Cell Design on Fast Charging Capabilities of Cylindrical Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 130505. [Google Scholar] [CrossRef]
- Vidal, D. Effect of the Positive Electrode and of Mechanical Stresses on the Cyclability of a Li-Ion Cell Containing Silicon-Based Negative Electrode. Ph.D. Thesis, Université Grenoble Alpes, Saint-Martin-d’Hères, France, 2022. [Google Scholar]
- Murashko, K.; Li, D.; Danilov, D.L.; Notten, P.H.L.; Pyrhonen, J.; Jokiniemi, J. Determination of Li-Ion Battery Degradation Mechanisms at High c-Rate Charging. In Proceedings of the 2019 IEEE Vehicle Power and Propulsion Conference, VPPC 2019—Proceedings, Hanoi, Vietnam, 14–17 October 2019; pp. 1–6. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Patel, A.N.; Zhang, C.; Amietszajew, T.; Kirkaldy, N.; Offer, G.J.; Marinescu, M. Internal Temperature Estimation for Lithium-Ion Batteries through Distributed Equivalent Circuit Network Model. J. Power Sources 2024, 611, 234701. [Google Scholar] [CrossRef]
- Wang, N.; Chen, A.; Zhao, W.; Zhu, R.; Duan, B. An Online Temperature Estimation for Cylindrical Lithium-Ion Batteries Based on Simplified Distribution Electrical-Thermal Model. J. Energy Storage 2022, 55, 105326. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Jiang, C.; Ji, Z.; Zhu, Z. Internal Temperature and Flame Jet Characteristics during Thermal Runaway Triggered by Nail Penetration for NCM811 Lithium-Ion Battery. J. Therm. Anal. Calorim. 2022, 147, 14925–14938. [Google Scholar] [CrossRef]
- Dubarry, M.; Anseán, D. Best Practices for Incremental Capacity Analysis. Front. Energy Res. 2022, 10, 1023555. [Google Scholar] [CrossRef]
- Nadimpalli, S.P.V.; Sethuraman, V.A.; Abraham, D.P.; Bower, A.F.; Guduru, P.R. Stress Evolution in Lithium-Ion Composite Electrodes during Electrochemical Cycling and Resulting Internal Pressures on the Cell Casing. J. Electrochem. Soc. 2015, 162, A2656. [Google Scholar] [CrossRef]
- Jamil, S.; Wang, G.; Yang, L.; Xie, X.; Cao, S.; Liu, H.; Chang, B.; Wang, X. Suppressing H2–H3 Phase Transition in High Ni–Low Co Layered Oxide Cathode Material by Dual Modification. J. Mater. Chem. A 2020, 8, 21306–21316. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material-Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Hahn, M.; Buqa, H.; Ruch, P.W.; Goers, D.; Spahr, M.E.; Ufheil, J.; Novák, P.; Kötz, R. A Dilatometric Study of Lithium Intercalation into Powder-Type Graphite Electrodes. Electrochem. Solid-State Lett. 2008, 11, A151. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMny CozO2 (NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Laszczynski, N.; Solchenbach, S.; Gasteiger, H.A.; Lucht, B.L. Understanding Electrolyte Decomposition of Graphite/NCM811 Cells at Elevated Operating Voltage. J. Electrochem. Soc. 2019, 166, A1853–A1859. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuck, L.; Ferreira, M.S.; Nascimento, M. Revealing a Correlation between Physical Parameters and Differential Voltage Analysis of a Commercial Li-Ion Battery Based on Fiber Optic Sensors. Batteries 2024, 10, 289. https://doi.org/10.3390/batteries10080289
Matuck L, Ferreira MS, Nascimento M. Revealing a Correlation between Physical Parameters and Differential Voltage Analysis of a Commercial Li-Ion Battery Based on Fiber Optic Sensors. Batteries. 2024; 10(8):289. https://doi.org/10.3390/batteries10080289
Chicago/Turabian StyleMatuck, Lucca, Marta S. Ferreira, and Micael Nascimento. 2024. "Revealing a Correlation between Physical Parameters and Differential Voltage Analysis of a Commercial Li-Ion Battery Based on Fiber Optic Sensors" Batteries 10, no. 8: 289. https://doi.org/10.3390/batteries10080289
APA StyleMatuck, L., Ferreira, M. S., & Nascimento, M. (2024). Revealing a Correlation between Physical Parameters and Differential Voltage Analysis of a Commercial Li-Ion Battery Based on Fiber Optic Sensors. Batteries, 10(8), 289. https://doi.org/10.3390/batteries10080289





