Experimental Investigation on Thermal Runaway of Lithium-Ion Batteries under Low Pressure and Low Temperature
Abstract
1. Introduction
2. Experimental Platform and Setup
2.1. Battery Samples
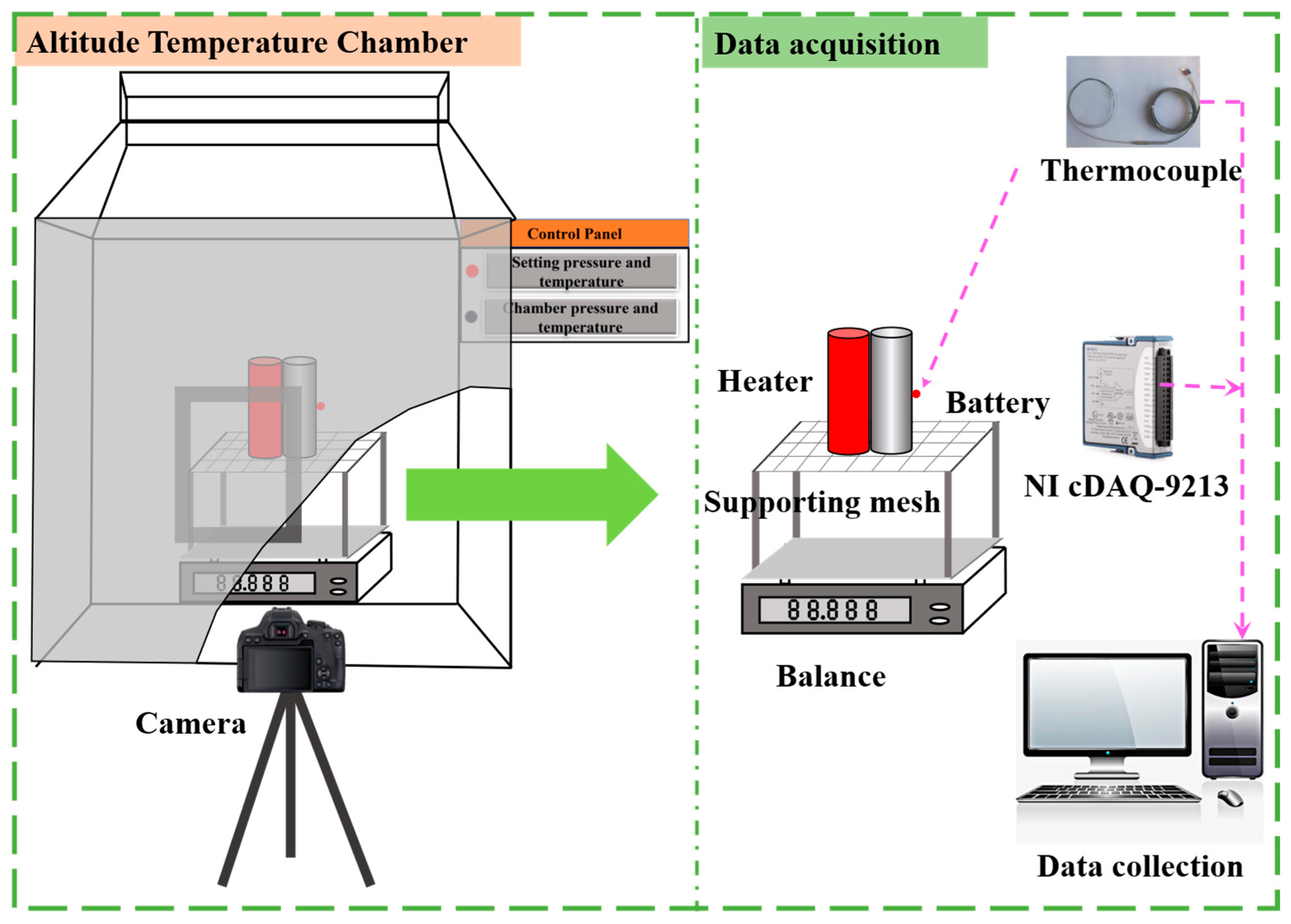
2.2. Experimental Setup
2.3. Experimental Design and Procedure
3. Results and Discussion
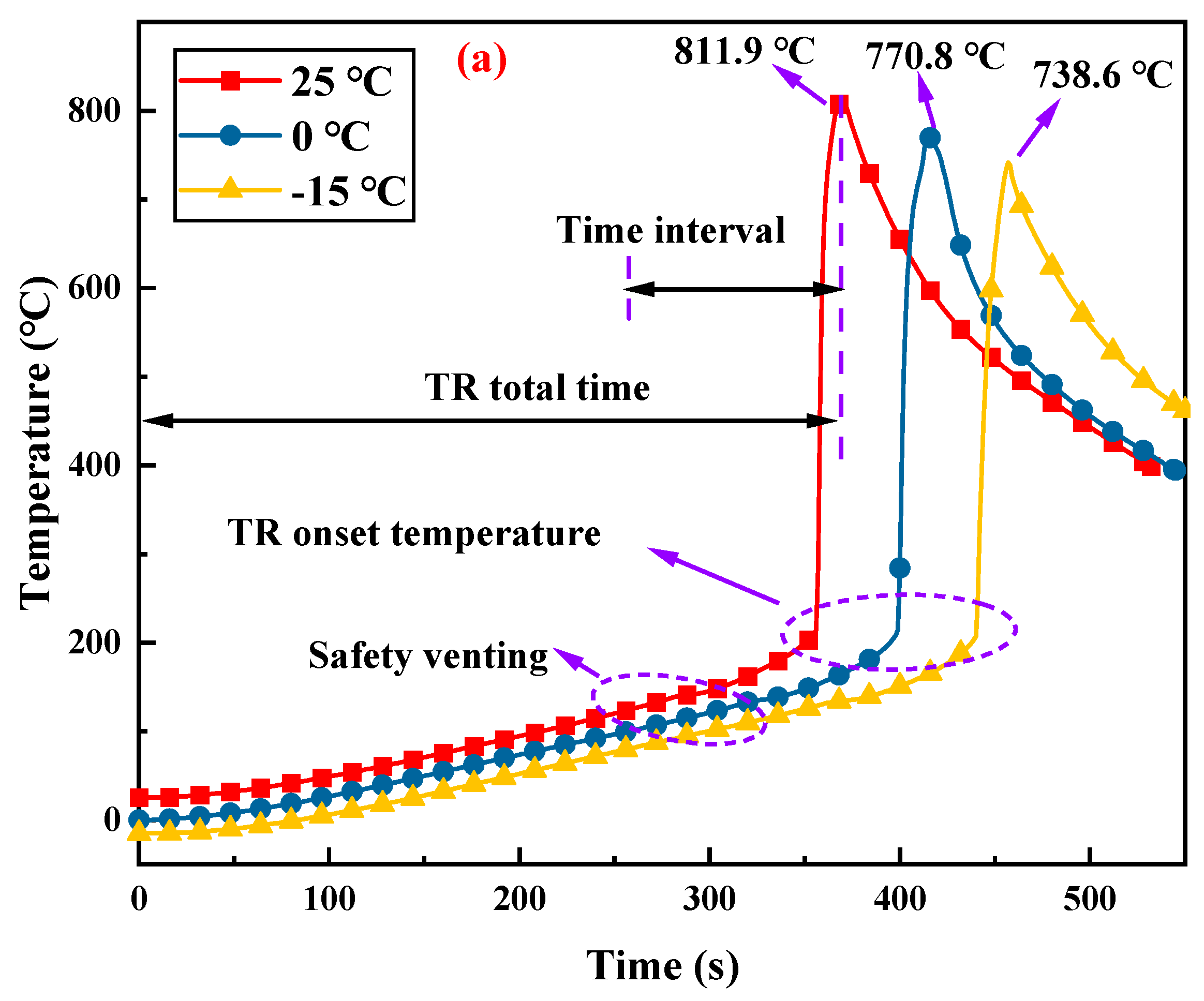
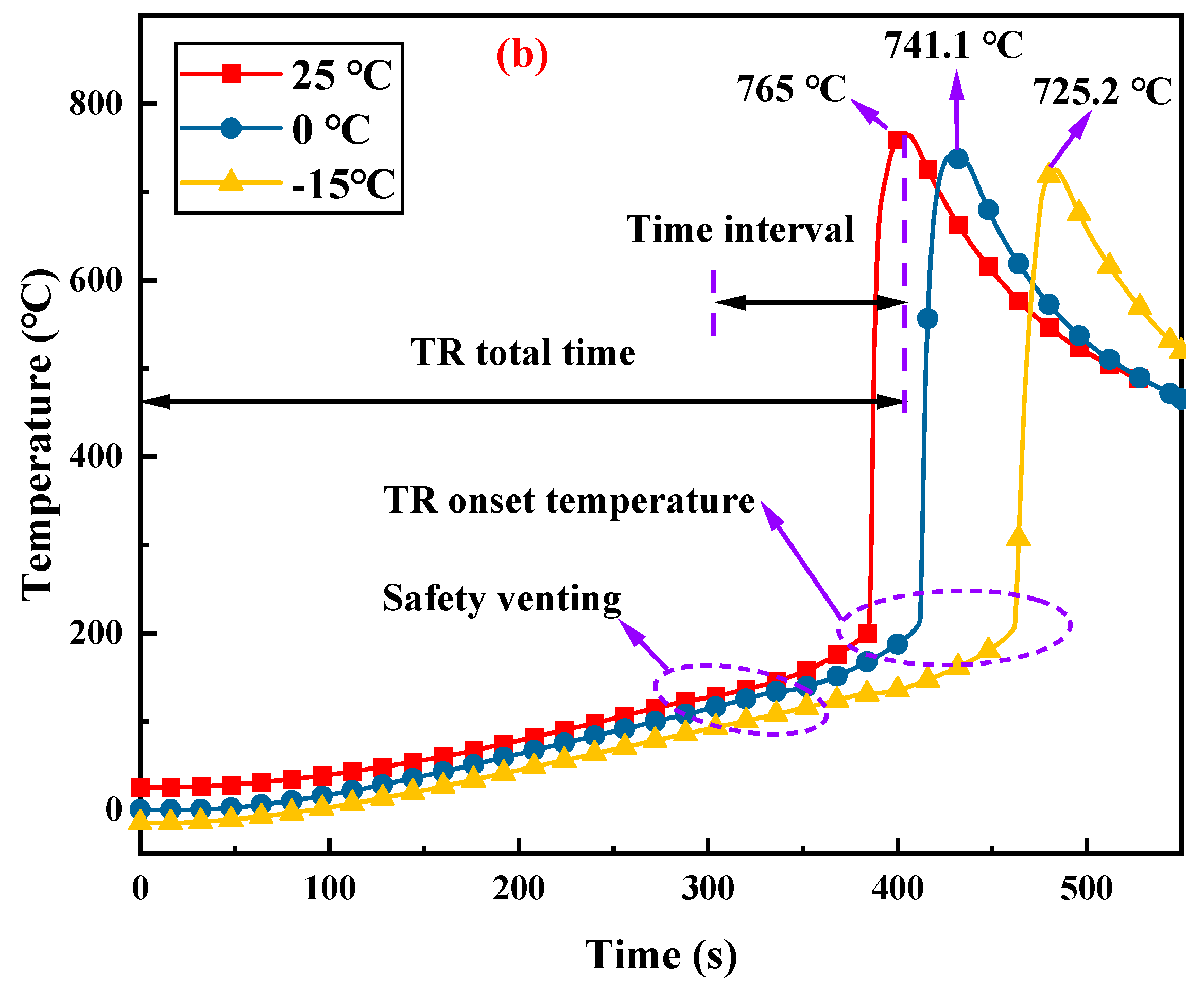
3.1. Thermal Runaway Process of Lithium-Ion Batteries
3.2. Effects of Ambient Pressure
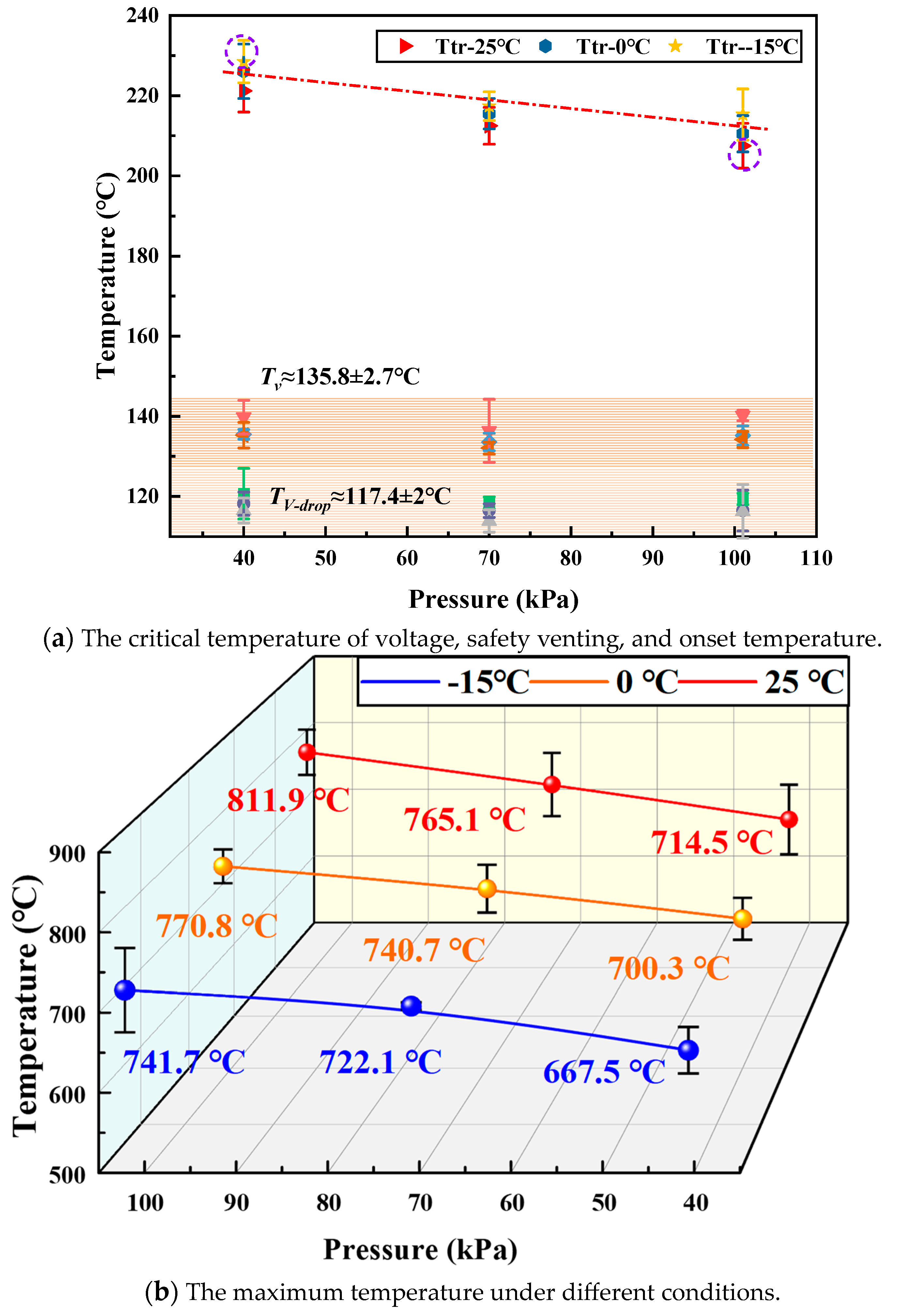
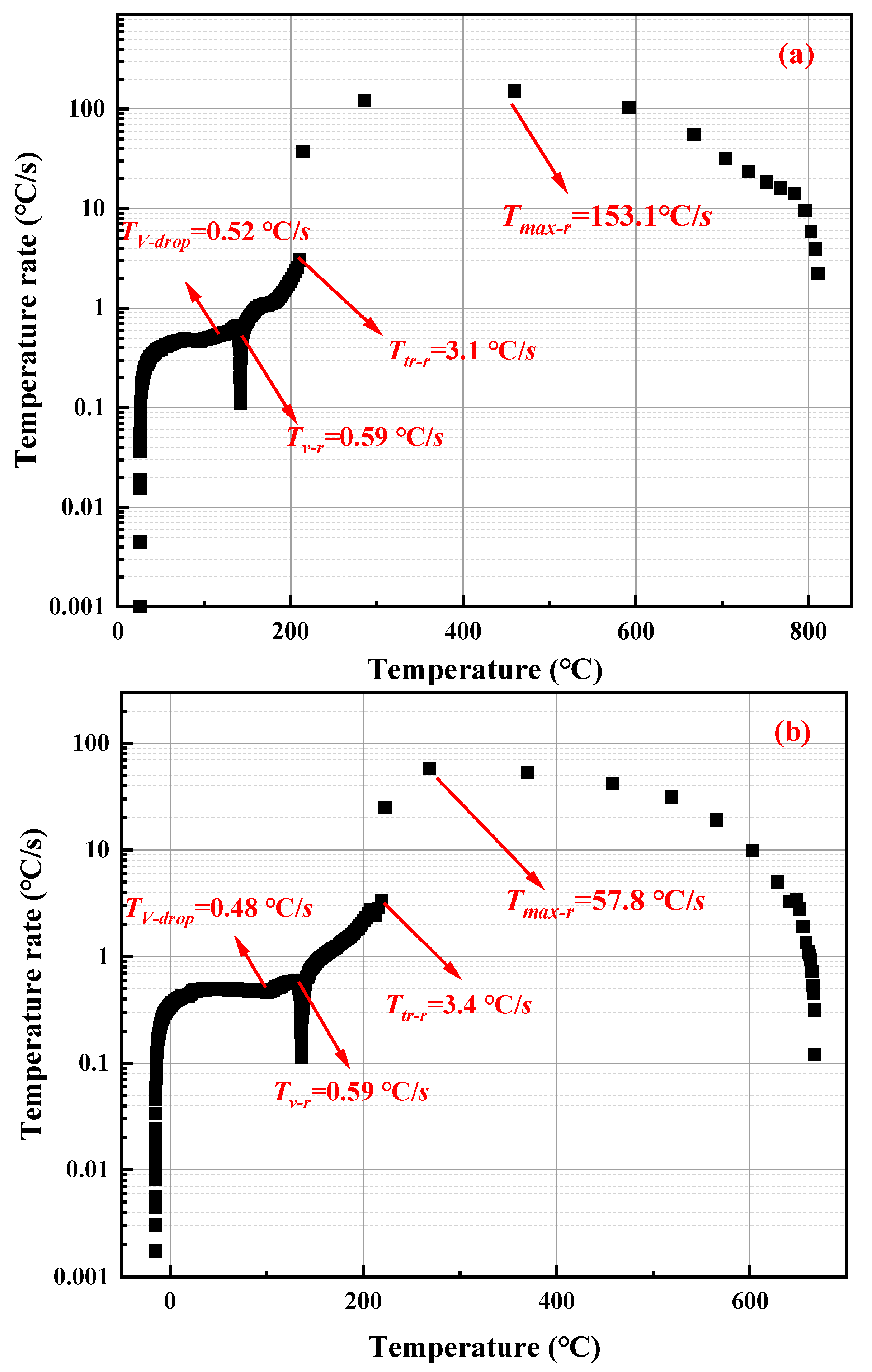
3.3. Thermal Runaway Critical Temperature
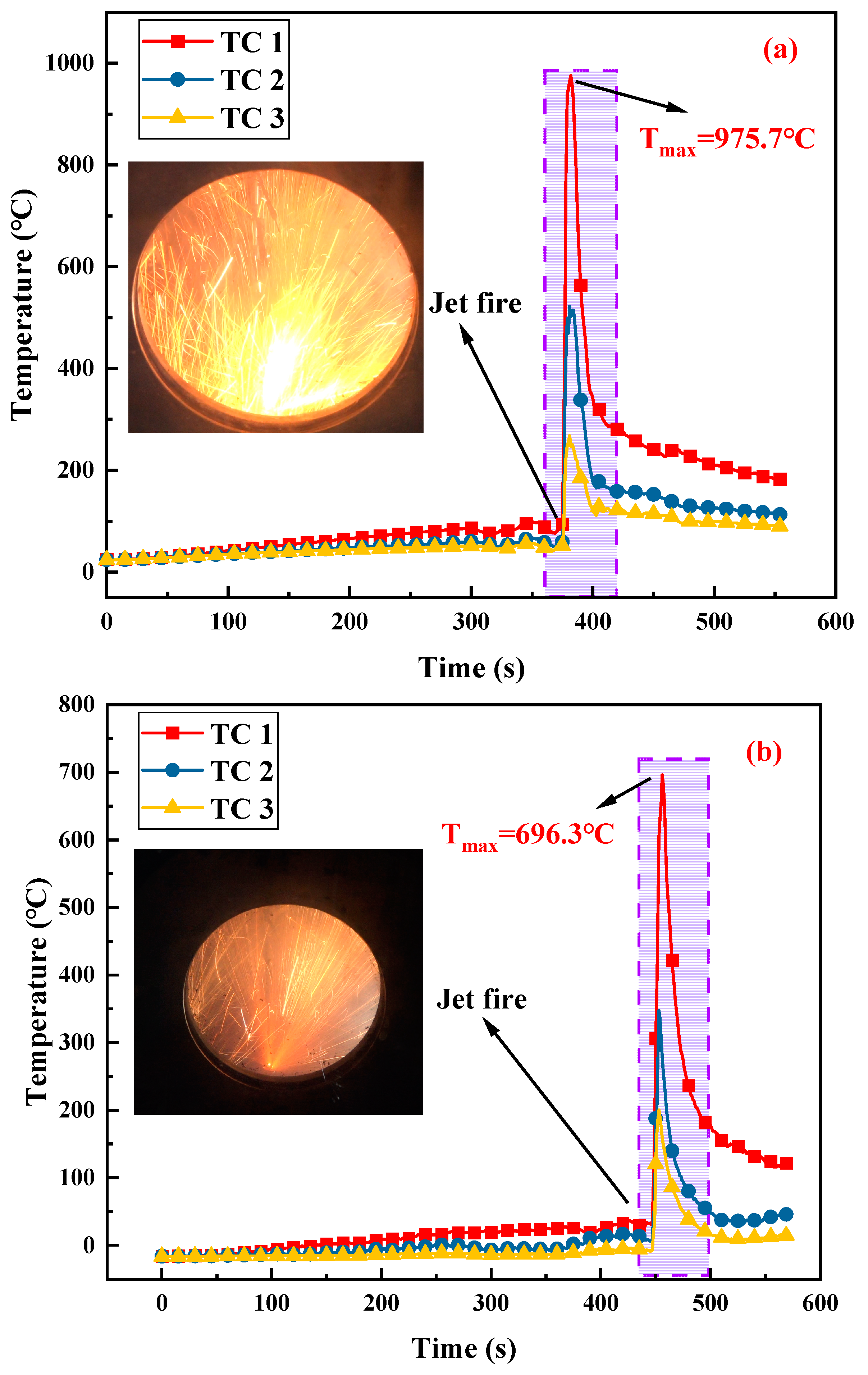
3.4. Flame Temperature Distribution
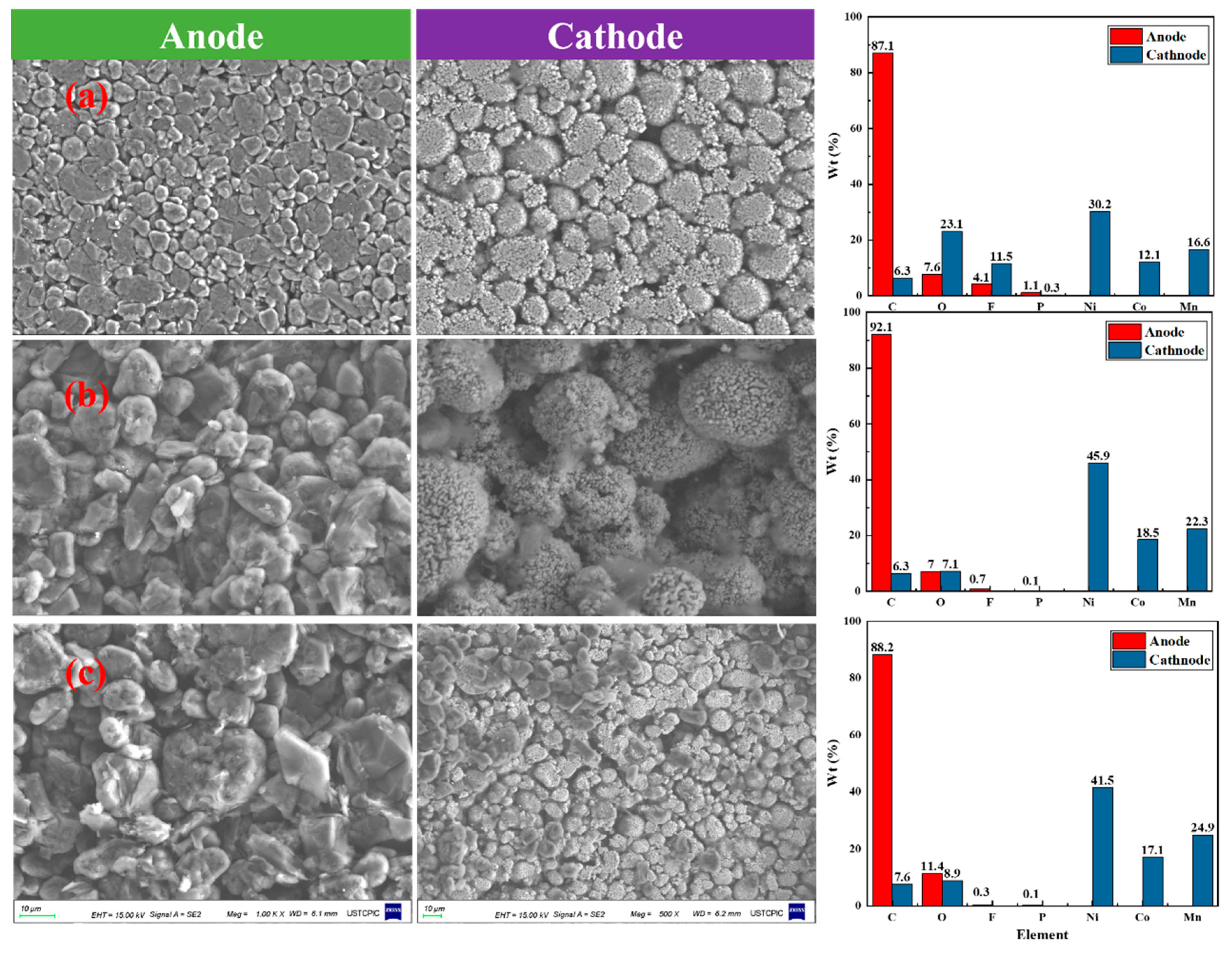
3.5. Physical and Chemical Characteristics of Debris
3.6. Mass Loss
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Epstein, A.; O’Flarity, S. Considerations for Reducing Aviation’s CO2 with Aircraft Electric Propulsion. J. Propuls. Power 2019, 35, 1–11. [Google Scholar] [CrossRef]
- Niu, H.; Chen, C.; Liu, Y.; Li, L.; Li, Z.; Ji, D.; Huang, X. Mitigating thermal runaway propagation of NCM 811 prismatic batteries via hollow glass microspheres plates. Process Saf. Environ. Prot. 2022, 162, 672–683. [Google Scholar] [CrossRef]
- Liu, P.; Sun, H.; Qiao, Y.; Sun, S.; Wang, C.; Jin, K.; Mao, B.; Wang, Q. Experimental study on the thermal runaway and fire behavior of LiNi0.8Co0.1Mn0.1O2 battery in open and confined spaces. Process Saf. Environ. Prot. 2022, 158, 711–726. [Google Scholar] [CrossRef]
- Xie, S.; Yang, X.; Sun, Q.; Wang, Z.; He, Y. Research progress and prospects on thermal safety of lithium-ion batteries in aviation low-temperature and low-pressure environments. J. Energy Storage 2024, 83, 110734. [Google Scholar] [CrossRef]
- Shao, Q.; Yang, M.; Xu, C.; Wang, H.; Liu, H. Fire Risk Analysis of Runway Excursion Accidents in High-Plateau Airport. IEEE Access 2020, 8, 204400–204416. [Google Scholar] [CrossRef]
- Mao, B.; Zhao, C.; Chen, H.; Wang, Q.; Sun, J. Experimental and modeling analysis of jet flow and fire dynamics of 18650-type lithium-ion battery. Appl. Energy 2021, 281, 116054. [Google Scholar] [CrossRef]
- Li, J.-H.; Wu, J.; Yu, Y.-X. Toward Large-Capacity and High-Stability Lithium Storages via Constructing Quinone–2D-MnO2-Pillared Structures. J. Phys. Chem. C 2021, 125, 3725–3732. [Google Scholar] [CrossRef]
- Pigłowska, M.; Kurc, B.; Rymaniak, Ł. Starch as the Flame Retardant for Electrolytes in Lithium-Ion Cells. Materials 2022, 15, 523. [Google Scholar] [CrossRef]
- Xie, S.; Ren, L.; Gong, Y.; Li, M.; Chen, X. Effect of Charging/Discharging Rate on the Thermal Runaway Characteristics of Lithium-Ion Batteries in Low Pressure. J. Electrochem. Soc. 2020, 167, 140503. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, X.; Chen, M.; Huang, Q.; Ding, C.; Wang, J. Study on the combustion behaviors and thermal stability of aging lithium-ion batteries with different states of charge at low pressure. Process Saf. Environ. Prot. 2023, 174, 391–402. [Google Scholar] [CrossRef]
- Meng, H.; Yang, Q.; Zio, E.; Xing, J. An integrated methodology for dynamic risk prediction of thermal runaway in lithium-ion batteries. Process Saf. Environ. Prot. 2023, 171, 385–395. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Song, C.; Gao, L. Characteristics of oxygenic-thermal coupled jet driven by concentration difference and temperature difference at high altitudes. Build. Environ. 2023, 228, 109897. [Google Scholar] [CrossRef]
- Bills, A.; Sripad, S.; Fredericks, W.L.; Singh, M.; Viswanathan, V. Performance Metrics Required of Next-Generation Batteries to Electrify Commercial Aircraft. ACS Energy Lett. 2020, 5, 663–668. [Google Scholar] [CrossRef]
- Warren, M.; Garbo, A.; Kotwicz Herniczek, M.T.; Hamilton, T.; German, B. Effects of Range Requirements and Battery Technology on Electric VTOL Sizing and Operational Performance. In Proceedings of the AIAA Scitech 2019 Forum: American Institute of Aeronautics and Astronautics, San Diego, CA, USA, 7–11 January 2019. [Google Scholar]
- Chen, M.; Zhou, D.; Chen, X.; Zhang, W.; Liu, J.; Yuen, R.; Wang, J. Investigation on the thermal hazards of 18650 lithium ion batteries by fire calorimeter. J. Therm. Anal. Calorim. 2015, 122, 755–763. [Google Scholar] [CrossRef]
- Chen, M.; Ouyang, D.; Weng, J.; Liu, J.; Wang, J. Environmental pressure effects on thermal runaway and fire behaviors of lithium-ion battery with different cathodes and state of charge. Process Saf. Environ. Prot. 2019, 130, 250–256. [Google Scholar] [CrossRef]
- Weng, J.; Yang, X.; Ouyang, D.; Chen, M.; Zhang, G.; Wang, J. Comparative study on the transversal/lengthwise thermal failure propagation and heating position effect of lithium-ion batteries. Appl. Energy 2019, 255, 113761. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; He, Y.; Yuen, R.; Wang, J. Study of the fire hazards of lithium-ion batteries at different pressures. Appl. Therm. Eng. 2017, 125, 1061–1074. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, S.; Shi, L.; Cheng, X.; Zhang, H. Ignition and combustion characteristics of lithium ion batteries under low atmospheric pressure. Energy 2018, 161, 38–45. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Xu, C.; Huang, X. Thermal runaway propagation in linear battery module under low atmospheric pressure. Appl. Therm. Eng. 2022, 216, 119086. [Google Scholar] [CrossRef]
- Jia, Z.; Huang, Z.; Zhai, H.; Qin, P.; Zhang, Y.; Li, Y.; Wang, Q. Experimental investigation on thermal runaway propagation of 18,650 lithium-ion battery modules with two cathode materials at low pressure. Energy 2022, 251, 123925. [Google Scholar] [CrossRef]
- Niu, H.; Chen, C.; Ji, D.; Li, L.; Li, Z.; Liu, Y.; Huang, X. Thermal-Runaway Propagation over a Linear Cylindrical Battery Module. Fire Technol. 2020, 56, 2491–2507. [Google Scholar] [CrossRef]
- Guo, L.S.; Wang, Z.R.; Wang, J.H.; Luo, Q.K.; Liu, J.J. Effects of the environmental temperature and heat dissipation condition on the thermal runaway of lithium ion batteries during the charge-discharge process. J. Loss Prev. Process Ind. 2017, 49, 953–960. [Google Scholar] [CrossRef]
- Ouyang, D.; Hu, J.; Chen, M.; Weng, J.; Huang, Q.; Liu, J.; Wang, J. Effects of abusive temperature environment and cycle rate on the homogeneity of lithium-ion battery. Thermochim. Acta 2019, 676, 241–248. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Jhu, C.-Y.; Wang, Y.-W.; Chiang, C.-C.; Shu, C.-M. Thermal runaway features of 18650 lithium-ion batteries for LiFePO4 cathode material by DSC and VSP2. J. Therm. Anal. Calorim. 2012, 109, 1297–1302. [Google Scholar] [CrossRef]
- Wang, Z.; He, T.; Bian, H.; Jiang, F.; Yang, Y. Characteristics of and factors influencing thermal runaway propagation in lithium-ion battery packs. J. Energy Storage 2021, 41, 102956. [Google Scholar] [CrossRef]
- Weng, J.; Ouyang, D.; Liu, Y.; Chen, M.; Li, Y.; Huang, X.; Wang, J. Alleviation on battery thermal runaway propagation: Effects of oxygen level and dilution gas. J. Power Sources 2021, 509, 230340. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Wang, Q.; Duan, Q.; Li, Y.; Li, L.; Wang, Q. Experimental investigation on thermal runaway propagation of large format lithium ion battery modules with two cathodes. Int. J. Heat Mass Transf. 2021, 172, 121077. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Jiang, L.; Zhao, C.; Sun, J.; Wang, Q. Refined study on lithium ion battery combustion in open space and a combustion chamber. Process Saf. Environ. Prot. 2020, 139, 133–146. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Chen, M.; Wang, J. What a role does the safety vent play in the safety of 18650-size lithium-ion batteries? Process Saf. Environ. Prot. 2022, 159, 433–441. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Li, Z.; Liu, J.; Xu, C.; Huang, X. Thermal runaway characteristics and failure criticality of massive ternary Li-ion battery piles in low-pressure storage and transport. Process Saf. Environ. Prot. 2021, 155, 486–497. [Google Scholar] [CrossRef]
- Meng, D.; Wang, X.; Chen, M.; Wang, J. Effects of environmental temperature on the thermal runaway of lithium-ion batteries during charging process. J. Loss Prev. Process Ind. 2023, 83, 105084. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Liu, Q.; Yi, X.; Han, X. Effect of Different Arrangement on Thermal Runaway Characteristics of 18650 Lithium Ion Batteries Under the Typical Pressure in Civil Aviation Transportation. Fire Technol. 2020, 56, 2509–2523. [Google Scholar]
- Yan, H.; Marr, K.C.; Ezekoye, O.A. Towards Fire Forensic Characteristics of Failed Cylindrical Format Lithium–Ion Cells and Batteries. Fire Technol. 2021, 57, 1723–1752. [Google Scholar] [CrossRef]
- Hu, J.; Liu, T.; Tang, Q.; Wang, X. Experimental investigation on thermal runaway propagation in the lithium ion battery modules under charging condition. Appl. Therm. Eng. 2022, 211, 118522. [Google Scholar] [CrossRef]
- Liu, T.; Hu, J.; Tao, C.; Zhu, X.; Wang, X. Effect of parallel connection on 18650-type lithium ion battery thermal runaway propagation and active cooling prevention with water mist. Appl. Therm. Eng. 2021, 184, 116291. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Chen, M.; Wang, J. Impact of Charging and Charging Rate on Thermal Runaway Behaviors of Lithium-Ion Cells. J. Electrochem. Soc. 2021, 168, 120510. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2(NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]


| Item | Specification |
|---|---|
| Cathode material | LiNi0.5Co0.2Mn0.3O2 (NCM523) |
| Anode | Graphite |
| Nominal capacity | 2600 mAh |
| Nominal voltage | 3.6 V |
| Voltage | Charge: 4.2 V Discharge: 2.75 V |
| Cell mass | 44.8 ± 0.2 g |
| Storage temperature | 1 year: −20~25 °C 3 months: −20~45 °C 1 month: −20~60 °C |
| Group No. | Test No. | Temperature (°C) | Pressure (kPa) | SOC (%) | Heating Power (W) |
|---|---|---|---|---|---|
| I | 1 | 25 | 101 | 100 | 100 |
| 2 | 70 | ||||
| 3 | 40 | ||||
| II | 4 | 0 | 101 | 100 | |
| 5 | 70 | ||||
| 6 | 40 | ||||
| III | 7 | −15 | 101 | 100 | |
| 8 | 70 | ||||
| 9 | 40 |
| Ambient Temperature (°C) | 101 kPa (s) | 70 kPa (s) | 40 kPa (s) |
|---|---|---|---|
| 25 | 370 ± 16.3 | 415 ± 16.7 | 460 ± 5.6 |
| 0 | 405 ± 13.9 | 428 ± 8.1 | 483 ± 9.5 |
| −15 | 429 ± 17.6 | 458 ± 11.9 | 503 ± 10.8 |
| Ambient Temperature (°C) | 101 kPa (s) | 70 kPa (s) | 40 kPa (s) |
|---|---|---|---|
| 25 | 75 ± 3.3 | 89 ± 2.7 | 98 ± 5.6 |
| 0 | 82 ± 1.9 | 94 ± 4.1 | 101 ± 3.5 |
| −15 | 88 ± 7.6 | 99 ± 3.9 | 105 ± 6.8 |
| Ambient Pressure (kPa) | 25 °C (°C·s−1) | 0 °C (°C·s−1) | −15 °C (°C·s−1) |
|---|---|---|---|
| 101 | 153.1 | 107.9 | 71.6 |
| 70 | 131.5 | 97.1 | 68.9 |
| 40 | 98.6 | 68.8 | 57.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, D.; Weng, J.; Wang, J. Experimental Investigation on Thermal Runaway of Lithium-Ion Batteries under Low Pressure and Low Temperature. Batteries 2024, 10, 243. https://doi.org/10.3390/batteries10070243
Meng D, Weng J, Wang J. Experimental Investigation on Thermal Runaway of Lithium-Ion Batteries under Low Pressure and Low Temperature. Batteries. 2024; 10(7):243. https://doi.org/10.3390/batteries10070243
Chicago/Turabian StyleMeng, Di, Jingwen Weng, and Jian Wang. 2024. "Experimental Investigation on Thermal Runaway of Lithium-Ion Batteries under Low Pressure and Low Temperature" Batteries 10, no. 7: 243. https://doi.org/10.3390/batteries10070243
APA StyleMeng, D., Weng, J., & Wang, J. (2024). Experimental Investigation on Thermal Runaway of Lithium-Ion Batteries under Low Pressure and Low Temperature. Batteries, 10(7), 243. https://doi.org/10.3390/batteries10070243







