Surface Modification Induces Oriented Zn(002) Deposition for Highly Stable Zinc Anode
Abstract
1. Introduction
2. Materials and Methods
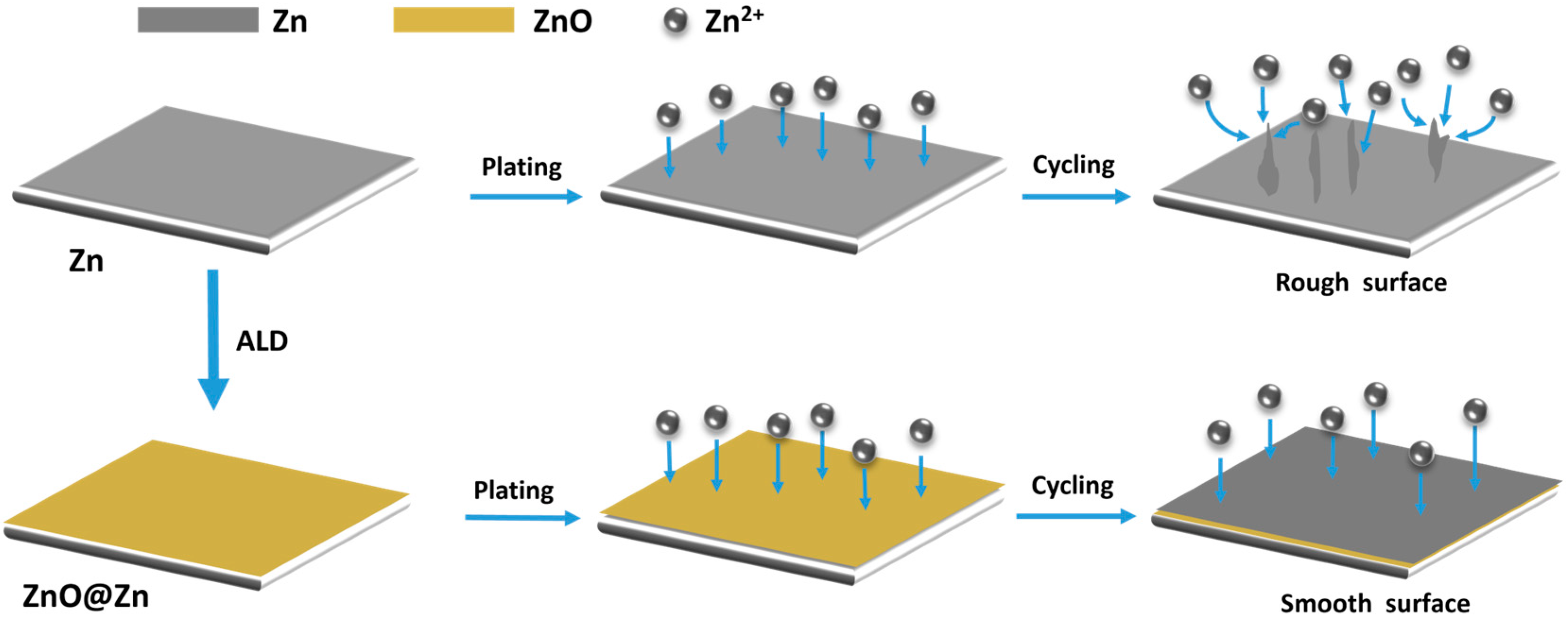
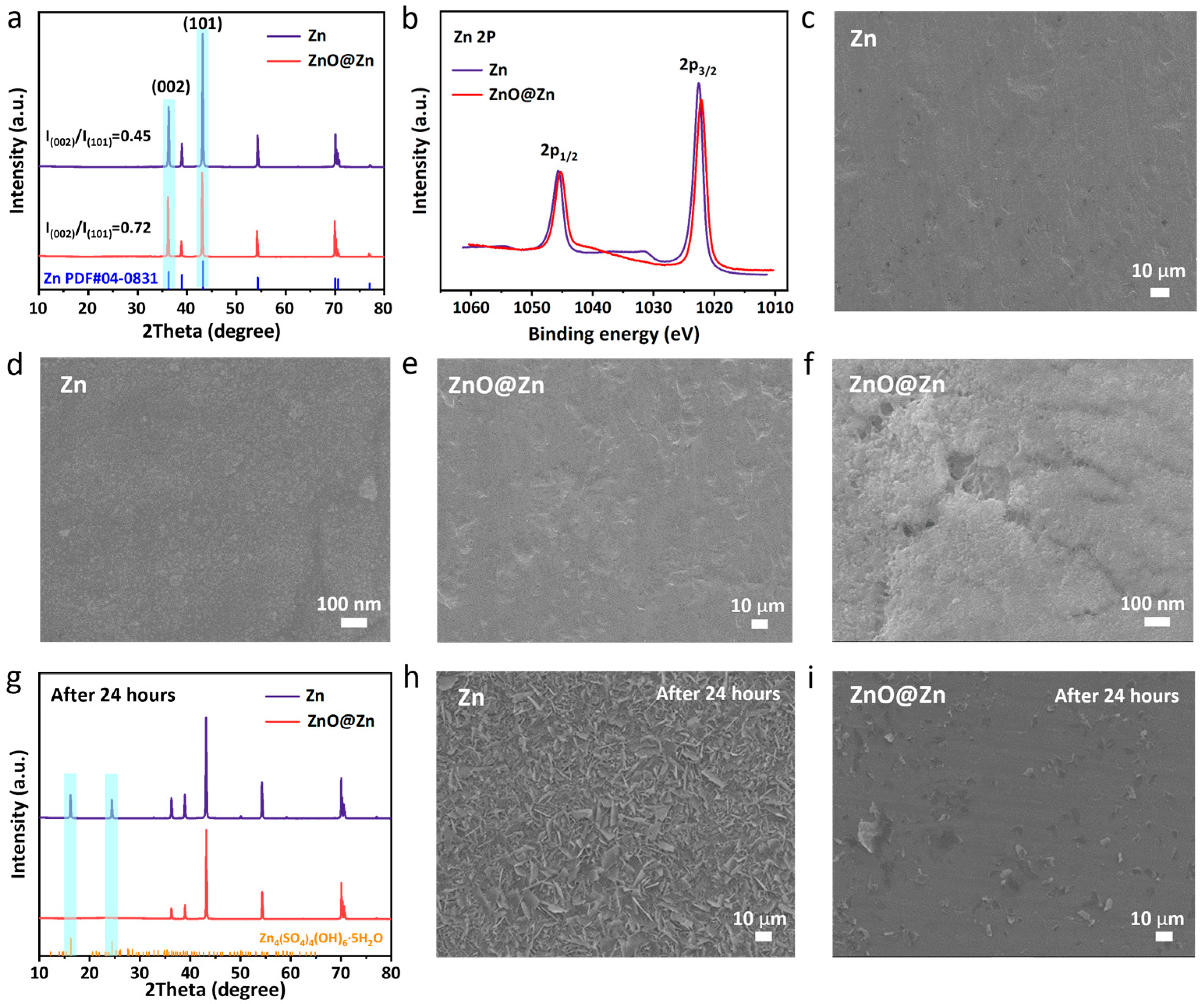
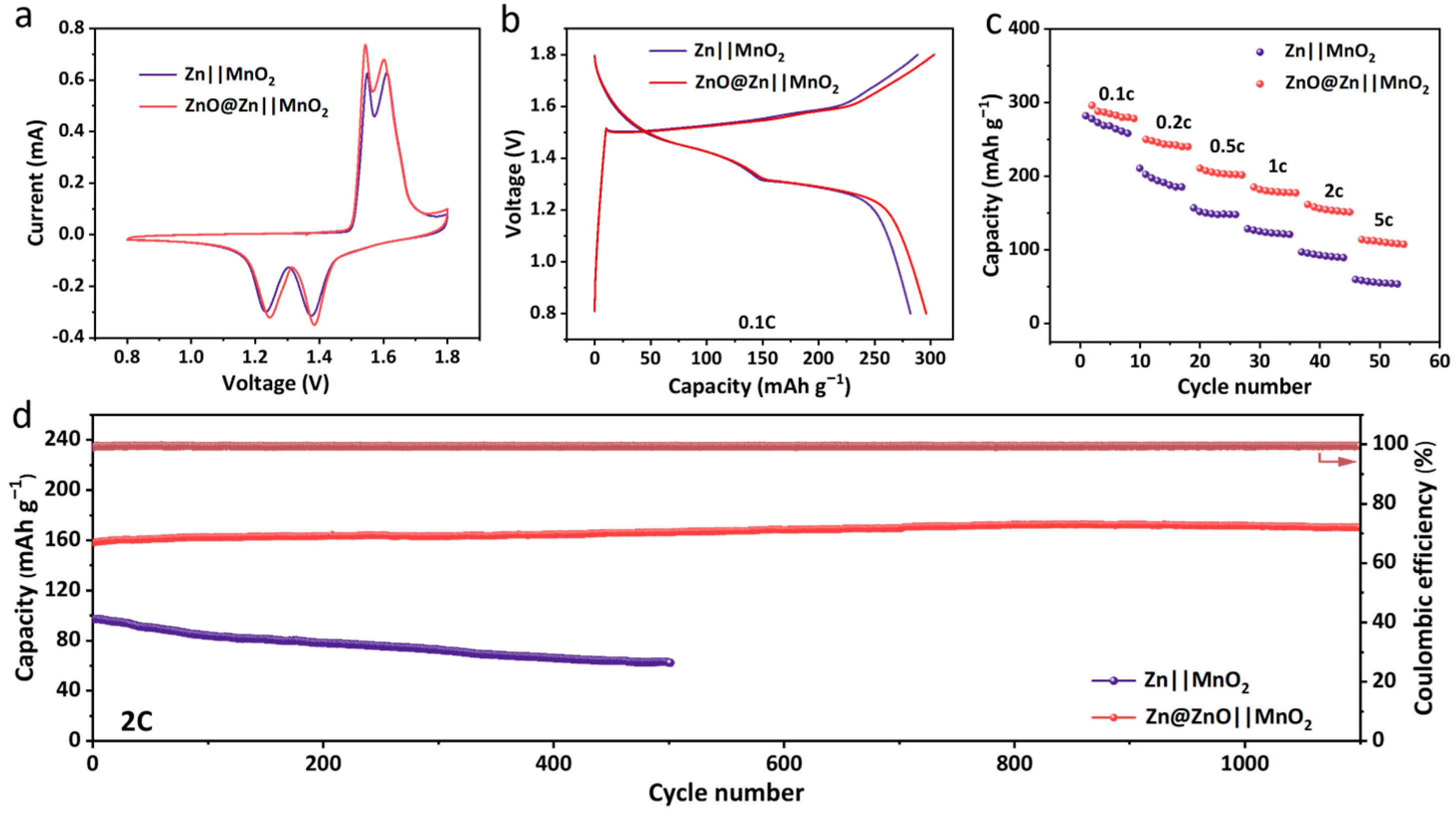
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Guo, S.; Zhou, H. Recent Advances in Manipulating Strategy of Aqueous Electrolytes for Zn Anode Stabilization. Energy Storage Mater. 2023, 56, 227–257. [Google Scholar] [CrossRef]
- Bai, L.; Hu, Z.; Hu, C.; Zhang, S.; Ying, Y.; Zhang, Y.; Li, L.; Zhang, H.; Li, N.; Shi, S.; et al. Utilizing Cationic Vacancies and Spontaneous Polarization on Cathode to Enhance Zinc-Ion Storage and Inhibit Dendrite Growth in Zinc-Ion Batteries. Angew. Chemie-Int. Ed. 2023, 62, 2301631. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Huang, J. Chemical Passivation Stabilizes Zn Anode. Adv. Mater. 2022, 34, 2109872. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, N.; Long, C.; Dong, B.; Fang, D.; Liu, Z.; Zhao, Y.; Li, X.; Fan, J.; Chen, S.; et al. Achieving Both High Voltage and High Capacity in Aqueous Zinc-Ion Battery for Record High Energy Density. Adv. Funct. Mater. 2019, 29, 1906142. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, R.; Wang, Y.; Hu, Z.; Wang, Y.; Zhang, A.; Wu, C.; Bai, Y. Insights on Artificial Interphases of Zn and Electrolyte: Protection Mechanisms, Constructing Techniques, Applicability, and Prospective. Adv. Funct. Mater. 2023, 33, 2213510. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, X.; Xu, W.; Chen, W.; Zhao, K.; Wang, Y.; Hong, S.; Wu, Q.; Li, M.C.; et al. Synergic Effect of Dendrite-Free and Zinc Gating in Lignin-Containing Cellulose Nanofibers-MXene Layer Enabling Long-Cycle-Life Zinc Metal Batteries. Adv. Sci. 2022, 9, 2202380. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Han, G.; Li, X.; Yuan, T.; Sun, H.; Gong, Y.; Chen, T. Recent Progress in Electrolyte Additives for Highly Reversible Zinc Anodes in Aqueous Zinc Batteries. Batteries 2023, 9, 284. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, S.; Li, Y.; Zheng, Z.; Dong, L. Sieve-Like Interface Built by ZnO Porous Sheets towards Stable Zinc Anodes. J. Colloid Interface Sci. 2023, 630, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jin, Q.; Jiang, X.; Dang, Z.M.; Zhang, D.; Jin, Y. Vertical Crystal Plane Matching between AgZn3 (002) and Zn(002) Achieving a Dendrite-Free Zinc Anode. Small 2022, 18, 2200131. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, Y.; Li, J.; Liao, Y.; Zeng, J.; Shen, Y.; Yuan, L.; Li, Z.; Huang, Y. Inducing the Preferential Growth of Zn(002) Plane for Long Cycle Aqueous Zn-Ion Batteries. Adv. Energy Mater. 2023, 13, 2203254. [Google Scholar] [CrossRef]
- Shangguan, M.; Wang, K.; Zhao, Y.; Xia, L. Tetraethylene Glycol Dimethyl Ether (TEGDME)-Water Hybrid Electrolytes Enable Excellent Cyclability in Aqueous Zn-Ion Batteries. Batteries 2023, 9, 462. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, Q.; Li, L.; Zhao, H.; Liu, R.; Guo, Z.; Wang, L. A Self-Growing 3D Porous Sn Protective Layer Enhanced Zn Anode. Batteries 2023, 9, 262. [Google Scholar] [CrossRef]
- Yang, H.; Chang, Z.; Qiao, Y.; Deng, H.; Mu, X.; He, P.; Zhou, H. Constructing a Super-Saturated Electrolyte Front Surface for Stable Rechargeable Aqueous Zinc Batteries. Angew. Chemie 2020, 132, 9463–9467. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, R.; Peng, C.; Chen, W.; Wu, T.; Hu, B.; Weng, W.; Yao, Y.; Zeng, J.; Chen, Z.; et al. Horizontally Arranged Zinc Platelet Electrodeposits Modulated by Fluorinated Covalent Organic Framework Film for High-Rate and Durable Aqueous Zinc Ion Batteries. Nat. Commun. 2021, 12, 6606. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, X.; Liu, Z.; Lei, H.; Cui, S.; Xie, X.; Hu, Y.; Ma, G. Alleviating Zn Dendrites by Growth of Ultrafine ZnO Nanowire Arrays through Horizontal Anodizing for High-Capacity, Long-Life Zn Ion Capacitors. ACS Appl. Mater. Interfaces 2023, 15, 4071–4080. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Yu, H.; Liu, W.; Kuang, G.; Mei, L.; Wu, Z.; Wei, W.; Ji, X.; Qu, B.; et al. A Multifunctional Artificial Interphase with Fluorine-Doped Amorphous Carbon Layer for Ultra-Stable Zn Anode. Adv. Funct. Mater. 2022, 32, 2205600. [Google Scholar] [CrossRef]
- Feng, J.; Li, X.; Cui, X.; Zhao, H.; Xi, K.; Ding, S. Periodically Alternating Electric Field Layers Induces the Preferential Growth of Zn(002) Plane for Ultralow Overpotential Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2204092. [Google Scholar] [CrossRef]
- Chen, M.; Yang, M.; Zhou, W.; Tian, Q.; Han, X.; Chen, J.; Zhang, P. Oriented Zn Plating Guided by Aligned ZnO Hexagonal Columns Realizing Dendrite-Free Zn Metal Electrodes. J. Colloid Interface Sci. 2023, 644, 368–377. [Google Scholar] [CrossRef]
- Xin, T.; Wang, Y.; Xu, Q.; Shang, J.; Yuan, X.; Song, W.; Liu, J. Forming an Amorphous ZnO Nanosheet Network by Confined Parasitic Reaction for Stabilizing Zn Anodes and Reducing Water Activity. ACS Appl. Energy Mater. 2022, 5, 2290–2299. [Google Scholar] [CrossRef]
- Hu, X.; Borowiec, J.; Zhu, Y.; Liu, X.; Wu, R.; Ganose, A.M.; Parkin, I.P.; Boruah, B.D. Dendrite-Free Zinc Anodes Enabled by Exploring Polar-Face-Rich 2D ZnO Interfacial Layers for Rechargeable Zn-Ion Batteries. Small 2023, 2306827, 2306827. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Yuan, H.; Xiong, F.; Liu, Q.; An, Y.; Zhang, J.; Wu, L.; Sun, J.; Zhang, Y.W.; et al. Achieving Highly Reversible Zinc Metal Anode via Surface Termination Chemistry. Sci. Bull. 2023, 68, 2993–3002. [Google Scholar] [CrossRef]
- Wang, R.; Xin, S.; Chao, D.; Liu, Z.; Wan, J.; Xiong, P.; Luo, Q.; Hua, K.; Hao, J.; Zhang, C. Fast and Regulated Zinc Deposition in a Semiconductor Substrate toward High-Performance Aqueous Rechargeable Batteries. Adv. Funct. Mater. 2022, 32, 2207751. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, H.; Ren, Y.; Mo, L.; He, Y.; Tan, P.; Huang, Y.; Li, Z.; Zhu, D.; Hu, L. Building Near-Unity Stacked (002) Texture for High-Stable Zinc Anode. Adv. Funct. Mater. 2023, 34, 2312506. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Liu, R.; Yang, Z.; Zhang, S.; Zhang, Y.; Wang, H.; Cao, Y.; Chen, A.; Sun, J. Manipulating the Zinc Deposition Behavior in Hexagonal Patterns at the Preferential Zn (100) Crystal Plane to Construct Surficial Dendrite-Free Zinc Metal Anode. Small 2022, 18, 2105978. [Google Scholar] [CrossRef]
- Li, T.; Hu, S.; Wang, C.; Wang, D.; Xu, M.; Chang, C.; Xu, X.; Han, C. Engineering Fluorine-Rich Double Protective Layer on Zn Anode for Highly Reversible Aqueous Zinc-Ion Batteries. Angew. Chemie Int. Ed. 2023, 62, 2314883. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Fu, K.; Yu, R.; Zhu, J.; Cai, H.; Zhang, X.; Wu, J.; Luo, W.; Mai, L. Ion Tunnel Matrix Initiated Oriented Attachment for Highly Utilized Zn Anodes. Adv. Mater. 2023, 35, 2302353. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, R.; Yu, L.; Kong, X.; Bao, S.; Tu, M.; Liu, X.; Xu, B. Engineering a Hierarchical Carbon Supported Magnetite Nanoparticles Composite from Metal Organic Framework and Graphene Oxide for Lithium-Ion Storage. J. Colloid Interface Sci. 2023, 630, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Allouche, A. Software News and Updates Gabedit—A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2012, 32, 174–182. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 Single Crystals with a Large Percentage of Reactive Facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Yin, H.; Liu, Y.; Zhu, Y.; Ye, F.; Xu, G.; Lin, M.; Kang, W. Bimetal-Initiated Concerted Zn Regulation Enabling Highly Stable Aqueous Zn-Ion Batteries. Batteries 2024, 10, 70. [Google Scholar] [CrossRef]
- Kim, E.; Vaynzof, Y.; Sepe, A.; Guldin, S.; Scherer, M.; Cunha, P.; Roth, S.V.; Steiner, U. Gyroid-Structured 3D ZnO Networks Made by Atomic Layer Deposition. Adv. Funct. Mater. 2014, 24, 863–872. [Google Scholar] [CrossRef]
- Fan, C.; Meng, W.; Li, D.; Jiang, L. Stratified Adsorption Strategy Facilitates Highly Stable Dendrite Free Zinc Metal Anode. Energy Storage Mater. 2023, 56, 468–477. [Google Scholar] [CrossRef]
- Han, Y.; Wang, F.; Zhang, B.; Yan, L.; Hao, J.; Zhu, C.; Zou, X.; Zhou, Y.; Xiang, B. Building Block Effect Induces Horizontally Oriented Bottom Zn(002) Deposition for a Highly Stable Zinc Anode. Energy Storage Mater. 2023, 62, 102928. [Google Scholar] [CrossRef]
- Bao, S.; Tu, M.; Huang, H.; Wang, C.; Chen, Y.; Sun, B.; Xu, B. Heterogeneous Iron Oxide Nanoparticles Anchored on Carbon Nanotubes for High-Performance Lithium-Ion Storage and Fenton-like Oxidation. J. Colloid Interface Sci. 2021, 601, 283–293. [Google Scholar] [CrossRef]
- Ke, X.; Li, L.; Wang, S.; Wang, A.; Jiang, Z.; Wang, F.R.; Kuai, C.; Guo, Y. Mn-Oxide Cathode Material for Aqueous Zn-Ion Battery: Structure, Mechanism, and Performance. Next Energy 2024, 2, 100095. [Google Scholar] [CrossRef]
- Liang, R.; Fu, J.; Deng, Y.P.; Pei, Y.; Zhang, M.; Yu, A.; Chen, Z. Parasitic Electrodeposition in Zn-MnO2 Batteries and Its Suppression for Prolonged Cyclability. Energy Storage Mater. 2021, 36, 478–484. [Google Scholar] [CrossRef]
- Xie, F.; Li, H.; Wang, X.; Zhi, X.; Chao, D.; Davey, K.; Qiao, S.Z. Mechanism for Zincophilic Sites on Zinc-Metal Anode Hosts in Aqueous Batteries. Adv. Energy Mater. 2021, 11, 2003419. [Google Scholar] [CrossRef]
- Zhao, R.; Dong, X.; Liang, P.; Li, H.; Zhang, T.; Zhou, W.; Wang, B.; Yang, Z.; Wang, X.; Wang, L.; et al. Prioritizing Hetero-Metallic Interfaces via Thermodynamics Inertia and Kinetics Zincophilia Metrics for Tough Zn-Based Aqueous Batteries. Adv. Mater. 2023, 35, 2209288. [Google Scholar] [CrossRef]
- Li, Y.; Jia, H.; Ali, U.; Liu, B.; Gao, Y.; Li, L.; Zhang, L.; Chai, F.; Wang, C. In-Situ Interfacial Layer with Ultrafine Structure Enabling Zinc Metal Anodes at High Areal Capacity. Chem. Eng. J. 2022, 450, 138374. [Google Scholar] [CrossRef]
- Tan, L.; Wei, C.; Zhang, Y.; An, Y.; Xiong, S.; Feng, J. Long-Life and Dendrite-Free Zinc Metal Anode Enabled by a Flexible, Green and Self-Assembled Zincophilic Biomass Engineered MXene Based Interface. Chem. Eng. J. 2022, 431, 134277. [Google Scholar] [CrossRef]
- Wang, M.; Wu, X.; Yang, D.; Zhao, H.; He, L.; Su, J.; Zhang, X.; Yin, X.; Zhao, K.; Wang, Y.; et al. A Colloidal Aqueous Electrolyte Modulated by Oleic Acid for Durable Zinc Metal Anode. Chem. Eng. J. 2023, 451, 138589. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Zhong, W.; Xie, C.; Xie, Y.; Zhang, Q.; Sun, D.; Tang, Y.; Wang, H. A Progressive Nucleation Mechanism Enables Stable Zinc Stripping-Plating Behavior. Energy Environ. Sci. 2021, 14, 5563–5571. [Google Scholar] [CrossRef]
- Qin, H.; Kuang, W.; Hu, N.; Zhong, X.; Huang, D.; Shen, F.; Wei, Z.; Huang, Y.; Xu, J.; He, H. Building Metal-Molecule Interface towards Stable and Reversible Zn Metal Anodes for Aqueous Rechargeable Zinc Batteries. Adv. Funct. Mater. 2022, 32, 2206695. [Google Scholar] [CrossRef]
- Li, Y.; Jia, H.; Ali, U.; Wang, H.; Liu, B.; Li, L.; Zhang, L.; Wang, C. Successive Gradient Internal Electric Field Strategy Toward Dendrite-Free Zinc Metal Anode. Adv. Energy Mater. 2023, 13, 2301643. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, T.; Yin, J.; Tian, Z.; Ma, Y.; Liu, Z.; Zhu, Y.; Alshareef, H.N. Controlled Deposition of Zinc-Metal Anodes via Selectively Polarized Ferroelectric Polymers. Adv. Mater. 2022, 34, 2106937. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.G.; Hua, W.; Sun, H.; Huyan, Y.; Tian, S.; Hou, Z.; Yang, J.; Wei, C.; Kang, F. Building Ohmic Contact Interfaces toward Ultrastable Zn Metal Anodes. Adv. Sci. 2021, 8, 2102612. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Xi, M.; Huang, Y.; Li, H.; Jin, H.; Ding, J.; Zhang, S.; Zhang, C.; Guo, Z. Interfacial Engineering of Zn Metal via a Localized Conjugated Layer for Highly Reversible Aqueous Zinc Ion Battery. Angew. Chemie Int. Ed. 2024, 63, 2319091. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, F.; Li, Z.; Gao, L.; Xu, B.; Wang, C. Surface Modification Induces Oriented Zn(002) Deposition for Highly Stable Zinc Anode. Batteries 2024, 10, 178. https://doi.org/10.3390/batteries10060178
Zhang H, Li F, Li Z, Gao L, Xu B, Wang C. Surface Modification Induces Oriented Zn(002) Deposition for Highly Stable Zinc Anode. Batteries. 2024; 10(6):178. https://doi.org/10.3390/batteries10060178
Chicago/Turabian StyleZhang, Hongfei, Fujie Li, Zijin Li, Liu Gao, Binghui Xu, and Chao Wang. 2024. "Surface Modification Induces Oriented Zn(002) Deposition for Highly Stable Zinc Anode" Batteries 10, no. 6: 178. https://doi.org/10.3390/batteries10060178
APA StyleZhang, H., Li, F., Li, Z., Gao, L., Xu, B., & Wang, C. (2024). Surface Modification Induces Oriented Zn(002) Deposition for Highly Stable Zinc Anode. Batteries, 10(6), 178. https://doi.org/10.3390/batteries10060178








