Efficient Leaching of Metal Ions from Spent Li-Ion Battery Combined Electrode Coatings Using Hydroxy Acid Mixtures and Regeneration of Lithium Nickel Manganese Cobalt Oxide
Abstract
1. Introduction
2. Experimental
2.1. Materials and Instrumentation
2.2. Analysis Procedure Using Atomic Absorption Spectroscopy
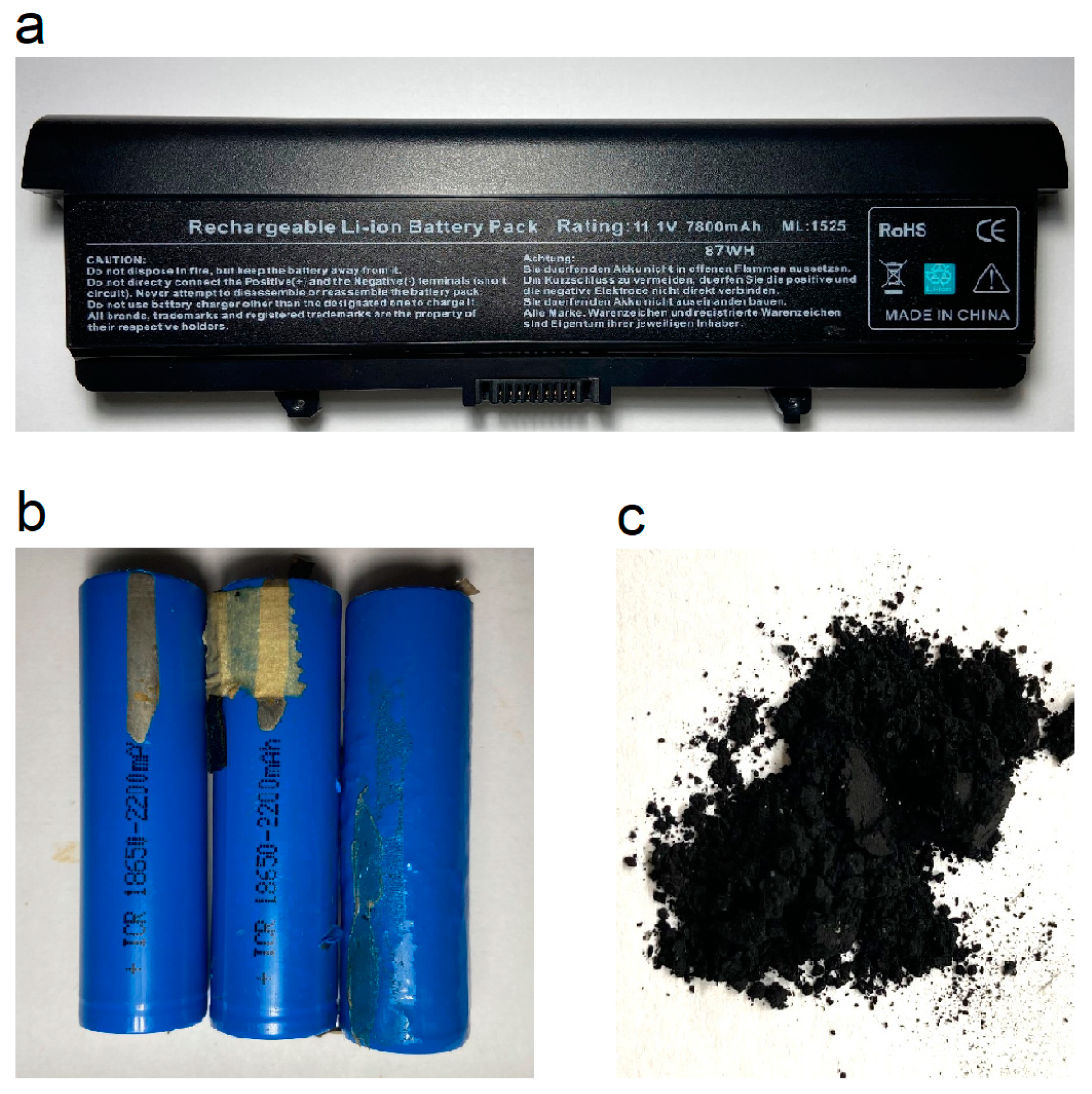
2.3. Collection of Lithium-Ion Battery Black Material (LiBBM), Preparation of Pyrolyzed Product (LiBBM-700), and Characterization
2.4. Composition of LiBBM-700 by Analysis of Li, Ni, Mn, and Co
2.5. General Procedure for Leaching Li, Ni, Mn, and Co from LiBBM-700 Using Aqueous Hydroxy Acid Solutions
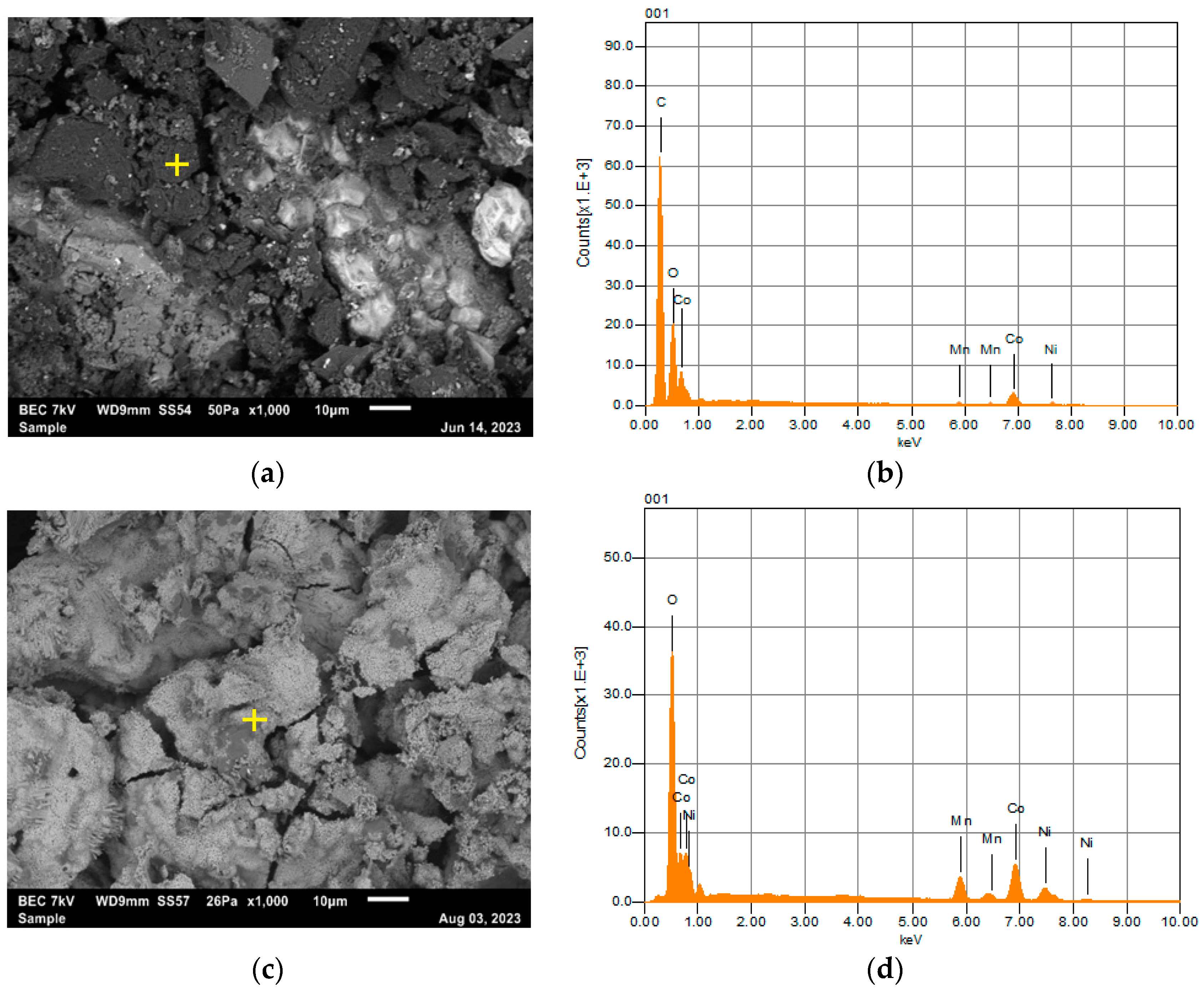

2.6. Regeneration of Lithium Nickel Manganese Cobalt Oxide through Pyrolysis of Chelate Gel
2.7. Analysis of Li, Ni, Mn, and Co in Regenerated Lithium Nickel Manganese Cobalt Oxide
3. Results and Discussion
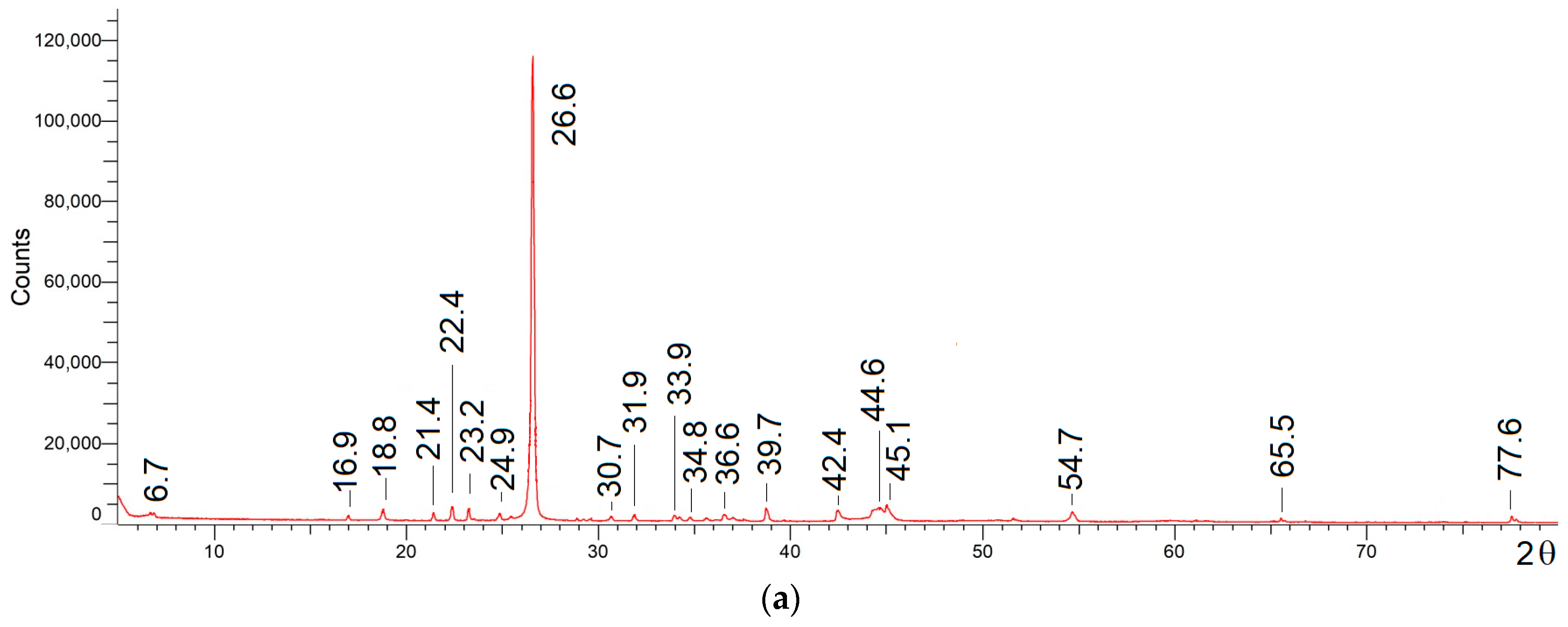
3.1. Collection and Preparation of Pyrolyzed Lithium-Ion Battery Black Material (LiBBM-700) and Characterization
3.2. Leaching Li, Ni, Mn, and Co from LiBBM-700 Using Aqueous Hydroxy Acid Solutions
3.3. Regeneration of Lithium Nickel Manganese Cobalt Oxide Using Leachate Solution from Entry 11 in Table 3 and Comparison with Lithium Nickel Manganese Cobalt Oxide in LiBBM-700
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, S.-J.; Zeng, X.-X.; Ma, Q.; Wu, X.-W.; Guo, Y.-G. Recent advancements in polymer-based composite electrolytes for rechargeable lithium batteries. Electrochem. Energy Rev. 2018, 1, 113–138. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Chen, D.; Rao, S.; Wang, D.; Cao, H.; Xie, W.; Liu, Z. Synergistic leaching of valuable metals from spent Li-ion batteries using sulfuric acid-L-ascorbic acid system. Chem. Eng. J. 2020, 388, 124321. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis. Hydrometallurgy 2020, 191, 105160. [Google Scholar] [CrossRef]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective recovery of valuable metals from spent lithium-ion batteries—Process development and kinetics evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Zheng, X.; Cao, H.; He, M.; Zhang, Y.; Yu, H.; Sun, Z. Selective Recovery of Lithium from Spent Lithium-Ion Batteries by Coupling Advanced Oxidation Processes and Chemical Leaching Processes. ACS Sustain. Chem. Eng. 2020, 8, 5165–5174. [Google Scholar] [CrossRef]
- Ning, P.; Meng, Q.; Dong, P.; Duan, J.; Xu, M.; Lin, Y.; Zhang, Y. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system. Waste Manag. 2020, 103, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Otsuki, A.; Chagnes, A. Investigation of the leaching mechanism of NMC 811 (LiNi 0.8 Mn 0.1 Co 0.1 O 2) by hydrochloric acid for recycling lithium ion battery cathodes. RSC Adv. 2019, 9, 38612–38618. [Google Scholar] [CrossRef]
- Fujita, T.; Chen, H.; Wang, K.-T.; He, C.-L.; Wang, Y.-B.; Dodbiba, G.; Wei, Y.-Z. Reduction, reuse and recycle of spent Li-ion batteries for automobiles: A review. Int. J. Miner. Metall. Mater. 2021, 28, 179–192. [Google Scholar] [CrossRef]
- Gao, W.; Liu, C.; Cao, H.; Zheng, X.; Lin, X.; Wang, H.; Zhang, Y.; Sun, Z. Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries. Waste Manag. 2018, 75, 477–485. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.S.; Guimarães, L.F.; Junior, A.B.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric car battery: An overview on global demand, recycling and future approaches towards sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef] [PubMed]
- Hantanasirisakul, K.; Sawangphruk, M. Sustainable Reuse and Recycling of Spent Li-Ion batteries from Electric Vehicles: Chemical, Environmental, and Economical Perspectives. Glob. Chall. 2023, 7, 2200212. [Google Scholar] [CrossRef] [PubMed]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-ion battery recycling—Overview of techniques and trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Hua, Y.; Zhou, S.; Huang, Y.; Liu, X.; Ling, H.; Zhou, X.; Zhang, C.; Yang, S. Sustainable value chain of retired lithium-ion batteries for electric vehicles. J. Power Sources 2020, 478, 228753. [Google Scholar] [CrossRef]
- Kang, D.H.P.; Chen, M.; Ogunseitan, O.A. Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ. Sci. Technol. 2013, 47, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Asadi Dalini, E.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A review on environmental, economic and hydrometallurgical processes of recycling spent lithium-ion batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 451–472. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Liang, Z.; Cai, C.; Peng, G.; Hu, J.; Hou, H.; Liu, B.; Liang, S.; Xiao, K.; Yuan, S.; Yang, J. Hydrometallurgical recovery of spent lithium ion batteries: Environmental strategies and sustainability evaluation. ACS Sustain. Chem. Eng. 2021, 9, 5750–5767. [Google Scholar] [CrossRef]
- Rautela, R.; Yadav, B.R.; Kumar, S. A review on technologies for recovery of metals from waste lithium-ion batteries. J. Power Sources 2023, 580, 233428. [Google Scholar] [CrossRef]
- Mishra, G.; Jha, R.; Meshram, A.; Singh, K.K. A review on recycling of lithium-ion batteries to recover critical metals. J. Environ. Chem. Eng. 2022, 10, 108534. [Google Scholar] [CrossRef]
- Sidiq, A.L.; Floweri, O.; Karunawan, J.; Abdillah, O.B.; Santosa, S.P.; Iskandar, F. NCM cathode active materials reproduced from end-of-life Li-ion batteries using a simple and green hydrometallurgical recycling process. Mater. Res. Bull. 2022, 153, 111901. [Google Scholar] [CrossRef]
- Lerchbammer, R.; Gerold, E.; Antrekowitsch, H. Gluconic Acid Leaching of Spent Lithium-Ion Batteries as an Environmentally Friendly Approach to Achieve High Leaching Efficiencies in the Recycling of NMC Active Material. Metals 2023, 13, 1330. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Jong, B.; Pozo-Gonzalo, C.; Banerjee, P.C. Current challenges and future opportunities toward recycling of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2022, 159, 112202. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Purcell, W. Reducing agents in the leaching of manganese ores: A comprehensive review. Hydrometallurgy 2019, 187, 168–186. [Google Scholar] [CrossRef]
- Cheng, Q.; Marchetti, B.; Chen, M.; Li, J.-T.; Wu, J.; Liu, X.; Zhou, X.-D. Novel approach for in situ recovery of cobalt oxalate from spent lithium-ion batteries using tartaric acid and hydrogen peroxide. J. Mater. Cycles Waste Manag. 2023, 25, 1534–1548. [Google Scholar] [CrossRef]
- Okonkwo, E.G.; Wheatley, G.; He, Y. The role of organic compounds in the recovery of valuable metals from primary and secondary sources: A mini-review. Resour. Conserv. Recycl. 2021, 174, 105813. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Martins, L.S.; Rovani, S.; Botelho Junior, A.B.; Romano Espinosa, D.C. Sustainable Approach for Critical Metals Recovery through Hydrometallurgical Processing of Spent Batteries Using Organic Acids. Ind. Eng. Chem. Res. 2023, 62, 18672–18682. [Google Scholar] [CrossRef]
- de Castro, R.H.; Romano Espinosa, D.C.; Gobo, L.A.; Kumoto, E.A.; Botelho Junior, A.B.; Tenorio, J.A.S. Design of Recycling Processes for NCA-Type Li-Ion Batteries from Electric Vehicles toward the Circular Economy. Energy Fuels 2024, 38, 5545–5557. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Herath, H.N.K.; Grady, T.L.; Gutierrez Reyes, C.D. Oxidation of glucose to glycolic acid using oxygen and pyrolyzed spent Li-ion battery electrode material as catalyst. Appl. Catal. A Gen. 2022, 648, 118920. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Pinzon, S.K.; Rockward, T.; Herath, H.N.K. Spent Li-Ion Battery Electrode Material with Lithium Nickel Manganese Cobalt Oxide as a Reusable Catalyst for Oxidation of Biofurans. ACS Sustain. Chem. Eng. 2022, 10, 12642–12650. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wang, D. Decarboxylative—Dimerization of levulinic acid using spent Li-ion battery electrode material with lithium nickel cobalt manganese oxide as a catalyst. Fuel Process. Technol. 2023, 250, 107913. [Google Scholar] [CrossRef]
- Paone, E.; Miceli, M.; Malara, A.; Ye, G.; Mousa, E.; Bontempi, E.; Frontera, P.; Mauriello, F. Direct Reuse of Spent Lithium-Ion Batteries as an Efficient Heterogeneous Catalyst for the Reductive Upgrading of Biomass-Derived Furfural. ACS Sustain. Chem. Eng. 2022, 10, 2275–2281. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, Q.; Kajiyoshi, K.; Yanagisawa, K.; Yang, X.; Makita, Y.; Kasaishi, S.; Ooi, K. Hydrothermal Syntheses of Layered Lithium Nickel Manganese Oxides from Mixed Layered Ni(OH)2−Manganese Oxides. Chem. Mater. 2002, 14, 3844–3851. [Google Scholar] [CrossRef]
- Manthiram, A.; Kim, J. Low temperature synthesis of insertion oxides for lithium batteries. Chem. Mater. 1998, 10, 2895–2909. [Google Scholar] [CrossRef]


| Cathode Material | Elemental Composition (w/w %) | Reference | |||
|---|---|---|---|---|---|
| Li | Ni | Mn | Co | ||
| LiCoO2 | 7.03 | - | - | 59.55 | [3] |
| LiMnO4 | 3.99 | - | 58.39 | - | [3] |
| LiNi0.3Mn0.3Co0.3O2 | 2.52 | 6.76 | 6.81 | 7.01 | [4] |
| LiNi0.3Mn0.3Co0.3O2 | 6.15 | 18.32 | 17.57 | 18.65 | [5] |
| LiNi0.5Mn0.3Co0.2O2 | 6.34 | 26.75 | 15.34 | 10.77 | [6] |
| LiNi0.6Mn0.2Co0.2O2 | 6.64 | 35.1 | 11.2 | 12.1 | [7] |
| LiNi0.8Mn0.1Co0.1O2 | 7.36 | 46.01 | 5.40 | 6.28 | [8] |
| Sample | Elemental Composition (w/w)% | Empirical Formula | |||||
|---|---|---|---|---|---|---|---|
| Li | Ni | Mn | Co | O | C | ||
| LiNMC in LiBBM-700 | 13.33 | 3.76 | 2.44 | 12.33 | 11.28 | 56.86 | LiNi0.03Mn0.02Co0.11O0.35 |
| Regenerated LiNMC (Table 3, Entry 11) | 32.60 | 9.06 | 5.58 | 29.93 | 22.83 | - | LiNi0.03Mn0.02Co0.11O0.30 |
| Entry | LiBBM-700 (g)/Leaching Solution (mL) | [GA] mol L−1 | [LA] mol L−1 | Leaching Efficiency of Metal Ions (%) | |||
|---|---|---|---|---|---|---|---|
| Li | Ni | Mn | Co | ||||
| 1 | 1:100 | 0.00 | 0.25 | 69 | 54 | 34 | 34 |
| 2 | 1:100 | 0.05 | 0.20 | 98 | 100 | 100 | 87 |
| 3 | 1:100 | 0.075 | 0.175 | 100 | 86 | 82 | 69 |
| 4 | 1:100 | 0.25 | 0.00 | 73 | 46 | 33 | 34 |
| 5 | 1:100 | 0.00 | 0.10 | 99 | 100 | 100 | 85 |
| 6 | 1:100 | 0.02 | 0.08 | 93 | 100 | 100 | 91 |
| 7 | 1:100 | 0.03 | 0.07 | 98 | 100 | 100 | 88 |
| 8 | 1:100 | 0.10 | 0.00 | 92 | 100 | 100 | 74 |
| 9 | 1:100 | 0.00 | 0.50 | 100 | 97 | 100 | 81 |
| 10 | 1:100 | 0.10 | 0.40 | 100 | 100 | 100 | 85 |
| 11 | 1:100 | 0.15 | 0.35 | 100 | 100 | 100 | 89 |
| 12 | 1:100 | 0.20 | 0.30 | 100 | 84 | 89 | 65 |
| 13 | 1:100 | 0.25 | 0.25 | 100 | 100 | 100 | 83 |
| 14 | 1:20 | 0.25 | 0.25 | 67 | 72 | 84 | 74 |
| 15 | 1:100 | 0.30 | 0.20 | 100 | 89 | 97 | 78 |
| 16 | 1:100 | 0.50 | 0.00 | 100 | 100 | 100 | 73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarasekara, A.S.; Wang, D.; Shrestha, A.B. Efficient Leaching of Metal Ions from Spent Li-Ion Battery Combined Electrode Coatings Using Hydroxy Acid Mixtures and Regeneration of Lithium Nickel Manganese Cobalt Oxide. Batteries 2024, 10, 170. https://doi.org/10.3390/batteries10060170
Amarasekara AS, Wang D, Shrestha AB. Efficient Leaching of Metal Ions from Spent Li-Ion Battery Combined Electrode Coatings Using Hydroxy Acid Mixtures and Regeneration of Lithium Nickel Manganese Cobalt Oxide. Batteries. 2024; 10(6):170. https://doi.org/10.3390/batteries10060170
Chicago/Turabian StyleAmarasekara, Ananda S., Deping Wang, and Ambar B. Shrestha. 2024. "Efficient Leaching of Metal Ions from Spent Li-Ion Battery Combined Electrode Coatings Using Hydroxy Acid Mixtures and Regeneration of Lithium Nickel Manganese Cobalt Oxide" Batteries 10, no. 6: 170. https://doi.org/10.3390/batteries10060170
APA StyleAmarasekara, A. S., Wang, D., & Shrestha, A. B. (2024). Efficient Leaching of Metal Ions from Spent Li-Ion Battery Combined Electrode Coatings Using Hydroxy Acid Mixtures and Regeneration of Lithium Nickel Manganese Cobalt Oxide. Batteries, 10(6), 170. https://doi.org/10.3390/batteries10060170






