Abstract
Cylindrical lithium-ion batteries are widely used in consumer electronics, electric vehicles, and energy storage applications. However, safety risks due to thermal runaway-induced fire and explosions have prompted the need for safety analysis methodologies. Though cylindrical batteries often incorporate safety devices, the safety of the battery also depends on its design and manufacturing processes. This study conducts a design and process failure mode and effect analysis (DFMEA and PFMEA) for the design and manufacturing of cylindrical lithium-ion batteries, with a focus on battery safety.
1. Introduction
As the demand for lithium-ion batteries has risen from use in portable electronics to electric vehicles, there has been a corresponding increase in the number of reported safety incidents worldwide. The number of battery fires in New York City alone rose from 104 in 2021 to 216 in 2022, killing six people in 2022 [1]. In 2016, Samsung had to recall 2.5 million Note 7 smartphones after complaints of overheating and exploding batteries [2].
In the mass production of cylindrical lithium-ion batteries, end-of-line testing is generally limited to capacity and open circuit voltage tests, which help in electrical screening but do not address the safety of the battery. While scanning electron microscopy, X-ray diffraction or nuclear magnetic response provide insights regarding the quality, performance, and safety of the battery, conducting these analyses on a large scale can be impractical due to the equipment costs and time required for sample preparation and analysis [3,4,5].
Addressing the safety issues of lithium-ion batteries is required in the design and manufacturing processes to reduce the frequency of failures and their consequences, if occurred. Failure mode and effect analysis (FMEA) is an engineering structured analysis of risks of potential failures [6]. The primary aim of conducting an FMEA is to avert the risk of a new design, process, or system not meeting the specified requirements, under certain conditions such as defined objectives and imposed limits. Its execution involves the analysis of failure modes, listing their possible causes and effects and suggesting corrective actions to alleviate the impact of those failure modes [6].
It involves systematically examining each element or process to determine the ways in which it may fail and the potential consequences of those failures. It assesses the risk of each failure mode based on their severity, likelihood of occurrence and detection. The failure modes with higher risk are prioritized and strategies like engineering controls, design modifications, process improvements and enhanced quality control measures are implemented to minimize the occurrence or impact of the failure mode [7].
Cylindrical lithium-ion batteries are complex systems with multi-step manufacturing processes. This introduces the possibility of diverse failure modes that detrimentally lead to a common effect, impacting the quality, reliability, and safety of the battery. Chris et al. conducted a failure mode, mechanisms mode and effect analysis, concentrating on design-related aspects, specifically material properties and design parameters [8]. However, the scope of this study was limited to the design, and a process failure mode and effect analysis has not been explored. While various researchers have examined the challenges within lithium-ion battery manufacturing processes, a significant gap remains in understanding the specific impact of each process on battery safety [9,10,11,12,13].
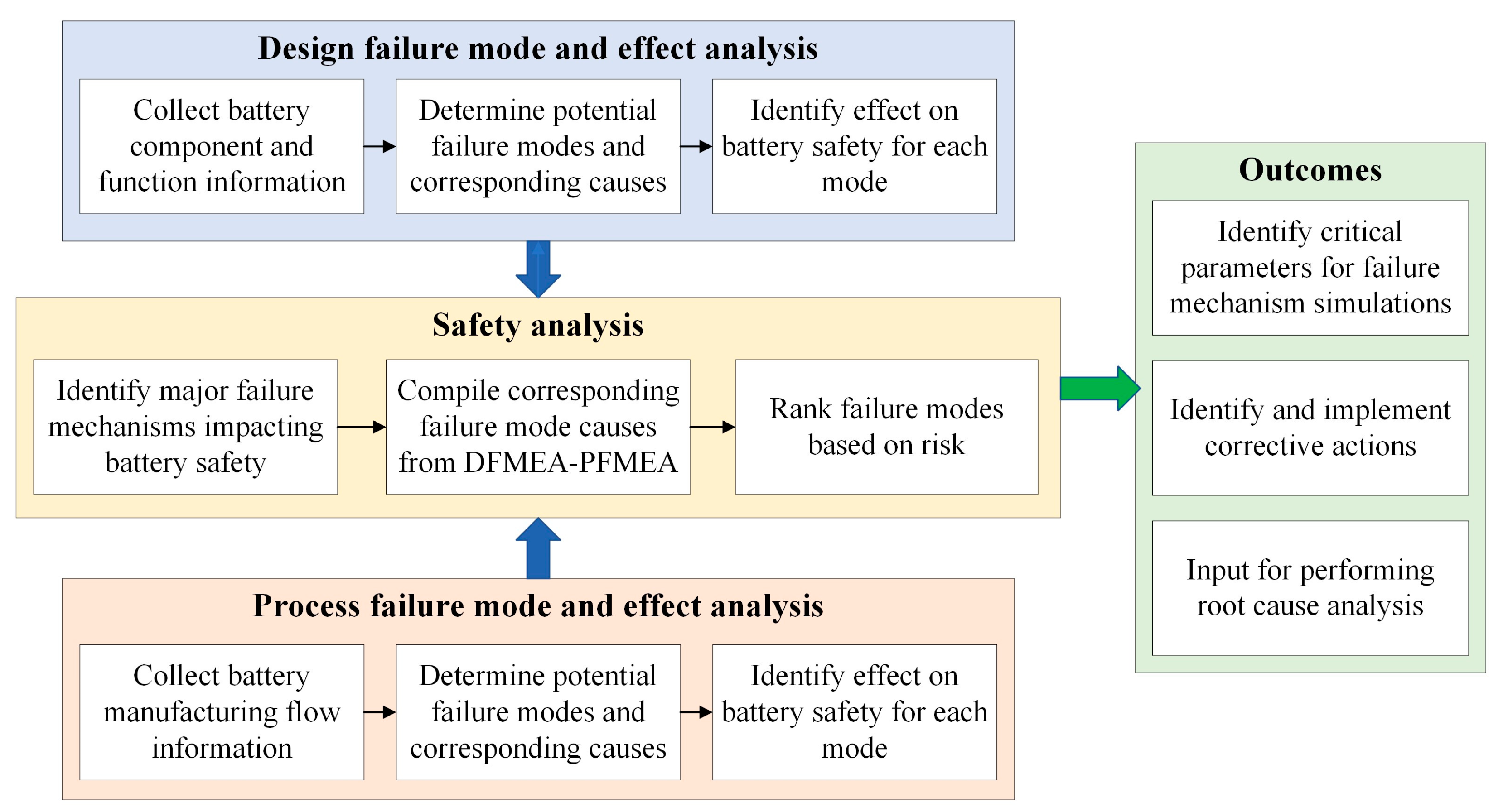
This paper presents an integrated safety analysis to address this challenge by consolidating the traditional DFMEA and PFMEA, as shown in Figure 1. Combining these two methodologies, the analysis considers the potential failure modes arising from the design elements and the manufacturing processes. The consolidation of failure mode causes that contribute to the same failure mechanisms facilitates a better understanding of the underlying mechanisms.
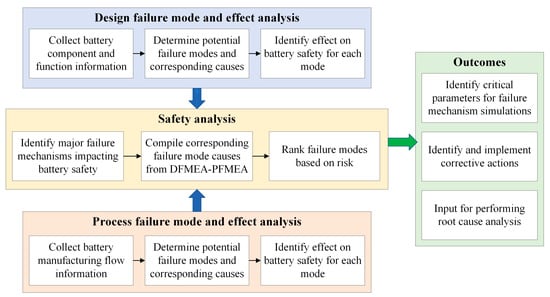
Figure 1.
Safety analysis approach using integrated DFMEA-PFMEA.
It is first important to comprehend the influence of each individual element and manufacturing process on battery safety. Section 2 and Section 3 provide an overview of the design elements and manufacturing processes, which serve as the foundation for identifying potential failure modes while performing the design and process failure mode and effect analysis (DFMEA and PFMEA), respectively. The corresponding modes, causes, and effects tables are listed in Appendix A. Section 4 presents the integrated DFMEA-PFMEA safety analysis, identifies four significant cause mechanisms, and compiles the corresponding causes to offer a comprehensive understanding. Section 5 serves as the concluding part of the paper, encapsulating the main findings and key insights derived from the preceding sections.
2. Design Failure Mode and Effect Analysis
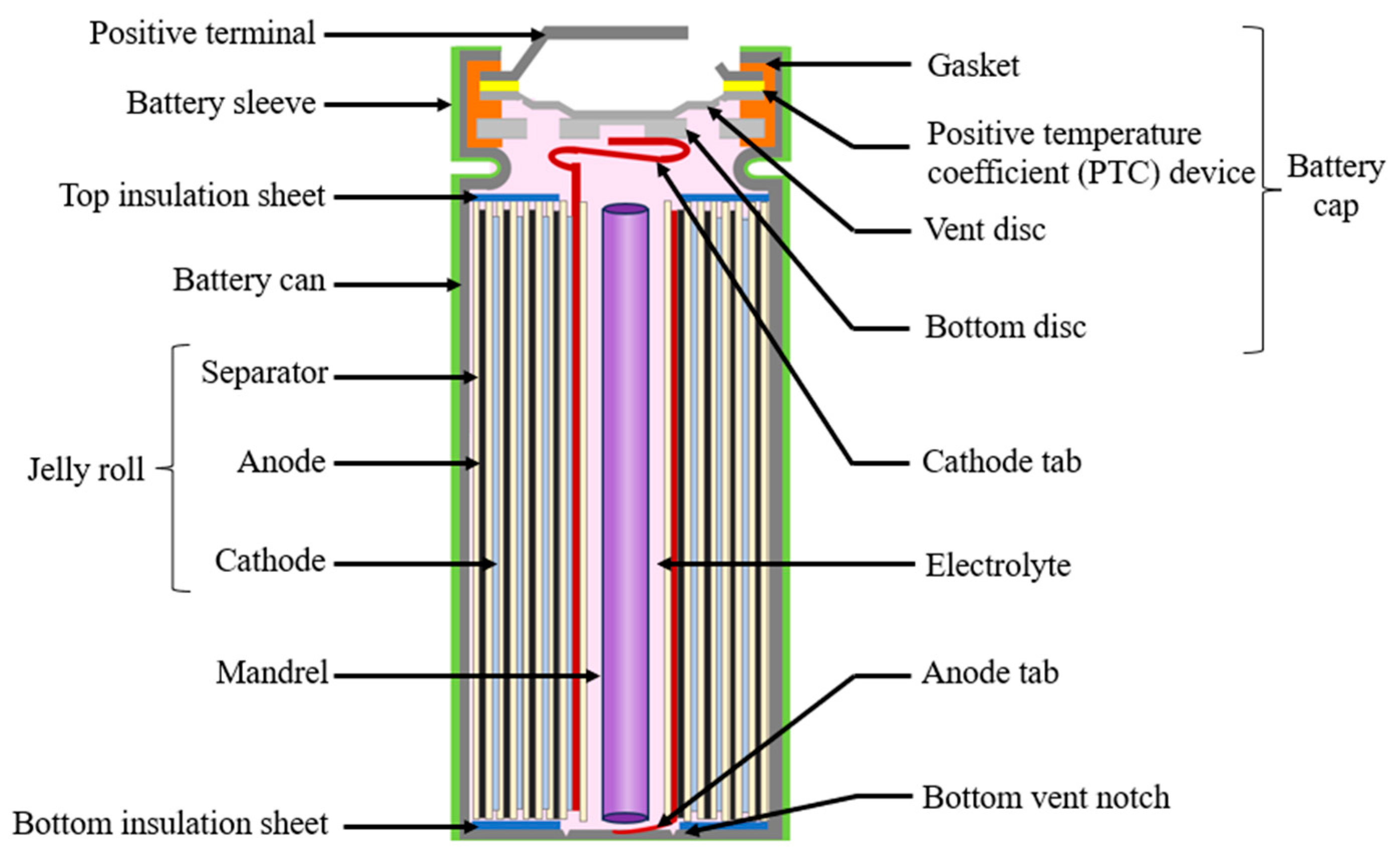
The design failure mode and effect analysis (DFMEA) provides a structured methodology to evaluate and address potential failure modes in various components and aspects of cylindrical lithium-ion batteries, including materials selection and design. Cylindrical batteries are composed of a rolled-up assembly called a jelly roll, which includes anode, cathode, and separator sheets tightly wound together and connected with electrical tabs. A schematic of a cylindrical lithium-ion battery is shown in Figure 2.
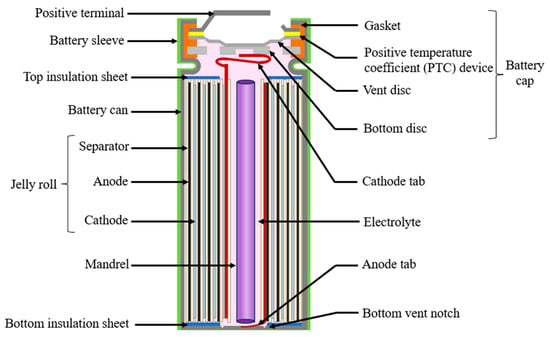
Figure 2.
Cylindrical battery structure.
The anode materials used, including graphite, silicon, germanium and Titanate, show good thermal stability [14]. However, graphite in the form of powder or combined with binder material is widely used [15]. The performance of the cell is affected by parameters like purity, particle size, particle size distribution, particle shapes, particle porosity, crystalline phase of carbon and degree of compaction [16,17].
The cathode materials consist of layered lithium cobalt dioxide (LCO), lithium iron phosphate (LFP), spinels like lithium manganese oxide (LMO), or mixed metal oxides like nickel cobalt aluminate (NCA) and nickel manganese cobaltite (NMC) [15]. The thermal stability of the battery is dependent on the cathode materials, which is affected by the nickel content [18]. Cathode materials undergo a phase transition and release oxygen in overcharge state along with heat generation and electrolyte decomposition, which can lead to a thermal runaway [19]. The amount and type of gases generated is directly proportional to the oxidation capability of the cathode materials, where LCO shows the highest oxidation capability, followed by LMO and LFP [20].
Thin foils of copper and aluminum are used as current collectors for anode and cathode material, respectively. A tab is welded to the current collector, which acts as a bridge that connects the electrode to the external circuit. The material, location, and number of tabs affect the performance of the cell in terms of uniform current distribution, heat generation and ohmic resistance [21]. The anode and cathode electrodes are separated by a porous polymer sheet called the separator. A shutdown separator can act as a safety device by closing the pores at high temperatures, blocking the ionic transport [22]. Properties to consider in the selection of a separator include mechanical strength, thermal and dimensional strength, permeability, porosity, chemical structure, surface energy with electrolyte and electrode materials [14].
The jelly roll is placed into a cylindrical metal can made of nickel-coated steel or aluminum followed by a mandrel. The internal cylindrical mandrel is an optional component that increases the mechanical stability and safety of the battery [23]. The liquid electrolyte, which is a mixture of organic carbonates and lithium salt, is then added. The mixture ratio of the solvent dictates the flammability and auto-ignition temperatures of the electrolyte [15]. Parameters like the salt-to-solvent ratio and the amount of additives added can impact the solid electrolyte interphase (SEI) layer formation, thereby impacting the stability and safety of the cell [24,25].
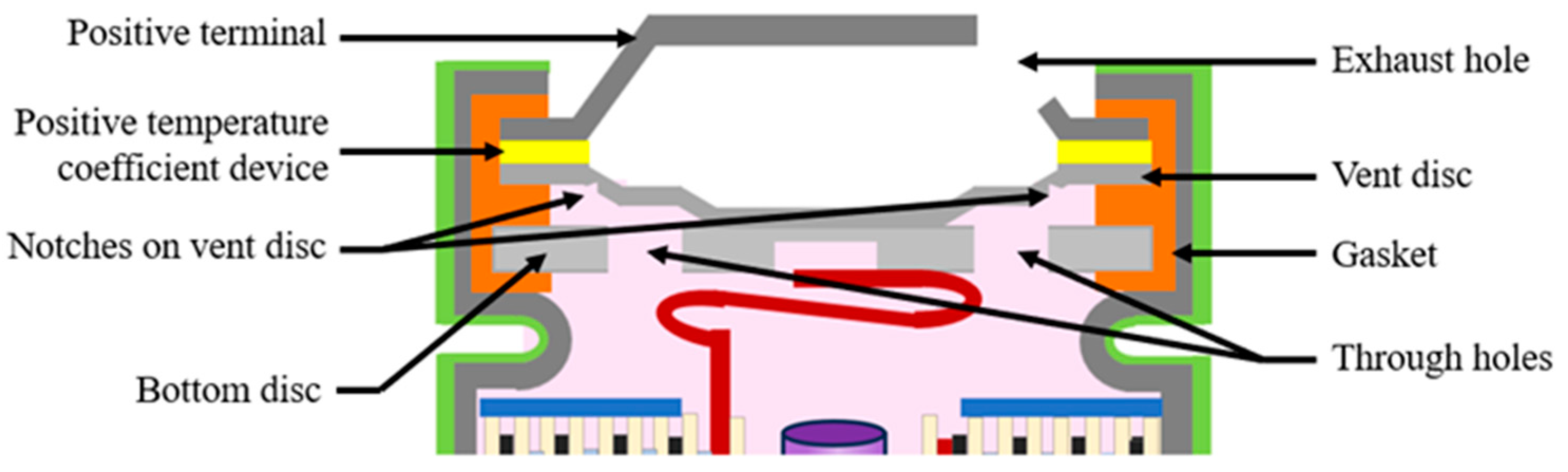
The battery is sealed with a cap located on the top of the cathode tab. A typical cylindrical battery cap structure is displayed in Figure 3. The battery cap is a vital component in the cylindrical battery as it often consists of safety devices to protect the cell from thermal runway and explosion [26]. The cap consists of conductive parts that include the positive temperature coefficient (PTC) thermistor, and the bottom and top disk that act as a current interrupt device (CID) [27]. The cap consists of a non-conductive plastic insert or gasket to insulate the positive terminal from the battery can. The cap provides an electrical connection between the cathode tab to the positive terminal, enabling the transfer of electrical current between the battery’s electrodes and external circuit.
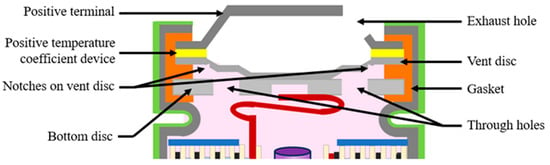
Figure 3.
Typical cylindrical battery cap structure.
The CID is designed to disconnect the battery’s internal current flow in case of excessive internal pressure, preventing thermal runaway. When the internal pressure of a cylindrical lithium-ion battery reaches a pre-determined level, typically between 1.0 and 1.2 MPa, the top disk will be pushed upwards to break the weak point, which is the welded connection between the central point of the top disk and the bottom disk. As a result, the electrical pathway between the current collector and the external load will be disconnected [28]. The function of safety vents is also to release the internal pressure of the battery by expelling the gases generated inside. The notch in the vent disc opens when the internal cell pressure increases beyond a limit, allowing for the gases to escape using the through holes in the bottom disc and the exhaust holes in the top terminal plate. For additional venting, a bottom vent is added to the battery can to prevent sidewall rupture [28].
Design failure mode and effect analysis (DFMEA) focuses on potential failure modes that are caused by the specifications and design parameters finalized in the design phase. While designing a lithium-ion battery, the general requirements for lithium-ion battery abuse tolerance also need to be considered [29]. Multiple lithium-ion battery industry standards encompass these requirements by formulating test conditions that simulate abuse scenarios capable of potentially triggering thermal runaway [30]. These standards generally classify testing into two categories: electrical abuse, involving operation beyond nominal voltage and current limits; and physical/environmental abuse, including extreme temperatures or mechanical stress [31]. Therefore, considering the potential abuse conditions when designing cell parameters is beneficial. Cells designed this way can have a certain safety margin or tolerate such abuse conditions, leading to predictable failure patterns and minimizing the damage caused. There can be more response time to take measures before the failure escalates.
Electrical abuse arises when a battery faces situations like overcharging, over-discharging, or an external short circuit, leading to adverse electrochemical reactions [29]. It is imperative to select electrolyte formulations with good thermal and electrochemical stability to prevent electrolyte decomposition and gas evolution during overcharge and over-discharge events [32]. Additionally, electrode structures should be designed to handle Li+ intercalation/deintercalation without experiencing degradation, such as electrode cracking or particle pulverization under high C-rate conditions [33].
In situations of thermal abuse, a battery can either encounter thermal shock or localized high temperatures [34]. The localized temperature rise within a battery is often a consequence of poor design [29]. Choosing cell materials with high thermal stability and low reactivity is paramount to mitigating the potential for exothermic reactions when exposed to elevated temperatures. The battery design should maintain structural integrity even when subjected to mechanical deformation. The outer casing of the battery should be designed to withstand mechanical forces without fracturing [29]. Using materials with high strength and durability can enhance the resilience of the casing. Ensuring the integrity of the separator is crucial to prevent direct contact between electrodes, which can lead to short circuits and thermal runaway. Designing separators with sufficient tear resistance can mitigate the risk of damage during mechanical abuse.
Table A1 presents the DFMEA for the design of a cylindrical lithium-ion battery, with a focus on safety. The design parameters for each element of the battery that influence its safety are consolidated and presented in Table 1.

Table 1.
Design parameters influencing battery safety.
3. Process Failure Mode and Effect Analysis
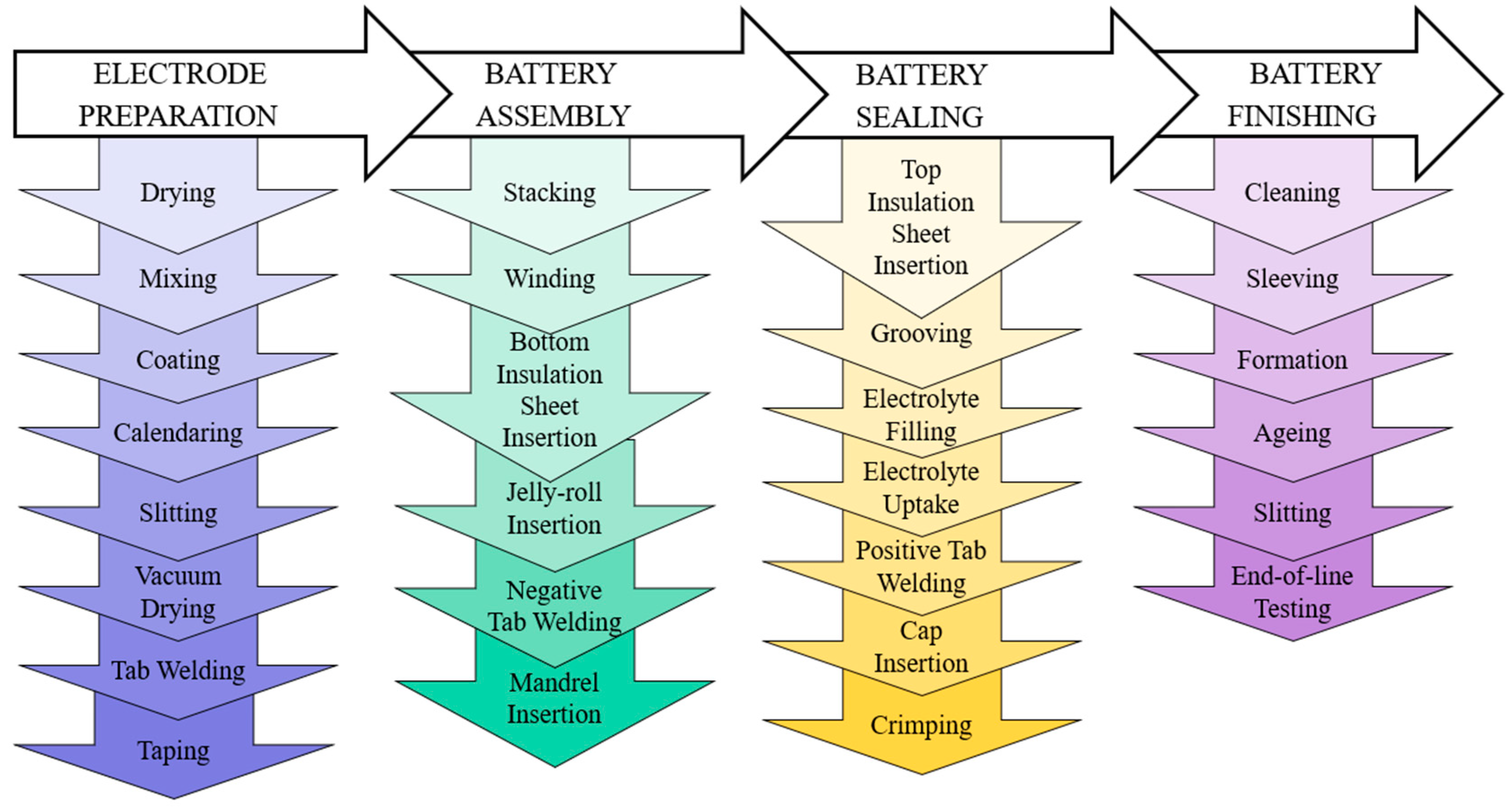
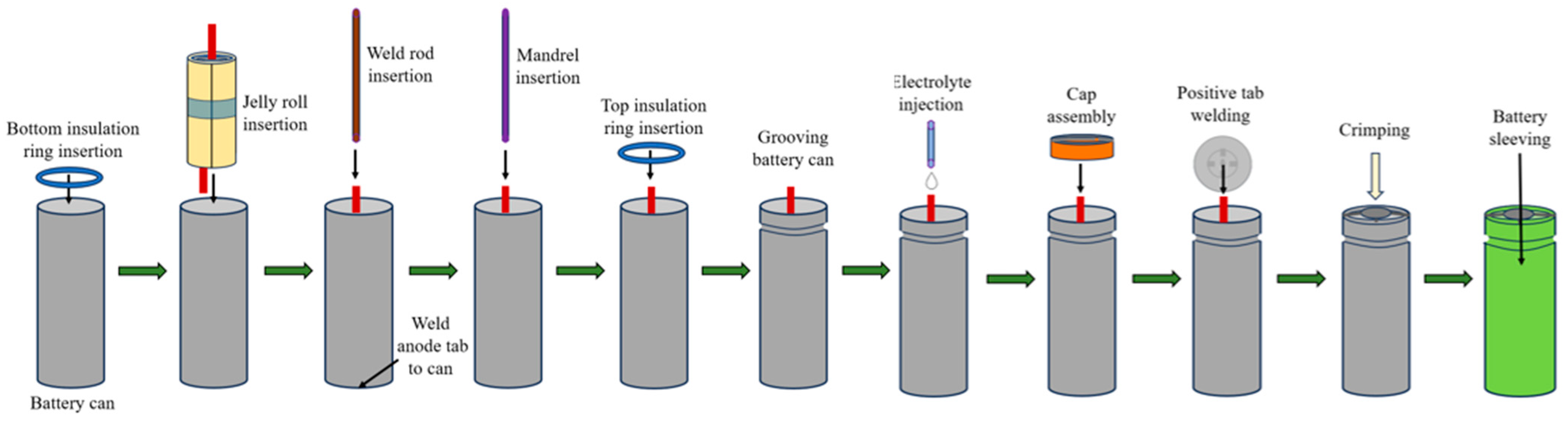
The primary input to a PFMEA is the process flow diagram, which describes the cylindrical battery manufacturing and assembly process. The manufacturing and assembly of a cylindrical battery involve the precise fabrication of battery cans and caps, the preparation of the electrode stack, its assembly into a jellyroll structure, followed by tab welding and assembly into battery can, and the sealing of the battery to ensure no leakage [9,12,35]. These steps, along with thorough quality checks, contribute to the production of reliable cylindrical batteries. To illustrate the overall manufacturing process of a cylindrical lithium-ion battery, Figure 4 provides a representation. This process involves four major steps: electrode preparation, cell assembly, cell sealing, and cell finishing [36].
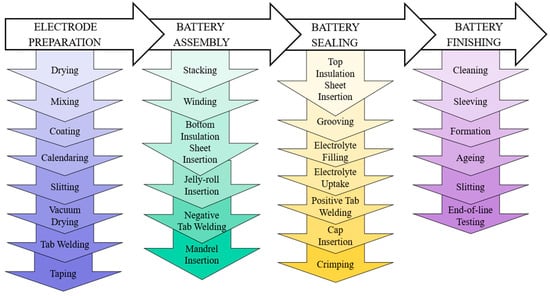
Figure 4.
Cylindrical battery manufacturing process.
The first step in the electrode fabrication process is the drying and mixing of the electrode materials. This involves mixing active materials (such as graphite, lithium cobalt oxide, or lithium iron phosphate) with conductive additives (such as carbon black) and a binder (such as polyvinylidene fluoride) in a solvent. The resulting slurry has a specific ratio of solids to solvent, which is crucial for the electrode’s performance. This process can be performed in a vacuum to avoid gas inclusions. The quality parameters that need to be considered are the homogeneity of the slurry, particle size, purity (the amount of foreign particles) and viscosity. These are influenced by the mixing and dispersing sequence, the filter systems, shear forces of the equipment, blending time and mixing temperature [12].
The most common method for coating the slurry mixture on the current collectors is the slot-die coating process [37]. Other techniques, such as spray coating or doctor-blade coating, may also be used. The coating thickness ranges from 70 µm to 350 µm and is measured using X-ray reflectivity (XRR) [21]. The coating speed, coating width and precision of the slurry pump define the thickness accuracy, homogeneity, and surface quality (blowholes, particles) of the coating [12].
After coating, the electrode sheet is dried to remove the solvent. The residual humidity and surface finish (cracks, inclusions) is determined by process parameters including temperature profile, drying speed and foil pretension [12]. Once the electrode is dry, it is calendared to improve its mechanical properties. Calendaring involves compressing the electrode using a pair of rollers, which increases its density and improves its adhesion to the current collector. The porosity, surface texture and adhesion between the coating and current collector is affected by line speed (30~100 m/min), roller diameters (600~1000 mm) and line load (500~1000 N/m) [38]. A high line load can cause fractures, which increases the moisture sorption of the active materials [39].
The fabricated electrode is slit into smaller sheets according to the design. Laser cutting is a widely applied shaping technology, where the cutting width and efficiency of the slitting process is controlled by laser power and scanning speed [9]. After slitting, the coils are vacuum dried for 12–30 h to remove residual moisture [12]. The drying time and temperature of the oven should be selected not to cause any cracks or fractures in the active materials while ensuring no residual moisture is present, as it can facilitate the generation of hydrogen fluoride gas [40]. The next step is welding the electrode tabs to the end of current collector, which is not coated by the active material. Resistance spot welding is usually used for cylindrical batteries; however, ultrasonic welding can be used on some occasions [38]. Low contact resistance and low mechanical and thermal stress must be ensured during the welding process. High resistance increases heat generation, resulting in cell degradation, and can cause thermal runway [41]. An insulation tape covers the welded tab to prevent electrical conduction and penetration through the separator [21].
The anode electrode, separator, and cathode electrode will be stacked together and rolled up to form the jelly roll. During the winding process, a center pin can be used to prevent deformation of the electrode assembly. This component is also referred to as a deformation prevention core and is removed once the winding process is complete [42]. An adhesive tape is used to secure the jelly roll. Winding speed, web tension and web edge control influence the quality of the jelly roll. The jelly roll is inserted into the battery can along with a bottom insulator, and the negative tab is welded to the internal surface of the battery can’s bottom. This is a challenging step as the weld should not penetrate the battery can [43].
A mandrel is inserted to furnish mechanical stability to the cell and facilitate pressure relief by offering an unobstructed route for the fluidized material and gases to migrate from the base to the cell’s crimp [44]. A top insulator is applied to the jelly roll structure. There is a hole in the insulator which allows the positive tab to go through it. A groove is made above the top insulation ring to host the battery cap. The grooving speed and depth should be controlled to avoid electrode deformation. After another drying process to remove the remaining moisture, the electrolyte is applied to the battery before the final sealing. Although the separator of a lithium-ion battery has a porous structure, an electrolyte uptake/wetting step is applied to facilitate the infusion of the electrolyte to wet the entire jelly roll structure to ensure a homogenous distribution of the electrolyte [45].
After the electrolyte filling, the positive tab is welded to the cap and the battery cap will sit on the groove. Once the cap and cell are aligned, the crimping process can begin. The crimping machine applies pressure to the edges of the cap, causing it to deform and grip the top edge of the battery. The pressure is carefully controlled to ensure that the cap is securely attached to the battery, but not so much that it causes damage or deformation of the battery. The battery is cleaned and then wrapped with a sleeve that is made of insulating material. The complete assembly process is shown in Figure 5.
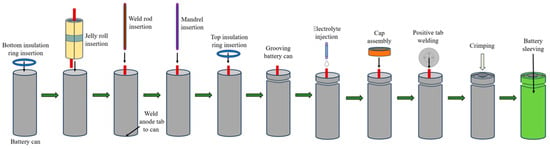
Figure 5.
Cylindrical battery assembly process.
The following electrical treatment is the formation process that will generate a solid electrolyte interphase (SEI) on the anode surface. High-current and high-temperature formation cycles produce a porous SEI layer that cannot prevent electrolyte decomposition due to contact of electrolyte with anode surface [46]. High currents can lead to lithium plating, reducing the safety of the cell [47]. Post the formation process, the battery is aged by high- and normal-temperature storage to verify its self-discharge characteristics. Then, the battery will undergo end-of-line testing to verify its performance and capacity. End-of-line testing involves visual inspection, capacity measurement, internal resistance measurement, and open circuit voltage testing to ensure that it meets the desired performance and safety criteria [12].
Process failure mode and effect analysis (PFMEA) focuses on potential failure modes of the process that are caused by manufacturing and assembly process deficiencies [48]. Table A2 consists of a PFMEA for the manufacturing process described above. The process parameters for each step of the manufacturing process that influence its safety are consolidated and presented in Table 2.

Table 2.
Process parameters influencing battery safety.
4. Discussion
Ensuring safety in lithium-ion batteries is often regarded as the stability of the battery in terms of abuse, including mechanical, electrical, and thermal [14]. Major safety concerns for lithium-ion batteries are thermal runaway and explosion. Thermal runaway is a phenomenon where exothermic reactions occur within the cell, leading to a rapid temperature increase, potentially causing the cell to catch fire [44]. When a lithium-ion battery experiences thermal runaway, it can lead to a buildup of pressure inside the battery, causing the cell to rupture or explode. Explosions can also occur due to increased gas generation in the battery [49].
From the integrated DFMEA–PFMEA, we have identified that localized heating and a short circuit increase the risk of thermal runaway, whereas increased gas generation due to moisture or electrolyte leakage increases the risk of explosion. Manufacturing and assembling defects in the safety devices also reduce the safety of the battery. Safety should, therefore, be a prime consideration in the initial development and material selection process. The following section describes each of the mechanisms affecting the safety of the battery.
4.1. Internal Short Circuit
The occurrence of internal short circuits in lithium-ion batteries can result in thermal runaway, as they generate sufficient heat to initiate a sequence of exothermic reactions [44]. Internal short circuits can occur due to lithium plating, lithium dendrites and contact between electrodes. The failure mechanisms, modes and causes of an internal short circuit from the DFMEA-PFMEA are mentioned in Table 3.

Table 3.
Failure mechanisms, modes, and causes of internal short circuit.
If the integrity of the separator is compromised due to presence of holes or a tear, it can lead to an internal short circuit. Poor puncture strength or tensile strength or thickness can increase the risk of tear because of the high particle size of active materials, metallic particles due to contamination, lithium dendrite growth and burrs [8].
Weak spots on the cell casing can result in breakage and damage to electrodes upon application of mechanical force. Inadequate thickness or an uneven distribution of the nickel plating may create areas of vulnerability where the underlying steel is exposed, compromising the structural integrity of the casing. Surface damage such as scratches or abrasions on the nickel-plated steel casing during the manufacturing process can serve as initiation points for stress concentration and corrosion. In case of damage to the shell casing, air may directly enter the battery system, triggering reactions with the internal active materials [50]. Finite element simulations performed on the cylindrical can casing have shown predictive fracture capabilities. Experimental observations corroborate theoretical predictions, with short circuits often originating near the end of the can, close to the battery electrode connection section [51].
Proper insulation during the alignment of electrode sheets and assembly of the battery is required. The design of wider separators with high thermal resistance is suggested to avoid a short circuit due to separator shrinkage when exposed to high temperatures [52].
Ensuring proper capacity balancing between the negative electrode and positive electrode is a critical aspect of designing lithium-ion batteries that can operate safely. This is achieved by maintaining a proper N/P (negative/positive) ratio. The N/P ratio is defined as the ratio of the reversible capacity of negative electrode and positive electrode and is controlled between 1.03 and 1.2 [14]. A low N/P ratio can cause the anode potential to drop to less than 0 V vs. Li/Li+ during charging, which could lead to lithium plating on the surface of the anode electrode. Lithium plating can lead to the formation of dendrite, which may pierce the separator and induce the cell to an internal short circuit, which can initiate a thermal runway [44].
Numerous methods have been employed by researchers to evaluate, detect, and study lithium plating, including analyzing voltage plateau signals [53], measuring cell thickness [54], and creating simulation models [55,56,57]. However, despite these efforts, accurately predicting the likelihood of internal short circuits resulting from lithium plating remains a difficult task. This challenge adds complexity to the safety design of lithium-ion batteries and further increases the importance of mitigating the failure modes that could potentially cause lithium plating during design and manufacturing.
4.2. Localized Heating
Heat is generated in the battery due to entropy change and Joule heat [58]. When the rate of heat generation exceeds that of dissipation, it results in a temperature rise. An inhomogeneous distribution of materials and the presence of fractures cause uneven temperature distribution due to different heat generation and heat dissipation conditions in the electrode [14]. This localized heating increases the risk of thermal runway due to the initiation of exothermic side reactions. The temperature hotspots can promote lithium metal growth as compared to the surrounding lower temperature area due to the locally enhanced surface exchange current density, leading to an internal short circuit [21]. The failure mechanisms, modes and causes of localized heating from the DFMEA-PFMEA are mentioned in Table 4.

Table 4.
Failure mechanisms, modes, and their causes for localized heating.
The poor quality of the materials and manufacturing processes can result in different heat generation and heat dissipation conditions in the electrode, which leads to an uneven temperature distribution. Reducing the porosity or increasing the electrode’s thickness can lead to an increase in ion concentration and potential gradient, which can influence the generation of Joule heat [59]. Electrode particle fracture due to calendaring and the vacuum drying process can cause local hotspots. Therefore, it becomes important to address the failure modes leading to localized heating and ensure that the design factors like porosity and electrode thickness are properly selected and tested. Proper process control during electrode fabrication can ensure that the design parameters are met consistently, thereby reducing the risk of localized heating due to manufacturing and improving the safety of the battery. Thermal simulations generally consider a lumped model [60], but the inclusion of local hotspots increases the accuracy of model prediction and aids in a better design of protection limits [61].
4.3. Increased Gas Generation
Gases generated in the battery increase the internal pressure, causing the battery to vent or rupture, eventually leading to thermal runway due to the reaction of hot flammable gases from the battery with ambient oxygen [62]. Gas generation in lithium-ion batteries is elevated by increased side reactions involving electrolyte decomposition, SEI layer formation, and moisture ingress. The failure mechanisms, modes and causes of increased gas generation from the DFMEA-PFMEA are mentioned in Table 5.

Table 5.
Failure mechanisms, modes, and causes of increased gas generation.
The poor thermal stability of cathodes and improper SEI layer growth can lead to electrolyte decomposition producing gases [18,63]. An improper composition of cathode material can cause increased gas generation during electrical abuse conditions like overcharge due to electrolyte decomposition at the cathode surface [64]. Coatings like transition metal oxide nanoparticles have shown potential in inhibiting lithium dendrites, enhancing stability and preventing side reactions during overcharge [65]. To facilitate Li+ diffusion and minimize overpotential, electrode porosity should be optimized, thereby reducing the side reactions [66].
The presence of moisture in a lithium-ion battery can lead to gas generation through a series of chemical reactions involving electrolyte, lithium salts, and moisture. When moisture enters the cell, it reacts with lithium salt to produce hydrogen fluoride (HF) and other byproducts. The hydrogen fluoride (HF) generated is highly reactive and can further react with the organic solvents in the electrolyte or the electrode materials, leading to more gas generation [67]. Moisture ingress can result from manufacturing defects and poor sealing due to improper design. The water content present in the anode during the manufacturing process drops from ~1000 ppm to ~200 ppm after vacuum drying, emphasizing the importance of the drying steps in the process [40]. Poor thermal stability of the gasket in the battery cap can lead to sagging during temperature cycling, leading to moisture ingress and electrolyte leakage [68].
Electrode crosstalk refers to a phenomenon wherein the byproducts generated at one electrode initiate adverse side reactions on the opposing electrode, leading to exothermic reactions and gas release [69]. This occurrence often stems from the dissolution of transition metals, the magnitude of which is influenced by factors such as cathode composition, electrolyte formulation, and the formation of a stable solid electrolyte interphase (SEI) [70]. Introducing aluminum doping into the transitional metal cathode material can mitigate the dissolution process [71], while incorporating appropriate electrolyte additives can suppress active material corrosion and oxygen evolution [69,72].
4.4. Malfunctioning of Safety Devices
When a lithium-ion battery goes into thermal runaway, the energy stored within the battery is often released in a matter of milliseconds [27]. Improper design and manufacturing processes can compromise the functionality of the safety devices, increasing the risk of cell failures or hazardous incidents. The failure mechanisms, modes and causes of malfunctioning of safety devices from the DFMEA-PFMEA are mentioned in Table 6.

Table 6.
Failure mechanisms, modes, and causes of malfunctioning of safety devices.
The PTC device is a temperature-sensitive resistor that limits the current flow when the battery’s temperature exceeds a specified threshold, protecting the battery from overheating. However, the improper design of PTC can increase the internal resistance of the battery, thereby increasing thermal loss [73]. PTC thermal mass and the heat dissipation coefficient affect its trip time, and choosing incorrect PTC material can cause the device to fail, leading to uncontrolled current flow and a higher risk of thermal runaway [74].
If the safety vent in a cylindrical battery becomes obstructed and the internal pressure is not released in a timely manner, the pressure may continue to build up and cause the battery case to rupture or even lead to explosions [75,76]. To mitigate this risk, a mandrel and a bottom vent are considered in certain models of cylindrical cells. This design enhancement increases the venting efficiency and reduces the thermal impact of a single battery rupture in a battery pack [28]. Without an internal mandrel, the electrode assembly can collapse, blocking the flow of gas, and increasing the risk of the cell reaching its burst pressure. A limitation of using the mandrel is that when gas flow rates are high, these may cause the mandrel to move independently from the electrode assembly, occasionally resulting in punctures to the crimp components [44].
It is important to choose the right activation pressure to ensure the CID is activated only when there is a risk of thermal runway or explosion and not during normal operation. Manufacturing issues, such as improper assembly, misalignment, or defects in the CID components, can cause it to fail or activate prematurely, compromising the battery’s safety and performance. The activation of the CID can be affected by the welding connection. If the welding connection is too strong, the top disk may not break the welded connection, which can prevent the CID from activating when needed. The trigger pressure of the safety vent in cylindrical batteries is typically higher than that of the pressure-responsive CID [77]. The vent opening area can determine the flow rate and burst pressure during venting [78]. Therefore, considering all the potential failure modes due to improper venting and simulating them to verify the design can help in improving the safety of the battery.
5. Conclusions
Enhancing the safety of lithium-ion batteries involves optimizing their design, ensuring high-quality manufacturing processes, and incorporating protective features to address potential safety incidents. Traditionally, this has been conducted in a haphazard manner. This study systematically examined all the safety factors within each design and manufacturing process element, using design and process FMEAs (DFMEA, PFMEA). Considering the multifaceted nature of factors contributing to failure causes, encompassing battery chemistry, design specifications, operating conditions, and intended use cases, a universal ranking was not provided. However, key areas of concern were found to be design and manufacturing processes that can exacerbate short circuits, localized heating, and abnormal gas generation within cells.
Of special concern is the contact between electrodes, which can result in a direct electrical pathway between electrodes and lead to short circuits within the battery. The design choice of a separator with poor puncture strength and thermal resistance increases the risk of tear and shrinkage, resulting in an internal short circuit. Maintaining a proper N/P ratio by choosing the correct quantities of anode and cathode active material, compaction densities and dimensions, lowers the risk of lithium plating.
An inhomogeneous distribution of active material in the coating process, reduced electrode porosity during calendaring process, and a non-uniform uptake of electrolyte in the wetting process cause non-uniform current distribution, leading to dendrite formation. Elevated rolling pressure during calendaring and elevated temperature and time during vacuum drying process can cause fractures or discontinuities within the electrode structure, leading to localized hotspots and potential thermal runaway. Considering these potential failure modes and their effects in the process of quality control by examining the electrode sheet before stacking to check for non-uniformity and fractures aids in the detection and elimination of jelly rolls that pose an elevated risk of causing thermal runaway.
It is imperative to ensure that the battery cap fulfills all the design requirements before its insertion. Qualification tests to consider encompass the CID pressure activation test for assessing burst pressure, the PTC trip temperature test to evaluate tripping temperature, response time and resistance, the temperature cycling test to inspect gasket performance, and the leakage test for verifying the seal of the cap.
The integrated FMEA-based safety analysis is useful in identifying design parameters that need to be considered while modeling a particular failure mechanism to predict an onset of failure. Identifying the process parameters and failure causes associated with these failures can provide guidance for designing quality checks in the manufacturing process. These quality checks, implemented as part of in-process quality control, can minimize costs and improve safety. This analysis can also be utilized for root cause analysis, aiming to determine the most probable explanation for a failure.
Author Contributions
Conceptualization, S.M. and L.K.; methodology, S.M. and M.P.; validation, L.K. and M.P.; formal analysis, S.M.; investigation, S.M.; writing—original draft preparation, S.M.; writing—review and editing, L.K. and M.P.; visualization, S.M.; supervision, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by more than 150 companies through the Center of Advanced Life Cycle Engineering, University of Maryland, College Park, MD, USA.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank the Center for Advanced Life Cycle Engineering (CALCE) and its over 150 funding companies, and the Centre for Advances in Reliability and Safety (CAiRS), Hong Kong SAR, China, admitted under AIR@InnoHK Research Cluster, for enabling research into advanced topics in reliability, safety, and sustainment.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Table A1 presents the DFMEA (design failure mode and effects analysis), delineating potential failure modes, their underlying causes, and the corresponding effects concerning battery safety. This analytical framework helps identify and mitigate design-related risks associated with the battery system, thereby enhancing its safety and reliability. Within Table A2 lies the PFMEA (process failure mode and effects analysis), which delves into potential failure modes, their root causes, and the resultant effects specific to battery safety. This examination scrutinizes the manufacturing and assembly processes involved in producing batteries, aiming to anticipate and address any process-related vulnerabilities that could compromise safety.

Table A1.
DFMEA for cylindrical lithium-ion battery.
Table A1.
DFMEA for cylindrical lithium-ion battery.
| Element | Potential Failure Mode | Potential Failure Causes | Effect on Battery Safety |
|---|---|---|---|
| Cathode | Poor thermal stability | Wrong choice of material Poor-quality material from the supplier | Increased gas generation, causing poor safety due to risk of explosion and thermal runaway |
| Poor overcharge safety | Wrong choice of material Poor-quality material from the supplier | Increased gas generation, causing poor safety due to risk of explosion | |
| High reactivity to electrolyte | Wrong composition of transition metals Wrong choice of binder | Increased gas generation and risk of thermal runaway due to crosstalk effect | |
| Metal contamination | Poor quality of material from supplier | Presence of metal particles can cause nucleation sites for formation of lithium dendrites, increasing risk of short circuit | |
| Improper N/P ratio | Wrong choice of material Poor-quality material from the supplier | N/P ratio is not maintained which can lead to lithium plating, increasing the risk of an internal short circuit | |
| Particle size is high | Wrong choice of blend time and temperature during design Wrong setting of equipment during manufacturing | Can result in tear of separator, causing an internal short circuit | |
| Specific area is too small | Improper design of cathode area for coating | Can result in severe electrochemical polarization, increasing the temperature at cathode, increasing risk of thermal runaway | |
| Peeling of cathode sheet | Improper binder chosen in design Poor quality binder from supplier | Non-uniform current distribution resulting in localized heating, increasing risk of thermal runaway | |
| Compaction density is high | Wrong choice settings for calendaring during design Improper settings of equipment during manufacturing | Can result in fractures causing non-uniform current distribution resulting in localized heating, increasing risk of thermal runaway | |
| Anode | Poor thermal stability | Wrong choice of material Poor-quality material from the supplier | Increase risk of thermal runaway |
| Metal contamination | Poor quality of material from supplier | Presence of metal particles can cause nucleation sites for formation of lithium dendrites, increasing risk of short circuit | |
| Compaction density is high | Wrong choice settings for calendaring during design Improper settings of equipment during manufacturing | Increase risk of lithium plating that could lead to an internal short circuit, causing thermal runaway | |
| Improper N/P ratio | Wrong choice of material Poor-quality material from the supplier | N/P ratio is not maintained which can lead to lithium plating, increasing the risk of an internal short circuit | |
| Particle size is high | Wrong choice of blend time and temperature during design Wrong setting of equipment during manufacturing | Can result in tear of separator, causing an internal short circuit | |
| Specific area is too small | Improper design of anode area for coating | Can result in severe electrochemical polarization, increasing the temperature at anode, increasing risk of thermal runaway | |
| Specific area is too large | Improper design of anode area for coating | Incomplete SEI layer formation resulting in increased gas generation, causing poor safety due to risk of explosion | |
| Peeling of anode sheet | Improper binder chosen in design Poor quality binder from supplier | Non-uniform current distribution resulting in localized heating, increasing risk of thermal runaway | |
| Electrolyte | Poor overcharge safety | Wrong choice of electrolyte composition Poor-quality material from the supplier | Increased gas generation, causing poor safety due to risk of explosion |
| Poor thermal stability | Wrong choice of electrolyte composition Poor-quality material from the supplier | Increased gas generation, causing poor safety due to risk of explosion | |
| Lack of electrolyte | Improper amount chosen during design Improper settings during manufacturing | Increased electrochemical polarization due to improper soaking, non-uniform current distribution, increasing risk of lithium plating, causing an internal short circuit | |
| Excess electrolyte | Improper amount chosen during design Improper settings during manufacturing | Improper seal, causing electrolyte leakage, leading to formation of flammable gas mixture, increasing the risk of explosion | |
| Micro short circuit | Wrong choice of electrolyte composition | Increasing risk of an internal short circuit, causing thermal runaway | |
| Conductivity of electrolyte is low/High ion diffusion resistance | Wrong choice of electrolyte composition Wrong amount of film forming additive is added Underuse of conductive agent | Can result in severe electrochemical polarization, increasing the temperature at anode, increasing risk of thermal runaway | |
| High corrosive nature | Wrong choice of electrolyte composition | Increased gas generation and risk of thermal runaway due to crosstalk effect | |
| Freezing point or viscosity is too high | Wrong choice of electrolyte composition | Can result in severe electrochemical polarization, increasing the temperature at anode, increasing risk of thermal runaway | |
| Current Collector | Tensile strength is low | Wrong choice of material Poor manufacturing quality from the supplier | Metal foil fracture, causing non-uniform current distribution, resulting in localized heating, increasing risk of thermal runaway |
| Poor elongation at break | Wrong choice of material Poor manufacturing quality from the supplier | Metal foil fracture, causing non-uniform current distribution, resulting in localized heating, increasing risk of thermal runaway | |
| Separator | Shutdown temperature of the separator is high | Wrong choice of separator Poor-quality material from the supplier | Delay in separator shutdown at elevated temperatures, increasing risk of thermal runaway. |
| Heat shrinkage is high | Wrong choice of separator Poor-quality material from the supplier | Increase risk of an internal short circuit leading to thermal runaway | |
| Puncture strength is low | Wrong choice of separator Poor-quality material from the supplier | Increase risk of an internal short circuit leading to thermal runaway | |
| Thickness is low | Wrong choice of separator Poor-quality material from the supplier | Increase risk of an internal short circuit leading to thermal runaway | |
| Poor elongation at break | Wrong choice of separator Poor-quality material from the supplier | Increase risk of an internal short circuit leading to thermal runaway | |
| Porosity is high | Wrong choice of separator Poor-quality material from the supplier | Reduction in mechanical strength, increasing the risk of tear, causing an internal short circuit, leading to thermal runaway | |
| Improper pore size distribution | Poor-quality material from the supplier | Non-uniform current distribution resulting in localized heating, lithium plating, increasing risk of thermal runaway | |
| Tensile strength is low | Wrong choice of separator Poor-quality material from the supplier | Increase risk of an internal short circuit leading to thermal runaway | |
| Cap | PTC base resistance is high | Wrong choice of PTC during design Poor-quality material from the supplier | Increased internal resistance, causing reduction incapacity |
| PTC temperature inflection point is high | Wrong choice of PTC during design Poor-quality material from the supplier | Delay in functioning of PTC, increasing risk of thermal runaway | |
| CID and vent activation pressure is high | Improper design of CID contact and vent disk Poor manufacturing quality from the supplier | Delay in activation of CID, increasing risk of internal pressure build up, causing thermal runway or explosion | |
| Insufficient air flow rate | Improper design of vent disk and exhaust holes Poor manufacturing quality from the supplier | Increased risk of internal pressure build up, causing thermal runway or explosion | |
| Gasket thermal stability is low | Wrong choice of gasket material during design Poor-quality material from the supplier | Improper seal, causing electrolyte leakage, leading to formation of flammable gas mixture, increasing the risk of explosion | |
| Gasket diffusion coefficient is high | Wrong choice of gasket material during design Poor-quality material from the supplier | Improper seal, causing electrolyte leakage, leading to formation of flammable gas mixture, increasing the risk of explosion | |
| Insulation ring thermal stability is low | Wrong choice of insulation ring material during design Poor-quality material from the supplier | Improper seal, causing electrolyte leakage, leading to formation of flammable gas mixture, increasing the risk of explosion | |
| Improper dimensions of PTC, insulation ring, vent disk, bottom disk, and gasket | Improper design of cap elements Poor manufacturing quality from the supplier | Improper seal, causing electrolyte leakage, leading to formation of flammable gas mixture, increasing the risk of explosion | |
| Top and Bottom Insulation Ring | Diameter of the ring is small | Improper design of top insulation ring Poor manufacturing quality from the supplier | Increased risk of an internal short circuit, causing thermal runway |
| Thickness is low | Improper design of top insulation ring Poor manufacturing quality from the supplier | Improper seal, causing electrolyte leakage, leading to formation of flammable gas mixture, increasing the risk of explosion | |
| Thickness is high | Improper design of top insulation ring Poor manufacturing quality from the supplier | Improper grooving and placement of cap forming an improper seal, causing electrolyte leakage, leading to formation of flammable gas mixture, increasing the risk of explosion | |
| Heat resistance is low | Wrong choice of material Poor manufacturing quality from the supplier | Deformation of the ring, increasing the risk of an internal short circuit, causing thermal runway | |
| Coefficient of thermal expansion does not match the can | Wrong choice of material Poor-quality material from the supplier | Improper seal at elevated temperatures, causing electrolyte leakage, leading to formation of flammable gas mixture, increasing risk of explosion | |
| Protective Tape | Heat resistance is low | Wrong choice of material Poor-quality material from the supplier | The tape could peel off at large current, causing an internal short circuit, increasing the risk of thermal runaway |
| Width/height is high | Wrong choice of dimensions during design Wrong setting of equipment during manufacturing | Reduction in capacity | |
| Width/height is low | Wrong choice of dimensions during design Wrong setting of equipment during manufacturing | Can result in tear of separator, causing an internal short circuit | |
| Tab | Improper hardness | Wrong choice of material Poor-quality material from the supplier | The tab could cut through the separator, causing an internal short circuit, increasing the risk of thermal runaway |
| Improper location and number of tabs | Improper design of tab location | Non-uniform current distribution resulting in localized heating, lithium plating, increasing risk of thermal runaway | |
| Increased electrical resistance | Improper design of tab size and composition | Localized heating, causing formation of local hotspots, increasing risk of thermal runaway | |
| Can | Thickness of nickel coating is low | Wrong thickness of coating chosen during design Wrong setting of coating during manufacturing | Generate weak areas on the battery casing and lead to case rupture during thermal runaway Increases risk of internal structure damage in mechanical abuse conditions |
| Diameter of can is large | Wrong choice of diameter during design Wrong setting of equipment during manufacturing | Loosening of jelly toll, causing non-uniform current distribution resulting in localized heating, increasing risk of thermal runaway | |
| Diameter of can is small | Wrong choice of diameter during design Wrong setting of equipment during manufacturing | Scratch of jelly roll during insertion could lead to an internal short circuit, increasing risk of thermal runaway | |
| Height of can is large | Wrong choice of height during design Wrong setting of equipment during manufacturing | Cell is discarded | |
| Height of can is small | Wrong choice of height during design Wrong setting of equipment during manufacturing | Improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | |
| Grooving depth is low | Wrong design of groove dimensions Poor quality of groove during manufacturing | Improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | |
| Grooving depth is high | Wrong design of groove dimensions Poor quality of groove during manufacturing | Electrode deformation can cause an internal short circuit | |
| Improper sealing compression | Wrong choice of compression pressure during design | Improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | |
| Mandrel and bottom vent | Thickness of mandrel is low | Wrong design of mandrel thickness Poor quality from supplier | Electrode deformation at core, increasing risk of an internal short circuit and thermal runaway |
| Thickness of mandrel is high | Wrong design of mandrel thickness Poor quality from supplier | Can scrape the electrode layers, increasing risk of an internal short circuit and thermal runaway | |
| Height of mandrel is low | Wrong design of mandrel height Poor quality from supplier | Can change the alignment of the mandrel, blocking the vent path, increasing risk of explosion | |
| Height of mandrel is high | Wrong design of mandrel height Poor quality from supplier | Increases the risk of production of projectiles due to force on the cap, when internal cell pressure increases | |
| Insufficient air flow rate | Improper design of mandrel and bottom vent | Increased risk of internal pressure build up, causing thermal runway or explosion |

Table A2.
PFMEA for manufacturing a cylindrical lithium-ion battery.
Table A2.
PFMEA for manufacturing a cylindrical lithium-ion battery.
| Process | Sub-Steps | Potential Failure Mode | Potential Failure Causes | Effect on Battery Safety |
|---|---|---|---|---|
| Electrode preparation | Mixing | Wrong material chosen for mixing | Error during procurement Wrong labeling | N/P ratio * is not maintained which can lead to lithium plating, increasing the risk of an internal short circuit [12] |
| Presence of moisture | Improper storage of raw material Improper warehouse humidity conditions | Increased gas generation, causing poor safety due to risk of explosion [13] | ||
| Presence of metal contaminants | Procurement of poor-quality material Improper storage of raw material | Presence of metal particles can cause nucleation sites for formation of lithium dendrites, increasing risk of short circuit [14] | ||
| Presence of dust contaminants | Procurement of poor-quality material Improper storage of raw material | Creation of discontinuities in electrode structure due to dust can result in local hotspots, increasing risk of thermal runaway [14] | ||
| Wrong composition of materials for the mixture | Quantity of materials not measured before mixing Error during measurement | N/P ratio is not maintained which can lead to lithium plating, increasing the risk of an internal short circuit | ||
| Presence of solid content in the mixture | Short blend time setting | Can result in tear of separator, causing an internal short circuit | ||
| Insufficient viscosity of the mixture | Improper blend time setting Improper temperature of mixture | Improper coating of the mixture, causing non-uniform current distribution and peeling of coated film, which results in localized heating, increasing risk of thermal runaway [16] | ||
| Coating | Non-uniform coating | Improper viscosity of the mixture Improper alignment of slot die Uneven flow rate of slot die Foil surface is uneven | Non-uniform current distribution resulting in localized heating and lithium plating, increasing risk of thermal runaway [17] | |
| Improper surface finish—presence of holes or voids | Improper viscosity of the mixture Improper alignment of slot die Uneven flow rate of slot die Foil surface is uneven | Non-uniform current distribution, resulting in localized heating, increasing risk of thermal runaway | ||
| Improper surface finish—non-uniform dispersion or presence of agglomerates | Improper blend time during mixing | Non-uniform current distribution, resulting in localized heating, increasing risk of thermal runaway Increase in electrical conductivity and polarization | ||
| Improper dimensions of coat—cathode width out of lower limit | Improper setting of equipment | |||
| Improper dimensions of coat—anode width out of lower limit | Improper setting of equipment | N/P ratio is lowered, which can lead to lithium plating, increasing the risk of an internal short circuit | ||
| Calendering | Thickness below lower limit Reduced electrode porosity | Improper setting of gap between the rollers Improper setting of force between the rollers | Low porosity of electrodes increases their diffusion resistance due to slow kinematics, which can result in lithium plating, increasing the risk of an internal short circuit | |
| Thickness above upper limit Increased electrode porosity | Improper setting of gap between the rollers | Cycle life performance degradation Jelly roll diameter above upper limit making assembly difficult | ||
| Increased surface roughness | Uneven surface of the rollers | Non-uniform current distribution resulting in localized heating, increasing risk of thermal runaway | ||
| Occurrence of fractures in the material | Improper setting of the rolling pressure Improper setting of force between the rollers | Cracks in cathode result in low N/P ratio which can lead to lithium plating, increasing risk of an internal short circuit. Fractures lead to increased moisture sorption leading to gas generation, increasing the risk of explosion | ||
| Warping of electrode sheets | Improper setting of the rolling pressure Improper setting of force between the rollers | Non-uniform current distribution resulting in localized heating, increasing risk of thermal runaway | ||
| Slitting | Improper width—cathode too narrow or anode too wide | Improper setting of equipment | ||
| Improper width—cathode too wide or anode too narrow | Improper setting of equipment | N/P ratio is lowered which can lead to lithium plating, increasing the risk of an internal short circuit | ||
| Improper width—separator too narrow | Improper setting of equipment | Increases the risk of an internal short circuit | ||
| Improper width—separator too wide | Improper setting of equipment | |||
| Improper height of electrodes and separator | Improper setting of equipment | Increases the risk of an internal short circuit | ||
| Presence of burrs | Wear of slitting knife Unclean edge of slitting knife | Increases the risk of an internal short circuit | ||
| Improper geometry of the cutting edges | Improper setting of equipment | Increases the risk of an internal short circuit | ||
| Presence of metallic foreign particles | Unclean equipment and workshop conditions Melted splatters from laser current/slitting knife | Presence of metal particles can cause nucleation sites for formation of lithium dendrites, increasing risk of short circuit | ||
| Vacuum Drying | Presence of moisture | Improper setting of room humidity level Insufficient setting of drying time | Increased gas generation, causing poor safety due to risk of explosion | |
| Occurrence of fractures in the material | Improper setting of room temperature Increased setting of drying time | Cracks in cathode result in low N/P ratio which can lead to lithium plating, increasing risk of an internal short circuit. Fractures lead to increased moisture sorption leading to gas generation, increasing the risk of explosion | ||
| Tab welding | Insufficient weld strength and improper weld tension | Improper setting of equipment Improper weld position | Increased contact resistance causes increased Joule heating creating thermal hotspots, increasing risk of thermal runaway | |
| Presence of burrs or protrusions | Improper setting of equipment | Can result in tear of separator, causing an internal short circuit | ||
| Over welding of tabs | Improper setting of equipment | Can damage the electrode sheet, causing an internal short circuit | ||
| Presence of dust contaminants | Improper maintenance of workshop environment | Creation of discontinuities in electrode structure due to dust can result in local hotspots, increasing risk of thermal runaway | ||
| Taping | Poor coverage of tab | Improper position settings in equipment | Can result in tear of separator, causing an internal short circuit | |
| Poor adhesion of tape | Poor quality procurement Presence of contaminants in the workshop | Can result in tear of separator, improper current distribution resulting in localized hotspots, causing an internal short circuit | ||
| Presence of dust contaminants | Improper maintenance of workshop environment | Creation of discontinuities in electrode structure due to dust can result in local hotspots, increasing risk of thermal runaway | ||
| Cell Assembly | Stacking and Winding | Presence of holes in separators | Poor quality of separator | Internal short circuit |
| Improper positioning of electrodes and separator | Operator fault | Internal short circuit | ||
| Improper rolling of jelly roll—loose winding | Improper tension settings in equipment | Non-uniform current distribution resulting in localized heating, increasing risk of thermal runaway | ||
| Improper rolling of jelly roll—tight winding | Improper tension settings in equipment | Internal short circuit | ||
| Improper rolling of jelly roll—winding spiral | Improper tension settings in equipment Improper removal of winding rod | Non-uniform current distribution resulting in localized heating, increasing risk of thermal runaway | ||
| Improper rolling of jelly roll—center collapse | Improper tension settings in equipment Improper removal of winding rod | Can scrape the electrode layers while welding the bottom tab, increasing the risk of an internal short circuit | ||
| Bottom Insulation Sheet and Jelly roll Insertion | Diameter of bottom insulation ring is missing or small | Operator fault Manufacturing defects | Short circuit between negative terminal and can (positive terminal) | |
| Improper alignment of jelly roll—inclined | Improper alignment of equipment | Weld rod can scrape the electrode layers while welding the bottom tab, the increasing risk of an internal short circuit | ||
| Improper alignment of jelly roll—positive and negative tab reversed | Operator fault Improper settings in equipment | External short circuit | ||
| Bottom Tab Welding | Insufficient weld strength and improper weld tension | Improper setting of equipment Improper weld position | Increased contact resistance causes increased Joule heating creating thermal hotspots, increasing risk of thermal runaway | |
| Presence of burrs or protrusions | Improper setting of equipment | Can result in tear of separator, causing an internal short circuit | ||
| Over welding of tab to can | Improper setting of equipment | Can damage the can, resulting in improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | ||
| Electrode damage due to weld rod | Improper alignment of equipment | Weld rod can scrape the electrode layers while welding the bottom tab, increasing risk of an internal short circuit | ||
| Mandrel Insertion | Mandrel is absent | Operator fault | Without an internal mandrel the electrode assembly can collapse, blocking the flow of gas, and increasing the risk of the of side wall rupture and the generation of high-speed projectiles | |
| Misalignment of mandrel | Improper alignment of equipment | Improper air flow path, increasing the risk of the of side wall rupture and the generation of high-speed projectiles | ||
| Cell sealing | Top Insulation Sheet Insertion | Diameter of top insulation ring is missing or small | Operator fault Manufacturing defects | Short circuit between negative terminal and can (positive terminal) |
| Improper alignment of insulation sheet | Improper alignment of equipment | |||
| Grooving | Improper groove height—low | Improper settings in equipment | Electrode deformation can cause an internal short circuit | |
| Improper groove height—high | Improper settings in equipment | Movement of jelly roll can increase risk of an internal short circuit | ||
| Improper crimping diameter—low | Improper settings in equipment | Electrode deformation can cause an internal short circuit | ||
| Improper crimping diameter—high | Improper settings in equipment | Improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | ||
| Presence of dust contaminants | Improper maintenance of workshop environment | Creation of discontinuities in electrode structure due to dust can result in local hotspots, increasing risk of thermal runaway | ||
| Electrolyte filling | Presence of contaminants in the electrolyte | Procurement of poor-quality material Improper storage of raw material | Increased gas generation, causing poor safety due to risk of explosion | |
| Presence of moisture | Improper setting of room humidity level | Increased gas generation, causing poor safety due to risk of explosion | ||
| Increased electrolyte quantity | Improper settings in equipment | Electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | ||
| Reduced electrolyte quantity | Improper settings in equipment | Inhomogeneous distribution of electrolyte, causing non-uniform current distribution, increasing risk of lithium plating, causing an internal short circuit | ||
| Electrolyte Uptake/Wetting | Incomplete/non-uniform soaking of electrolyte | Insufficient soaking time settings | Inhomogeneous distribution of electrolyte, causing non-uniform current distribution, increasing risk of lithium plating, causing an internal short circuit | |
| Presence of moisture | Improper setting of room humidity level | Increased gas generation, causing poor safety due to risk of explosion | ||
| Contamination of the electrolyte | Improper maintenance of workshop environment | Increased gas generation, causing poor safety due to risk of explosion | ||
| Positive Tab Welding | Insufficient weld strength and improper weld tension | Improper setting of equipment Improper weld position | Increased contact resistance causes increased Joule heating creating thermal hotspots, increasing risk of thermal runaway | |
| Presence of burrs or protrusions | Improper setting of equipment | Can result in tear of separator, causing an internal short circuit | ||
| Cap Insertion | Improper cap diameter—low | Manufacturing defects | Improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | |
| Improper cap diameter—high | Manufacturing defects | |||
| Misalignment of cap | Improper alignment of equipment | Improper activation of safety devices, improper sealing, causing electrolyte leakage leading to formation of flammable gases, increasing risk of explosion | ||
| PTC resistance out of specification | Manufacturing defects | PTC does not activate when temperature is out of limits, increasing risk of thermal runaway | ||
| CID activation pressure out of specification | Manufacturing defects | CID fails to activate, increasing risk of thermal runaway and explosion | ||
| Crimping and sealing | Improper crimping force—low | Improper equipment settings | Improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | |
| Improper crimping force—high | Improper equipment settings | Electrode deformation can cause an internal short circuit | ||
| Misaligned crimp | Improper alignment of equipment | Improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | ||
| Improper seal due to gasket | Poor gasket quality like improper, sagging due to thermal stress, crack in the gasket, wrong size | Improper seal, causing electrolyte leakage leading to formation of flammable gas mixture, increasing risk of explosion | ||
| Cell Finishing | Sleeving | Improper sleeve thickness—low | Manufacturing defects | Insufficient wear resistance can cause external short circuit |
| Improper sleeve thickness—high | Manufacturing defects | Poor heat dissipation, increasing risk of thermal runaway | ||
| Improper sleeve direction—positive and negative side reversed | Improper loading into equipment | External short circuit | ||
| Formation and aging | Improper SEI layer formation | High currents during formation cycle | Increased gas generation and risk of thermal runaway due to crosstalk effect | |
| Improper cell activation | High currents during formation cycle Short formation cycles | |||
| Improper aging conditions | Improper setting of aging time and conditions |
* N/P ratio is the ratio of negative electrode capacity to positive electrode capacity.
References
- Linhorst, M. What It Takes for an E-Bike Battery to Explode. Slate, 20 February 2023. [Google Scholar]
- Dolcourt, J. Samsung Galaxy Note 7 Recall: Here’s What Happens Now. CNET, 16 April 2017. [Google Scholar]
- Lee, S.-H.; Ko, I.-H. Failure Analysis of Swelling in Prismatic Lithium-Ion Batteries During Their Cycle Life After Long-Term Storage. J. Fail. Anal. Prev. 2018, 18, 554–561. [Google Scholar] [CrossRef]
- Wu, Y.; Saxena, S.; Xing, Y.; Wang, Y.; Li, C.; Yung, W.; Pecht, M. Analysis of Manufacturing-Induced Defects and Structural Deformations in Lithium-Ion Batteries Using Computed Tomography. Energies 2018, 11, 925. [Google Scholar] [CrossRef]
- Iturrondobeitia, A.; Aguesse, F.; Genies, S.; Waldmann, T.; Kasper, M.; Ghanbari, N.; Wohlfahrt-Mehrens, M.; Bekaert, E. Post-Mortem Analysis of Calendar-Aged 16 Ah NMC/Graphite Pouch Cells for EV Application. J. Phys. Chem. C 2017, 121, 21865–21876. [Google Scholar] [CrossRef]
- Bartolomé, E.; Benítez, P. Failure Mode and Effect Analysis (FMEA) to Improve Collaborative Project-Based Learning: Case Study of a Study and Research Path in Mechanical Engineering. Int. J. Mech. Eng. Educ. 2022, 50, 291–325. [Google Scholar] [CrossRef]
- Card, A.J.; Ward, J.R.; Clarkson, P.J. Beyond FMEA: The Structured What-If Technique (SWIFT). J. Healthc. Risk Manag. 2012, 31, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, C.; Williard, N.; Mathew, S.; Pecht, M. A Failure Modes, Mechanisms, and Effects Analysis (FMMEA) of Lithium-Ion Batteries. J. Power Sources 2015, 297, 113–120. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and Future Lithium-Ion Battery Manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef]
- Baazouzi, S.; Feistel, N.; Wanner, J.; Landwehr, I.; Fill, A.; Birke, K.P. Design, Properties, and Manufacturing of Cylindrical Li-Ion Battery Cells—A Generic Overview. Batteries 2023, 9, 309. [Google Scholar] [CrossRef]
- Günther, T.; Billot, N.; Schuster, J.; Schnell, J.; Spingler, F.B.; Gasteiger, H.A. The Manufacturing of Electrodes: Key Process for the Future Success of Lithium-Ion Batteries. Adv. Mater. Res. 2016, 1140, 304–311. [Google Scholar] [CrossRef]
- Heimes, H.H.; Kampker, A.; Lienemann, C.; Locke, M.; Offermanns, C.; Michaelis, S.; Rahimzei, E. Lithium-Ion Battery Cell Production Process; PEM der RWTH Aachen University and VDMA: Aachen/Frankfurt am Main, Germany, 2018; ISBN 978-3-947920-03-7. [Google Scholar]
- Wood, D.L.; Li, J.; An, S.J. Formation Challenges of Lithium-Ion Battery Manufacturing. Joule 2019, 3, 2884–2888. [Google Scholar] [CrossRef]
- Wu, X.; Song, K.; Zhang, X.; Hu, N.; Li, L.; Li, W.; Zhang, L.; Zhang, H. Safety Issues in Lithium Ion Batteries: Materials and Cell Design. Front. Energy Res. 2019, 7, 65. [Google Scholar] [CrossRef]
- Mikolajczak, C.; Kahn, M.; White, K.; Long, R.T. Lithium-Ion Batteries Hazard and Use Assessment; Springer Briefs in Fire; Springer: Boston, MA, USA, 2011; ISBN 978-1-4614-3485-6. [Google Scholar]
- Ogumi, Z.; Inaba, M. Carbon Anodes. In Advances in Lithium-Ion Batteries; Van Schalkwijk, W.A., Scrosati, B., Eds.; Springer: Boston, MA, USA, 2002; pp. 79–101. ISBN 978-0-306-47356-2. [Google Scholar]
- Nazri, G.A.; Yebka, B. Reactivity and Safety Aspects of Carbonaceous Anodes Used in Lithium-Ion Batteries—Correlation of Structural Parameters and Reactivity. In Materials for Lithium-Ion Batteries; Julien, C., Stoynov, Z., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 161–178. ISBN 978-0-7923-6651-5. [Google Scholar]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Nie, M.; Xia, J.; Dahn, J.R. A Systematic Study on the Reactivity of Different Grades of Charged Li[NixMnyCoz]O2 with Electrolyte at Elevated Temperatures Using Accelerating Rate Calorimetry. J. Power Sources 2016, 327, 145–150. [Google Scholar] [CrossRef]
- Kong, W.; Li, H.; Huang, X.; Chen, L. Gas Evolution Behaviors for Several Cathode Materials in Lithium-Ion Batteries. J. Power Sources 2005, 142, 285–291. [Google Scholar] [CrossRef]
- Yao, X.-Y.; Pecht, M.G. Tab Design and Failures in Cylindrical Li-Ion Batteries. IEEE Access 2019, 7, 24082–24095. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.J. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Bailey, J.J.; Vo, N.T.; Magdysyuk, O.V.; Drakopoulos, M.; et al. Identifying the Cause of Rupture of Li-Ion Batteries during Thermal Runaway. Adv. Sci. 2018, 5, 1700369. [Google Scholar] [CrossRef]
- Suo, L.; Hu, Y.-S.; Li, H.; Armand, M.; Chen, L. A New Class of Solvent-in-Salt Electrolyte for High-Energy Rechargeable Metallic Lithium Batteries. Nat. Commun. 2013, 4, 1481. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Li, H.; Nuli, Y.; Wang, J. Electrolytes for Advanced Lithium Ion Batteries Using Silicon-Based Anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety Mechanisms in Lithium-Ion Batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Kong, L.; Li, C.; Jiang, J.; Pecht, M. Li-Ion Battery Fire Hazards and Safety Strategies. Energies 2018, 11, 2191. [Google Scholar] [CrossRef]
- Xu, B.; Kong, L.; Wen, G.; Pecht, M.G. Protection Devices in Commercial 18650 Lithium-Ion Batteries. IEEE Access 2021, 9, 66687–66695. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- O’Hara, T. Navigating the Regulatory Maze of Lithium Battery Safety. Intertek. [Online]. Available online: https://evwest.com/support/BMSv2.2.pdf (accessed on 15 February 2024).
- Goodman, J.K.S.; Miller, J.T.; Kreuzer, S.; Forman, J.; Wi, S.; Choi, J.M.; Oh, B.; White, K. Lithium-ion cell response to mechanical abuse: Three-point bend. J. Energy Storage 2020, 28, 101244. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Q.; Li, K.; Ping, P.; Jiang, L.; Sun, J. A self-cooling and flame-retardant electrolyte for safer lithium ion batteries. Sustain. Energy Fuels 2018, 2, 1323–1331. [Google Scholar] [CrossRef]
- Lain, M.J.; Kendrick, E. Understanding the limitations of lithium ion batteries at high rates. J. Power Sources 2021, 493, 229690. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Li, Y.; Wang, G.; Wang, J. Thermal runaway and fire behaviors of large-scale lithium ion batteries with different heating methods. J. Hazard. Mater. 2019, 379, 120730. [Google Scholar] [CrossRef] [PubMed]
- Lienemann, C.; Maiser, D.; Michaelis, D.; Kampker, P.; Heimes, H.; Wessel, S.; Thielmann, A.; Sauer, A.; Hettesheimer, T. Battery Production Equipment 2030; VDMA Battery Production: Frankfurt am Main, Germany, 2016. [Google Scholar]
- Yoshio, M.; Brodd, R.J.; Kozawa, A. (Eds.) Lithium-Ion Batteries: Science and Technologies; Springer: New York, NY, USA, 2009; ISBN 978-0-387-34444-7. [Google Scholar]
- Schmitt, M.; Baunach, M.; Wengeler, L.; Peters, K.; Junges, P.; Scharfer, P.; Schabel, W. Slot-Die Processing of Lithium-Ion Battery Electrodes—Coating Window Characterization. Chem. Eng. Process. Process Intensif. 2013, 68, 32–37. [Google Scholar] [CrossRef]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current Status and Challenges for Automotive Battery Production Technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Huttner, F.; Diener, A.; Heckmann, T.; Eser, J.C.; Abali, T.; Mayer, J.K.; Scharfer, P.; Schabel, W.; Kwade, A. Increased Moisture Uptake of NCM622 Cathodes after Calendering Due to Particle Breakage. J. Electrochem. Soc. 2021, 168, 090539. [Google Scholar] [CrossRef]
- Kosfeld, M.; Westphal, B.; Kwade, A. Moisture Behavior of Lithium-Ion Battery Components along the Production Process. J. Energy Storage 2023, 57, 106174. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, X.; Shang, B.; Li, G. Unbalanced Discharging and Aging Due to Temperature Differences among the Cells in a Lithium-Ion Battery Pack with Parallel Combination. J. Power Sources 2016, 306, 733–741. [Google Scholar] [CrossRef]
- Kim, J.K.; Saito, A.; Kim, K. Jelly-Roll Type Electrode Assembly, Lithium Secondary Battery Having the Same, and Method for Manufacturing the Same. U.S. Patent 20060073380A1, 28 October 2008. [Google Scholar]
- Lee, J.; Kim, T.; Jung, H.; Shin, B. Method for Welding Metal Tab of Electrode Layer for Cable Battery and Electrode Manufactured Thereby. U.S. Patent 20160211500A1, 17 October 2014. Available online: https://patents.google.com/patent/US20160211500A1/en (accessed on 17 February 2024).
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Bailey, J.J.; Malik, R.; Vo, N.T.; Magdysyuk, O.V.; et al. Characterising Thermal Runaway within Lithium-Ion Cells by Inducing and Monitoring Internal Short Circuits. Energy Environ. Sci. 2017, 10, 1377–1388. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Jin, C.; Wood Iii, D.L.; Singler, T.J.; Li, J. On Electrolyte Wetting through Lithium-Ion Battery Separators. Extreme Mech. Lett. 2020, 40, 100960. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Alpas, A.T. Micromechanisms of Solid Electrolyte Interphase Formation on Electrochemically Cycled Graphite Electrodes in Lithium-Ion Cells. Carbon 2012, 50, 5359–5371. [Google Scholar] [CrossRef]
- Mao, C.; An, S.J.; Meyer, H.M.; Li, J.; Wood, M.; Ruther, R.E.; Wood, D.L. Balancing Formation Time and Electrochemical Performance of High Energy Lithium-Ion Batteries. J. Power Sources 2018, 402, 107–115. [Google Scholar] [CrossRef]
- Sharma, K.D.; Srivastava, S. Failure Mode and Effect Analysis (FMEA) Implementation: A Literature Review. J. Adv. Res. Aeronaut. Space Sci. 2018, 5, 1–17. [Google Scholar]
- Doughty, D.H.; Roth, E.P. A General Discussion of Li Ion Battery Safety. Electrochem. Soc. Interface 2012, 21, 37. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, J.; Cao, L.; Wu, Z.; Santhanagopalan, S. Constitutive behavior and progressive mechanical failure of electrodes in lithium-ion batteries. J. Power Sources 2017, 357, 126–137. [Google Scholar] [CrossRef]
- Greve, L.; Fehrenbach, C. Mechanical testing and macro-mechanical finite element simulation of the deformation, fracture, and short circuit initiation of cylindrical Lithium ion battery cells. J. Power Sources 2012, 214, 377–385. [Google Scholar] [CrossRef]
- Huang, X. Separator Technologies for Lithium-Ion Batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Gao, S.; Feng, X.; Lu, L.; Kamyab, N.; Du, J.; Coman, P.; White, R.E.; Ouyang, M. An Experimental and Analytical Study of Thermal Runaway Propagation in a Large Format Lithium Ion Battery Module with NCM Pouch-Cells in Parallel. Int. J. Heat Mass Transf. 2019, 135, 93–103. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal Runaway Features of Large Format Prismatic Lithium Ion Battery Using Extended Volume Accelerating Rate Calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Parhizi, M.; Ahmed, M.B.; Jain, A. Determination of the Core Temperature of a Li-Ion Cell during Thermal Runaway. J. Power Sources 2017, 370, 27–35. [Google Scholar] [CrossRef]
- Akolkar, R. Modeling Dendrite Growth during Lithium Electrodeposition at Sub-Ambient Temperature. J. Power Sources 2014, 246, 84–89. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; White, R.E. Mathematical Modeling of the Lithium Deposition Overcharge Reaction in Lithium-Ion Batteries Using Carbon-Based Negative Electrodes. J. Electrochem. Soc. 1999, 146, 3543–3553. [Google Scholar] [CrossRef]
- Oh, K.-Y.; Epureanu, B.I. A Novel Thermal Swelling Model for a Rechargeable Lithium-Ion Battery Cell. J. Power Sources 2016, 303, 86–96. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Q.; Yuca, N.; Ling, M.; Higa, K.; Battaglia, V.S.; Parkinson, D.Y.; Srinivasan, V.; Liu, G. A Convenient and Versatile Method To Control the Electrode Microstructure toward High-Energy Lithium-Ion Batteries. Nano Lett. 2016, 16, 4686–4690. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Lu, L.; Wu, P.; Kulp, C.; Prasser, S. Thermal Runaway Propagation Model for Designing a Safer Battery Pack with 25 Ah LiNi Co Mn O2 Large Format Lithium Ion Battery. Appl. Energy 2015, 154, 74–91. [Google Scholar] [CrossRef]
- Kim, G.-H.; Pesaran, A.; Spotnitz, R. A Three-Dimensional Thermal Abuse Model for Lithium-Ion Cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Mishra, D.; Zhao, P.; Jain, A. Thermal Runaway Propagation in Li-ion Battery Packs Due to Combustion of Vent Gases. J. Electrochem. Soc. 2022, 169, 100520. [Google Scholar] [CrossRef]
- Bryngelsson, H.; Stjerndahl, M.; Gustafsson, T.; Edström, K. How Dynamic Is the SEI? J. Power Sources 2007, 174, 970–975. [Google Scholar] [CrossRef]
- Arai, J.; Okada, Y.; Sugiyama, T.; Izuka, M.; Gotoh, K.; Takeda, K. In Situ Solid State 7 Li NMR Observations of Lithium Metal Deposition during Overcharge in Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A952–A958. [Google Scholar] [CrossRef]
- Lee, H.; Ren, X.; Niu, C.; Yu, L.; Engelhard, M.H.; Cho, I.; Ryou, M.H.; Jin, H.S.; Kim, H.T.; Liu, J.; et al. Suppressing Lithium Dendrite Growth by Metallic Coating on a Separator. Adv. Funct. Mater. 2017, 27, 1704391. [Google Scholar] [CrossRef]
- Beuse, T.; Fingerle, M.; Wagner, C.; Winter, M.; Börner, M. Comprehensive Insights into the Porosity of Lithium-Ion Battery Electrodes: A Comparative Study on Positive Electrodes Based on LiNi0.6Mn0.2Co0.2O2 (NMC622). Batteries 2021, 7, 70. [Google Scholar] [CrossRef]
- Schneider, M. Water Uptake of Tape-Cast Cathodes for Lithium Ion Batteries. J. Ceram. Sci. Tech. 2013, 4, 69–76. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.J.; Ku, C.H. Cap Assembly for Preventing Gasket Sag, and Cylindrical Secondary Battery Having the Same. EU Patent 2475026B1, 31 December 2009. Available online: https://patents.google.com/patent/EP2475026B1/en (accessed on 17 February 2024).
- Song, Y.; Wang, L.; Sheng, L.; Ren, D.; Liang, H.; Li, Y.; Wang, A.; Zhang, H.; Xu, H.; He, X. The significance of mitigating crosstalk in lithium-ion batteries: A review. Energy Environ. Sci. 2023, 16, 1943–1963. [Google Scholar] [CrossRef]
- Zhan, C.; Wu, T.; Lu, J.; Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes—A critical review. Energy Environ. Sci. 2018, 11, 243–257. [Google Scholar] [CrossRef]
- Li, W.; Kim, U.H.; Dolocan, A.; Sun, Y.K.; Manthiram, A. Formation and Inhibition of Metallic Lithium Microstructures in Lithium Batteries Driven by Chemical Crossover. ACS Nano 2017, 11, 5853–5863. [Google Scholar] [CrossRef]
- Yim, T.; Woo, S.G.; Lim, S.H.; Cho, W.; Song, J.H.; Han, Y.K.; Kim, Y.J. 5V-class high-voltage batteries with over-lithiated oxide and a multi-functional additive. J. Mater. Chem. A 2015, 3, 6157–6167. [Google Scholar] [CrossRef]
- Asare, E.; Evans, J.; Newton, M.; Peijs, T.; Bilotti, E. Effect of Particle Size and Shape on Positive Temperature Coefficient (PTC) of Conductive Polymer Composites (CPC)—A Model Study. Mater. Des. 2016, 97, 459–463. [Google Scholar] [CrossRef]
- Pesaran, A.A.; Kim, G.H.; Smith, K.; Darcy, E. Designing Safe Lithium-Ion Battery Packs Using Thermal Abuse Models (Presentation). Available online: https://digital.library.unt.edu/ark:/67531/metadc931995/m1/2/ (accessed on 9 July 2023).
- Kong, L.; Hu, X.; Gui, G.; Su, Y.; Pecht, M. Computed Tomography Analysis of Li-Ion Battery Case Ruptures. Fire Technol. 2020, 56, 2565–2578. [Google Scholar] [CrossRef]
- Yao, X.-Y.; Kong, L.; Pecht, M.G. Reliability of Cylindrical Li-Ion Battery Safety Vents. IEEE Access 2020, 8, 101859–101866. [Google Scholar] [CrossRef]
- Xu, B.; Lee, J.; Kwon, D.; Kong, L.; Pecht, M. Mitigation Strategies for Li-Ion Battery Thermal Runaway: A Review. Renew. Sustain. Energy Rev. 2021, 150, 111437. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Hargather, M.; Mier, F.A.; Ferreira, S.R. Determining the Internal Pressure in 18650 Format Lithium Ion Batteries under Thermal Abuse; Sandia National Lab (SNL-NM): Albuquerque, NM, USA, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).