Multi-Scale Heterogeneity of Electrode Reaction for 18650-Type Lithium-Ion Batteries during Initial Charging Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Test and Sample Preparation
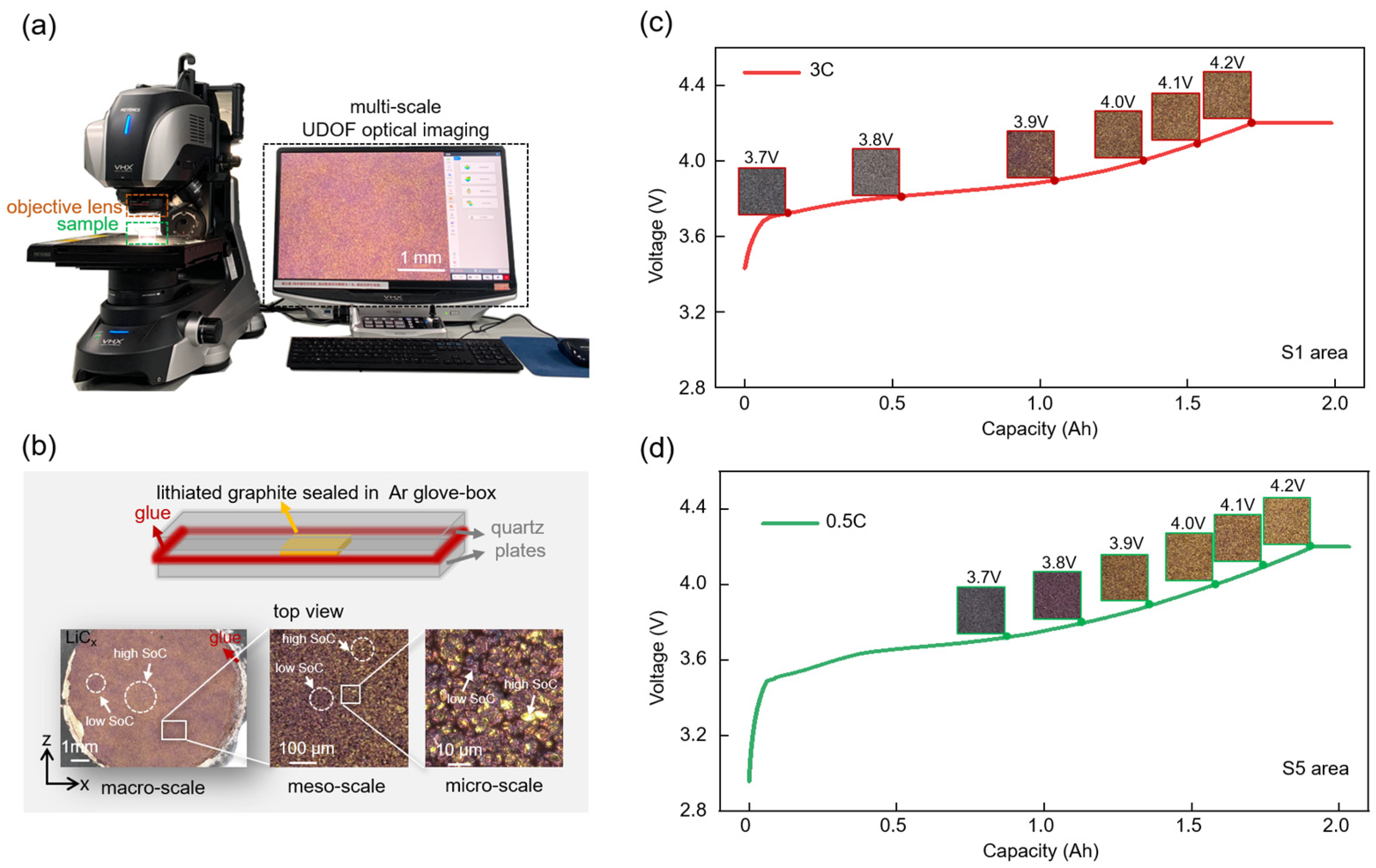
2.2. Multi-Scale Characterizations
3. Results and Discussion
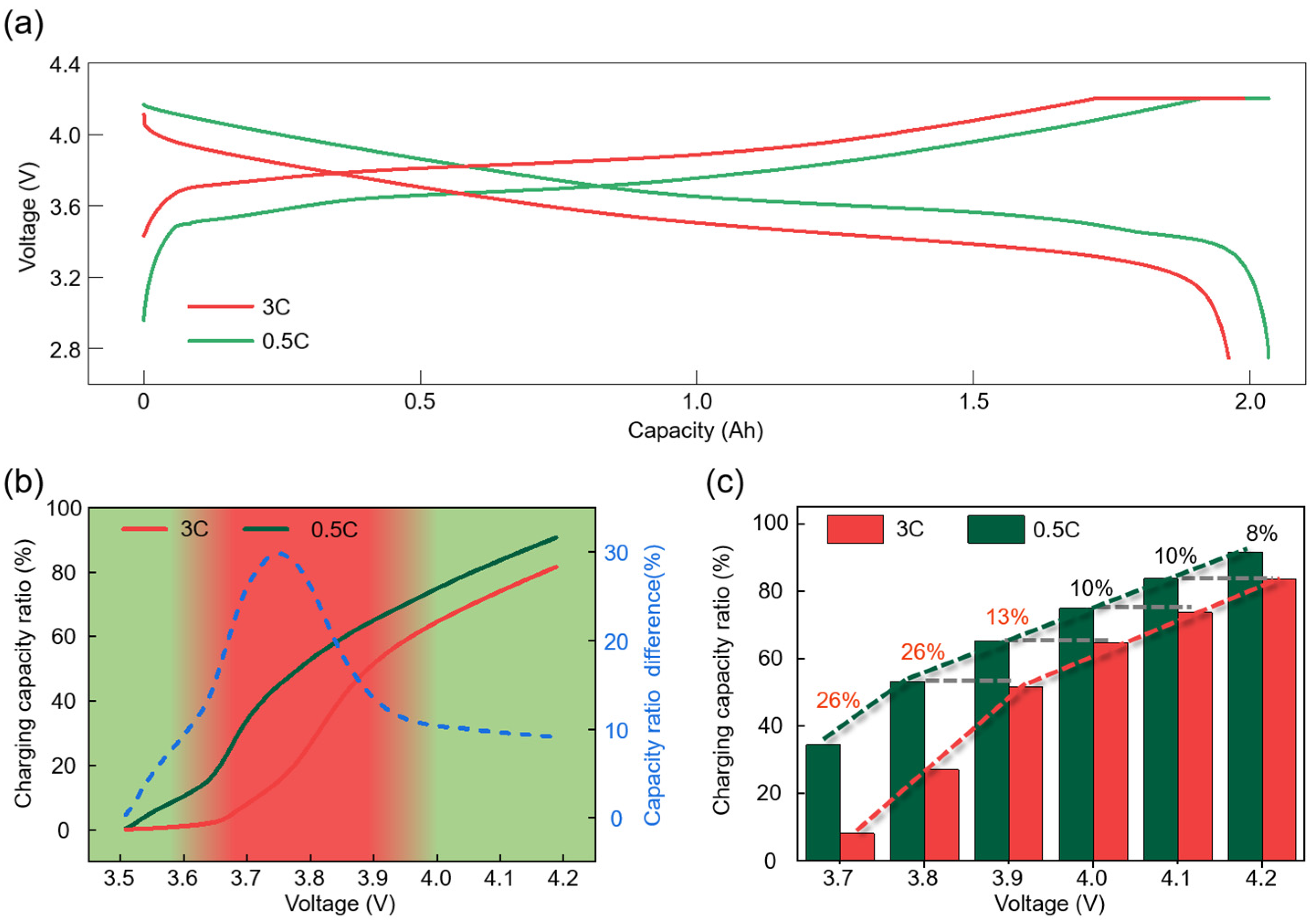
3.1. Electrochemical Heterogeneity of 18650-Type Cells
3.2. Structural Non-Uniformity of 18650-Type Cells and Its Impact on Initial Charging
3.3. Quasi-Operando Charging Behavior of Graphite Anode
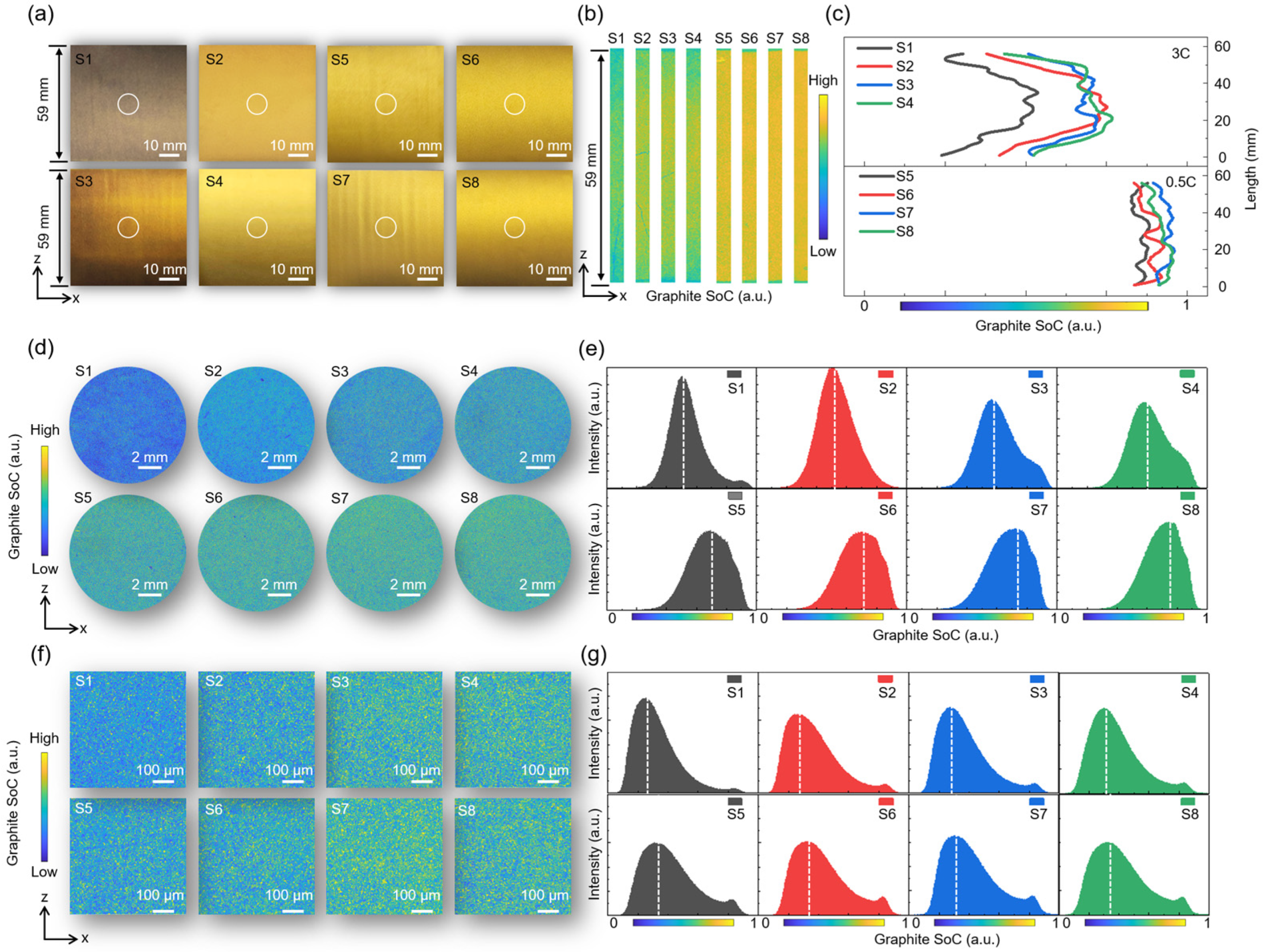
3.4. Multi-Scale Reaction Heterogeneity of Graphite Anode
3.5. Electrode Reaction Heterogeneity and Lithium Island Deposition in a Single-Coated Anode Section
3.6. Electrode Reaction Heterogeneity of Cross-Sectional NCM523 Cathode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries–Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Raza, M.Y. Research on China’s renewable energy policies under the dual carbon goals: A political discourse analysis. Energy Strat. Rev. 2023, 48, 101118. [Google Scholar] [CrossRef]
- Li, T.; Ma, L.; Liu, Z.; Yi, C.; Liang, K. Dual carbon goal-based quadrilateral evolutionary game: Study on the new energy vehicle industry in China. Int. J. Environ. Res. Public Health 2023, 20, 3217. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhao, F.Q.; Hao, H.; Liu, Z.W. Opportunities, challenges and strategies for developing electric vehicle energy storage systems under the carbon neutrality goal. World Electr. Veh. J. 2023, 14, 170. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of lithium battery technologies for electric vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Yu, A.; Wei, Y.Q.; Chen, W.W.; Peng, N.J.; Peng, L.H. Life cycle environmental impacts and carbon emissions: A case study of electric and gasoline vehicles in China. Transp. Res. Part D Transp. Environ. 2018, 65, 409–420. [Google Scholar] [CrossRef]
- Yu, R.J.; Cong, L.Z.; Li, Y.M.; Ran, C.J.; Zhao, D.C.; Li, P.; Van Mierlo, J. Prospects of passenger vehicles in China to meet dual carbon goals and bottleneck of critical materials from a fleet evolution perspective. World Electr. Veh. J. 2024, 15, 14. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; He, Q.Q.; He, H.M.; Xue, S. Development of new-energy vehicles under the carbon peaking and carbon neutrality strategy in China. Sustainability 2023, 15, 7725. [Google Scholar] [CrossRef]
- Li, M.; Feng, M.; Luo, D.; Chen, Z.W. Fast charging Li-ion batteries for a new era of electric vehicles. Cell Rep. Phys. Sci. 2020, 1, 100212. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Kang, Y.Q.; Zhao, Y.; Wang, L.; Liu, J.L.; Li, Y.X.; Liang, Z.; He, X.M.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Deng, J.; Bae, C.; Marcicki, J.; Masias, A.; Miller, T. Safety modelling and testing of lithium-ion batteries in electrified vehicles. Nat. Energy 2018, 3, 261–266. [Google Scholar] [CrossRef]
- Chakraborty, P.; Parker, R.; Hoque, T.; Cruz, J.; Du, L.L.; Wang, S.; Bhunia, S. Addressing the range anxiety of battery electric vehicles with charging en route. Sci. Rep. 2022, 12, 5588. [Google Scholar] [CrossRef] [PubMed]
- Kempton, W. Electric vehicles: Driving range. Nat. Energy 2016, 1, 16131. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.Y.; Feng, X.N.; O’Kane, S.; Liu, X.H.; Chen, J.Y.; Ji, C.Z.; Endler, E.; Li, R.H.; Liu, L.S.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Zhu, G.L.; Zhao, C.Z.; Huang, J.Q.; He, C.X.; Zhang, J.; Chen, S.H.; Xu, L.; Yuan, H.; Zhang, Q. Fast charging lithium batteries: Recent progress and future prospects. Small 2019, 15, e1805389. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhu, Y.Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Weng, S.T.; Yang, G.J.; Zhang, S.M.; Liu, X.Z.; Zhang, X.; Liu, Z.P.; Cao, M.Y.; Ates, M.N.; Li, Y.J.; Chen, L.Q.; et al. Kinetic limits of graphite anode for fast-charging lithium-ion batteries. Nano-Micro Lett. 2023, 15, 215. [Google Scholar] [CrossRef]
- Yao, Y.X.; Chen, X.; Yao, N.; Gao, J.H.; Xu, G.; Ding, J.F.; Song, C.L.; Cai, W.L.; Yan, C.; Zhang, Q. Unlocking charge transfer limitations for extreme fast charging of Li-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202214828. [Google Scholar] [CrossRef]
- Huang, W.X.; Ye, Y.S.; Chen, H.; Vilá, R.A.; Xiang, A.; Wang, H.X.; Liu, F.; Yu, Z.A.; Xu, J.W.; Zhang, Z.W.; et al. Onboard early detection and mitigation of lithium plating in fast-charging batteries. Nat. Commun. 2022, 13, 7091. [Google Scholar] [CrossRef]
- Mao, C.Y.; Ruther, R.E.; Li, J.L.; Du, Z.J.; Belharouak, I. Identifying the limiting electrode in lithium ion batteries for extreme fast charging. Electrochem. Commun. 2018, 97, 37–41. [Google Scholar] [CrossRef]
- Li, J.Z.; Sharma, N.; Jiang, Z.S.; Yang, Y.; Monaco, F.; Xu, Z.R.; Hou, D.; Ratner, D.; Pianetta, P.; Cloetens, P.; et al. Dynamics of particle network in composite battery cathodes. Science 2022, 376, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Rahman, M.M.; Mu, L.Q.; Liu, Y.J.; Lin, F. Chemomechanical behaviors of layered cathode materials in alkali metal ion batteries. J. Mater. Chem. 2018, 6, 21859–21884. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast charging of lithium-ion batteries: A review of materials aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Yue, X.Y.; Zhang, J.; Dong, Y.T.; Chen, Y.M.; Shi, Z.Q.; Xu, X.J.; Li, X.L.; Liang, Z. Reversible li plating on graphite anodes through electrolyte engineering for fast-charging batteries. Angew. Chem. Int. 2023, 62, e202302285. [Google Scholar] [CrossRef] [PubMed]
- Logan, E.R.; Dahn, J.R. Electrolyte design for fast-charging Li-ion batteries. Trends Chem. 2020, 2, 354–366. [Google Scholar] [CrossRef]
- Mancini, M.; Martin, J.; Ruggeri, I.; Drewett, N.; Axmann, P.; Wohlfahrt-Mehrens, M. Enabling fast-charging lithium-ion battery anodes: Influence of spheroidization on natural graphite. Batt. Supercaps 2022, 5, e202200109. [Google Scholar] [CrossRef]
- Cai, W.L.; Yan, C.; Yao, Y.X.; Xu, L.; Xu, R.; Jiang, L.L.; Huang, J.Q.; Zhang, Q. Rapid lithium diffusion in order@disorder pathways for fast-charging graphite anodes. Small Struct. 2020, 1, 2000010. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhu, T.Y.; McShane, E.; McCloskey, B.D.; Chen, G.Y. Single-crystal LiNixMnyCo1−x−yO2 cathodes for extreme fast charging. Small 2022, 18, 2105833. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, Y.E.; Kim, H. Improved fast charging capability of graphite anodes via amorphous Al2O3 coating for high power lithium ion batteries. J. Power Sources 2019, 422, 18–24. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Simulation and optimization of the dual lithium ion insertion cell. J. Electrochem. Soc. 1994, 141, 1–10. [Google Scholar] [CrossRef]
- Wang, F.; Tang, M. Thermodynamic origin of reaction non-uniformity in battery porous electrodes and its mitigation. J. Electrochem. Soc. 2020, 167, 120543. [Google Scholar] [CrossRef]
- Kuang, Y.D.; Chen, C.J.; Kirsch, D.; Hu, L.B. Thick electrode batteries: Principles, opportunities, and challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhu, J.E.; Finegan, D.P.; Zhao, H.B.; Lu, X.K.; Li, W.; Hoffman, N.; Bertei, A.; Shearing, P.; Bazant, M.Z. Guiding the design of heterogeneous electrode microstructures for Li-ion batteries: Microscopic imaging, predictive modeling, and machine learning. Adv. Energy Mater. 2021, 11, 2003908. [Google Scholar] [CrossRef]
- Lu, X.K.; Bertei, A.; Finegan, D.P.; Tan, C.; Daemi, S.R.; Weaving, J.S.; O’Regan, K.B.; Heenan, T.M.M.; Hinds, G.; Kendrick, E.; et al. 3D microstructure design of lithium-ion battery electrodes assisted by X-ray nano-computed tomography and modelling. Nat. Commun. 2020, 11, 2079. [Google Scholar] [CrossRef]
- Xue, Z.C.; Sharma, N.; Wu, F.X.; Pianetta, P.; Lin, F.; Li, L.X.; Zhao, K.J.; Liu, Y.J. Asynchronous domain dynamics and equilibration in layered oxide battery cathode. Nat. Commun. 2023, 14, 8394. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; de Vasconcelos, L.S.; Hassan, S.; Zhao, K.J. Asynchronous-to-synchronous transition of Li reactions in solid-solution cathodes. Nano Lett. 2022, 22, 5883–5890. [Google Scholar] [CrossRef]
- Kazyak, E.; Chen, K.H.; Chen, Y.X.; Cho, T.H.; Dasgupta, N.P. Enabling 4C fast charging of lithium-ion batteries by coating graphite with a solid-state electrolyte. Adv. Energy Mater. 2022, 12, 2102618. [Google Scholar] [CrossRef]
- Lu, L.L.; Lu, Y.Y.; Zhu, Z.X.; Shao, J.X.; Yao, H.B.; Wang, S.G.; Zhang, T.W.; Ni, Y.; Wang, X.X.; Yu, S.H. Extremely fast-charging lithium ion battery enabled by dual-gradient structure design. Sci. Adv. 2022, 8, eabm6624. [Google Scholar] [CrossRef]
- Chen, K.H.; Goel, V.; Namkoong, M.J.; Wied, M.; Müller, S.; Wood, V.; Sakamoto, J.; Thornton, K.; Dasgupta, N.P. Enabling 6C fast charging of Li-ion batteries with graphite/hard carbon hybrid anodes. Adv. Energy Mater. 2021, 11, 2003336. [Google Scholar] [CrossRef]
- Vishnugopi, B.S.; Verma, A.; Mukherjee, P.P. Fast charging of lithium-ion batteries via electrode engineering. J. Electrochem. Soc. 2020, 167, 090508. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.J.; Mijailovic, A.; Wang, G.Y.; Xiong, J.; Mathew, K.; Lu, W.Q.; Sheldon, B.W.; Wu, Q.L. Gradient porosity electrodes for fast charging lithium-ion batteries. J. Mater. Chem. A 2022, 10, 12114–12124. [Google Scholar] [CrossRef]
- Bridgewater, G.; Capener, M.J.; Brandon, J.; Lain, M.J.; Copley, M.; Kendrick, E. A comparison of lithium ion cell performance across three different cell formats. Batteries 2021, 7, 38. [Google Scholar] [CrossRef]
- Sturm, J.; Frank, A.; Rheinfeld, A.; Erhard, S.V.; Jossen, A. Impact of electrode and cell design on fast charging capabilities of cylindrical lithium-ion batteries. J. Electrochem. Soc. 2020, 167, 130505. [Google Scholar] [CrossRef]
- Waldmann, T.; Scurtu, R.G.; Richter, K.; Wohlfahrt-Mehrens, M. 18650 vs. 21700 Li-ion cells—A direct comparison of electrochemical, thermal, and geometrical properties. J. Power Sources 2020, 472, 228614. [Google Scholar] [CrossRef]
- Parmananda, M.; Norris, C.; Roberts, S.A.; Mukherjee, P.P. Probing the role of multi-scale heterogeneity in graphite electrodes for extreme fast charging. ACS Appl. Mater. Interfaces 2022, 14, 18335–18352. [Google Scholar] [CrossRef]
- Günter, F.J.; Burgstaller, C.; Konwitschny, F.; Reinhart, G. Influence of the electrolyte quantity on lithium-ion cells. J. Electrochem. Soc. 2019, 166, A1709–A1714. [Google Scholar] [CrossRef]
- Shodiev, A.; Primo, E.; Arcelus, O.; Chouchane, M.; Osenberg, M.; Hilger, A.; Manke, I.; Li, J.L.; Franco, A.A. Insight on electrolyte infiltration of lithium ion battery electrodes by means of a new three-dimensional-resolved lattice Boltzmann model. Energy Storage Mater. 2021, 38, 80–92. [Google Scholar] [CrossRef]
- Mühlbauer, M.J.; Petz, D.; Baran, V.; Dolotko, O.; Hofmann, M.; Kostecki, R.; Senyshyn, A. Inhomogeneous distribution of lithium and electrolyte in aged Li-ion cylindrical cells. J. Power Sources 2020, 475, 228690. [Google Scholar] [CrossRef]
- Lu, X.K.; Lagnoni, M.; Bertei, A.; Das, S.; Owen, R.E.; Li, Q.; O’Regan, K.; Wade, A.; Finegan, D.P.; Kendrick, E.; et al. Multiscale dynamics of charging and plating in graphite electrodes coupling operando microscopy and phase-field modelling. Nat. Commun. 2023, 14, 5127. [Google Scholar] [CrossRef] [PubMed]
- Sui, T.; Song, B.H.; Dluhos, J.; Lu, L.; Korsunsky, A.M. Nanoscale chemical mapping of Li-ion battery cathode material by FIB-SEM and TOF-SIMS multi-modal microscopy. Nano Energy 2015, 17, 254–260. [Google Scholar] [CrossRef]
- Bessette, S.; Paolella, A.; Kim, C.; Zhu, W.; Hovington, P.; Gauvin, R.; Zaghib, K. Nanoscale lithium quantification in LiXNiyCowMnzO2 as cathode for rechargeable batteries. Sci. Rep. 2018, 8, 17575. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, D.; Ma, Z.; Li, L. Multi-Scale Heterogeneity of Electrode Reaction for 18650-Type Lithium-Ion Batteries during Initial Charging Process. Batteries 2024, 10, 109. https://doi.org/10.3390/batteries10030109
Meng D, Ma Z, Li L. Multi-Scale Heterogeneity of Electrode Reaction for 18650-Type Lithium-Ion Batteries during Initial Charging Process. Batteries. 2024; 10(3):109. https://doi.org/10.3390/batteries10030109
Chicago/Turabian StyleMeng, Dechao, Zifeng Ma, and Linsen Li. 2024. "Multi-Scale Heterogeneity of Electrode Reaction for 18650-Type Lithium-Ion Batteries during Initial Charging Process" Batteries 10, no. 3: 109. https://doi.org/10.3390/batteries10030109
APA StyleMeng, D., Ma, Z., & Li, L. (2024). Multi-Scale Heterogeneity of Electrode Reaction for 18650-Type Lithium-Ion Batteries during Initial Charging Process. Batteries, 10(3), 109. https://doi.org/10.3390/batteries10030109






