Tuning of Ionic Liquid–Solvent Electrolytes for High-Voltage Electrochemical Double Layer Capacitors: A Review
Abstract
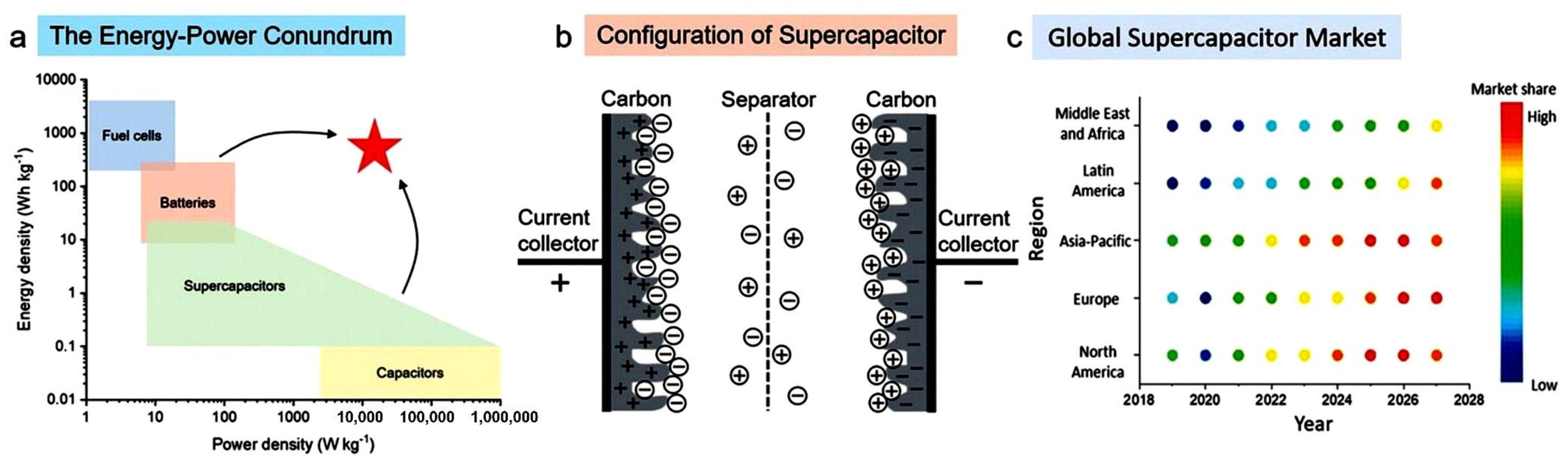
1. Introduction
2. Electrolytes for EDLCs
3. Tuning of IL–Solvent Electrolytes for EDLCs
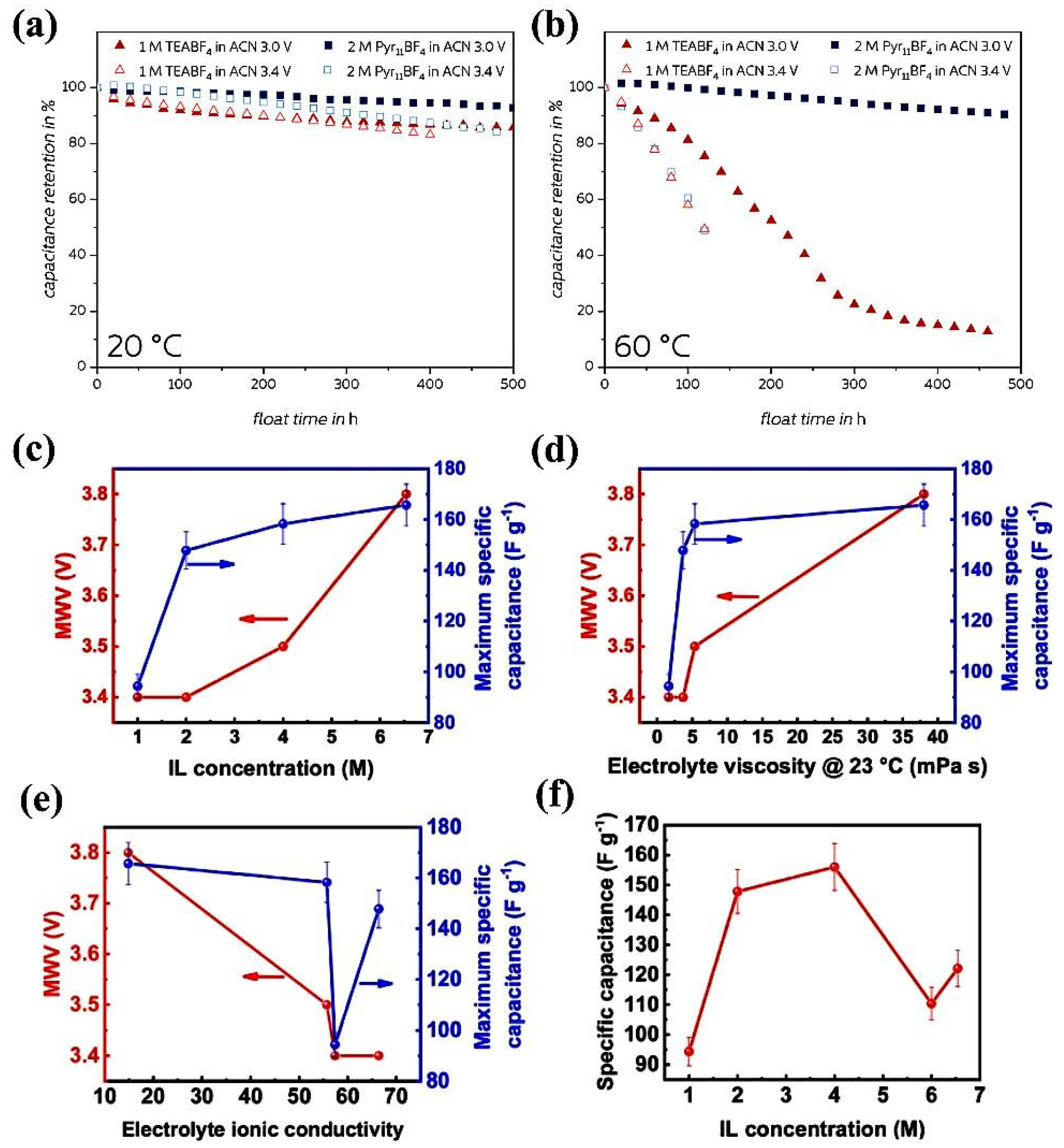
3.1. Higher Salt Concentration for High Voltage
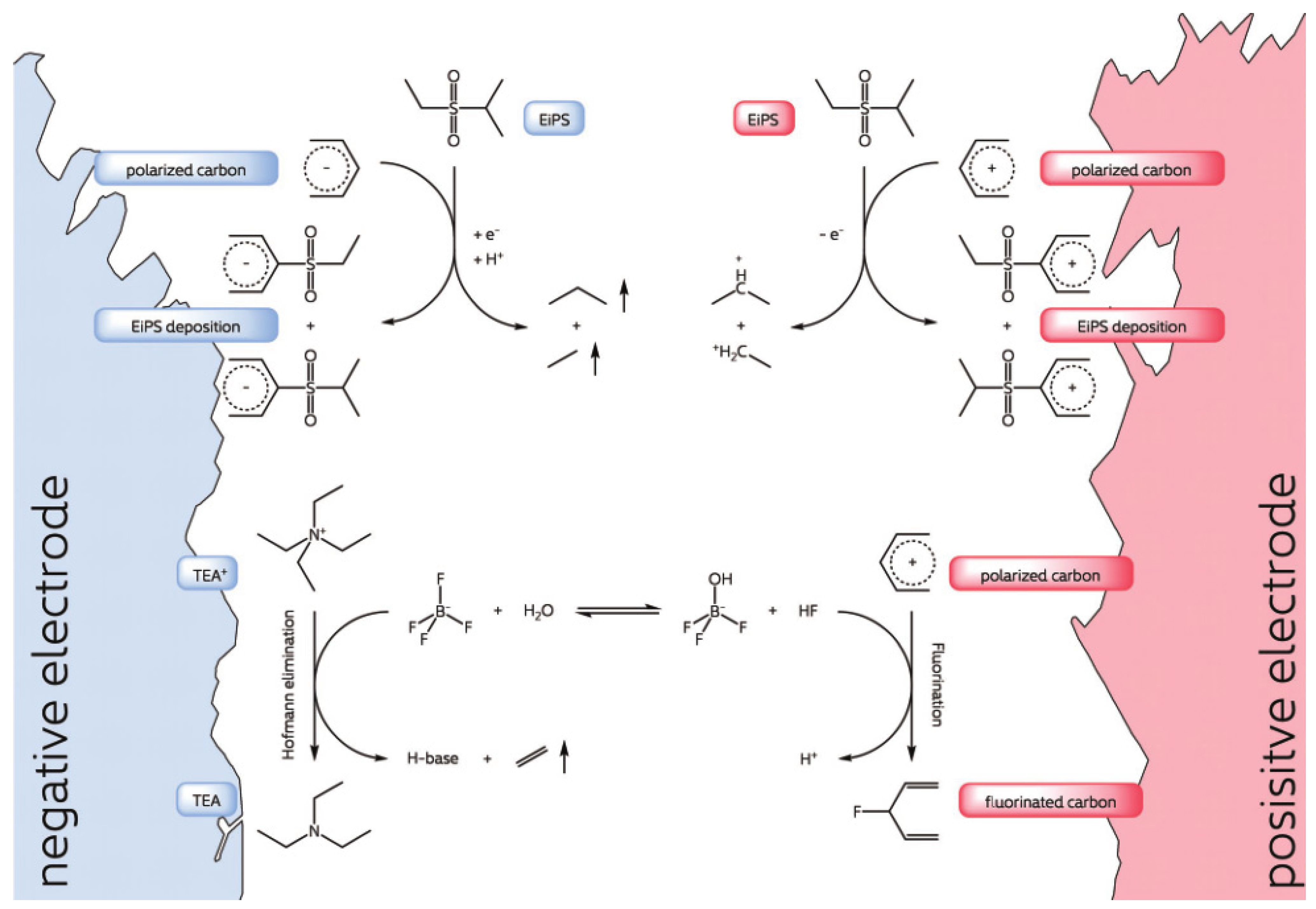
3.2. The Use of Single-Substitute Solvent with High Electrochemical Stability
3.3. Adding Co-Solvents to AN or PC for High Voltage
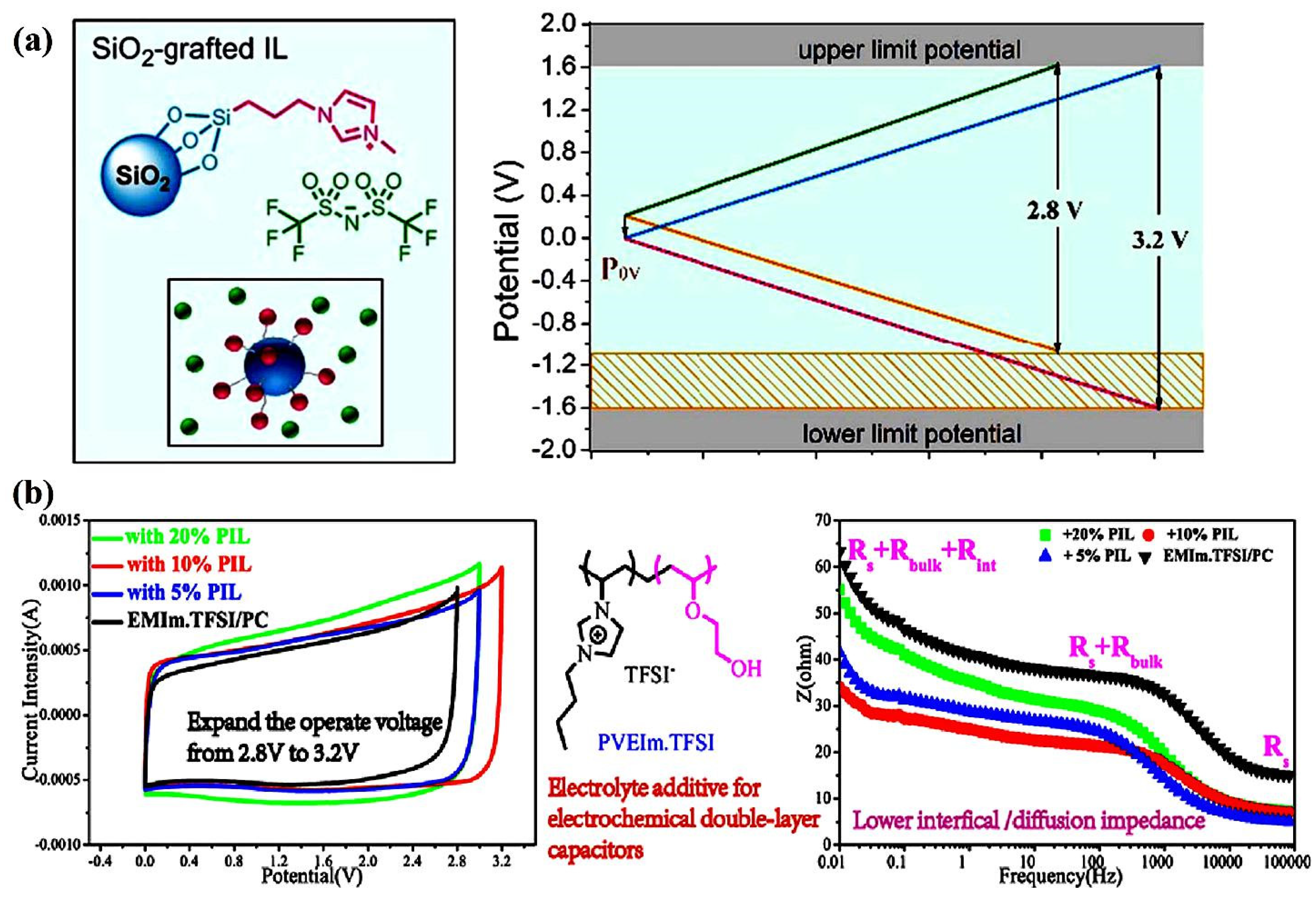
3.4. The Use of Solute Salt Additives for High Voltage
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Zuo, D.; Cheng, S.; Chen, S.; Liu, Y.; Bao, W.; Deng, S.; Harris, S.J.; Wan, J. Ultrafast materials synthesis and manufacturing techniques for emerging energy and environmental applications. Chem. Soc. Chem. 2023, 52, 1103. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, T.; Zhou, Q.; Sun, Y.; Qu, M.; Zeng, Z.; Ju, Y.; Li, L.; Wang, K.; Chi, F. A review of technologies and applications on versatile energy storage systems. Renew. Sustain. Energy Rev. 2021, 148, 111263. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y.; You, Y.; Yuan, J.; Xu, Q.; Xie, H.; Chen, Y. Rational design of electrode materials for advanced supercapacitors: From lab research to commercialization. Adv. Funct. Mater. 2023, 33, 2213095. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Review on Supercapacitors: Technologies and performance evaluation. J. Energ. Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every bite of supercap: A brief review on construction and enhancement of supercapacitor. J. Energy Storage 2022, 50, 104699. [Google Scholar] [CrossRef]
- Pathak, M.; Bhatt, D.; Bhatt, R.C.; Bohra, B.S.; Tattari, G.; Rana, S.; Arya, M.C.; Sahoo, N.G. High Energy Density Supercapacitors: An Overview of Efficient Electrode Materials, Electrolytes, Design, and Fabrication. Chem. Rec. 2024, 24, e202300236. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Song, G.; Li, C.; Wang, T.; Hu, K.L.F.; Cheng, S.; Hondo, E.; Liu, S.; Kawi, S. Hierarchical hollow carbon particles with encapsulation of carbon nanotubes for high performance supercapacitors. Small 2023, 20, 2305517. [Google Scholar] [CrossRef]
- Luo, L.; Lan, Y.; Zhang, Q.; Deng, J.; Luo, L.; Zeng, Q.; Gao, H.; Zhao, W. A review on biomass-derived activated carbon as electrode materials foe energy storage supercapacitors. J. Energy Storage 2022, 55, 105839. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, J.; Jiang, G.; Ni, K.; Shu, N.; Taberna, P.; Zhu, Y.; Simon, P. Electrochemical characterization of single layer graphene/electrolyte interface: Effect of solvent on the interfacial capacitance. Angew. Chem. Int. Ed. 2021, 60, 13317–13322. [Google Scholar] [CrossRef]
- Tareen, A.K.; Khan, K.; Iqbal, M.; Zhang, Y.; Long, J.; Nazeer, F.; Mahmood, A.; Mahmood, N.; Shi, Z.; Ma, C.; et al. Recent advanced in novel graphene: New horizons in renewable energy storage technologies. J. Mater. Chem. C 2022, 10, 11472–11531. [Google Scholar] [CrossRef]
- Das, S.; Manuraj, M.; Rakhi, R.B.; Ajayaghosh, A. High-frequency electrochemical double layer capacitor based in carbon nanotubes ink coated eggshell membrane electrodes. J. Energy Storage 2022, 45, 103799. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Fang, H.; Xu, Y.; Sun, W.; Chen, S.; Wang, Y.; Lv, L. Redox-active tetramino-benzoquinone π-π stacking and H-bonding onto multiwalled carbon nanotubes toward a high-performance asymmetric supercapacitor. ACS Appl. Energy Mater. 2022, 5, 8112–8122. [Google Scholar] [CrossRef]
- Martín-Illán, J.; Sierram, L.; Ocón, P.; Zamora, F. Electrochemical double-layer capacitor based on carbon@ covalent organic framework aerogels. Angew. Chem. Int. Ed. 2022, 61, e202213106. [Google Scholar] [CrossRef]
- Shalkh, J.S.; Shalkh, N.S.; Mishra, Y.K.; Pawar, S.S.; Parveen, N.; Shewale, P.M.; Sabale, S.; Kanjanaboos, P.; Praserthdam, S.; Lokhande, C.D. The implementation of graphene-based aerogel in the field of supercapacitor. Nanotechnology 2021, 32, 362001. [Google Scholar]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Schütter, C.; Pohlmann, S.; Balducci, A. Industrial requirements of materials for electrical double layer capacitors: Impact on current and future applications. Adv. Energy Mater. 2019, 9, 1900334. [Google Scholar] [CrossRef]
- Tao, R.; Yang, T.; Zhang, J.; Wu, Z.; Qiu, L. Design strategies of covalent organic framework-based electrodes for supercapacitor application. Chem. Commun. 2023, 59, 3175–3192. [Google Scholar] [CrossRef] [PubMed]
- Balducci, A. Electrolytes for high voltage electrochemical double layer capacitors: A perspective article. J. Power Sources 2016, 326, 534–540. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar]
- Lim, J.M.; Jang, Y.S.; Nguyen, H.V.T.; Kim, J.S.; Yoon, Y.; Park, B.J.; Seo, D.H.; Lee, K.; Han, Z.; Ostrikov, K.; et al. Advances in high-voltage supercapacitors for energy storage systems: Materials and electrolyte tailoring to implementation. Nanoscale Adv. 2023, 5, 615–626. [Google Scholar] [CrossRef]
- Kim, E.; Han, J.; Ryu, S.; Choi, Y.; Yoo, J. Ionic liquid electrolyte for electrochemical energy storage devices. Materials 2021, 14, 4000. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Saruhan, B. Application of ionic liquids for batteries and supercapacitors. Materials 2021, 14, 2942. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Ionic liquids for supercapacitive energy storage: A mini-review. Energy Fuels 2021, 35, 8443–8455. [Google Scholar] [CrossRef]
- Gerdroodbar, A.E.; Alihemmati, H.; Safavi-Mirmahaleh, S.; Golshan, M.; Damircheli, R.; Eliseeva, S.N.; Salami-Kalajahi, M. A review on transport pathways and coordination chemistry between ions and electrolytes in energy storage devices. J. Energy Storage 2023, 74, 109311. [Google Scholar] [CrossRef]
- Xia, L.; Yu, L.; Hu, D.; Chen, G.Z. Electrolytes for electrochemical energy storage. Mater. Chem. Front. 2017, 1, 584–618. [Google Scholar] [CrossRef]
- Venâncio, R.; Vicentini, R.; Pinzón C., M.J.; Corrêa, D.A.; Miranda, A.N.; Queiroz, A.C.; Degasperi, F.T.; Siqueira, L.J.A.; Da Silva, L.M.; Zanin, H. Combining electrochemical, molecular simulation and operando techniques to investigate the stability of electrodes and organic electrolytes used in EDLCs. Energy Storage Mater. 2023, 62, 102943. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zuo, D.; Xu, J.; Wang, Q.; Zhang, H. A supramolecular gel polymer electrolyte based on poly(vinyl alcohol) and tannic acid for flexible electrical double layer capacitors. J. Energy Storage 2023, 72, 108618. [Google Scholar] [CrossRef]
- Gajewski, P.; Lewandowska, A.; Szczesniak, K.; Voelkel, A.; Marcinkowska, A. Optimization of ionogel polymer electrolytes composition for their best performance in electric double layer capacitor. ChemElectroChem 2022, 27, e202200745. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Hashmi, S.A.; Khan, M.; Choi, D.; Qurashi, A. Frontiers and recent developments on supercapacitor’s materials, design, and applications: Transport and power system applications. J. Energy Storage 2023, 58, 106104. [Google Scholar] [CrossRef]
- Lu, S.; Song, Y.; Guo, K.; Chen, X.; Xu, J.; Zhao, L. Effect of aqueous electrolytes on the electrochemical behaviors of ordered mesoporous carbon composites after KOH activation as supercapacitors electrodes. J. Electroanal. Chem. 2018, 818, 58–67. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, C.; Kao, E.; Warren, R.; Zhang, R.; Teh, K.S.; Zhong, J.; Wei, M.; Li, B.; Chu, Y.; et al. Titanium Disulfide Coated Carbon Nanotube Hybrid Electrodes Enable High Energy Density Symmetric Pseudocapacitors. Adv. Mater. 2018, 30, 1704754. [Google Scholar] [CrossRef]
- Tian, Z.; Deng, W.; Wang, X.; Liu, C.; Li, C.; Chen, J.; Xue, M.; Li, R.; Pan, F. Superconcentrated aqueous electrolyte to enhance energy density for advanced supercapacitors. Funct. Mater. Lett. 2017, 10, 1750081. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, L.; Li, Z.; Dou, H.; Zhang, X. Design strategies and research progress for Water-in-Salt electrolytes. Energy Storage Mater. 2022, 44, 10–28. [Google Scholar] [CrossRef]
- Xiao, D.; Wu, Q.; Liu, X.; Dou, Q.; Liu, L.; Yan, B.; Yu, H. High-voltage and high-capacitance aqueous supercapacitor using carbon nanorods electrodes and water-in-salt electrolyte. ChemElectroChem 2019, 6, 439–443. [Google Scholar] [CrossRef]
- Martins, V.L.; Mantovi, P.S.; Torresi, R.M. Suppressing early capacitance fade of electrochemical capacitors with water-in-salt electrolytes. Electrochim. Acta 2021, 372, 137854. [Google Scholar] [CrossRef]
- Lannelongue, P.; Bouchal, R.; Mourad, E.; Bodin, C.; Olarte, M.; Vot, S.; Favier, F.; Fontaine, O. “Water-in-Salt” for Supercapacitors: A Compromise between Voltage, Power Density, Energy Density and Stability. J. Electrochem. Soc. 2018, 165, A657–A663. [Google Scholar] [CrossRef]
- Samanta, P.; Ghosh, S.; Kundu, A.; Samanta, P.; Murmu, N.C.; Kulia, T. Review a strategic way of high-performance energy storage device development with envirmentally viable “Water-in-salt” electrolytes. J. Energy Chem. 2023, 78, 350–373. [Google Scholar] [CrossRef]
- Laheäär, A.; Jänes, A.; Lust, E. NaClO4 and NaPF6 as potential non-aqueous electrolytes salts for electrical double layer capacitor application. Electrochim. Acta 2012, 82, 309–313. [Google Scholar] [CrossRef]
- Chidiac, J.; Timperman, L.; Anouti, M. Role of FTFSI anion asymmetry on physical properties of AFTFSI (A=Li, Na and K) based electrolytes and consequences on supercapacitor application. ChemPhysChem 2021, 22, 1863–1879. [Google Scholar] [CrossRef]
- Wang, X.; Mahandzhiyski, A.Y.; Arstad, B.; Van Aken, K.L.; Mathis, T.S.; Gallegos, A.; Tian, Z.; Ren, D.; Sheridan, E.; Grimes, B.A.; et al. Selective charging behavior in an ionic mixture electrolyte supercapacitor system for higher energy and power. J. Am. Chem. Soc. 2017, 139, 18681–18687. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Sugimoto, T.; Yamagata, M.; Kikuta, M.; Kono, M.; Ishikawa, M. A neat ionic liquid electrolyte based on FSI anion for electric double layer capacitor. J. Power Sources 2008, 185, 1585–1588. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Teberna, P.L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Rennie, A.J.R.; Martins, V.L.; Torresi, R.M.; Hall, P.J. Ionic liquids containing sulfonium cations as electrolytes for electrochemical double layer capacitors. J. Phys. Chem. C 2015, 119, 23865–23874. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, J.; Lee, A.S.; Na, W.; Yoon, H.; Kim, W.; Koo, C.M. High-voltage ionic liquid electrolytes based on ether functionalized pyrrolidinium for electric double-layer capacitors. Electrochim. Acta 2016, 222, 1847–1852. [Google Scholar] [CrossRef]
- Martins, V.L.; Rennie, A.J.R.; Sanchez-Ramirez, N.; Torresi, R.M.; Hall, P.J. Improved performance of ionic liquid supercapacitors by using tetracyanoborate anions. ChemElectroChem 2018, 5, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Aken, K.L.V.; Beidaghi, M.; Gogotsi, Y. Formulation of ionic-liquid electrolyte to expand the voltage window of supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 4806–4809. [Google Scholar] [CrossRef]
- Lin, R.; Taberna, P.L.; Fantini, S.; Presser, V.; Pérez, C.R.; Malbosc, F.; Rupesinghe, N.L.; Teo, K.B.K.; Gogosti, Y.; Simon, P. Capacitive energy storage from −50 to 100 °C using an ionic liquid electrolyte. J. Phys. Chem. Lett. 2011, 2, 2396–2401. [Google Scholar] [CrossRef]
- Newell, R.; Faure-Vincent, J.; Iliev, B.; Schubert, T.; Aradilla, D. A new high performance ionic liquid mixture electrolyte for large temperature range supercapacitor application (−70 °C to 80 °C) operating at 3.5 V cell voltage. Electrochim. Acta 2018, 267, 15–19. [Google Scholar] [CrossRef]
- Krummacher, J.; Schütter, C.; Passerini, S.; Balducci, A. Characterization of different conductive salts in CAN-based electrolytes for electrochemical double-layer capacitors. ChemElectroChem 2013, 4, 353–361. [Google Scholar] [CrossRef]
- Balducci, A. High voltage electrochemical double layer capacitor containing mixtures of ionic liquids and organic carbonate as electrolytes. Electrochem. Commun. 2011, 13, 814–817. [Google Scholar] [CrossRef]
- Wong, S.I.; Lin, H.; Sunarso, J.; Wong, B.T.; Jia, B. Optimization of ionic-liquid based electrolyte concentration for high-energy density graphene supercapacitors. Appl. Mater. Today 2020, 18, 100522. [Google Scholar] [CrossRef]
- Schütter, C.; Bothe, A.; Balducci, A. Mixtures of acetonitrile and ethyl isopropyl sulfone as electrolytes foe electrochemical double layer capacitors. Electrochim. Acta 2020, 331, 135421. [Google Scholar] [CrossRef]
- Koh, A.R.; Hwang, B.; Roh, K.C.; Kim, K. The effect of the ionic size of small quaternary ammonium BF4 salts on electrochemical double layer capacitors. Phys. Chem. Chem. Phys. 2014, 16, 15146–15151. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, B.; Koo, Y.M.; Macfarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.; Dhariwal, J.; Saha, M.; Trivedi, S.; Banjare, M.K.; Kanaoujiya, R.; Behera, K. Ionic liquids: Environmentally sustainable materials for energy conversion and storage applications. Environ. Sci. Pollut. Res. 2023. [CrossRef] [PubMed]
- Wang, X.; Zhou, H.; Sheridan, E.; Walmsley, J.C.; Ren, D.; Chen, D. Geometrically confined favorable ion packing for high gravimetric capacitance in carbon-ionic liquid supercapacitors. Energy Environ. Sci. 2016, 9, 232–239. [Google Scholar] [CrossRef]
- Ali, E. Supercapacitors utilising ionic liquids. Energy Storage Mater. 2017, 9, 47–69. [Google Scholar]
- Lethesh, K.C.; Bamgbopa, M.O.; Susantyoko, R.A. Prospects and design insights of neat ionic liquids as supercapacitor electrolytes. Front. Energy Res. 2021, 9, 741772. [Google Scholar] [CrossRef]
- Pohlmann, S.; Olschläger, T.; Giidrich, P.; Jacquemin, J.A.V.J.; Balducci, A. Mixtures of azepanium based ionic liquids and propylene cabonates as high voltage electrolytes for supercapacitors. Electrochim. Acta 2015, 153, 426–432. [Google Scholar] [CrossRef]
- Kasprzak, D.; Stepniak, I.; Galinski, M. Acetate- and lactate-based ionic liquids: Synthesis, characterisation and electrochemical properties. J. Mol. Liq. 2018, 264, 233–241. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Lee, S.; Kwak, K.; Lee, K.K. Bis(oxalate)borate-containing electrolytes for high voltage electric double-layer capacitors: A comparative study. Electrochim. Acta 2019, 321, 134649. [Google Scholar] [CrossRef]
- Mishra, A.; Shetti, N.P.; Basu, S.; Reddy, K.R.; Aminabhavi, T.M. Chapter 7- Recent developments in ionic liquid-based electrolytes for energy storage supercapacitors and rechargeable batteries. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 199–221. [Google Scholar]
- Feng, J.; Wang, Y.; Xu, Y.; Sun, Y.; Tang, Y.; Yan, X. Ion regulation of ionic liquid electrolytes for supercapacitors. Energy Environ. Sci. 2021, 14, 2859. [Google Scholar] [CrossRef]
- Krummacher, J.; Schütter, C.; Hess, L.H.; Balducci, A. Non-aqueous electrolytes for electrochemical capacitors. Curr. Opin. Electrochem. 2018, 9, 64–69. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.D.; Hu, W.B.; Qiao, J.L.; Zhang, L.; Zhang, J.J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Shahzad, S.; Shah, A.; Kowsari, E.; Iftikhar, F.J.; Nawab, A.; Piro, B.; Akhter, M.S.; Rana, U.A.; Zou, Y. Ionic liquids as environmentally benign electrolytes for high-performance supercapacitors. Glob. Chall. 2019, 3, 1800023. [Google Scholar] [CrossRef]
- Pan, S.; Yao, M.; Zhang, J.; Li, B.; Xing, C.; Song, X.; Su, P.; Zhang, H. Recognition of ionic liquids as high-voltage electrolytes for supercapacitors. Front. Chem. 2020, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Stettner, T.; Balducci, A. Protic ionic liquids in energy storage devices: Past, present and future perspective. Energy Storage Mater. 2021, 40, 402–444. [Google Scholar] [CrossRef]
- Sun, L.; Zhuo, K.; Chen, Y.; Du, Q.; Zhang, S.; Wang, J. Ionic liquid-based redox active electrolytes for supercapacitors. Adv. Funct. Mater. 2022, 32, 2203611. [Google Scholar] [CrossRef]
- Zhou, T.; Gui, C.; Sun, L.; Hu, Y.; Lyu, H.; Wang, Z.; Song, Z.; Yu, G. Energy application of ionic liquids: Recent developments and future prospects. Chem. Rev. 2023, 123, 12170–12253. [Google Scholar] [CrossRef]
- Bittner, A.M.; Zhu, M.; Yang, Y.; Waibel, H.F.; Konuma, M.; Starke, U.; Weber, C.J. Ageing of electrochemical double layer capacitors. J. Power Sources 2012, 203, 262–273. [Google Scholar] [CrossRef]
- Kurzweil, P.; Schottenbauer, J.; Schell, C. Past, present and future of electrochemical capacitors: Pseudocapacitance, aging mechanisms and service life estimation. J. Energy Storage 2021, 35, 102311. [Google Scholar] [CrossRef]
- Zheng, J.P. The limitations of energy density of battery/double-layer capacitor asymmetric cells. J. Electrochem. Soc. 2003, 150, A484–A492. [Google Scholar] [CrossRef]
- Köps, L.; Kreth, F.A.; Bothe, A.; Balducci, A. High voltage electrochemical capacitors operating at elevated temperature besed on 1,1-dimethylpyrrolidinium tetrafluoroborate. Energy Storage Mater. 2022, 44, 66–72. [Google Scholar] [CrossRef]
- Pohlmann, S.; Balducci, A. A new conducting salt for high voltage propylene carbonated-based electrochemical double layer capacitors. Electrochim. Acta 2013, 110, 221–227. [Google Scholar] [CrossRef]
- Palm, R.; Kurig, H.; Tõnurist, K.; Jänes, A.; Lust, E. Influence of different organic solvent additives on 1-ethyl-3-methylimidazolium tetrafluoroborate electrolyte based electrical double layer capacitors. J. Electrochem. Soc. 2013, 160, A1741–A1745. [Google Scholar] [CrossRef]
- Scalia, A.; Varzi, A.; Moretti, A.; Ruschaupt, P.; Lamberti, A.; Tress, E.; Passerini, S. Electrolytes based on N-butyl-N-methyl-pyrrolidium 4,5-dicyano-2-(trifluoromethyl) imidazole for high voltage electrochemical double layer capacitors. ChemElectroChem. 2019, 6, 552–557. [Google Scholar] [CrossRef]
- Brandt, A.; Balducci, A. Theoretical and practical energy limitations of organic and ionic liquid-based electrolytes for high voltage electrochemical double layer capacitors. J. Power Sources 2014, 250, 343–351. [Google Scholar] [CrossRef]
- Schütter, C.; Husch, T.; Korth, M.; Balducci, A. Toward new solvents for EDLCs: From computational screening to electrochemical validation. J. Phys. Chem. C 2015, 119, 13413–13424. [Google Scholar] [CrossRef]
- Chiba, K.; Ueda, T.; Yamaguchi, Y.; Oki, Y.; Shimodate, F.; Naoi, K. Electrolyte systems for high withstand voltage and durability I. linear sulfones for electric double-layer capacitors. J. Electrochem. Soc. 2011, 158, A872. [Google Scholar] [CrossRef]
- Senoh, H.; Sakaebe, H.; Sano, H.; Yao, M.; Kuratani, K.; Takeichi, N.; Kiyobayashi, T. Sulfone-based electrolyte solutions for rechargeable magnesium batteries using 2,5-dimethoxy-1,4-benzoquinone positive electrode. J. Electrochem. Soc. 2014, 161, A1315. [Google Scholar] [CrossRef]
- Köps, L.; Kreth, F.A.; Leitenschneider, D.; Schutjajew, K.; Glä, R.; Oschatz, M.; Balducci, A. Improving the stability of supercapacitors at high voltages and high temperature by implementation of ethyl isopropyl sulfone as electrolyte solvent. Adv. Energy Mater. 2023, 13, 2203821. [Google Scholar] [CrossRef]
- Chiba, K.; Ueda, T.; Yamaguchi, Y.; Oki, Y.; Saiki, F.; Naoi, K. Electrolyte systems for high withstand voltage and durability II: Alkylated cyclic carbonates for electric double-layer capacitors. J. Electrochem. Soc. 2011, 158, A1320–A1327. [Google Scholar] [CrossRef]
- Hess, L.H.; Balducci, A. 1,2-butylene carbonate as solvent for EDLCs. Electrochimica Acta 2018, 281, 437–444. [Google Scholar] [CrossRef]
- Rumble, J.R.; David, R.L.; Thomas, J.B. CRC Handbook of Chemistry and Physics: A Ready Reference Book of Chemical and Physical Data, 99th ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Nguyen, H.V.T.; Faheem, A.B.; Kwak, K.; Lee, K. Propionitrile as a single organic solvent for high voltage electric double-layer capacitors. J. Power Sources 2020, 463, 228134. [Google Scholar] [CrossRef]
- Bothe, A.; Pourhosseini, S.E.M.; Ratajczak, P.; Beguin, F.; Balducci, A. Towards understanding the impact of operating voltage on the stability of adiponitrile-based electrical double-layer capacitors. J. Power Sources 2021, 496, 229841. [Google Scholar] [CrossRef]
- Tabarov, F.S.; Galimzyanov, R.R.; Krechetov, I.S.; Kalashnik, A.T.; Galimzyanov, T.R.; Boboev, I.R.; Lisitsin, A.V.; Stakhanova, S.V. Vinylene carbonate, toluene and diethyl ether as electrolyte additives for a wide-temperature range operating of EDLCs. J. Power Sources 2023, 560, 232658. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Qi, Y.; Zhang, C.; Huang, Y.; Yan, J.; Cen, K.; Xiong, G.; Bo, Z.; Ostrikov, K. A strong-weak binary solvation structure for unimpeded low-temperature ion transport in nanoporous energy storage materials. J. Mater. Chem. A 2023, 11, 16995–18006. [Google Scholar] [CrossRef]
- Lang, J.; Zhang, X.; Liu, L.; Yang, B.; Yang, J.; Yan, X. Highly enhanced energy density of supercapacitors at extremely low temperatures. J. Power Sources 2019, 423, 271–279. [Google Scholar] [CrossRef]
- Yao, J.; Shi, M.; Li, W.; Han, Q.; Wu, M.; Yang, W.; Wang, E.; Lu, X. Fluorinated ether-based electrolyte for supercapacitors with increased working voltage and suppressed self-discharge. ChemElectroChem 2022, 9, e202200223. [Google Scholar] [CrossRef]
- Lu, H.; He, L.; Li, X.; Zhang, W.; Che, J.; Liu, X.; Hou, Z.; Du, H.; Qu, Y. Ionic liquid-solvent mixture of propylene carbonate and 1,2-dimethoxyethane as electrolyte for electric double-layer capacitor. J. Mater. Sci. Mater. Electron. 2019, 30, 13933–13938. [Google Scholar] [CrossRef]
- Nambu, N.; Kobayashi, D.; Sasaki, Y. Physical and electrolytic properties of different cyclic carbonates as solvents for electric double-layer capacitors. Electrochemistry 2013, 81, 814–816. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Wang, C.; Shi, Z. A novel electrolyte used in high working voltage application for electrical double-layer capacitor using spiro-(1,1′)-bipyrridinium tetrafluoroborate in mixtures solvents. Electrochim. Acta 2015, 182, 1166–1174. [Google Scholar] [CrossRef]
- Jänes, A.; Thomberg, T.; Eskusson, J.; Lust, E. Fluoroethylene carbonate as co-solvent for propylene carbonate based electrical double layer capacitors. J. Electrochem. Soc. 2013, 160, A1025. [Google Scholar] [CrossRef]
- Vatamanu, J.; Borodin, O.; Olguin, M.; Yushin, G.; Bedrov, D. Charge storage at the nanoscale: Understanding the trends from the molecular scale perspective. J. Mater. Chem. A 2017, 5, 21049–21076. [Google Scholar] [CrossRef]
- Azmi, S.; Klimek, A.; Frackowiak, E. Why electrochemical capacitor electrolytes should not be ignored? Electrochim. Acta 2023, 452, 142347. [Google Scholar] [CrossRef]
- Murayama, I.; Yoshimoto, N.; Egashira, M.; Morita, M.; Kobayashi, Y.; Ishiikawa, M. Characteristics of electric double layer capacitors with an ionic liquid electrolyte containing Li ion. Electrochemistry 2005, 73, 600–602. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tanaka, R.; Ishikawa, M. Beneficial effects of a Li salt on electrode behavior in an ionic liquid for electric double layer capacitors. Electrochemistry 2007, 75, 589–591. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Li, H.; Feng, R.; He, L.; Lu, H. Electrochemical double-layer capacitor containing mixtures of ionic liquid, lithium salt, and organic solvent as an electrolyte. Front. Mater. 2021, 8, 633460. [Google Scholar] [CrossRef]
- Krause, F.C.; Jones, J.; Smart, M.C.; Chin, K.B.; Brandon, E.J. Screening electrolytes designed for high voltage electrochemical capacitors. Electrochim. Acta 2021, 374, 137898. [Google Scholar] [CrossRef]
- Kim, M.K.K.; Kim, S. Conducting and interface characterization of carbonate-type organic electrolyte containing EMImBF4 as an additive against activated carbon electrode. Carbon Lett. 2015, 16, 51–56. [Google Scholar] [CrossRef]
- Kwon, H.; Jang, S.; Kang, Y.; Roh, K. The effect of ILs as co-salts in electrolytes for high voltage supercapacitors. Sci. Rep. 2019, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Zhang, X.; Huang, Z.; Huang, Y.; Yan, J.; Cen, K.; Yang, H. Binary ionic liquids hybrid electrolyte based supercapacitors with high energy & power density. RSC Adv. 2023, 13, 15762. [Google Scholar] [PubMed]
- Dou, Q.; Lian, C.; Lei, S.; Chen, J.; Liu, H.; Yan, X. Silica-grafted ionic liquid for maximizing the operational voltage of electrical double-layer capacitors. Energy Storage Mater. 2019, 18, 253–259. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, K.; Zhang, X.; Zhang, X.; Ma, P.; Yang, B.; Xu, S.; Lang, J. High-voltage electrochemical double layer capacitors enabled by polymeric ionic liquid. Electrochim. Acta 2023, 441, 141829. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, X.; Xie, Y.; Wang, Z.; Huang, J.; Qu, Y.; Mu, D.; Zhang, X.; Yang, W.; Zhang, H. Additive engineering enables ionic-liquid electrolyte-based supercapacitors to deliver simultaneously high energy and power density. ACS Sustain. Chem. Eng. 2023, 11, 5685–5695. [Google Scholar] [CrossRef]



| Types | Electrolytes | Operating Voltage (V) | References |
|---|---|---|---|
| Aqueous electrolyte | 6M KOH,6M NaOH, 1M HCl, 0.5M H2SO4 | 0.9 | [33] |
| 1M NaCl, 1M KCl, 0.5M Na2SO4, 0.5M K2SO4 | 1.7 | [34] | |
| 0.5M H2SO4 | 1.2 | [34] | |
| LiCl (1M, 10M, 20M) | 1.4, 1.5, 2.35 | [34] | |
| KCH3COO | 2.0 | [38] | |
| WIS (21M LiTFSI) | 2.2 | [35] | |
| WIS (21M LiTFSI) | 2.6 | [36] | |
| WIS (31.3M LiTFSI) | 2.4 | [37] | |
| Solvent-free ILs | EMImBF4 | 3.5 | [41] |
| EMImFSI | 2.0 | [42] | |
| EMImTFSI | 3.0 | [43] | |
| Pyr14TFSI | 3.7 | [44] | |
| MPImFSI | 3.5 | [45] | |
| MPPyrFSI | 3.5 | [45] | |
| MeoMPyrFSI | 3.5 | [45] | |
| Pip14B(CN)4 | 3.7 | [46] | |
| Pyr14B(CN)4 | 3.7 | [46] | |
| Mixed ILs | EMImTFSI + EMImBF4 | 3.5 | [47] |
| Pyr14FSI + PIP13FSI | 3.7 | [48] | |
| EMImBF4 + TMABF4 | 3.5 | [41] | |
| EMImTFSI + MPPyrTFSI | 3.5 | [49] | |
| IL–solvent | Pyr14TFSI/AN | 3.1 | [50] |
| Pyr14BF4/AN | 3.1 | [50] | |
| Pyr14BF4/PC | 3.2 | [51] | |
| TEABF4/AN or PC | 2.5–2.8 | [52] | |
| SBPBF4/AN or PC | 2.8–3.2 | [53] | |
| (TEMABF4; DEDMABF4; TMPABF4; TMEABF4)/AN | 3.0 | [54] |
| Tuning Methods | Electrolytes | Voltage (V) | Ref. | Challenges |
|---|---|---|---|---|
| High salt concentration | 2M Pyr11BF4/AN | 3.4 | [75] | High viscosity and high cost limit the commercial application |
| 2.3M Pyr14TFSI/PC | 3.5 | [51] | ||
| EMIMBF4/AN or PC or γ-butyrolactone (0.005–1.0 M solvent) | 3.2 | [77] | ||
| High stable solvent | TEABF4/EiPS | 3.4 | [83] | Accompanied by a reduction in power output |
| SBPBF4/2,3-BC | 3.5 | [84] | ||
| Pyr14BF4/1,2-BC | 3.15 | [85] | ||
| SBPBF4/PN | 3.5 | [87] | ||
| DMPBF4/PN | 3.3 | [87] | ||
| Pyr14BF4/ADN | 3.4 | [88] | ||
| Co-solvent | TEMABF4/EiPS/AN | 3.0 | [53] | The increase of voltage window is limited |
| EMImBF4/MA/AN | 3.5 | [91] | ||
| TEABF4/TTE/AN | 3.6 | [92] | ||
| SBPBF4/DME/PC | 3.2 | [95] | ||
| (C2H5)3CH3NBF4/FEC/PC | 3.0 | [96] | ||
| Solute salt additive | EMImBF4/LiPF6/PC/DMC | 3.0 | [101] | The designing and selecting suitable additives lack systematic guidance |
| SBPBF4/LiDFOB/AN | 4.0 | [102] | ||
| TEABF4/EMImBF4/PC | 3.7 | [104] | ||
| Pyr14TFSI/TMPATFSI/AN | 3.1 | [105] | ||
| EMImTFSI/SiO2-IL/PC | 3.2 | [106] | ||
| EMImTFSI/PVEImTFSI/PC | 3.2 | [107] | ||
| EMImBF4/Ti3C2CTAB/PC | 3.0 | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xue, K.; Yan, C.; Li, Y.; Zhang, X.; Su, K.; Ma, P.; Wan, S.; Lang, J. Tuning of Ionic Liquid–Solvent Electrolytes for High-Voltage Electrochemical Double Layer Capacitors: A Review. Batteries 2024, 10, 54. https://doi.org/10.3390/batteries10020054
Wang Y, Xue K, Yan C, Li Y, Zhang X, Su K, Ma P, Wan S, Lang J. Tuning of Ionic Liquid–Solvent Electrolytes for High-Voltage Electrochemical Double Layer Capacitors: A Review. Batteries. 2024; 10(2):54. https://doi.org/10.3390/batteries10020054
Chicago/Turabian StyleWang, Yan, Kaiyuan Xue, Changzeng Yan, Yuehui Li, Xingyun Zhang, Kailimai Su, Pengjun Ma, Shanhong Wan, and Junwei Lang. 2024. "Tuning of Ionic Liquid–Solvent Electrolytes for High-Voltage Electrochemical Double Layer Capacitors: A Review" Batteries 10, no. 2: 54. https://doi.org/10.3390/batteries10020054
APA StyleWang, Y., Xue, K., Yan, C., Li, Y., Zhang, X., Su, K., Ma, P., Wan, S., & Lang, J. (2024). Tuning of Ionic Liquid–Solvent Electrolytes for High-Voltage Electrochemical Double Layer Capacitors: A Review. Batteries, 10(2), 54. https://doi.org/10.3390/batteries10020054






