N-Doped Graphene (N-G)/MOF(ZIF-8)-Based/Derived Materials for Electrochemical Energy Applications: Synthesis, Characteristics, and Functionality
Abstract
1. Introduction
2. N-G/ZIF-8-Based/Derived Materials as Battery Electrodes
2.1. Synthesis Trends and Characteristics of N-G/ZIF-8-Based/Derived Battery Electrodes
2.2. Performance and Functionalities of N-G/ZIF-8-Based/Derived Battery Electrodes
3. N-G/ZIF-8-Based/Derived Materials for Electrochemical Capacitors
3.1. Synthesis Trends and Characteristics of N-G/ZIF-8-Based/Derived Electrode Materials for Electrochemical Capacitors
3.2. Performance and Functionalities of N-G/ZIF-8-Based/Derived Electrode Materials for Electrochemical Capacitors
4. N-G/ZIF-8Based/Derived Materials as Electrocatalysts
4.1. Synthesis Trends and Characteristics of N-G/ZIF-8-Based/Derived Electrocatalysts
4.2. Performance and Functionalities of N-G/ZIF-8-Based/Derived Electrocatalyst
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedman, J.S.; Girdhar, A.; Gelfand, R.M.; Memik, G.; Mohseni, H.; Taflove, A.; Wessels, B.W.; Leburton, J.-P.; Sahakian, A. V Cascaded Spintronic Logic with Low-Dimensional Carbon. Nat. Commun. 2017, 8, 15635. [Google Scholar] [CrossRef]
- Senthil, T.; Divakaran, N.; Kale, M.B.; Mubarak, S.; Dhamodharan, D.; Wu, L.; Bensingh, R.J.; Kader, M.A.; Dutta, K. Chapter 2—Low-Dimensional Carbon-Based Nanomaterials for Energy Conversion and Storage Applications. In Nanostructured, Functional, and Flexible Materials for Energy Conversion and Storage Systems; Pandikumar, A., Rameshkumar, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–68. ISBN 978-0-12-819552-9. [Google Scholar]
- Castro, K.P.R.; Colombo, R.N.P.; Iost, R.M.; da Silva, B.G.R.; Crespilho, F.N. Low-Dimensionality Carbon-Based Biosensors: The New Era of Emerging Technologies in Bioanalytical Chemistry. Anal. Bioanal. Chem. 2023, 415, 3879–3895. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, M.; Fu, X.; Gao, J.; Huang, C.; Li, Y. Research of Low-Dimensional Carbon-Based Magnetic Materials. ACS Appl. Electron. Mater. 2022, 4, 3263–3277. [Google Scholar] [CrossRef]
- Das, C.M.; Kang, L.; Ouyang, Q.; Yong, K.-T. Advanced Low-Dimensional Carbon Materials for Flexible Devices. InfoMat 2020, 2, 698–714. [Google Scholar] [CrossRef]
- Wang, Q.H.; Bellisario, D.O.; Drahushuk, L.W.; Jain, R.M.; Kruss, S.; Landry, M.P.; Mahajan, S.G.; Shimizu, S.F.E.; Ulissi, Z.W.; Strano, M.S. Low Dimensional Carbon Materials for Applications in Mass and Energy Transport. Chem. Mater. 2014, 26, 172–183. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yu, J.; Li, B.; Ding, B. Multi-Functional Flexible 2D Carbon Nanostructured Networks. Nat. Commun. 2020, 11, 5134. [Google Scholar] [CrossRef]
- Meunier, V.; Souza Filho, A.G.; Barros, E.B.; Dresselhaus, M.S. Physical Properties of Low-Dimensional ${sp}^{2}$-Based Carbon Nanostructures. Rev. Mod. Phys. 2016, 88, 25005. [Google Scholar] [CrossRef]
- Eaton, A.L.; Fielder, M.; Nair, A.K. Mechanical and Thermal Properties of Carbon-Based Low-Dimensional Materials. MRS Bull. 2022, 47, 1001–1010. [Google Scholar] [CrossRef]
- Bouzid, A.-H.; Das, S.K. High Temperature Aged Leakage Relaxation Screening Tests on Confined Flexible Graphite Gaskets. In Proceedings of the ASME 2021 Pressure Vessels and Piping Conference, Virtual, 13–15 July 2021. [Google Scholar]
- Ali, A.; Liang, F.; Zhu, J.; Shen, P.K. The Role of Graphene in Rechargeable Lithium Batteries: Synthesis, Functionalisation, and Perspectives. Nano Mater. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Chen, X.; Tian, Y. Review of Graphene in Cathode Materials for Lithium-Ion Batteries. Energy Fuels 2021, 35, 3572–3580. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Kaner, R.B. Scalable Fabrication of High-Power Graphene Micro-Supercapacitors for Flexible and on-Chip Energy Storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Ryu, Y.K.; Boscá, A.; Ladrón-de-Guevara, A.; Hunt, E.; Zuo, J.; Pedrós, J.; Calle, F.; Martinez, J. Recent Trends in Graphene Supercapacitors: From Large Area to Microsupercapacitors. Sustain. Energy Fuels 2021, 5, 1235–1254. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Zhuang, S.; Ingis, B.; Nunna, B.B.; Lee, E.S. Carbon-Based Catalysts for Oxygen Reduction Reaction: A Review on Degradation Mechanisms. Carbon 2019, 151, 160–174. [Google Scholar] [CrossRef]
- Talukder, N.; Wang, Y.; Nunna, B.B.; Lee, E.S. Nitrogen-Doped Graphene Nanomaterials for Electrochemical Catalysis/Reactions: A Review on Chemical Structures and Stability. Carbon 2021, 185, 198–214. [Google Scholar] [CrossRef]
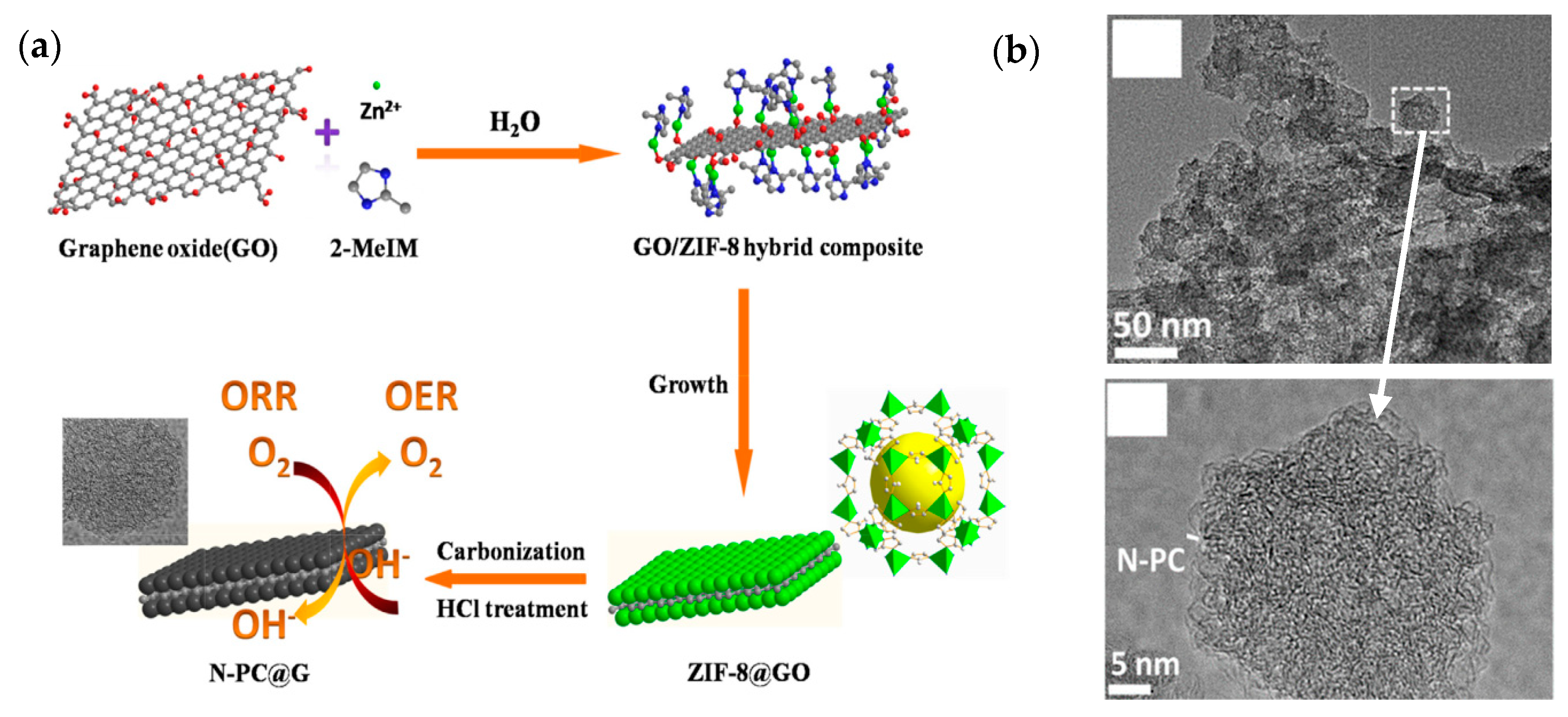
- Deokar, G.; Jin, J.; Schwingenschlögl, U.; Costa, P.M.F.J. Chemical Vapor Deposition-Grown Nitrogen-Doped Graphene’s Synthesis, Characterization and Applications. npj 2D Mater. Appl. 2022, 6, 14. [Google Scholar] [CrossRef]
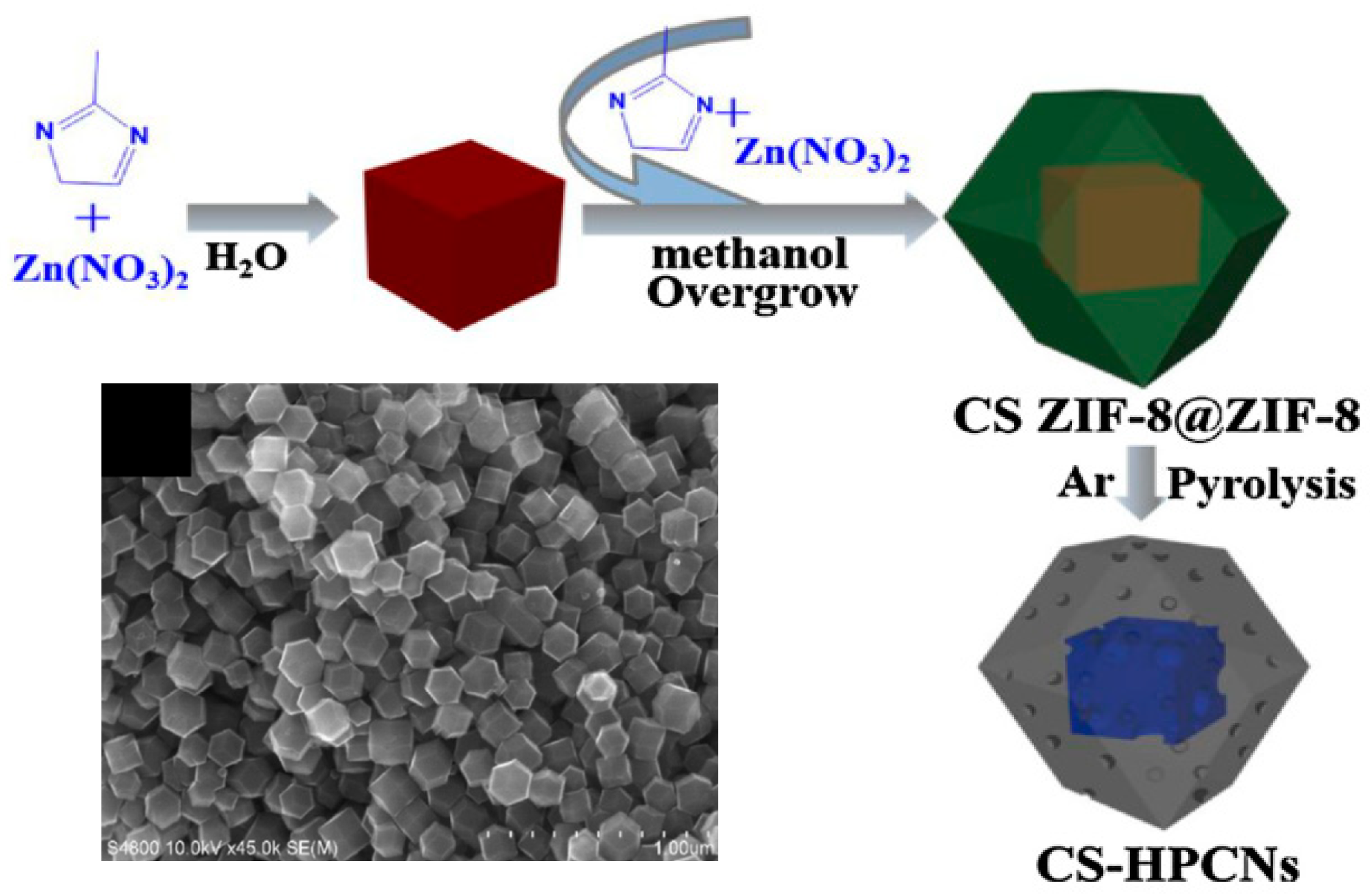
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-Doped Graphene and Its Electrochemical Applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S.; Cui, C.; Qian, W.; Jin, Y. Comprehensive Review on Nitrogen-Doped Graphene: Structure Characterization, Growth Strategy, and Capacitive Energy Storage. Energy Fuels 2023, 37, 902–918. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L.; Jin, Z. Nitrogen-Doped Graphene: Synthesis, Characterizations and Energy Applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef]
- Ullah, S.; Shi, Q.; Zhou, J.; Yang, X.; Ta, H.Q.; Hasan, M.; Ahmad, N.M.; Fu, L.; Bachmatiuk, A.; Rümmeli, M.H. Advances and Trends in Chemically Doped Graphene. Adv. Mater. Interfaces 2020, 7, 2000999. [Google Scholar] [CrossRef]
- Zhuang, S.; Nunna, B.B.; Mandal, D.; Lee, E.S. A Review of Nitrogen-Doped Graphene Catalysts for Proton Exchange Membrane Fuel Cells-Synthesis, Characterization, and Improvement. Nano-Struct. Nano-Objects 2018, 15, 140–152. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Qu, H.; Huang, L.; Han, Z.; Wang, Y.; Zhang, Z.; Wang, Y.; Chang, Q.; Wei, N.; Kipper, M.J.; Tang, J. A Review of Graphene-Oxide/Metal–Organic Framework Composites Materials: Characteristics, Preparation and Applications. J. Porous Mater. 2021, 28, 1837–1865. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, Y.; Uliana, A.; Zhu, J.; Liu, J.; der Bruggen, B. Zeolitic Imidazolate Framework/Graphene Oxide Hybrid Nanosheets Functionalized Thin Film Nanocomposite Membrane for Enhanced Antimicrobial Performance. ACS Appl. Mater. Interfaces 2016, 8, 25508–25519. [Google Scholar] [CrossRef]
- Wang, K.; Hui, K.N.; San Hui, K.; Peng, S.; Xu, Y. Recent Progress in Metal–Organic Framework/Graphene-Derived Materials for Energy Storage and Conversion: Design, Preparation, and Application. Chem. Sci. 2021, 12, 5737–5766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent Advances and Challenges of Metal–Organic Framework/Graphene-Based Composites. Compos. B Eng. 2022, 230, 109532. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-Organic Frameworks/Graphene-Based Materials: Preparations and Applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, D.; Wang, C.; You, B.; Li, B.; Han, J.; Jiang, S.; Zhang, C.; He, S. Zeolitic Imidazolate Framework-67 and Its Derivatives for Photocatalytic Applications. Coord. Chem. Rev. 2024, 502, 215612. [Google Scholar] [CrossRef]
- Talukder, N.; Wang, Y.; Nunna, B.B.; Tong, X.; Lee, E.S. An Investigation on the Structural Stability of ZIF-8 in Water versus Water-Derived Oxidative Species in Aqueous Environment. Microporous Mesoporous Mater. 2024, 366, 112934. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Lalonde, M.B.; Bury, W.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T. Opening ZIF-8: A Catalytically Active Zeolitic Imidazolate Framework of Sodalite Topology with Unsubstituted Linkers. J. Am. Chem. Soc. 2012, 134, 18790–18796. [Google Scholar] [CrossRef]
- Xue, W.; Zhou, Q.; Li, F.; Ondon, B.S. Zeolitic Imidazolate Framework-8 (ZIF-8) as Robust Catalyst for Oxygen Reduction Reaction in Microbial Fuel Cells. J. Power Sources 2019, 423, 9–17. [Google Scholar] [CrossRef]
- Tian, K.; Wang, J.; Cao, L.; Yang, W.; Guo, W.; Liu, S.; Li, W.; Wang, F.; Li, X.; Xu, Z.; et al. Single-Site Pyrrolic-Nitrogen-Doped Sp 2-Hybridized Carbon Materials and Their Pseudocapacitance. Nat. Commun. 2020, 11, 3884. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Zhao, Q.; Zhang, X.; Liu, R.; Ge, X.; Wang, G.; Zhao, H.; Cai, W. Metal-Organic Framework Derived Nitrogen-Doped Porous Carbon@graphene Sandwich-like Structured Composites as Bifunctional Electrocatalysts for Oxygen Reduction and Evolution Reactions. Carbon 2016, 106, 74–83. [Google Scholar] [CrossRef]
- Cao, P.; Liu, Y.; Quan, X.; Zhao, J.; Chen, S.; Yu, H. Nitrogen-Doped Hierarchically Porous Carbon Nanopolyhedras Derived from Core-Shell ZIF-8@ZIF-8 Single Crystals for Enhanced Oxygen Reduction Reaction. Catal. Today 2019, 327, 366–373. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Y.; Liang, Y.; Kong, B.; Zhang, J.; Song, J.; Bao, Q.; Simon, G.P.; Jiang, S.P.; Wang, H. Nitrogen-Doped Nanoporous Carbon/Graphene Nano-Sandwiches: Synthesis and Application for Efficient Oxygen Reduction. Adv. Funct. Mater. 2015, 25, 5768–5777. [Google Scholar] [CrossRef]
- Zhuang, S.; Nunna, B.B.; Lee, E.S. Metal Organic Framework-Modified Nitrogen-Doped Graphene Oxygen Reduction Reaction Catalyst Synthesized by Nanoscale High-Energy Wet Ball-Milling Structural and Electrochemical Characterization. MRS Commun. 2018, 8, 40–48. [Google Scholar] [CrossRef]
- Deguenon, L.; Yamegueu, D.; Moussa kadri, S.; Gomna, A. Overcoming the Challenges of Integrating Variable Renewable Energy to the Grid: A Comprehensive Review of Electrochemical Battery Storage Systems. J. Power Sources 2023, 580, 233343. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, D.; Lee, M.; Nam, K.W. Challenges and Possibilities for Aqueous Battery Systems. Commun. Mater. 2023, 4, 37. [Google Scholar] [CrossRef]
- Tanim, T.R.; Dufek, E.J.; Sazhin, S. V Challenges and Needs for System-Level Electrochemical Lithium-Ion Battery Management and Diagnostics. MRS Bull. 2021, 46, 420–428. [Google Scholar] [CrossRef]
- He, J.; Manthiram, A. A Review on the Status and Challenges of Electrocatalysts in Lithium-Sulfur Batteries. Energy Storage Mater. 2019, 20, 55–70. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Guo, Z. Challenges and Future Perspectives on Sodium and Potassium Ion Batteries for Grid-Scale Energy Storage. Mater. Today 2021, 50, 400–417. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Jun, S.C. Challenges and Strategies toward Cathode Materials for Rechargeable Potassium-Ion Batteries. Adv. Mater. 2021, 33, 2004689. [Google Scholar] [CrossRef]
- Deshmukh, A.; Thripuranthaka, M.; Chaturvedi, V.; Das, A.K.; Shelke, V.; Shelke, M.V. A Review on Recent Advancements in Solid State Lithium–Sulfur Batteries: Fundamentals, Challenges, and Perspectives. Prog. Energy 2022, 4, 42001. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Martín-Jimeno, F.J.; Suárez-García, F.; Paredes, J.I.; Enterría, M.; Pereira, M.F.R.; Martins, J.I.; Figueiredo, J.L.; Martínez-Alonso, A.; Tascón, J.M.D. A “Nanopore Lithography” Strategy for Synthesizing Hierarchically Micro/Mesoporous Carbons from ZIF-8/Graphene Oxide Hybrids for Electrochemical Energy Storage. ACS Appl. Mater. Interfaces 2017, 9, 44740–44755. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Fan, Z.; Lin, Q.; Wang, J.; Chang, Z.; Li, T.; Henzie, J.; Kim, J.; Dou, H.; Zhang, X.; et al. Confined Pyrolysis of ZIF-8 Polyhedrons Wrapped with Graphene Oxide Nanosheets to Prepare 3D Porous Carbon Heterostructures. Small Methods 2019, 3, 1900277. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Zhao, J.; Zheng, J.; Wang, J.; Huang, J. A Facile In-situ Synthesis of ZIF-8 Nanoparticles Anchored on Reduced Graphene Oxide as a Sulfur Host for Li-S Batteries. Mater. Res. Bull. 2021, 133, 111061. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, Q.; Gong, J.; Chen, Z.; Wu, Z.; Li, S.; Zheng, Q.; Wu, X.; Lam, K.; Lin, D. Multilevel Spatial Confinement of Transition Metal Selenides Porous Microcubes for Efficient and Stable Potassium Storage. J. Colloid Interface Sci. 2023, 644, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, C.; Xu, G.; Cheng, F. Synergetic-Modified Porous Co2SiO4 Conductive Network with ZIF-8 Derived Carbon and Reduced Graphene Oxide for High-Rate Lithium-Ion Battery Anode. J. Electroanal. Chem. 2023, 941, 117530. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Electrochemical Capacitors: Performance Metrics and Evaluation by Testing and Analysis. Adv. Energy Mater. 2021, 11, 2002192. [Google Scholar] [CrossRef]
- He, X.; Zhang, X. A Comprehensive Review of Supercapacitors: Properties, Electrodes, Electrolytes and Thermal Management Systems Based on Phase Change Materials. J. Energy Storage 2022, 56, 106023. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical Capacitors: Mechanism, Materials, Systems, Characterization and Applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilp, W.; Hu, M.; Wang, H.; Huang, H.-S.; Fujita, T.; Wu, K.C.-W.; Chen, L.-C.; Yamauchi, Y.; Ariga, K. Nanoporous Carbons through Direct Carbonization of a Zeolitic Imidazolate Framework for Supercapacitor Electrodes. Chem. Commun. 2012, 48, 7259–7261. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, C.; Zhao, Y.; Song, L.; Zhang, J.; Huang, R.; Qu, L. Decoration of Graphene Network with Metal–Organic Frameworks for Enhanced Electrochemical Capacitive Behavior. Carbon 2014, 78, 231–242. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wang, H.; Jiao, X.; Ouyang, Y.; Xia, X.; Lei, W.; Hao, Q. ZIF-8 Nanocrystals Derived N-Doped Carbon Decorated Graphene Sheets for Symmetric Supercapacitors. Electrochim. Acta 2018, 289, 494–502. [Google Scholar] [CrossRef]
- Khakpour, F.; Ghafouri Taleghani, H.; Soleimani Lashkenari, M. Metal–Organic Framework/Sulfur-Doped Graphene Oxide Nanocomposite for High Efficiency Electrochemical Double-Layer Capacitors. Appl. Organomet. Chem. 2023, 37, e6969. [Google Scholar] [CrossRef]
- Xiao, P.; Cao, L.; Wang, H.; Yan, G.; Chen, Q. Rational Design of Three-Dimensional Metal-Organic Framework-Derived Active Material/Graphene Aerogel Composite Electrodes for Alkaline Battery-Supercapacitor Hybrid Device. Surf. Interfaces 2022, 33, 102266. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, C.; Zhao, L.; Zhang, X.; Wang, J. Preparation of Porous Layered Cobalt-Zinc Sulfide Nanostructures Based on Graphene Oxide Supported ZIF-8 Template for High-Performance Supercapacitors. J. Solid. State Chem. 2022, 316, 123581. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Li, B.; Zhou, Y.; Gong, T.; Li, J. Preparation of Nitrogen-Doped Hierarchical Porous Carbon Electrodes for High Performance Capacitive Deionization. Ionics 2023, 29, 2935–2945. [Google Scholar] [CrossRef]
- Sun, Y.; Polani, S.; Luo, F.; Ott, S.; Strasser, P.; Dionigi, F. Advancements in Cathode Catalyst and Cathode Layer Design for Proton Exchange Membrane Fuel Cells. Nat. Commun. 2021, 12, 5984. [Google Scholar] [CrossRef]
- Shao, Y.; Dodelet, J.-P.; Wu, G.; Zelenay, P. PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges. Adv. Mater. 2019, 31, 1807615. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, S. Recent Advances in Cathode Catalyst Architecture for Lithium–Oxygen Batteries. eScience 2023, 3, 100123. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Xu, Q.; Niu, J.; Xia, Z. N-doped Graphene as Catalysts for Oxygen Reduction and Oxygen Evolution Reactions: Theoretical Considerations. J. Catal. 2014, 314, 66–72. [Google Scholar] [CrossRef]
- Zhuang, S.; Lei, L.; Nunna, B.; Lee, E.S. New Nitrogen-Doped Graphene/MOF-Modified Catalyst for Fuel Cell Systems. ECS Trans. 2016, 72, 149. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Zhuang, S.; Nunna, B.B.; Lei, L.; Lee, E.S. Synthesis of Nitrogen-Doped Graphene Catalyst by Wet Ball Milling for Electrochemical Systems. In Proceedings of the 251st ACS National Meeting & Exposition, San Diego, CA, USA, 13–17 March 2016. [Google Scholar]
- Lemes, G.; Sebastián, D.; Pastor, E.; Lázaro, M.J. N-doped Graphene Catalysts with High Nitrogen Concentration for the Oxygen Reduction Reaction. J. Power Sources 2019, 438, 227036. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, H.; Qu, J.; Dai, L. Nitrogen-Doped Graphene by Ball-Milling Graphite with Melamine for Energy Conversion and Storage. 2D Mater. 2015, 2, 44001. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Parvez, K.; Yang, S.; Hernandez, Y.; Winter, A.; Turchanin, A.; Feng, X.; Müllen, K. Nitrogen-Doped Graphene and Its Iron-Based Composite as Efficient Electrocatalysts for Oxygen Reduction Reaction. ACS Nano 2012, 6, 9541–9550. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, L.; Yadav, R.M.; Yang, Y.; Zhang, X.; Vajtai, R.; Lou, J.; Ajayan, P.M. Nitrogen-Doped Graphene with Pyridinic Dominance as a Highly Active and Stable Electrocatalyst for Oxygen Reduction. ACS Appl. Mater. Interfaces 2015, 7, 14763–14769. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A Review of Oxygen Reduction Mechanisms for Metal-Free Carbon-Based Electrocatalysts. npj Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Aijaz, A.; Fujiwara, N.; Xu, Q. From Metal–Organic Framework to Nitrogen-Decorated Nanoporous Carbons: High CO2 Uptake and Efficient Catalytic Oxygen Reduction. J. Am. Chem. Soc. 2014, 136, 6790–6793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, Z.; Jiang, F.; Yang, L.; Qian, J.; Zhou, Y.; Li, W.; Hong, M. Highly Graphitized Nitrogen-Doped Porous Carbon Nanopolyhedra Derived from ZIF-8 Nanocrystals as Efficient Electrocatalysts for Oxygen Reduction Reactions. Nanoscale 2014, 6, 6590–6602. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, J.; Zhang, Y.; Xu, W.; Xing, W.; Xu, D.; Zhang, Y.; Zhang, X. ZIF-8 Derived Graphene-Based Nitrogen-Doped Porous Carbon Sheets as Highly Efficient and Durable Oxygen Reduction Electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 14235–14239. [Google Scholar] [CrossRef]
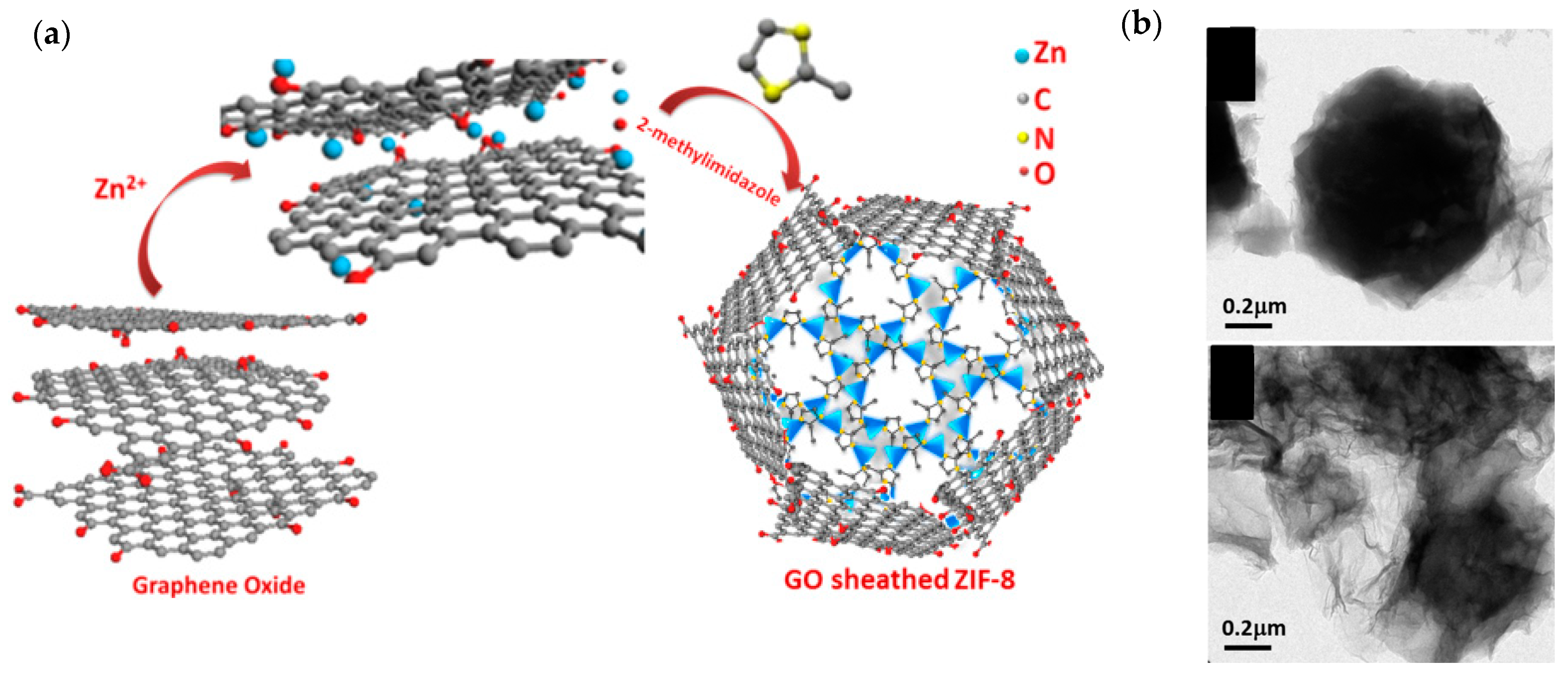
- Thomas, M.; Illathvalappil, R.; Kurungot, S.; Nair, B.N.; Mohamed, A.A.P.; Anilkumar, G.M.; Yamaguchi, T.; Hareesh, U.S. Graphene Oxide Sheathed ZIF-8 Microcrystals: Engineered Precursors of Nitrogen-Doped Porous Carbon for Efficient Oxygen Reduction Reaction (ORR) Electrocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 29373–29382. [Google Scholar] [CrossRef]
- Zhang, P.-Y.; Yang, X.-H.; Jiang, Q.-R.; Cui, P.-X.; Zhou, Z.-Y.; Sun, S.-H.; Wang, Y.-C.; Sun, S.-G. General Carbon-Supporting Strategy to Boost the Oxygen Reduction Activity of Zeolitic-Imidazolate-Framework-Derived Fe/N/Carbon Catalysts in Proton Exchange Membrane Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 30724–30734. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Wang, S.; Xuan, J.; Xiong, D.; Zhou, L.; Zhou, J.; Wang, J.; Yang, Y.; Du, Y. Hierarchical Porous Yolk-Shell Co-N-C Nanocatalysts Encaged Ingraphene Nanopockets for High-Performance Zn-Air Battery. Nano Res. 2023, 16, 8893–8901. [Google Scholar] [CrossRef]
- Yu, G.; Xia, J.; Zhang, F.; Wang, Z. Hierarchical and Hybrid RGO/ZIF-8 Nanocomposite as Electrochemical Sensor for Ultrasensitive Determination of Dopamine. J. Electroanal. Chem. 2017, 801, 496–502. [Google Scholar] [CrossRef]
- Yang, S.; Yang, M.; Yao, X.; Fa, H.; Wang, Y.; Hou, C. A Zeolitic Imidazolate Framework/Carbon Nanofiber Nanocomposite Based Electrochemical Sensor for Simultaneous Detection of Co-Existing Dihydroxybenzene Isomers. Sens. Actuators B Chem. 2020, 320, 128294. [Google Scholar] [CrossRef]
- Li, K.; Kang, J.; Zhan, T.; Cao, W.; Liu, X.; Gao, H.; Si, C.; She, X. Electrochemical Sensing Platform for Naphthol Isomers Based on in Situ Growth of ZIF-8 on Reduced Graphene Oxide by a Reaction-Diffusion Technique. J. Colloid Interface Sci. 2021, 581, 576–585. [Google Scholar] [CrossRef]
- Rezvani Jalal, N.; Madrakian, T.; Afkhami, A.; Ahmadi, M. Ni/Co Bimetallic Metal–Organic Frameworks on Nitrogen-Doped Graphene Oxide Nanoribbons for Electrochemical Sensing of Doxorubicin. ACS Appl. Nano Mater. 2022, 5, 11045–11058. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Li, Z.; Tao, H.; Qu, L.; Li, J.; Zhu, M.; Zha, Q. A Graphene Oxide/Zn-Metal Organic Framework Electrochemical Sensor for Acetaminophen Detection. Surf. Interfaces 2023, 39, 102910. [Google Scholar] [CrossRef]
- Sun, S.; Wang, B.; Qing, M.; Tian, D.; Jiang, L.; Zhu, L. Electrochemical Dopamine Detection Using Electrochemically Reduced Graphene Oxide/Silver/Metal Organic Framework Composites. ChemistrySelect 2023, 8, e202204720. [Google Scholar] [CrossRef]
- Liu, Q.; Xing, Y.; Pang, X.; Zhan, K.; Sun, Y.; Wang, N.; Hu, X. Electrochemical Immunosensor Based on MOF for Rapid Detection of 6-Benzyladenine in Bean Sprouts. J. Food Compos. Anal. 2023, 115, 105003. [Google Scholar] [CrossRef]
- Xie, J.; Yang, H.; Wang, X.; Gao, F. ZIF-8/Electro-Reduced Graphene Oxide Nanocomposite for Highly Electrocatalytic Oxidation of Hydrazine in Industrial Wastewater. Microchem. J. 2021, 168, 106521. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Erk, E.E.; Soylak, M.; Erk, N.; Karimi, F. Metal–Organic Framework Based Electrochemical Immunosensor for Label-Free Detection of Glial Fibrillary Acidic Protein as a Biomarker. Ind. Eng. Chem. Res. 2023, 62, 4532–4539. [Google Scholar] [CrossRef]
- Abdul Aziz, S.F.N.; Rahim, A.S.M.A.; Normi, Y.M.; Alang Ahmad, S.A.; Salleh, A.B. Rational Design of Mini Protein Mimicking Uricase: Encapsulation in ZIF-8 for Uric Acid Detection. Proteins Struct. Funct. Bioinform. 2023, 91, 967–979. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, Q.; Xiang, X.; Jiang, L.; Wang, S.; Duan, L.; Wang, S. Metal-Organic Framework Hybrid Materials of ZIF-8/RGO for Immobilization of D-Amino Acid Dehydrogenase. Nano Res. 2023, 17, 290–296. [Google Scholar] [CrossRef]
- Kim, D.W.; Eum, K.; Kim, H.; Kim, D.; de Mello, M.D.; Park, K.; Tsapatsis, M. Continuous ZIF-8/Reduced Graphene Oxide Nanocoating for Ultrafast Oil/Water Separation. Chem. Eng. J. 2019, 372, 509–515. [Google Scholar] [CrossRef]
- Shen, L.; Wang, X.; Zhang, Z.; Jin, X.; Jiang, M.; Zhang, J. Design and Fabrication of the Evolved Zeolitic Imidazolate Framework-Modified Polylactic Acid Nonwoven Fabric for Efficient Oil/Water Separation. ACS Appl. Mater. Interfaces 2021, 13, 14653–14661. [Google Scholar] [CrossRef] [PubMed]
- Kamali, Z.; Khashehchi, M.; Zarafshan, P.; Pipelzadeh, E. Enhanced Desalination Performance of Capacitive Deionization Using ZIF-8/Graphene Nanocomposite Electrode. Appl. Water Sci. 2020, 10, 239. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Yusop, M.Z.M.; Hamdan, R.; Aziz, F.; Awang, N.A.; Ismail, A.F. Synthesis of Zeolitic Imidazolate Framework-8 Modified Graphene Oxide Composite and Its Application for Lead Removal. J. Chem. Technol. Biotechnol. 2023, 98, 2668–2676. [Google Scholar] [CrossRef]
- Kim, D.; Kim, D.W.; Hong, W.G.; Coskun, A. Graphene/ZIF-8 Composites with Tunable Hierarchical Porosity and Electrical Conductivity. J. Mater. Chem. A 2016, 4, 7710–7717. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Misdan, N.; Nazri, N.A.M. Modified ZIF-8 Mixed Matrix Membrane for CO2/CH4 Separation. AIP Conf. Proc. 2017, 1891, 020091. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Su, P.; Xu, Z.; Zhang, G.; Shen, C.; Meng, Q. Metal–Organic Framework Channelled Graphene Composite Membranes for H2/CO2 Separation. J. Mater. Chem. A 2016, 4, 18747–18752. [Google Scholar] [CrossRef]
- Jiang, M.; Li, H.; Zhou, L.; Xing, R.; Zhang, J. Hierarchically Porous Graphene/ZIF-8 Hybrid Aerogel: Preparation, CO2 Uptake Capacity, and Mechanical Property. ACS Appl. Mater. Interfaces 2018, 10, 827–834. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Yin, Z.; Yang, Y.; Wang, Z. ZIF-8/GO Sandwich Composite Membranes through a Precursor Conversion Strategy for H2/CO2 Separation. J. Memb. Sci. 2022, 647, 120291. [Google Scholar] [CrossRef]
- Arifin, N.F.T.; Yusof, N.; Nordin, N.A.H.M.; Jaafar, J.; Ismail, A.F.; Salleh, W.N.W.; Aziz, F. An Improved Hybrid Nanocomposites of Rice Husk Derived Graphene (GRHA)/Zeolitic Imidazolate Framework-8 for Hydrogen Adsorption. Int. J. Hydrogen Energy 2021, 46, 24864–24876. [Google Scholar] [CrossRef]
- Lv, C.; Kang, W.; Liu, S.; Yang, P.; Nishina, Y.; Ge, S.; Bianco, A.; Ma, B. Growth of ZIF-8 Nanoparticles In Situ on Graphene Oxide Nanosheets: A Multifunctional Nanoplatform for Combined Ion-Interference and Photothermal Therapy. ACS Nano 2022, 16, 11428–11443. [Google Scholar] [CrossRef] [PubMed]
- Sanaei-Rad, S.; Ghasemzadeh, M.A.; Razavian, S.M.H. Synthesis of a Novel Ternary ZIF-8/GO/MgFe2O4 Nanocomposite and Its Application in Drug Delivery. Sci. Rep. 2021, 11, 18734. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Liu, W.; Zhu, H.; Chen, L.; Liao, H.; Chen, H. Click-Chemistry and Ionic Cross-Linking Induced Double Cross-Linking Ionogel Electrolyte for Flexible Lithium-Ion Batteries. J. Energy Storage 2023, 72, 108509. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Y.; Chen, Q. High Lithium Anodic Performance of Highly Nitrogen-Doped Porous Carbon Prepared from a Metal-Organic Framework. Nat. Commun. 2014, 5, 5261. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Shi, M.; Chong, S.; Chen, Y.; Shu, C.; Dai, X.; Tan, Q.; Liu, Y. N-Doped ZIF-8-Derived Carbon (NC-ZIF) as an Anodic Material for Lithium-Ion Batteries. J. Alloys Compd. 2019, 800, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Liang, W.; Li, S.; Zou, F.; Bhaway, S.M.; Qiang, Z.; Gao, M.; Vogt, B.D.; Zhu, Y. A Nitrogen Doped Carbonized Metal–Organic Framework for High Stability Room Temperature Sodium–Sulfur Batteries. J. Mater. Chem. A 2016, 4, 12471–12478. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Xing, Y.; Wang, S.; Yang, P.; Li, H. MOF-Derived, N-Doped Porous Carbon Coated Graphene Sheets as High-Performance Anodes for Lithium-Ion Batteries. New J. Chem. 2016, 40, 9679–9683. [Google Scholar] [CrossRef]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-Dimensional Hierarchically Porous Nitrogen-Doped Carbon from Water Hyacinth as Selenium Host for High-Performance Lithium–Selenium Batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Shiraishi, S. Chapter 27—Electric Double Layer Capacitors. In Carbon Alloys; Yasuda, E., Inagaki, M., Kaneko, K., Endo, M., Oya, A., Tanabe, Y., Eds.; Elsevier Science: Oxford, UK, 2003; pp. 447–457. ISBN 978-0-08-044163-4. [Google Scholar]
- Tao, Y.; Kanoh, H.; Groen, J.C.; Kaneko, K. Characterization of Alkaline Post-Treated ZSM-5 Zeolites by Low Temperature Nitrogen Adsorption. In Characterization of Porous Solids VII; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 279–286. [Google Scholar]
- Valyon, J.; Ötvös, Z.; Onyestyák, G.; Rees, L.V.C. The Sorption Dynamics of Propane, i-Butane and Neopentane in Carbon Nanotubes. In Characterization of Porous Solids VII; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 439–446. [Google Scholar]
- Devi, M.; Rawat, S.; Sharma, S. A Comprehensive Review of the Pyrolysis Process: From Carbon Nanomaterial Synthesis to Waste Treatment. Oxf. Open Mater. Sci. 2021, 1, itab014. [Google Scholar] [CrossRef]
- Velmurugan, V. Review of Research and Development on Pyrolysis Process. Mater. Today Proc. 2022, 49, 3679–3686. [Google Scholar] [CrossRef]
- Jiang, H.; Shao, J.; Zhu, Y.; Yu, J.; Cheng, W.; Yang, H.; Zhang, X.; Chen, H. Production Mechanism of High-Quality Carbon Black from High-Temperature Pyrolysis of Waste Tire. J. Hazard. Mater. 2023, 443, 130350. [Google Scholar] [CrossRef]
- Jäckel, N.; Simon, P.; Gogotsi, Y.; Presser, V. Increase in Capacitance by Subnanometer Pores in Carbon. ACS Energy Lett. 2016, 1, 1262–1265. [Google Scholar] [CrossRef]
- Sylla, N.F.; Ndiaye, N.M.; Ngom, B.D.; Momodu, D.; Madito, M.J.; Mutuma, B.K.; Manyala, N. Effect of Porosity Enhancing Agents on the Electrochemical Performance of High-Energy Ultracapacitor Electrodes Derived from Peanut Shell Waste. Sci. Rep. 2019, 9, 13673. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing Carbon Supercapacitor Electrodes with Hierarchical Porous Structures. J. Mater. Chem. A 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Yang, S.; Zhang, Z.; Liu, H.; Xiao, J.; Wan, L.; Luo, J.; Wang, S.; Liu, Y. Scalable Synthesis of Freestanding Sandwich-Structured Graphene/Polyaniline/Graphene Nanocomposite Paper for Flexible All-Solid-State Supercapacitor. Sci. Rep. 2015, 5, 9359. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhang, H.; Xing, T.; Chen, G. A Facile Approach to Prepare a Flexible Sandwich-Structured Supercapacitor with RGO-Coated Cotton Fabric as Electrodes. RSC Adv. 2019, 9, 4180–4189. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gong, Y.; Wang, M.; Pan, C. Preparation of Sandwich-like NiCo2O4/RGO/NiO Heterostructure on Nickel Foam for High-Performance Supercapacitor Electrodes. Nanomicro Lett. 2016, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Y.; Zhou, J.; Sun, D.; Li, H. Facile Self-Assembly of Sandwich-like MXene Layered Multiscale Structure Nanocomposite. 2D Mater. 2022, 10, 15014. [Google Scholar] [CrossRef]
- Li, Z.; Chen, G.; Deng, J.; Li, D.; Yan, T.; An, Z.; Shi, L.; Zhang, D. Creating Sandwich-like Ti3C2/TiO2/RGO as Anode Materials with High Energy and Power Density for Li-Ion Hybrid Capacitors. ACS Sustain. Chem. Eng. 2019, 7, 15394–15403. [Google Scholar] [CrossRef]
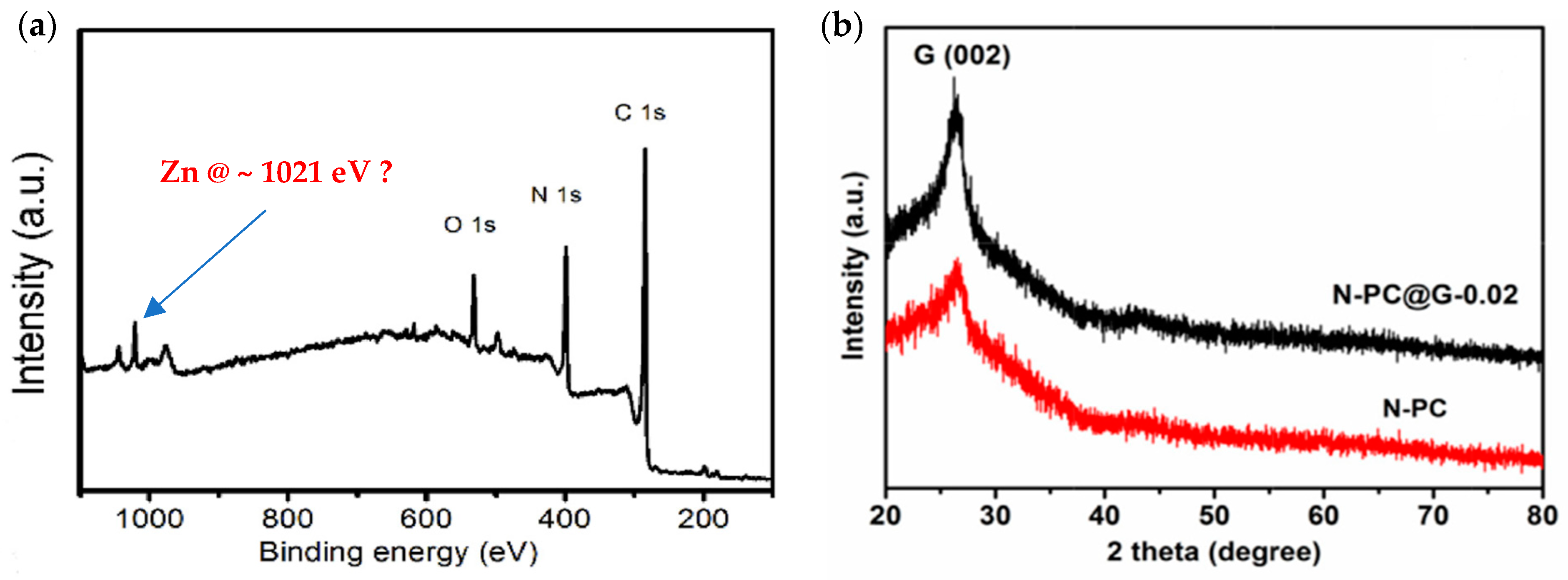
- Zhuang, S.; Singh, H.; Nunna, B.B.; Mandal, D.; Boscoboinik, J.A.; Lee, E.S. Nitrogen-Doped Graphene-Based Catalyst with Metal-Reduced Organic Framework: Chemical Analysis and Structure Control. Carbon 2018, 139, 933–944. [Google Scholar] [CrossRef]
- Singh, H.; Zhuang, S.; Nunna, B.B.; Lee, E.S. Thermal Stability and Potential Cycling Durability of Nitrogen-Doped Graphene Modified by Metal-Organic Framework for Oxygen Reduction Reactions. Catalysts 2018, 8, 607. [Google Scholar] [CrossRef]
- Zhuang, S.; Lee, E.S.; Lei, L.; Nunna, B.B.; Kuang, L.; Zhang, W. Synthesis of Nitrogen-Doped Graphene Catalyst by High-Energy Wet Ball Milling for Electrochemical Systems. Int. J. Energy Res. 2016, 40, 2136–2149. [Google Scholar] [CrossRef]
- Korusenko, P.M.; Bolotov, V.V.; Nesov, S.N.; Povoroznyuk, S.N.; Khailov, I.P. Changes of the Electronic Structure of the Atoms of Nitrogen in Nitrogen-Doped Multiwalled Carbon Nanotubes under the Influence of Pulsed Ion Radiation. Nucl. Instrum. Methods Phys. Res. B 2015, 358, 131–135. [Google Scholar] [CrossRef]
- Lherbier, A.; Blase, X.; Niquet, Y.-M.; Triozon, F.; Roche, S. Charge Transport in Chemically Doped 2D Graphene. Phys. Rev. Lett. 2008, 101, 36808. [Google Scholar] [CrossRef]
- Usachov, D.; Vilkov, O.; Gruneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.K.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; et al. Nitrogen-Doped Graphene: Efficient Growth, Structure, and Electronic Properties. Nano Lett. 2011, 11, 5401–5407. [Google Scholar] [CrossRef]
- Talukder, N.; Wang, Y.; Nunna, B.B.; Tong, X.; Boscoboinik, J.A.; Lee, E.S. Investigation on Electrocatalytic Performance and Material Degradation of an N-Doped Graphene-MOF Nanocatalyst in Emulated Electrochemical Environments. Ind. Chem. Mater. 2023, 1, 360–375. [Google Scholar] [CrossRef]
- Son, S.; Lim, D.; Nam, D.; Kim, J.; Shim, S.E.; Baeck, S.-H. N, S-Doped Nanocarbon Derived from ZIF-8 as a Highly Efficient and Durable Electro-Catalyst for Oxygen Reduction Reaction. J. Solid. State Chem. 2019, 274, 237–242. [Google Scholar] [CrossRef]
- Rong, H.; Zhan, T.; Sun, Y.; Wen, Y.; Liu, X.; Teng, H. ZIF-8 Derived Nitrogen, Phosphorus and Sulfur Tri-Doped Mesoporous Carbon for Boosting Electrocatalysis to Oxygen Reduction in Universal PH Range. Electrochim. Acta 2019, 318, 783–793. [Google Scholar] [CrossRef]
- Shirzadi Jahromi, H.; Saxena, S.; Sridhar, S.; Ghantasala, M.K.; Guda, R.; Rozhkova, E.; Suthar, K. Synthesis and Characterization of Nitrogen Doped Reduced Graphene Oxide Based Cobalt-ZIF-8 Catalysts for Oxygen Reduction Reaction. ECS Trans. 2021, 104, 59. [Google Scholar] [CrossRef]
- Xue, S.; Yu, Y.; Wei, S.; Xu, B.; Lei, J.; Ban, R.; Wu, Q.; Zheng, M.; Li, J.; Kang, J. Nitrogen-Doped Porous Carbon Derived from ZIF-8 as a Support of Electrocatalyst for Enhanced Oxygen Reduction Reaction in Acidic Solution. J. Taiwan Inst. Chem. Eng. 2018, 91, 539–547. [Google Scholar] [CrossRef]
- Sui, X.-L.; Zhang, L.-M.; Zhao, L.; Gu, D.-M.; Huang, G.-S.; Wang, Z.-B. Nitrogen-Doped Graphene Aerogel with an Open Structure Assisted by in-Situ Hydrothermal Restructuring of ZIF-8 as Excellent Pt Catalyst Support for Methanol Electro-Oxidation. Int. J. Hydrogen Energy 2018, 43, 21899–21907. [Google Scholar] [CrossRef]
- Xu, X.; Dong, X.; Li, D.; Qi, M.; Huang, H. ZIF-8-Derived Three-Dimensional Nitrogen-Doped Porous Carbon as a Pt Catalyst Support for Electrocatalytic Oxidation of Glucose in a Glucose Fuel Cell. ACS Appl. Energy Mater. 2023, 6, 2886–2896. [Google Scholar] [CrossRef]
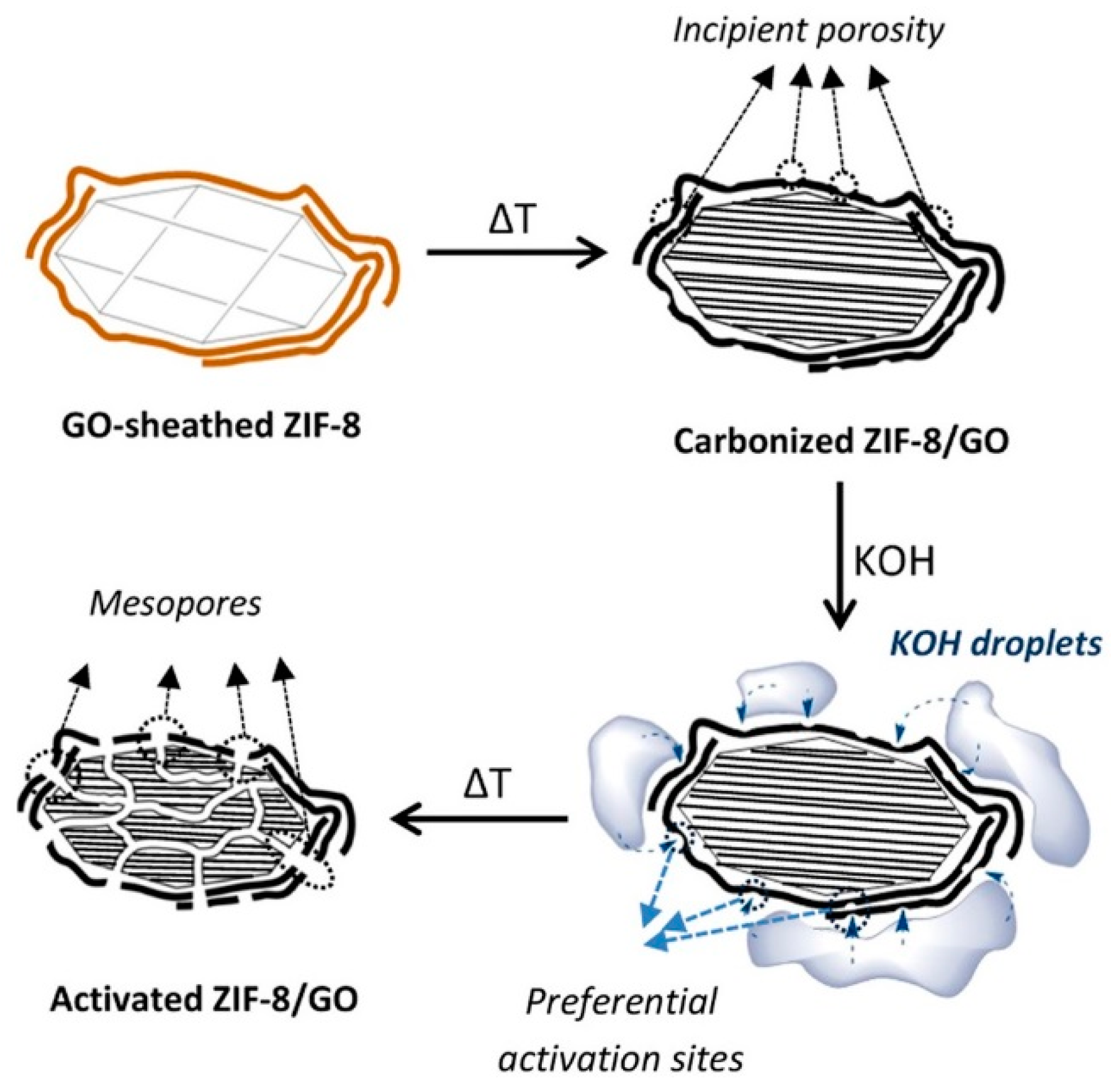
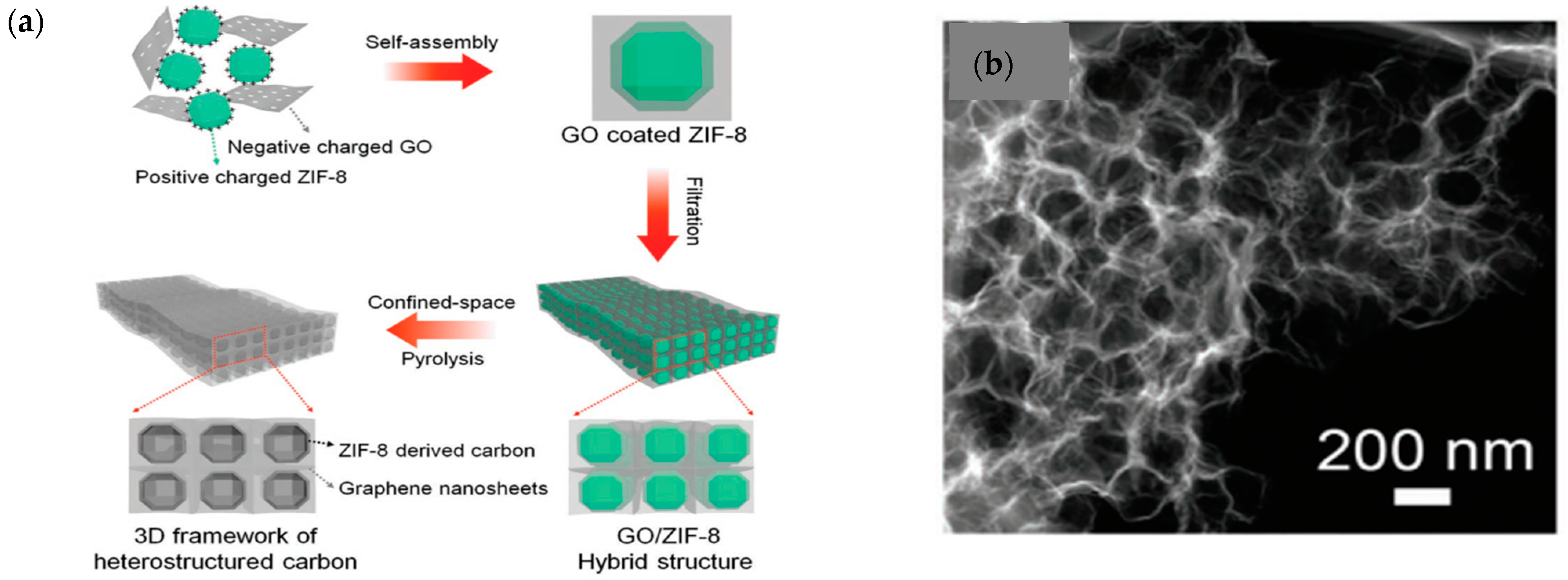
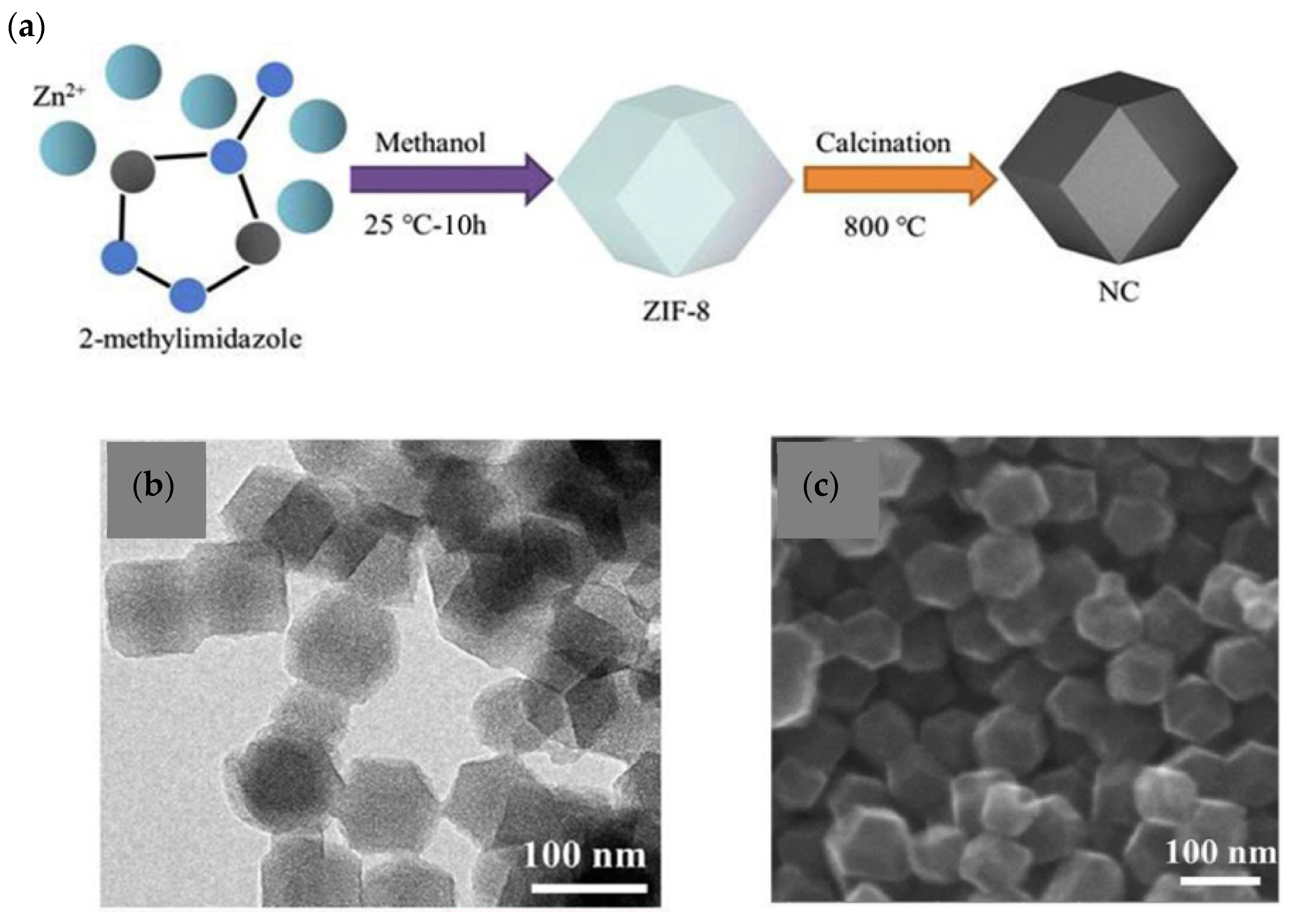
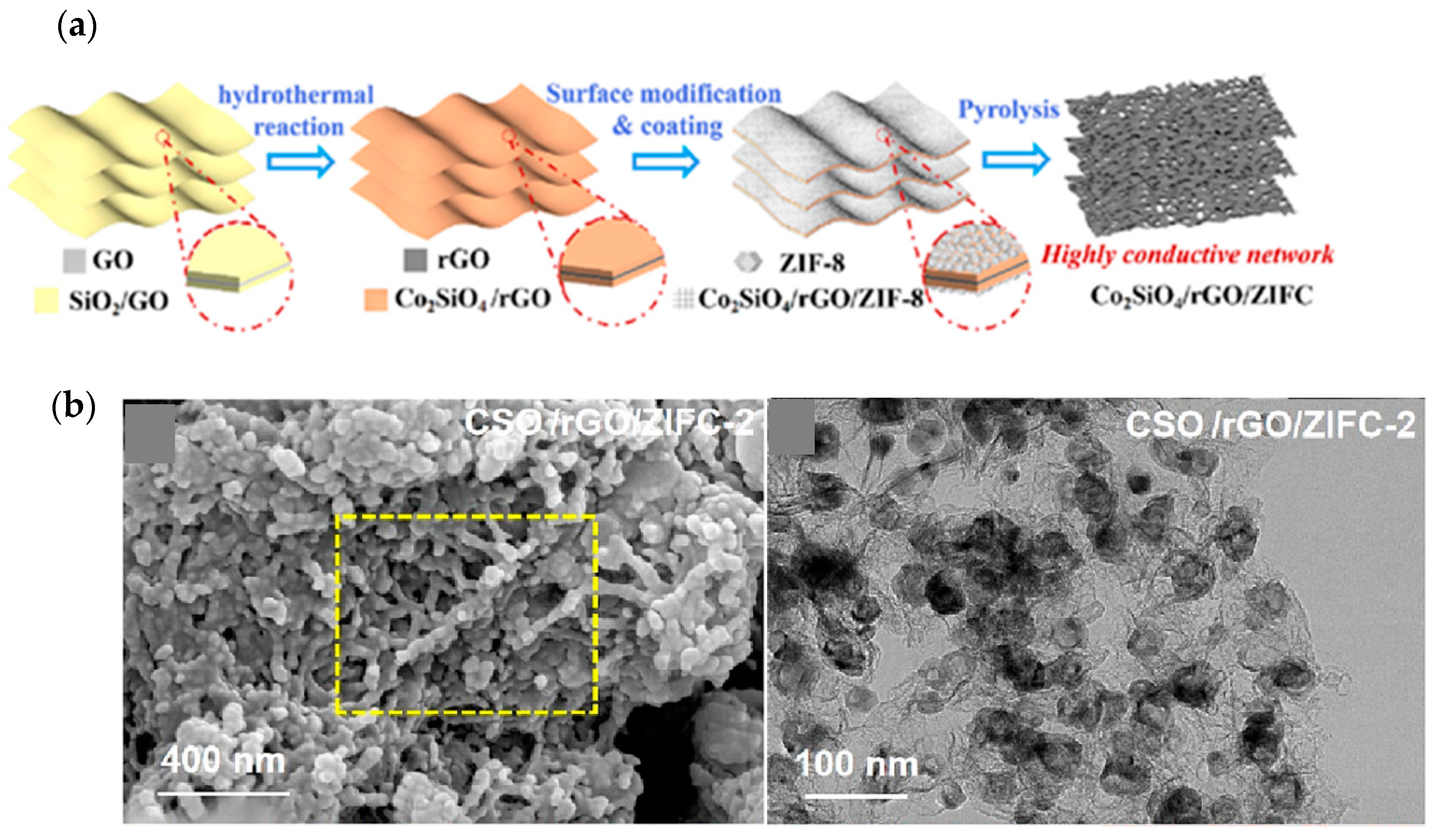
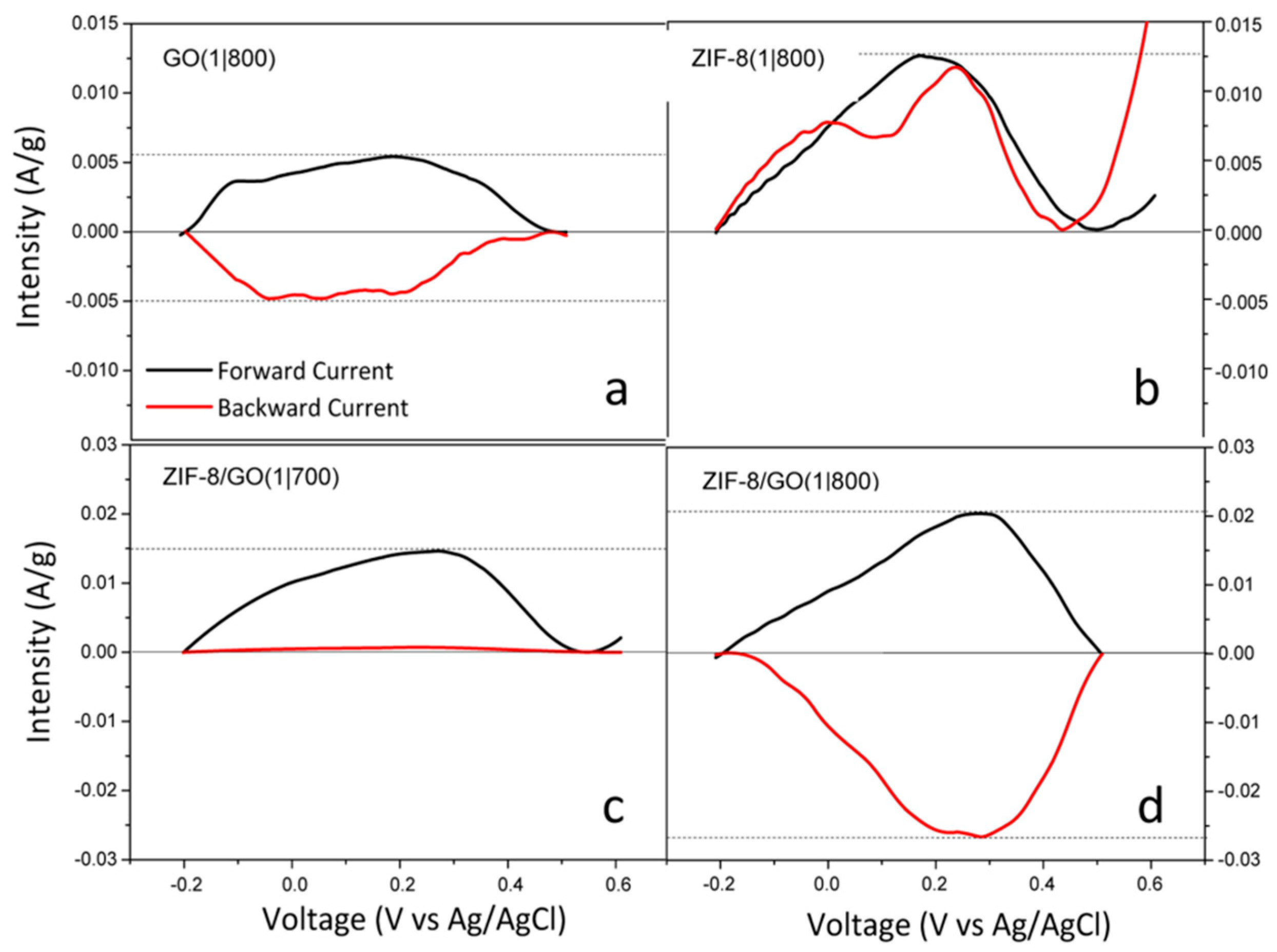
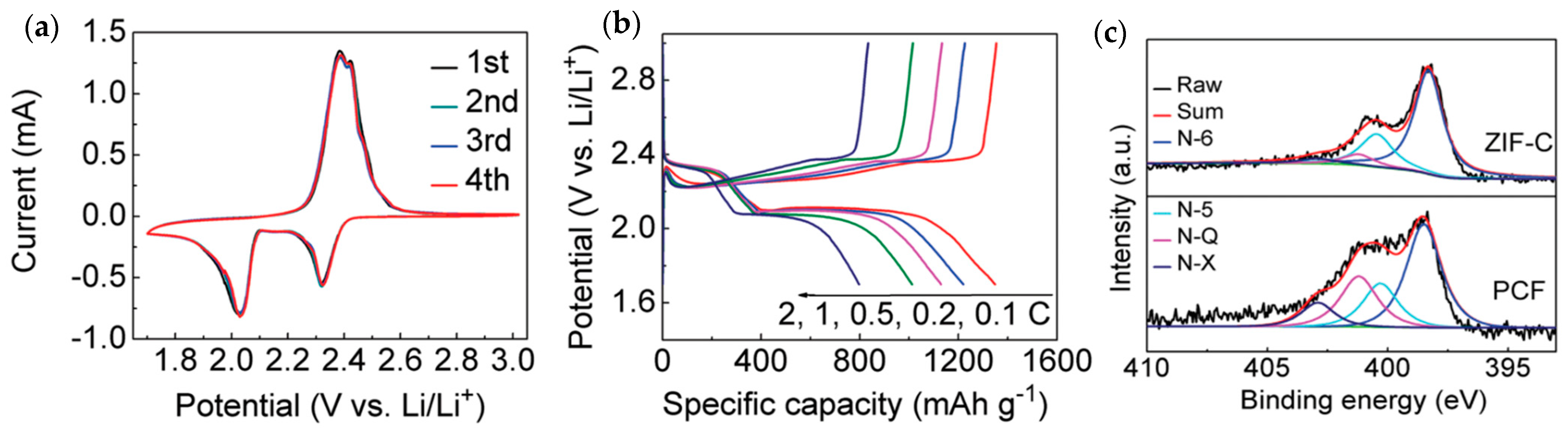
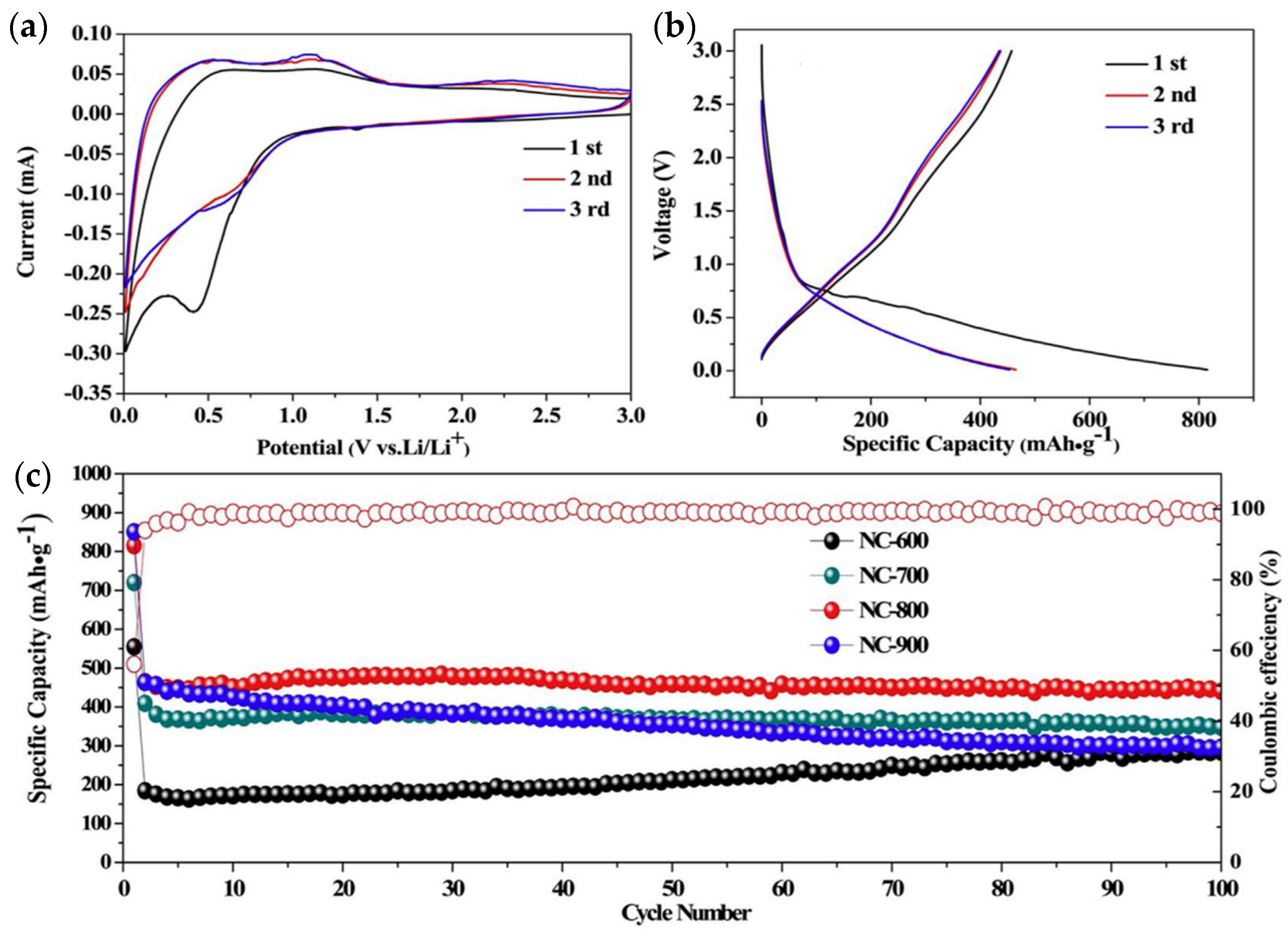

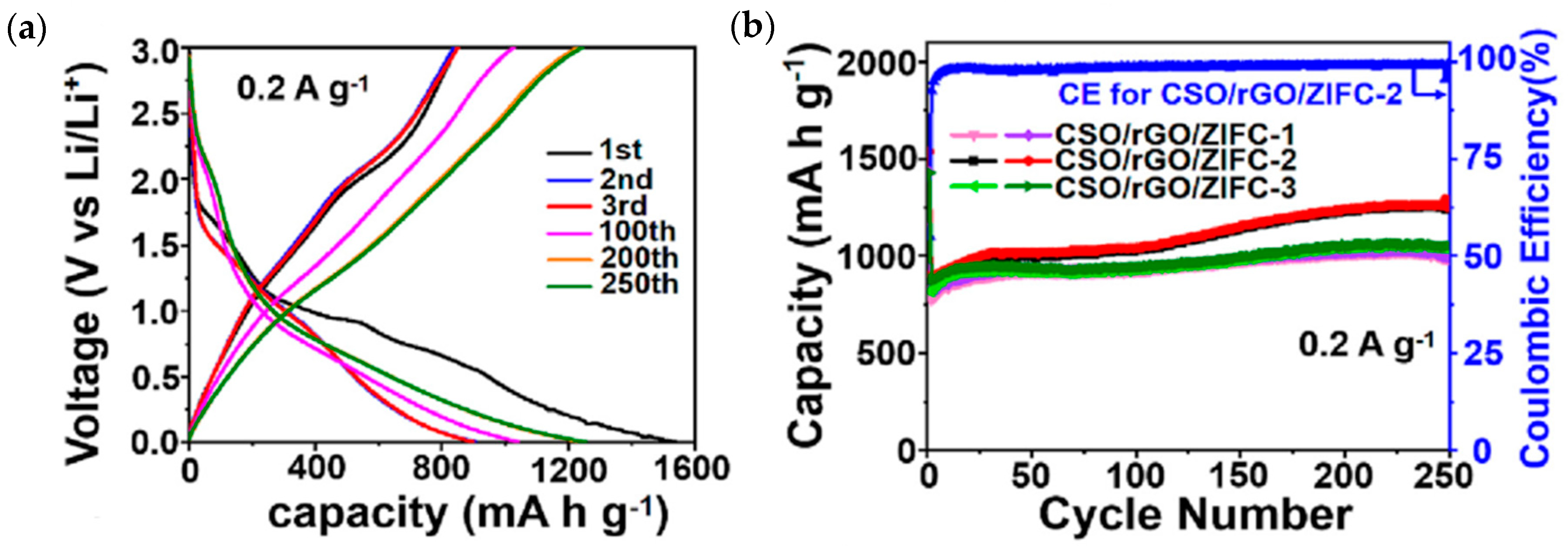
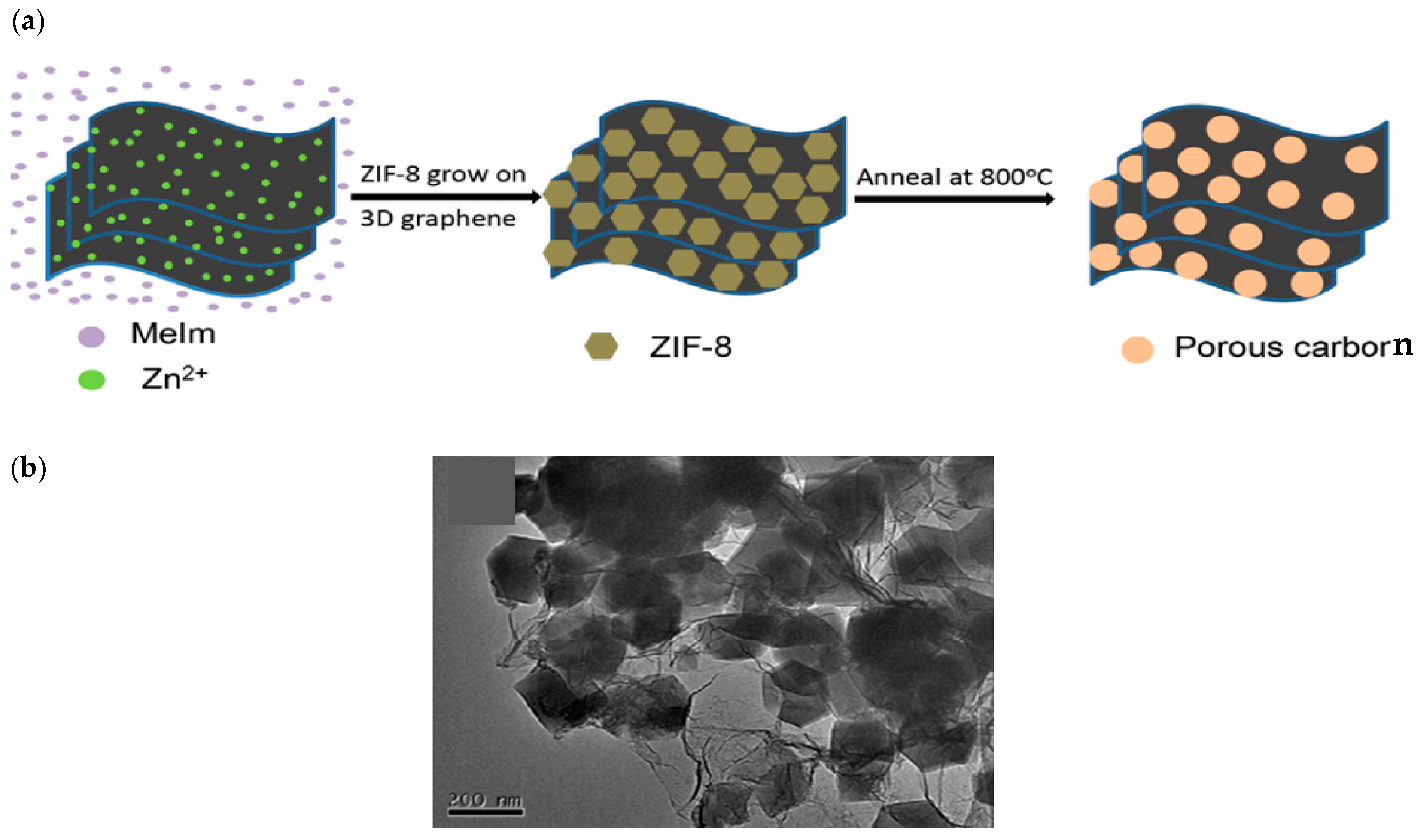
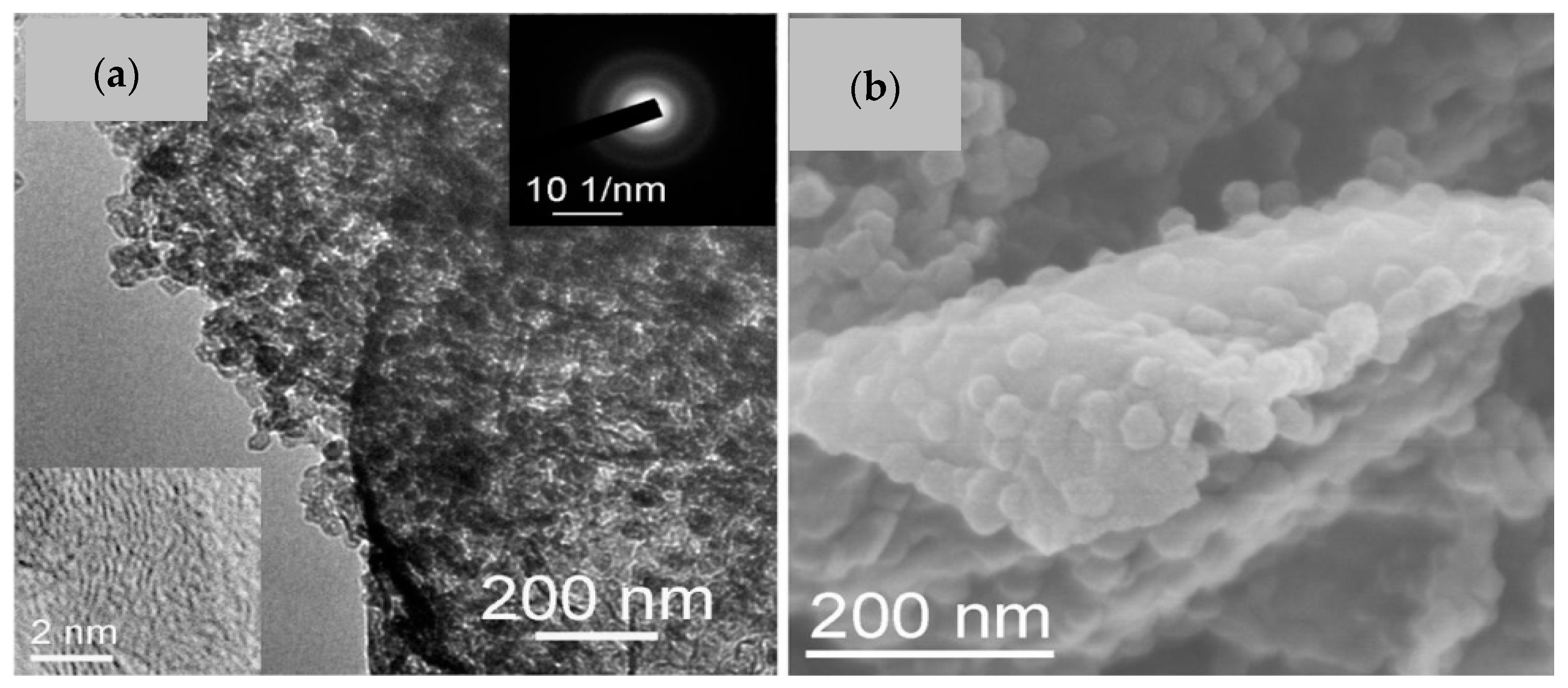
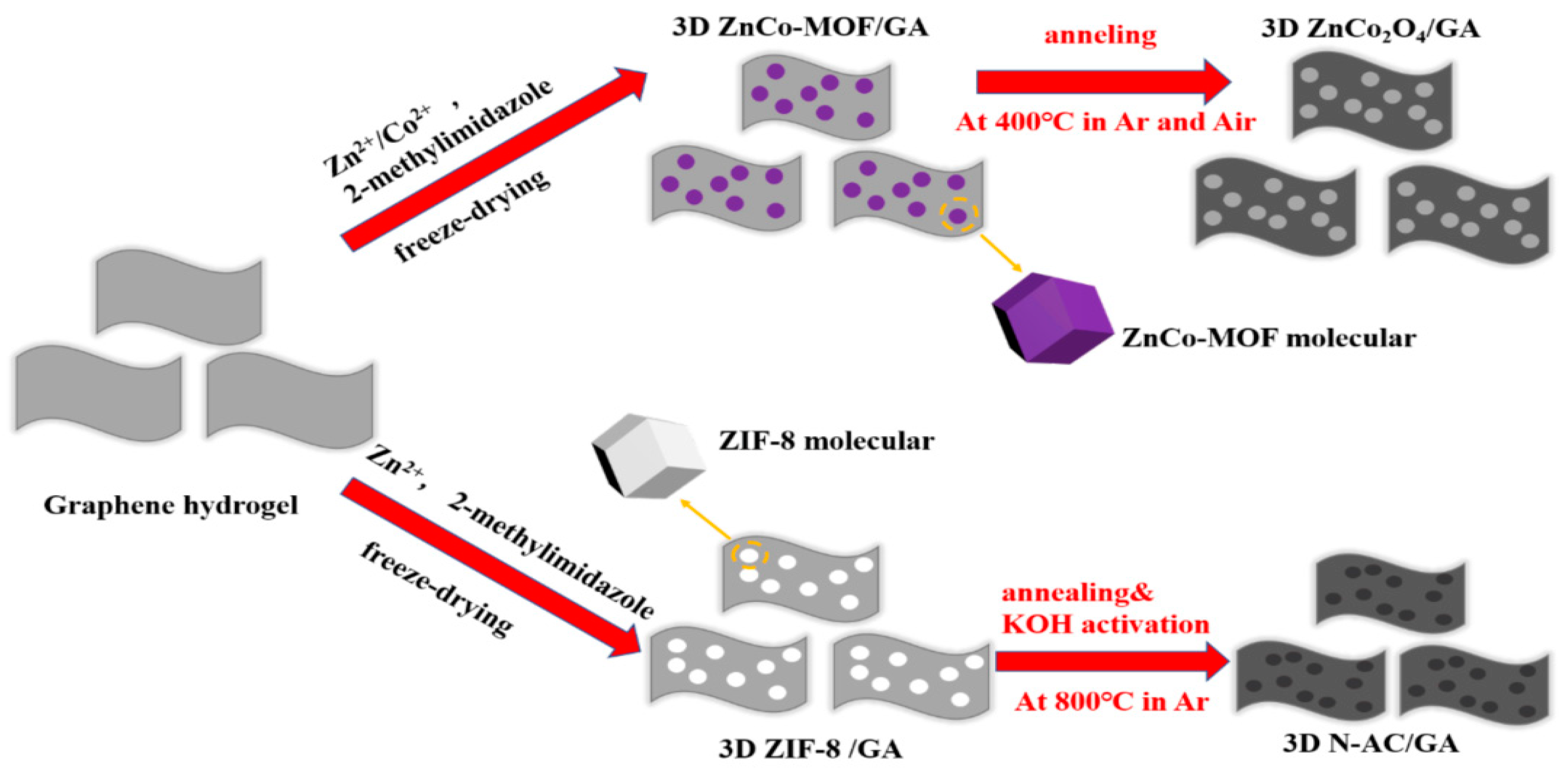
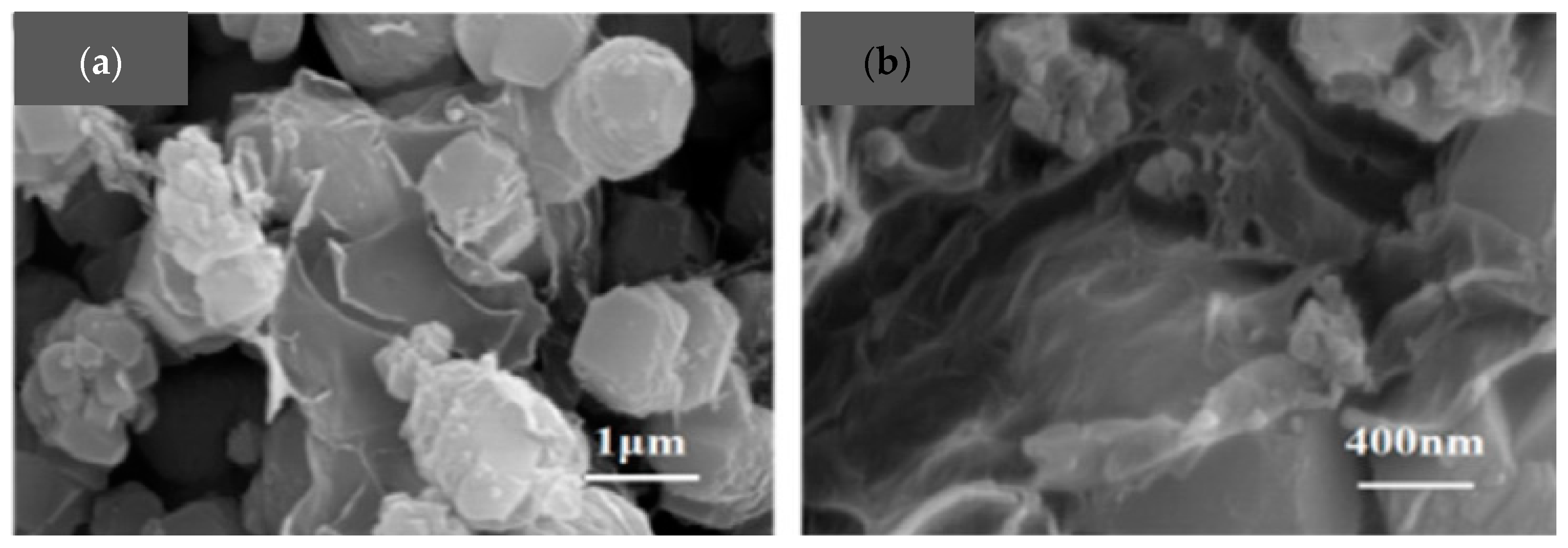
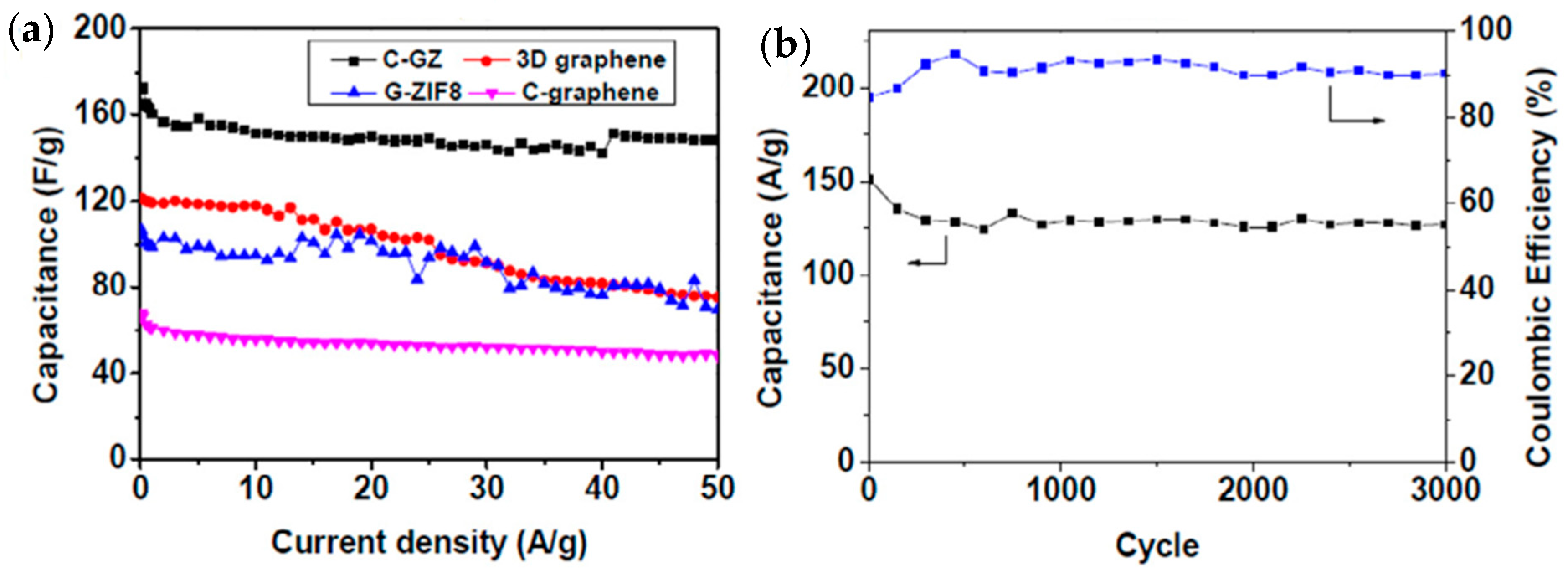
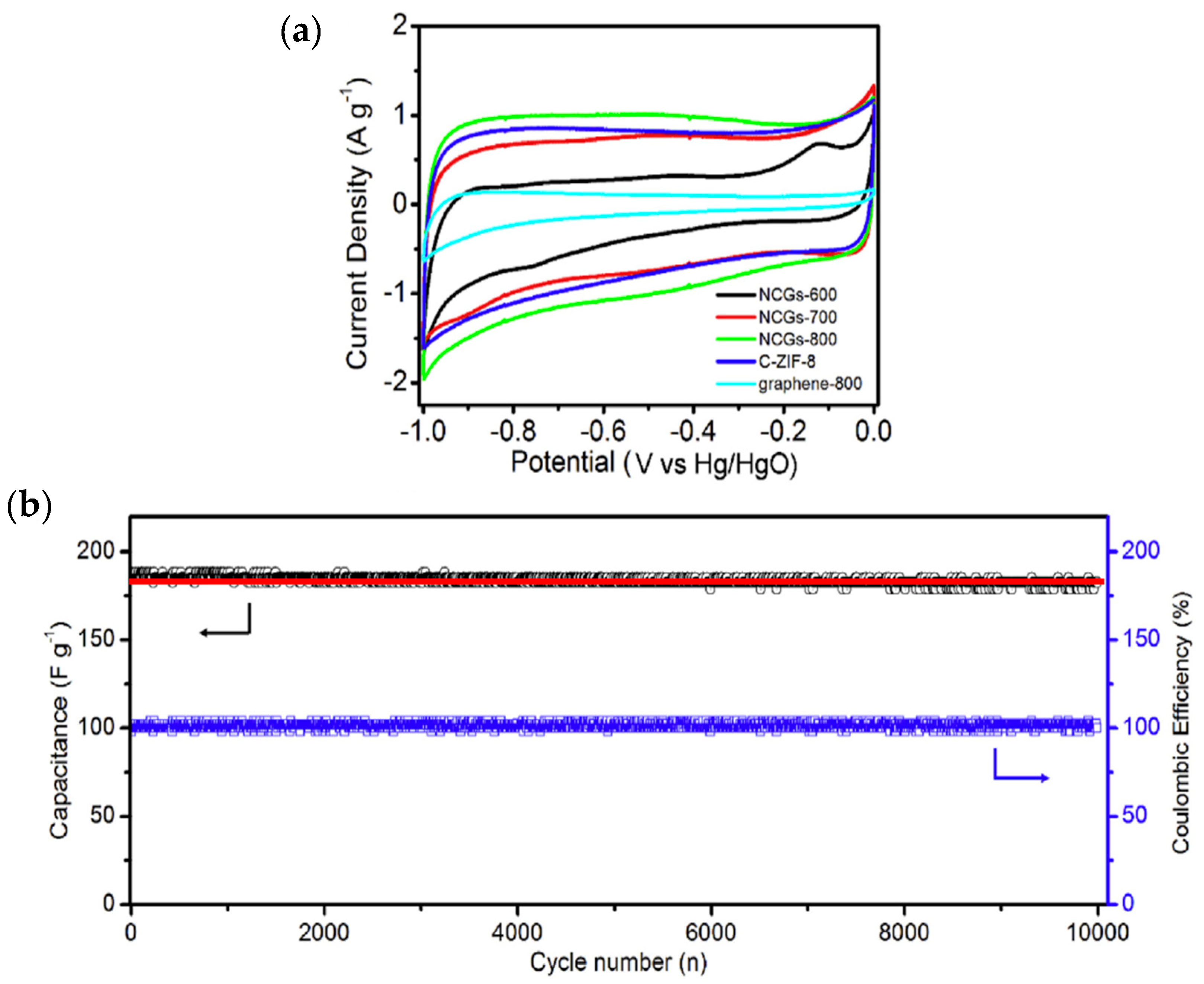
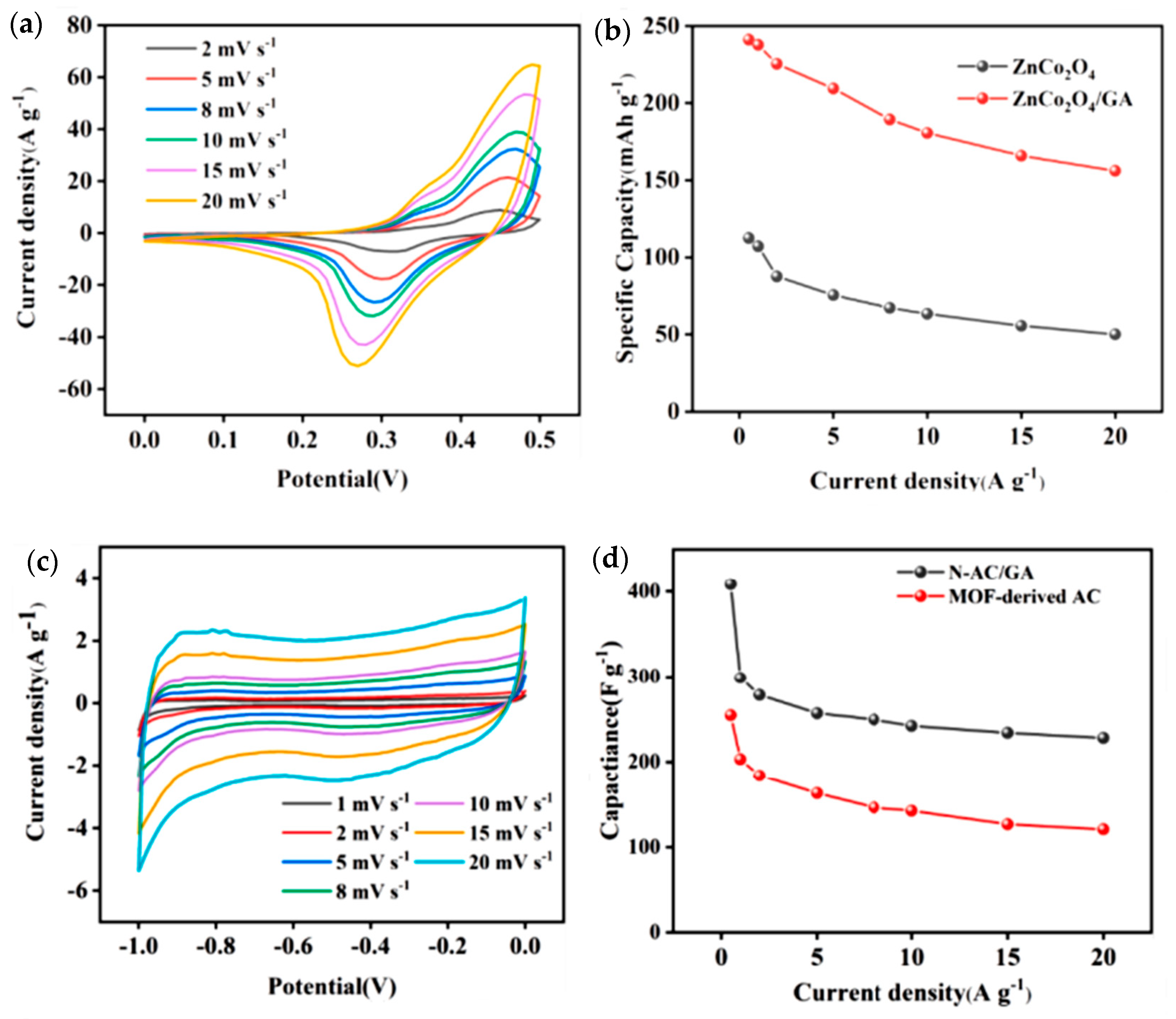
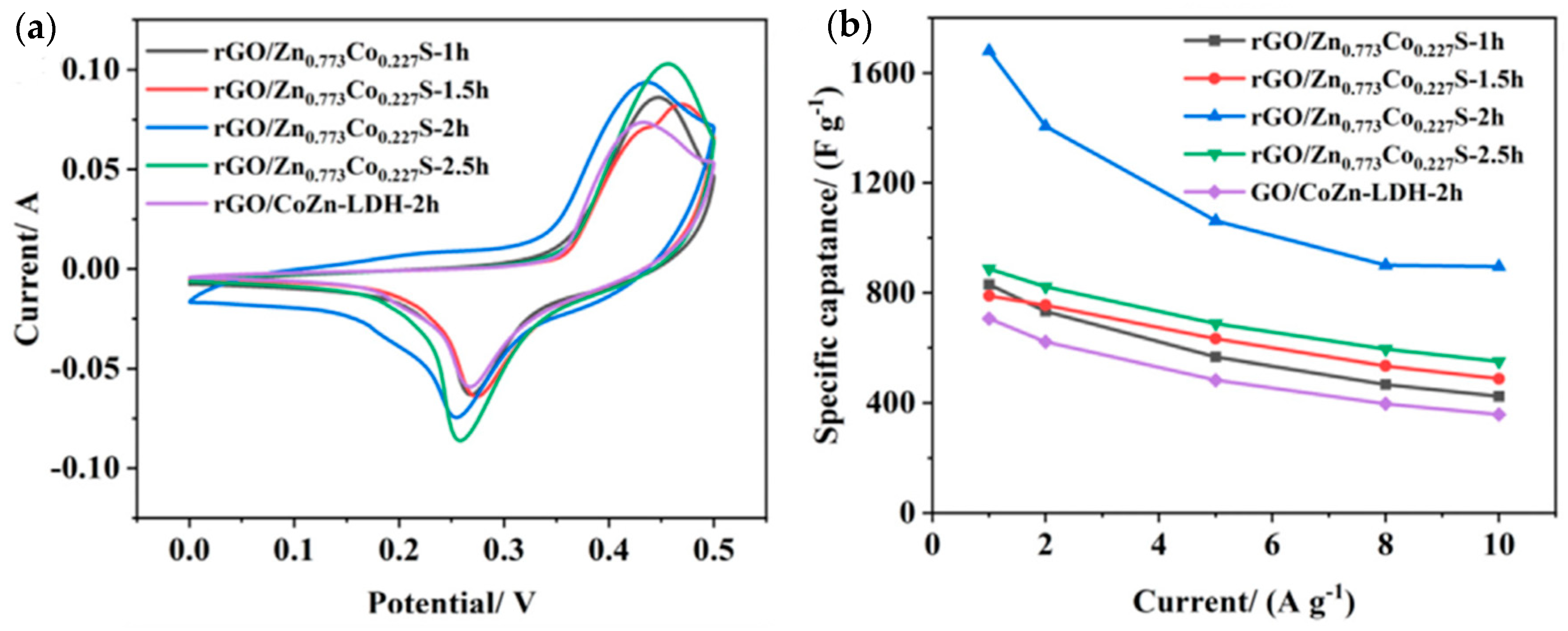




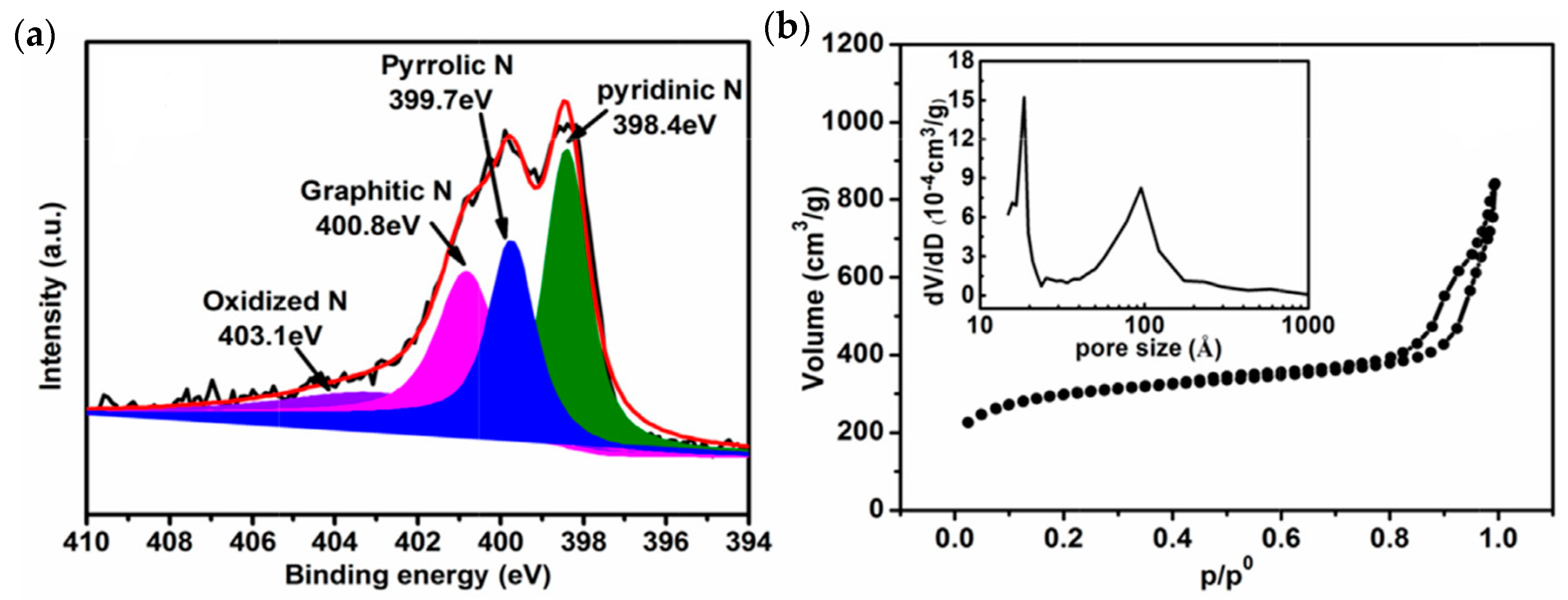

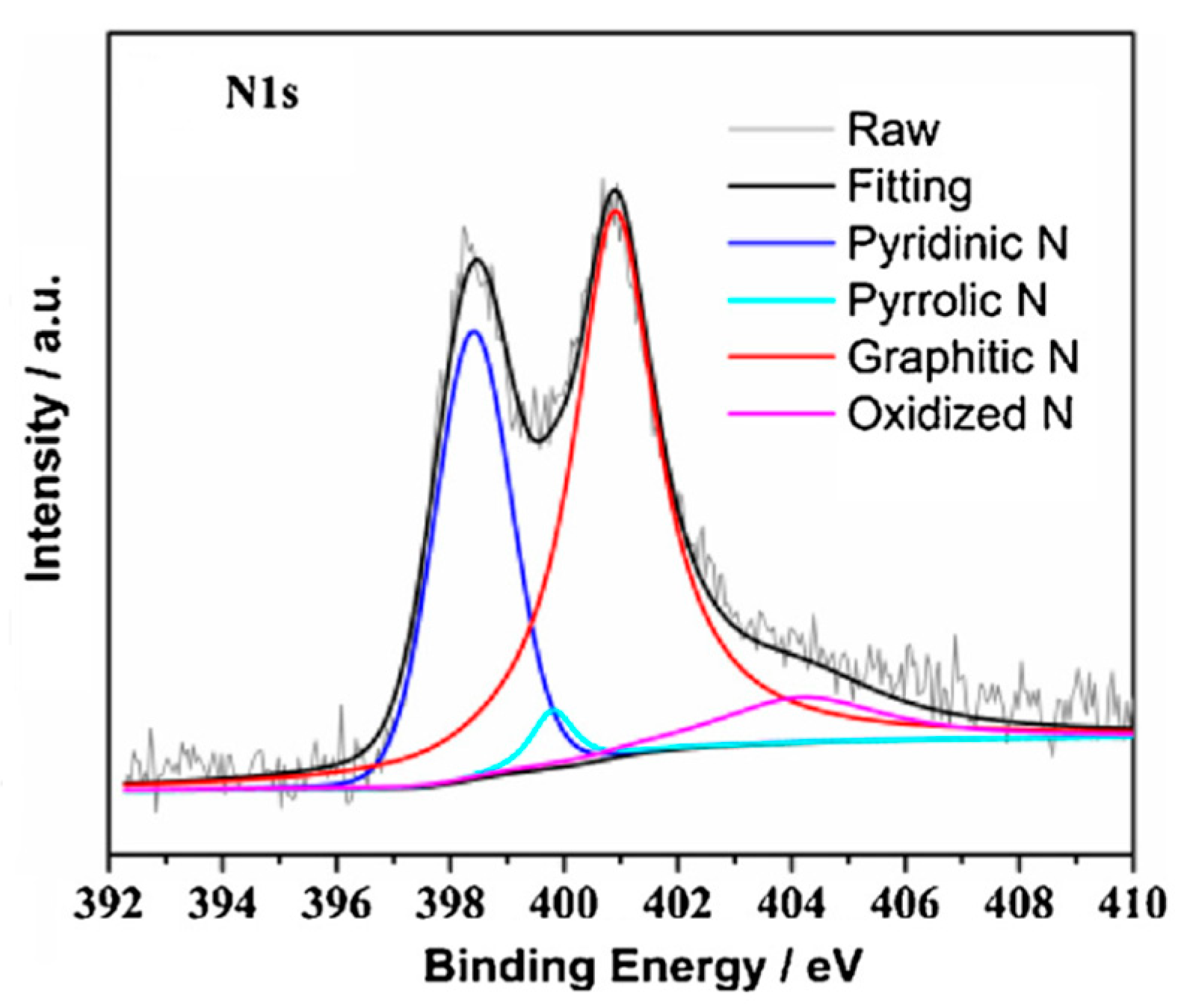
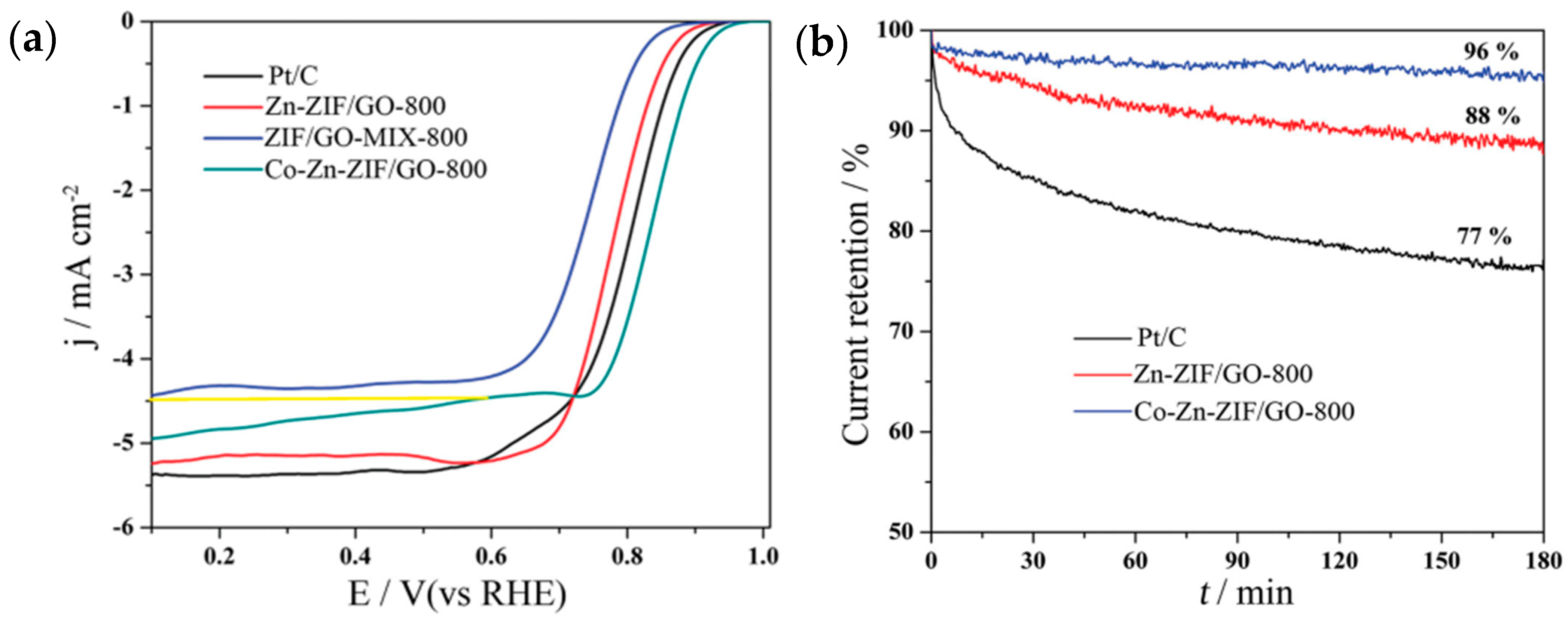
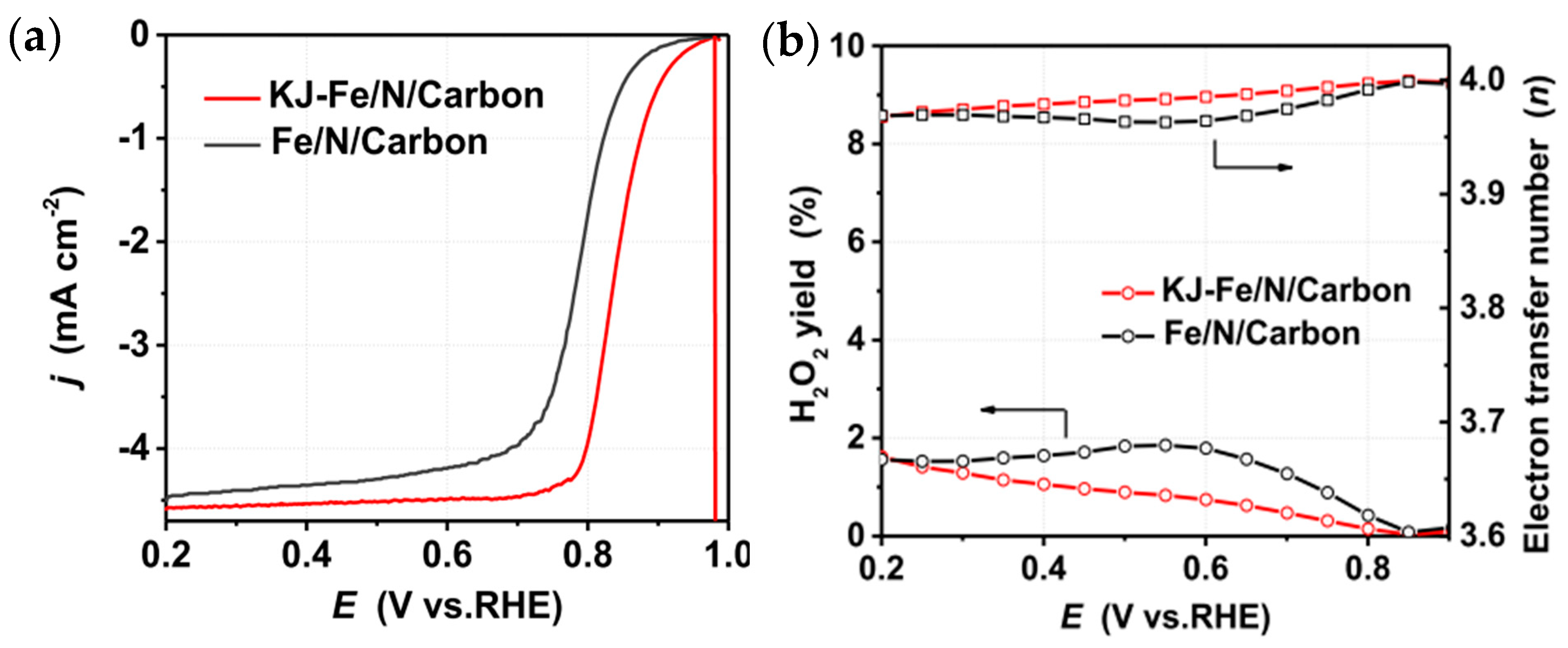
| Material | Discharge Capacitive Performance (of the Best Sample) |
|---|---|
| N-doped graphene analogousp articles (Anode materials for LIBs) (Zheng et al.—2014 [107]) |
|
| Activated ZIF-8/GO (General energy storage materials) (Martín-Jimeno et al.—2017 [48]) |
|
| Three-dimensional porous carbon framework (PCF) (Host material for Li−S batteries) (Ding et al.—2019 [49]) |
|
| N-doped ZIF-8-derived carbon (NC-ZIF) (Anode materials for LIBs) (Tai et al.—2019 [108]) |
|
| ZIF-8 nanocrystals attached to reduced graphene oxide/sulfur (ZIF-8@rGO/S) (Cathode material for Li-S batteries) (Wang et al.—2021 [50]) |
|
| Zinc–cobalt bimetallic selenide (ZnSe/Co0.85Se@NC@C@rGO) (Anode material for PIBs) (Zhang et al.—2023 [51]) |
|
| Three-dimensional porous Co2SiO4/rGO/ZIFC conductive network (Anode materials for LIBs) (Guo et al.—2023 [52]) |
|
| N-doped nanoporous carbon matrix with sulfur (cZIF-8/S). (Cathode materials for Na-S batteries) (Chen et al.—2016 [109]) |
|
| N-doped porous carbon-coated graphene (rGO) sheets (NPCGSs). (Anode materials for LIBs) (Liu et al.—2016 [110]) |
|
| Material | Pore Diameter (nm) | Pore Volume (cm3/g) | BET Surface Area (m2/g) |
|---|---|---|---|
| N-doped graphene analogous particles (Zheng et al. [107]) | 2.02 | 0.32 | 634.6 |
| Activated ZIF-8/GO (Martín-Jimeno et al. [48]) | <1 to 4 | 1.219 | 1304 |
| Three-dimensional porous carbon framework (PCF) (Ding et al. [49]) | ~0.6 | 0.35 | 643 |
| N-doped ZIF-8-derived carbon (NC-ZIF) (Tai et al. [108]) | ~1.8 to 2.0< | ~0.3 | 815.8 |
| ZIF-8 nanocrystals attached to reduced graphene oxide/sulfur (ZIF-8@rGO/S) (Wang et al. [50]) | 3.4 to 15.5 | 0.329 | 905.6 |
| Zinc–cobalt bimetallic selenide (ZnSe/Co0.85Se@NC@C@rGO) (Zhang et al. [51]) | ~4.0 | ~0.12 | 232.51 |
| Three-dimensional porous Co2SiO4/rGO/ZIFC conductive network (Guo et al. [52]) | Not specified | 0.50 | 210 |
| N-doped nanoporous carbon matrix with sulfur (cZIF-8/S) (Chen et al. [109]) | ~0.5 | Not specified | 627 |
| N-doped porous carbon-coated graphene (rGO) sheets (NPCGSs) (Liu et al. [110]) | 0.55, 1.5 | Not specified | 781.3 |
| Material | Capacitive Performance (of the Best Sample) |
|---|---|
| Nanoporous carbon derived from ZIF-8 (Chaikittisilp et al.—2012 [57]) |
|
| ZIF-8 supported on 3D graphene (Li et al.—2014 [58]) |
|
| ZIF-8 nanocrystal-derived N-doped carbon-decorated graphene sheets (Wang et al.—2018 [59]) |
|
| Sulfur-doped graphene oxide/ZIF-8 composite (Khakpour et al.—2022 [60]) |
|
| 3D ZnCo2O4/graphene aerogel (GA) (cathode); ZIF-8-derived N-doped active carbon/graphene aerogel (anode) (Xiao et al.—2022 [61]) |
|
| rGO/Zn0.773Co0.227S composite (Cui et al.—2022 [62]) |
|
| ZIF-8, rGO, and CNT-derived N-doped porous carbon material; NPC/CNT-RG (Liu et al.—2023 [63]) |
|
| Material | Pore Diameter (nm) | Pore Volume (cm3/g) | BET Surface Area (m2/g) |
|---|---|---|---|
| Nanoporous carbon derived from ZIF-8 (Chaikittisilp et al. [57]) | 1.02 to 1.17 | 0.57 | 1075 |
| ZIF-8 supported on 3D graphene (Li et al. [58]) | 4 | Not specified | 352.8 |
| ZIF-8 nanocrystal-derived N-doped carbon-decorated graphene sheets (Wang et al. [59]) | Not specified | Not specified | 816.4 |
| Sulfur-doped graphene oxide/ZIF-8 composite (Khakpour et al. [60]) | 2.66 | 0.68 | 1030.4 |
| Three-dimensional ZnCo2O4/graphene aerogel (GA) (cathode); ZIF-8-derived N-doped active carbon/graphene aerogel (anode) (Xiao et al. [61]) | Cathode: ~8 Anode: ~3 | 0.20 0.12 | 90 156.82 |
| rGO/Zn0.773Co0.227S composite (Cui et al. [62]) | 4 to 6 | Not specified | 32.58 |
| ZIF-8, rGO, and CNT-derived N-doped porous carbon material; NPC/CNT-RG. (Liu et al. [63]) | 3.74 to ~26.54 | Not specified | 436.8 |
| Materials | Precursors | Synthesis Steps | ORR Catalytic Performance (of the Best Sample) |
|---|---|---|---|
| N-decorated nanoporous carbon (NC) (Aijaz et al.—2014 [77]) | ZIF-8; furfuryl alcohol (FA); and ammonium hydroxide (NH4OH). | Chemical mixing of ZIF-8 and FA. FA polymerization. High-temperature calcinated (between 600–1000 °C) under a stream of nitrogen gas. | Measured in O2-saturated 0.1 M KOH. Catalyst loading: 33.95 µg/cm2 (2.4 µg catalyst on 3 mm diameter electrode surface). ORR onset potential 0.83 V vs. RHE. Current density of 4.9 mA/cm2 at 0.1 V vs. RHE (at 2500 rpm; scan rate: 10 mV/s). |
| Nitrogen-doped graphitic porous carbon (NGPC) (Zhang et al.—2014 [78]) | ZIF-8 nanocrystals. | Single-step high-temperature carbonization of ZIF-8 (between 600–1000 °C)—in a nitrogen-filled environment. | Measured in O2-saturated 0.1 M KOH. Catalyst loading: 102 µg/cm2. ORR onset potential: 0.883 V vs. RHE (−0.02 V vs. Ag/AgCl). Current density: 4.3 mA/cm2 at 0.3 V vs. RHE (−0.60 V vs. Ag/AgCl) (at 1600 rpm; scan rate: 5 mV/s). |
| Graphene-based nitrogen-doped porous carbon sheets (GNPCSs) (Zhong et al.—2014 [79]) | Polyvinyl pyrrolidone (PVP); graphene oxide (GO) sheets; zinc nitrate salt (Zn(NO3)2·6H2O); 2-methylimidazole. | PVP layering on GO sheets; chemically. ZIF-8 growth on PVP-layered GO to form ZIF-8/GO. High-temperature calcination of ZIF-8/GO (in the range of 650–950 °C). | Measured in O2-saturated 0.1 M KOH. Catalyst loading: 200 µg/cm2. ORR onset potential: 0.957 V vs. RHE. Current density: 6.0 mA/cm2 at 0.45 V vs. RHE (at 1600 rpm; scan rate: 5 mV/s). |
| Graphene oxide-sheathed ZIF-8 microcrystals (GZs) (Thomas et al.—2016 [80]) | Graphene oxide (GO); zinc nitrate salt (Zn(NO3)2·6H2O); 2-methylimidazole. | Ultrasonication of GO and zinc salt solution; then, introduce 2-methylimidazole to form GO/ZIF-8. Carbonization of GO/ZIF-8 at 950 °C in a nitrogen atmosphere. | Measured in O2-saturated 0.1 M KOH. Catalyst loading: ~170 µg/cm2. ORR onset potential: 0.88 V vs. RHE. Current density: ~4.5 mA/cm2 at 0.4 V vs. RHE (at 1600 rpm; scan rate: 5 mV/s). |
| N-doped porous carbon@graphene (N-PC@G) (Liu et al.—2016 [36]) | Graphene oxide (GO); 2-methylimidazole; zinc nitrate salt (Zn(NO3)2·6H2O). | Ultrasonic dispersion of GO solution; then, introduce 2-methylimidazole solution; finally, zinc salt is introduced to form ZIF-8@GO. Pyrolysis of ZIF-8@GO at 900 °C in a nitrogen atmosphere. | Measured in O2-saturated 0.1 M KOH. Catalyst loading: ~407 µg/cm2. ORR onset potential: 1.01 V vs. RHE. Current density: ~4.4 mA/cm2 at 0.6 V vs. RHE (at 1600 rpm; scan rate: 10 mV/s). |
| Metal organic framework-modified nitrogen-doped graphene (N-G/MOF) (Zhuang et al.—2016 [39,68,127,128]) | Graphene oxide (GO); melamine; ZIF-8. | Nitrogen-doped graphene (N-G) synthesized from GO and melamine by a nanoscale high-energy wet ball milling process. By the same process, N-G and ZIF-8 were integrated to produce N-G/MOF. | Measured in O2-saturated 0.1 M KOH. Catalyst loading: 37.3 µg/cm2. ORR onset potential: ~0.73 V vs. RHE. Limiting current density: 5.02 mA/cm2 (at 1600 rpm; scan rate: 10 mV/s). |
| Core–shell hierarchically porous carbon nanopolyhedras (CS-HPCNs) (Cao et al.—2019 [37]) | Zinc nitrate salt (Zn(NO3)2·6H2O); 2-methylimidazole. | Chemical synthesis of ZIF-8 core crystal, chemically grown ZIF-8 shell over the core forming ZIF-8@ZIF-8; high-temperature treatment of core–shell ZIF-8@ZIF-8 (in the range of 900–1100 °C)—in nitrogen flow environment. | Measured in O2-saturated 0.1 M KOH. Catalyst loading: 100 µg/cm2. ORR onset potential: 0.709 V vs. RHE. Current density: ~3.75 mA/cm2 at −0.09 V vs. RHE (at 1600 rpm; scan rate: 10 mV/s). |
| N-doped nanoporous carbon/graphene nanosheets (Zn-ZIF/GO and Co-Zn-ZIF/GO) (Wei et al.—2015 [38]) | Graphene oxide (GO); zinc nitrate salt (Zn(NO3)2·6H2O); 2-methylimidazole; cobalt nitrate salt (Co(NO3)2·6H2O) | Chemical synthesis of ZIF-8 nanocrystal; then, add the GO solution to produce Zn-ZIF/GO; add cobalt nitrate salt to the Zn-ZIF/GO solution to produce Co-Zn-ZIF/GO. | Zn-ZIF/GO and Co-Zn-ZIF/GO in alkaline electrolyte: measured in O2-saturated 0.1 M KOH. Catalyst loading: 200 µg/cm2. ORR onset potential: 0.92 V and 0.96 V vs. RHE. Current density: 5.2 mA/cm2 and ~4.5 mA/cm2 at 0.6 V vs. RHE (at 1600 rpm; scan rate: 10 mV/s). Co-Zn-ZIF/GO in acidic electrolyte: measured in O2-saturated 0.1 M HClO4. Catalyst loading: 300 µg/cm2. ORR onset potential: 0.85 V vs. RHE. Current density: 4.2 mA/cm2 at 0.4 V vs. RHE (at 1600 rpm; scan rate: 10 mV/s). |
| Ketjenblack added zeolitic imidazolate framework-derived Fe/N/carbon (KJ-Fe/N/carbon) (Zhang et al.—2022 [81]) | Zinc nitrate salt (Zn(NO3)2·6H2O); 2-methylimidazole; iron(III) chloride hexahydrate (FeCl3·6H2O); Ketjenblack EC600J (KJ600). | KJ600-Fe-ZIF-8 production by solvent technique; pyrolysis of KJ600-Fe-ZIF-8 at 950 °C in an Ar/NH3 environment. | Measured in O2-saturated 0.1 M H2SO4. Catalyst loading: 600 µg/cm2. ORR: E1/2 0.834 V vs. RHE. (onset potential: ~0.98 V vs. RHE). Liming current density: ~4.6 mA/cm2 at (at 900 rpm; scan rate: 10 mV/s). |
| MOF-derived yolk–shell Co-N-C combined with 3D macroporous graphene nanopockets (yolk–shell Co-N-C@GNP) (Liu et al.—2023 [82]) | Zinc nitrate salt (Zn(NO3)2·6H2O); 2-methylimidazole; GO; cobalt nitrate salt (Co(NO3)2·6H2O). | Mix ZIF-8 (produced from zinc salt and 2-methylimidazole) and graphene nanopockets (prepared from GO); then, add cobalt salt solution. Calcination of the product at 950 °C in an Ar atmosphere. | Measured in O2-saturated 0.1 M KOH. Catalyst loading: 450 µg/cm2. ORR onset potential: 1.01 V vs. RHE. (E1/2 0.86 V vs. RHE). Limiting current density: 5.8 mA/cm2 (at 1600 rpm; scan rate: 5 mV/s). |
| Nitrogen and sulfur co-doped porous carbon (NSDPC) (Son et al.—2019 [134]) | ZIF-8 and Thiourea (CH4N2S). | Chemical synthesis of ZIF-8. Solution of ZIF-8 and thiourea at different weight ratios. Dry out the solution. Carbonization of the ZIF-8/thiourea mixture at 900 °C for 5 h in an N2-saturated environment. | Measured in O2-saturated 0.1 M KOH. Catalyst loading: ~530 µg/cm2 (~235 µg NSDPC, ~295 µg Ketjen black). Limiting current density: ~5.9 mA/cm2 (at 1600 rpm; scan rate: 5 mV/s). |
| N, P, and S tri-doped mesoporous carbon (NPSpC) (Rong et al.—2019 [135]) | ZIF-8; sodium phytate (C6H6Na12O24P6); dodecyl mercaptan (C12H26S). | Chemical synthesis of ZIF-8. ZIF-8 and sodium phytate solution prepared in ethanol at room temperature. Add dodecyl mercaptan dropwise for 30 min and stir for 24 h. Then, wash with ethanol and dry out overnight in a vacuum environment at 50 °C. Carbonize at 900 °C for 4 h in an N2 environment. | In alkaline electrolyte: measured in O2-saturated 0.1 M KOH. Catalyst loading: ~1125 µg/cm2 (with Nafion). Onset potential: 0.923 V vs. RHE. Half-wave potential: 0.821 V vs. RHE. Limiting current density: 4.89 mA/cm2. In acidic electrolyte: measured in O2-saturated 0.5 M H2SO4. Catalyst loading: ~1289.1 µg/cm2 (with Nafion). Onset potential: 0.899 V vs. RHE. Half-wave potential: 0.757 V vs. RHE. Limiting current density: 5.20 mA/cm2. |
| Material | Pore Diameter (nm) | Pore Volume (cm3/g) | BET Surface Area (m2/g) |
|---|---|---|---|
| N-decorated nanoporous carbon (NC) (Aijaz et al. [77]) | 1 to 3 | 1.58 | 2747 |
| Nitrogen-doped graphitic porous carbon (NGPC) (Zhang et al. [78]) | 1.2 to 1.6 | 0.99 | 932 |
| Graphene-based nitrogen-doped porous carbon sheets (GNPCSs) (Zhong et al. [79]) | Not specified | Not specified | 911 |
| Graphene oxide sheathed ZIF-8 microcrystals (GZs) (Thomas et al. [80]) | 0.2 to 0.5 | 0.7 | 502 |
| N-doped porous carbon@graphene (N-PC@G) (Liu et al. [36]) | 1.8, 10 | 0.907 | 1094.3 |
| Metal organic framework-modified nitrogen-doped graphene (N-G/MOF) (Zhuang et al. [39,68,127,128]) | 1.6 | 0.404 | 1103 |
| Core–shell hierarchically porous carbon nanopolyhedras (CS-HPCNs) (Cao et al. [37]) | 0.5, 1, and 10 to 17 | 1.374 | 991 |
| N-doped nanoporous carbon/graphene nanosheets (Zn-ZIF/GO and Co-Zn-ZIF/GO) (Wei et al. [38]) | Not specified | Not specified | 1170 |
| Ketjenblack-added zeolitic imidazolate framework-derived Fe/N/carbon (KJ-Fe/N/carbon) (Zhang et al. [81]) | 2, 100 | Not specified | 125, 405 |
| MOFs-derived yolk–shell Co-N-C combined with 3D macroporous graphene nanopockets (yolk–shell Co-N-C@GNP) (Liu et al. [82]) | Not specified | ~0.66 | 178.20 |
| Nitrogen and sulfur-co-doped porous carbon (NSDPC) (Son et al. [134]) | ~10 | 0.17 | 114 |
| N, P, and S tri-doped mesoporous carbon (NPSpC) (Rong et al. [135]) | ~2.6 | 1.957 | 1641 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talukder, N.; Wang, Y.; Nunna, B.B.; Lee, E.S. N-Doped Graphene (N-G)/MOF(ZIF-8)-Based/Derived Materials for Electrochemical Energy Applications: Synthesis, Characteristics, and Functionality. Batteries 2024, 10, 47. https://doi.org/10.3390/batteries10020047
Talukder N, Wang Y, Nunna BB, Lee ES. N-Doped Graphene (N-G)/MOF(ZIF-8)-Based/Derived Materials for Electrochemical Energy Applications: Synthesis, Characteristics, and Functionality. Batteries. 2024; 10(2):47. https://doi.org/10.3390/batteries10020047
Chicago/Turabian StyleTalukder, Niladri, Yudong Wang, Bharath Babu Nunna, and Eon Soo Lee. 2024. "N-Doped Graphene (N-G)/MOF(ZIF-8)-Based/Derived Materials for Electrochemical Energy Applications: Synthesis, Characteristics, and Functionality" Batteries 10, no. 2: 47. https://doi.org/10.3390/batteries10020047
APA StyleTalukder, N., Wang, Y., Nunna, B. B., & Lee, E. S. (2024). N-Doped Graphene (N-G)/MOF(ZIF-8)-Based/Derived Materials for Electrochemical Energy Applications: Synthesis, Characteristics, and Functionality. Batteries, 10(2), 47. https://doi.org/10.3390/batteries10020047








