Spherical Graphite Anodes: Influence of Particle Size Distribution and Multilayer Structuring in Lithium-Ion Battery Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Slurry Preparation and Manufacturing of the Electrodes
2.2. Mechanical, Electrical, and Structural Characterization of the Anode
2.2.1. SEM Analysis
2.2.2. Adhesion Strength
2.2.3. Electrical Conductivity
2.2.4. Pore Size Distribution and Porosity
2.3. Color Measurement by Spectrophotometry
2.4. Battery Cell Production and Electrochemical Characterization
2.4.1. Charge-Rate Test and Cell Ageing
2.4.2. Electrochemical Impedance Spectroscopy and Ionic Resistance
3. Results and Discussion
3.1. SEM Analysis Results
3.2. Mechanical Properties of Two-Layer Anodes
3.3. Structural Properties of the Multilayer Anodes
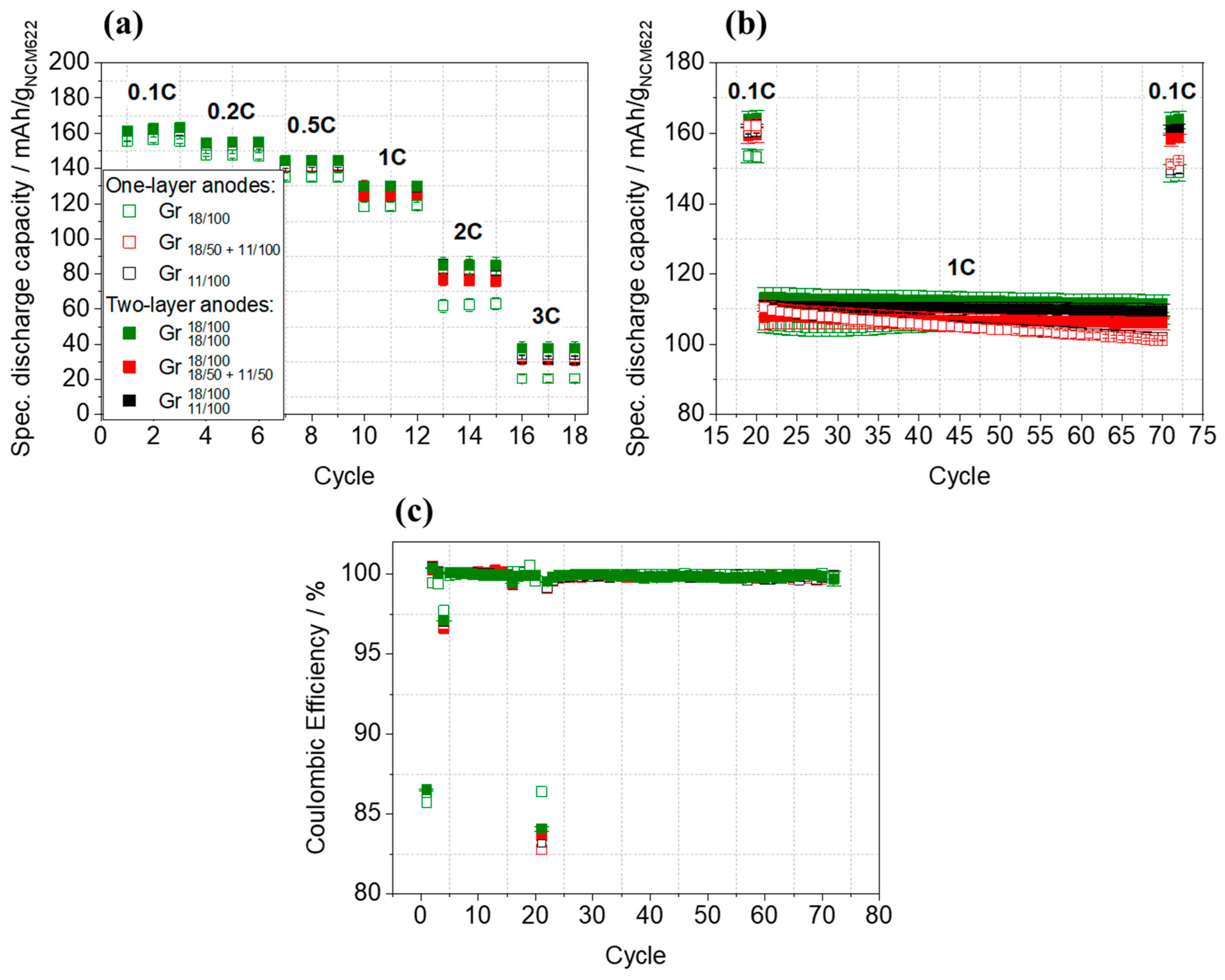
3.4. Electrochemical Characterization of Multilayer Anodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hannan, M.A.; Lipu, M.S.H.; Hussain, A.; Mohamed, A. A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: Challenges and recommendations. Renew. Sustain. Energy Rev. 2017, 78, 834–854. [Google Scholar] [CrossRef]
- Ku, T.-T.; Li, C.-S. Implementation of Battery Energy Storage System for an Island Microgrid with High PV Penetration. IEEE Trans. Ind. Appl. 2021, 57, 3416–3424. [Google Scholar] [CrossRef]
- Eftekhari, A. Lithium Batteries for Electric Vehicles: From Economy to Research Strategy. ACS Sustain. Chem. Eng. 2019, 7, 5602–5613. [Google Scholar] [CrossRef]
- European Environment Agency. Greenhouse Gas Emissions from Transport in Europe. Available online: https://www.eea.europa.eu/ims/greenhouse-gas-emissions-from-transport (accessed on 22 May 2023).
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Azam, M.A.; Safie, N.E.; Ahmad, A.S.; Yuza, N.A.; Zulkifli, N.S.A. Recent advances of silicon, carbon composites and tin oxide as new anode materials for lithium-ion battery: A comprehensive review. J. Energy Storage 2021, 33, 102096. [Google Scholar] [CrossRef]
- Wu, J.; Cao, Y.; Zhao, H.; Mao, J.; Guo, Z. The critical role of carbon in marrying silicon and graphite anodes for high-energy lithium-ion batteries. Carbon Energy 2019, 1, 57–76. [Google Scholar] [CrossRef]
- Larcher, D.; Beattie, S.; Morcrette, M.; Edström, K.; Jumas, J.-C.; Tarascon, J.-M. Recent findings and prospects in the field of pure metals as negative electrodes for Li-ion batteries. J. Mater. Chem. 2007, 17, 3759. [Google Scholar] [CrossRef]
- Naoki, N.; Gleb, Y. High-Capacity Anode Materials for Lithium-Ion Batteries: Choice of Elements and Structures for Active Particles. Part. Part. Syst. Charact. 2014, 31, 317–336. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Zhao, Q.; Dou, S.; Liu, H.; Huang, Y.; Hu, X. Si-containing precursors for Si-based anode materials of Li-ion batteries: A review. Energy Storage Mater. 2016, 4, 92–102. [Google Scholar] [CrossRef]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High Rate Capability of Graphite Negative Electrodes for Lithium-Ion Batteries. J. Power Sources 2005, 152, A474. [Google Scholar] [CrossRef]
- Simon, B.; Flandrois, S.; Guerin, K.; Fevrier-Bouvier, A.; Teulat, I.; Biensan, P. On the choice of graphite for lithium ion batteries. J. Power Sources 1999, 81–82, 312–316. [Google Scholar] [CrossRef]
- Yazami, R.; Touzain, P. A reversible graphite-lithium negative electrode for electrochemical generators. J. Power Sources 1983, 9, 365–371. [Google Scholar] [CrossRef]
- Han, Y.-J.; Kim, J.; Yeo, J.-S.; An, J.C.; Hong, I.-P.; Nakabayashi, K.; Miyawaki, J.; Jung, J.-D.; Yoon, S.-H. Coating of graphite anode with coal tar pitch as an effective precursor for enhancing the rate performance in Li-ion batteries: Effects of composition and softening points of coal tar pitch. Carbon 2015, 94, 432–438. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Wissler, M. Graphite and carbon powders for electrochemical applications. J. Power Sources 2006, 156, 142–150. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Umeno, T.; Abe, T.; Ogumi, Z. Improvement of natural graphite as a lithium-ion battery anode material, from raw flake to carbon-coated sphere. J. Mater. Chem. 2004, 14, 1754–1758. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Nichols, Handbook of Mineralogy: Volume I. Elements, Sulfides, Sulfosalts. Tucson, Arizona (Mineral Data Publishing), 1990. viii + 588 pp. Price $82.50 + $5.00 shipping and handling. Mineral. Mag. 1991, 55, 146–147. [Google Scholar] [CrossRef]
- Wu, Y.P.; Rahm, E.; Holze, R. Carbon anode materials for lithium ion batteries. J. Power Sources 2003, 114, 228–236. [Google Scholar] [CrossRef]
- Wu, Y.P.; Jiang, C.; Wan, C.; Holze, R. Anode materials for lithium ion batteries by oxidative treatment of common natural graphite. Solid State Ion. 2003, 156, 283–290. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Hara, Y.; Adachi, Y. Effect of Carbon Coating on Electrochemical Performance of Treated Natural Graphite as Lithium-Ion Battery Anode Material. J. Electrochem. Soc. 2000, 147, 1245. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, Y.; Li, J.; Song, Y.; Shi, J.; Guo, Q.; Liu, L. Synthesis and electrochemical properties of artificial graphite as an anode for high-performance lithium-ion batteries. Carbon 2013, 64, 553–556. [Google Scholar] [CrossRef]
- Zaghib, K.; Song, X.; Guerfi, A.; Rioux, R.; Kinoshita, K. Purification process of natural graphite as anode for Li-ion batteries: Chemical versus thermal. J. Power Sources 2003, 119–121, 8–15. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, K.H.; Jeon, Y.J.; Lim, S.H.; Lee, S.M. Si/C composite lithium-ion battery anodes synthesized using silicon nanoparticles from porous silicon. Electrochim. Acta 2014, 133, 73–81. [Google Scholar] [CrossRef]
- Endo, M.; Kim, C.; Nishimura, K.; Fujino, T.; Miyashita, K. Recent development of carbon materials for Li ion batteries. Carbon 2000, 38, 183–197. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamonds and Fullerenes: Processing, Properties and Applications; William Andrew: Norwich, NY, USA, 2012; ISBN 9780815517399. [Google Scholar]
- Xing, B.; Zhang, C.; Cao, Y.; Huang, G.; Liu, Q.; Zhang, C.; Chen, Z.; Yi, G.; Chen, L.; Yu, J. Preparation of synthetic graphite from bituminous coal as anode materials for high performance lithium-ion batteries. Fuel Process. Technol. 2018, 172, 162–171. [Google Scholar] [CrossRef]
- Yan, L.; Fang, Y.; Deng, J.; Zhu, Y.; Zhang, Y.; Cheng, J.; Zhao, X. Preparation and Characterization of Mesocarbon Microbeads by the Co-Polycondensation of High-Temperature Coal Tar Pitch and Coal Pyrolytic Extracts. Materials 2022, 15, 5136. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Park, K.; Song, J.; Hong, J.; Zhou, L.; Mai, Y.-W.; Huang, H.; Goodenough, J.B. Hollow carbon-nanotube/carbon-nanofiber hybrid anodes for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 16280–16283. [Google Scholar] [CrossRef]
- Park, T.-H.; Yeo, J.-S.; Seo, M.-H.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Enhancing the rate performance of graphite anodes through addition of natural graphite/carbon nanofibers in lithium-ion batteries. Electrochim. Acta 2013, 93, 236–240. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Ramakrishnan, S.; Park, B.-H.; Han, M.-K.; Yoo, D.J. Enhanced electrochemical performance and long-term durability of composite membranes through a binary interface with sulfonated unzipped graphite nanofibers for polymer electrolyte fuel cells operating under low relative humidity. Appl. Surf. Sci. 2022, 593, 153407. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K. Spherical carbon-coated natural graphite as a lithium-ion battery-anode material. Angew. Chem. Int. Ed Engl. 2003, 42, 4203–4206. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Doose, S.; Müller, J.; Höfels, C.; Kwade, A. Impact of Spheroidization of Natural Graphite on Fast-Charging Capability of Anodes for LIB. Batteries 2023, 9, 305. [Google Scholar] [CrossRef]
- Lämmerer, W.; Flachberger, H. Wissenswertes zur Charakterisierung und Aufbereitung von Rohgrafiten. Berg. Huettenmaenn. Monatsh. 2017, 162, 336–344. [Google Scholar] [CrossRef]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Wang, H.; Ikeda, T.; Fukuda, K.; Yoshio, M. Effect of milling on the electrochemical performance of natural graphite as an anode material for lithium-ion battery. J. Power Sources 1999, 83, 141–147. [Google Scholar] [CrossRef]
- Diehm, R.; Kumberg, J.; Dörrer, C.; Müller, M.; Bauer, W.; Scharfer, P.; Schabel, W. In-Situ Investigations of Simultaneous Two-Layer Slot Die Coating of Component Graded Anodes for improved High Energy Li-Ion Batteries. Energy Technol. 2020, 8, 1901251. [Google Scholar] [CrossRef]
- Kumberg, J.; Müller, M.; Diehm, R.; Spiegel, S.; Wachsmann, C.; Bauer, W.; Scharfer, P.; Schabel, W. Drying of Lithium-Ion Battery Anodes for Use in High-Energy Cells: Influence of Electrode Thickness on Drying Time, Adhesion, and Crack Formation. Energy Technol. 2019, 7, 1900722. [Google Scholar] [CrossRef]
- Chen, L.-C.; Liu, D.; Liu, T.-J.; Tiu, C.; Yang, C.-R.; Chu, W.-B.; Wan, C.-C. Improvement of lithium-ion battery performance using a two-layered cathode by simultaneous slot-die coating. J. Energy Storage 2016, 5, 156–162. [Google Scholar] [CrossRef]
- Gottschalk, L.; Oertel, C.; Strzelczyk, N.; Müller, J.; Krüger, J.; Haselrieder, W.; Kwade, A. Improving the Performance of Lithium-Ion Batteries Using a Two-Layer, Hard Carbon-Containing Silicon Anode for Use in High-Energy Electrodes. Energy Technol. 2022, 5, 2200858. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Meyer, C.; Bockholt, H.; Haselrieder, W.; Kwade, A. Characterization of the calendering process for compaction of electrodes for lithium-ion batteries. J. Mater. Process. Technol. 2017, 249, 172–178. [Google Scholar] [CrossRef]
- Haselrieder, W.; Westphal, B.; Bockholt, H.; Diener, A.; Höft, S.; Kwade, A. Measuring the coating adhesion strength of electrodes for lithium-ion batteries. Int. J. Adhes. Adhes. 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Westphal, B.G.; Mainusch, N.; Meyer, C.; Haselrieder, W.; Indrikova, M.; Titscher, P.; Bockholt, H.; Viöl, W.; Kwade, A. Influence of high intensive dry mixing and calendering on relative electrode resistivity determined via an advanced two point approach. J. Energy Storage 2017, 11, 76–85. [Google Scholar] [CrossRef]
- Froboese, L.; Titscher, P.; Westphal, B.; Haselrieder, W.; Kwade, A. Mercury intrusion for ion- and conversion-based battery electrodes—Structure and diffusion coefficient determination. Mater. Charact. 2017, 133, 102–111. [Google Scholar] [CrossRef]
- Weber, M.; Schoo, A.; Sander, M.; Mayer, J.K.; Kwade, A. Introducing Spectrophotometry for Quality Control in Lithium-Ion-Battery Electrode Manufacturing. Energy Technol. 2023, 11, 57. [Google Scholar] [CrossRef]
- Landesfeind, J.; Hattendorff, J.; Ehrl, A.; Wall, W.A.; Gasteiger, H.A. Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy. J. Electrochem. Soc. 2016, 163, A1373–A1387. [Google Scholar] [CrossRef]
- Morasch, R.; Landesfeind, J.; Suthar, B.; Gasteiger, H.A. Detection of Binder Gradients Using Impedance Spectroscopy and Their Influence on the Tortuosity of Li-Ion Battery Graphite Electrodes. J. Electrochem. Soc. 2018, 165, A3459–A3467. [Google Scholar] [CrossRef]
- Marks, T.; Trussler, S.; Smith, A.J.; Xiong, D.; Dahn, J.R. A Guide to Li-Ion Coin-Cell Electrode Making for Academic Researchers. J. Electrochem. Soc. 2011, 158, A51. [Google Scholar] [CrossRef]
- Laue, V.; Röder, F.; Krewer, U. Joint structural and electrochemical modeling: Impact of porosity on lithium-ion battery performance. Electrochim. Acta 2019, 314, 20–31. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Du, Z.; Daniel, C.; Dunlop, A.R.; Polzin, B.J.; Jansen, A.N.; Krumdick, G.K.; Wood, D.L. Impact of secondary particle size and two-layer architectures on the high-rate performance of thick electrodes in lithium-ion battery pouch cells. J. Power Sources 2021, 515, 230429. [Google Scholar] [CrossRef]
- Neidhart, L.; Fröhlich, K.; Eshraghi, N.; Cupid, D.; Winter, F.; Jahn, M. Aqueous Manufacturing of Defect-Free Thick Multi-Layer NMC811 Electrodes. Nanomaterials 2022, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Parikh, D.; Christensen, T.; Li, J. Correlating the influence of porosity, tortuosity, and mass loading on the energy density of LiNi0.6Mn0.2Co0.2O2 cathodes under extreme fast charging (XFC) conditions. J. Power Sources 2020, 474, 228601. [Google Scholar] [CrossRef]
- Parikh, D.; Christensen, T.; Hsieh, C.-T.; Li, J. Elucidation of Separator Effect on Energy Density of Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A3377–A3383. [Google Scholar] [CrossRef]
- Ogihara, N.; Kawauchi, S.; Okuda, C.; Itou, Y.; Takeuchi, Y.; Ukyo, Y. Theoretical and Experimental Analysis of Porous Electrodes for Lithium-Ion Batteries by Electrochemical Impedance Spectroscopy Using a Symmetric Cell. J. Electrochem. Soc. 2012, 159, A1034–A1039. [Google Scholar] [CrossRef]
- da Silva, M.T.Q.S.; do Rocio Cardoso, M.; Veronese, C.M.P.; Mazer, W. Tortuosity: A brief review. Mater. Today Proc. 2022, 58, 1344–1349. [Google Scholar] [CrossRef]
- Gottschalk, L.; Strzelczyk, N.; Adam, A.; Kwade, A. Influence of different anode active materials and blends on the performance and fast-charging capability of lithium-ion battery cells. J. Energy Storage 2023, 68, 107706. [Google Scholar] [CrossRef]
- Huber, K.; Adam, A.; Grießl, D.; Kwade, A. Understanding slurry mixing effects on the fast charging capability of lithium-ion battery cells: Methodology and case study. J. Power Sources 2022, 536, 231455. [Google Scholar] [CrossRef]
- Boyce, A.M.; Cumming, D.J.; Huang, C.; Zankowski, S.P.; Grant, P.S.; Brett, D.J.L.; Shearing, P.R. Design of Scalable, Next-Generation Thick Electrodes: Opportunities and Challenges. ACS Nano 2021, 15, 18624–18632. [Google Scholar] [CrossRef] [PubMed]

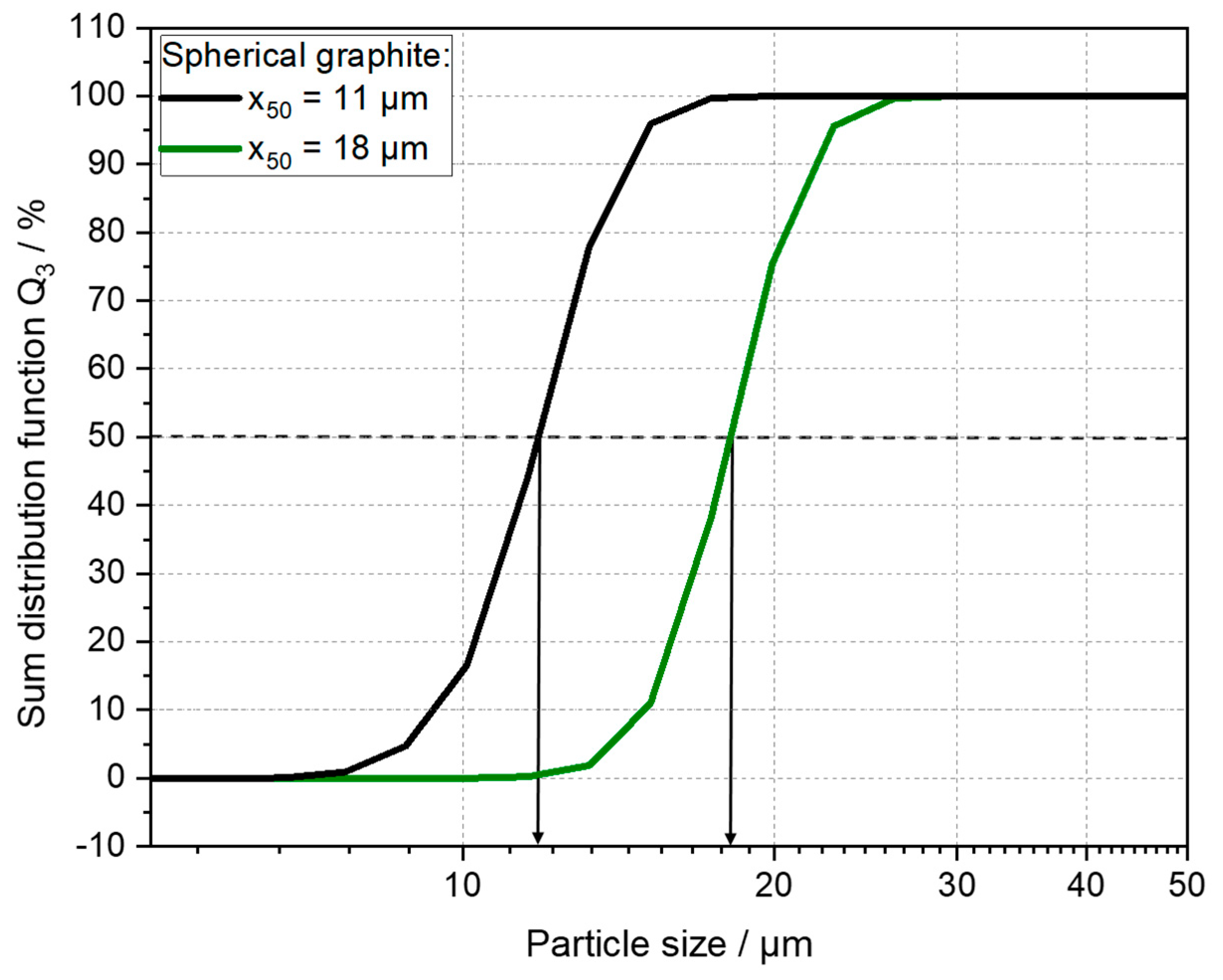

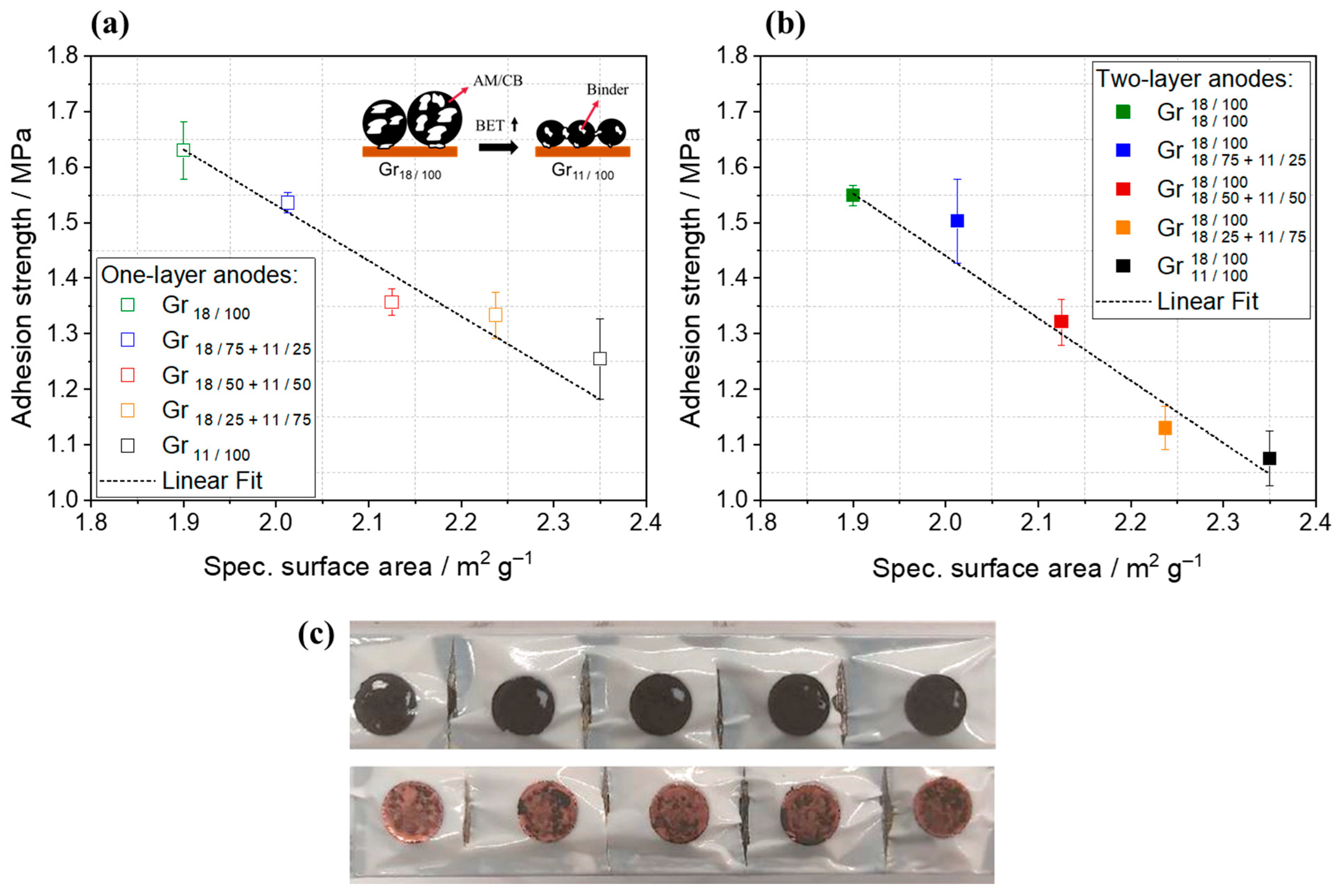
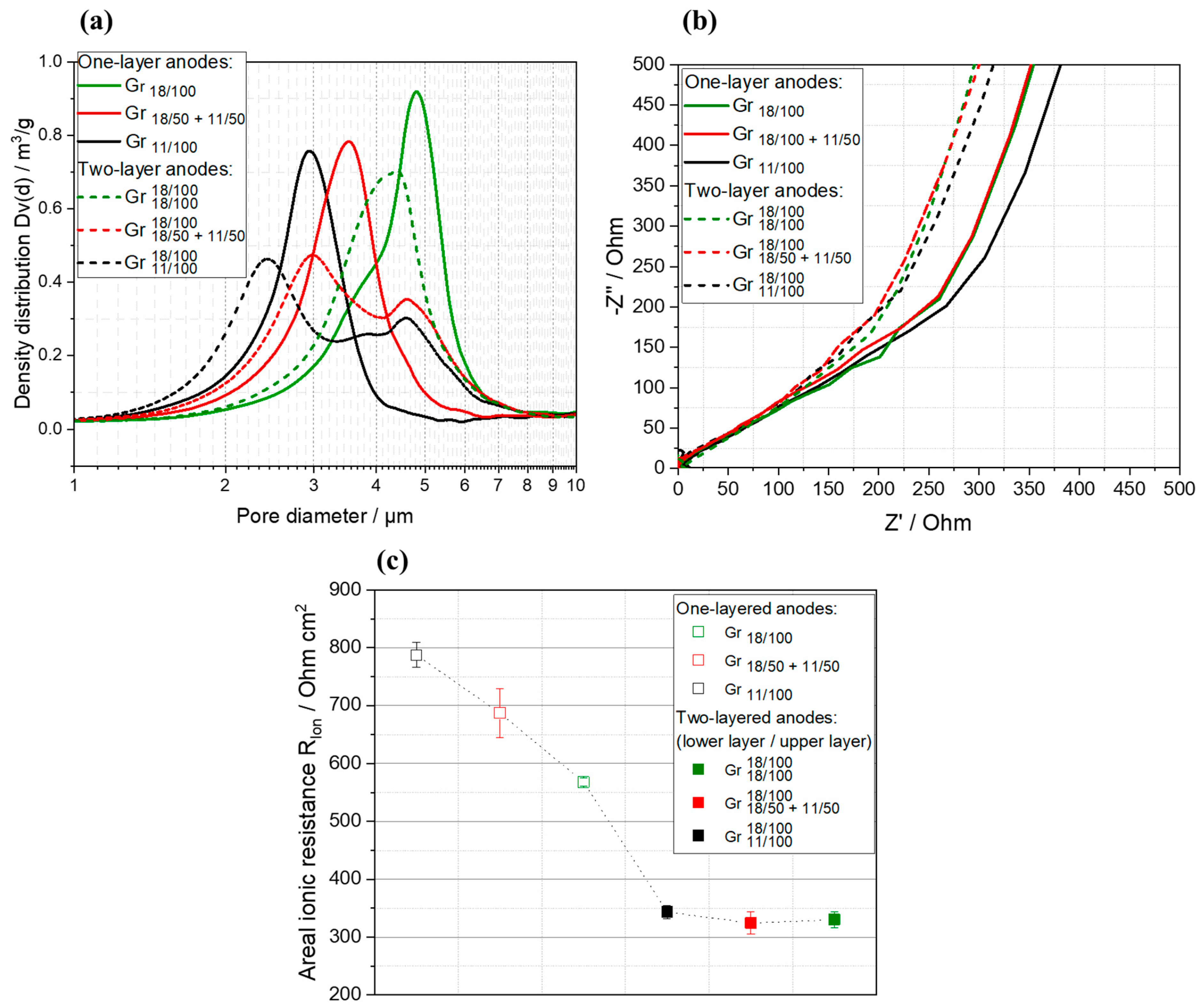
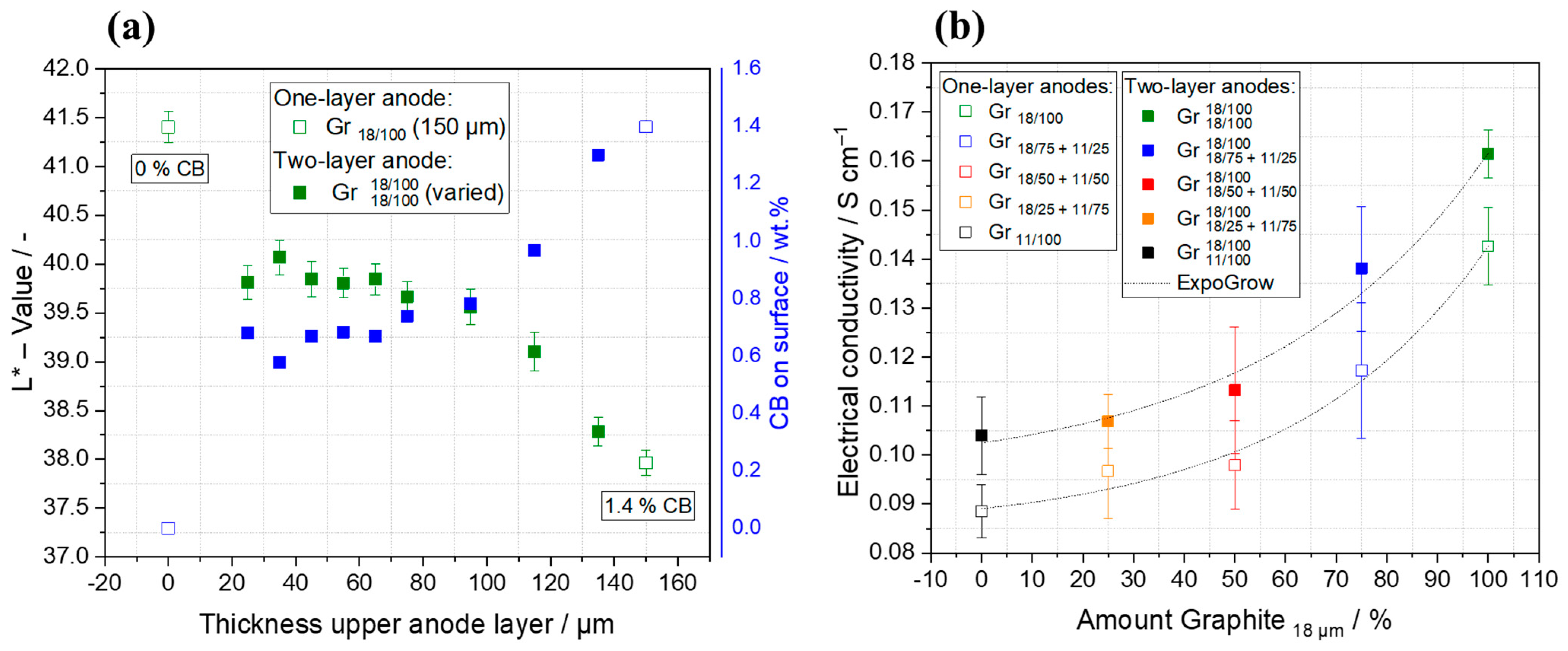
| Anode Active Material | Abbreviation | Particle Size x50 [µm] | Surface Area [m2/g] | Density [g/cm ³] | Capacity 1 [mAh/g] |
|---|---|---|---|---|---|
| Graphite x50 = 18 µm | Gr 18 | 17.6 | 1.9 | 2.20 | 364.8 |
| Graphite x50 = 11 µm | Gr 11 | 10.8 | 2.35 | 2.24 | 363.3 |
| Name | Ratio Gr 18:Gr 11 [%] | Gr 18 [wt. %] | Gr 11 [wt. %] | Carbon Black [wt. %] | Binder (Na-CMC + SBR) [wt. %] |
|---|---|---|---|---|---|
| Gr 18/100 | 100:0 | 93.00 | - | 1.40 | 5.60 |
| Gr 18/75+11/25 | 75:25 | 69.75 | 23.25 | 1.40 | 5.60 |
| Gr 18/50+11/50 | 50:50 | 46.50 | 46.50 | 1.40 | 5.60 |
| Gr 18/25+11/75 | 75:25 | 23.25 | 69.75 | 1.40 | 5.60 |
| Gr 11/100 | 0:100 | - | 93.00 | 1.40 | 5.60 |
| Anode | One-Layered | Anode Thickness [µm] | Anode Density [g/cm³] | Theoretical Porosity [%] |
| Gr 18/100 | One-layered | 135 | 0.95 | 57 |
| Gr 18/75+11/25 | One-layered | 127 | 1.02 | 54 |
| Gr 18/50+11/50 | One-layered | 128 | 1.01 | 55 |
| Gr 18/25+11/75 | One-layered | 126 | 1.03 | 54 |
| Gr 11/100 | One-layered | 125 | 1.03 | 54 |
| Two-Layered | Anode Thickness [µm] | Anode density [g/cm³] | Theoretical Porosity [%] | |
| Two-layered | 130 | 0.99 | 55 | |
| Two-layered | 126 | 1.03 | 54 | |
| Two-layered | 123 | 1.05 | 53 | |
| Two-layered | 122 | 1.06 | 52 | |
| Two-layered | 122 | 1.03 | 54 |
| Electrode | NCM622 [wt. %] | Conductive Graphite [wt. %] | Carbon Black [wt. %] | Binder (PVDF) [wt. %] |
|---|---|---|---|---|
| Cathode | 95.5 | 0.75 | 1.5 | 2.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottschalk, L.; Müller, J.; Schoo, A.; Baasch, E.; Kwade, A. Spherical Graphite Anodes: Influence of Particle Size Distribution and Multilayer Structuring in Lithium-Ion Battery Cells. Batteries 2024, 10, 40. https://doi.org/10.3390/batteries10020040
Gottschalk L, Müller J, Schoo A, Baasch E, Kwade A. Spherical Graphite Anodes: Influence of Particle Size Distribution and Multilayer Structuring in Lithium-Ion Battery Cells. Batteries. 2024; 10(2):40. https://doi.org/10.3390/batteries10020040
Chicago/Turabian StyleGottschalk, Laura, Jannes Müller, Alexander Schoo, Ernesto Baasch, and Arno Kwade. 2024. "Spherical Graphite Anodes: Influence of Particle Size Distribution and Multilayer Structuring in Lithium-Ion Battery Cells" Batteries 10, no. 2: 40. https://doi.org/10.3390/batteries10020040
APA StyleGottschalk, L., Müller, J., Schoo, A., Baasch, E., & Kwade, A. (2024). Spherical Graphite Anodes: Influence of Particle Size Distribution and Multilayer Structuring in Lithium-Ion Battery Cells. Batteries, 10(2), 40. https://doi.org/10.3390/batteries10020040







