Manipulating Electrolyte Interface Chemistry Enables High-Performance TiO2 Anode for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Characterization
2.3. Electrochemical Measurements
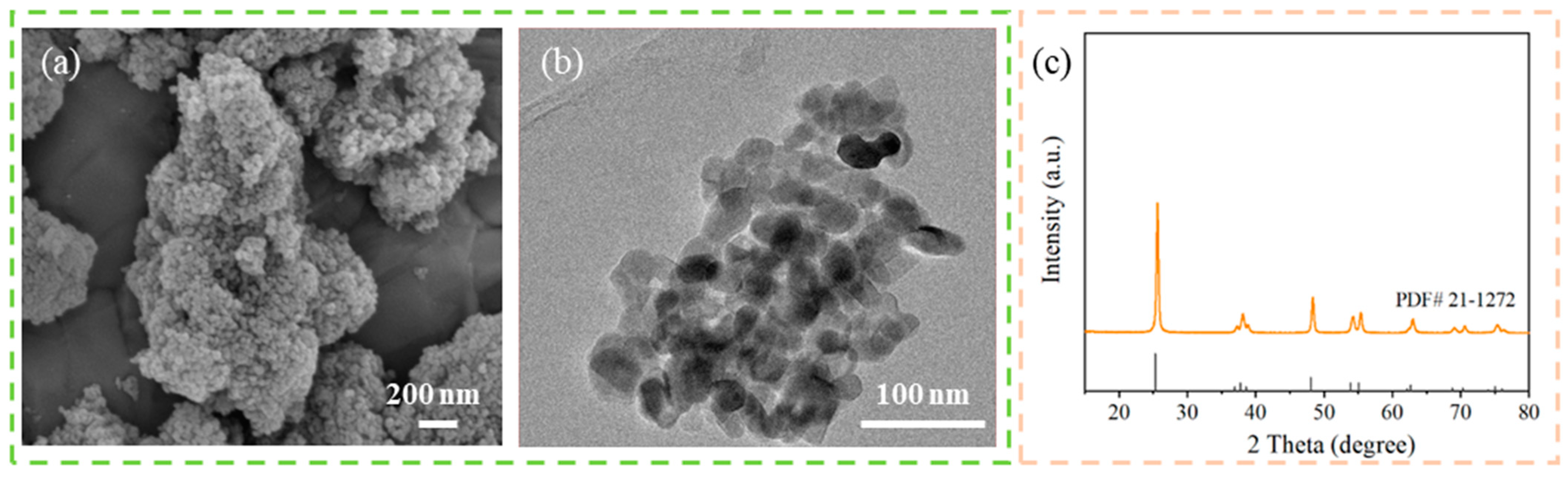
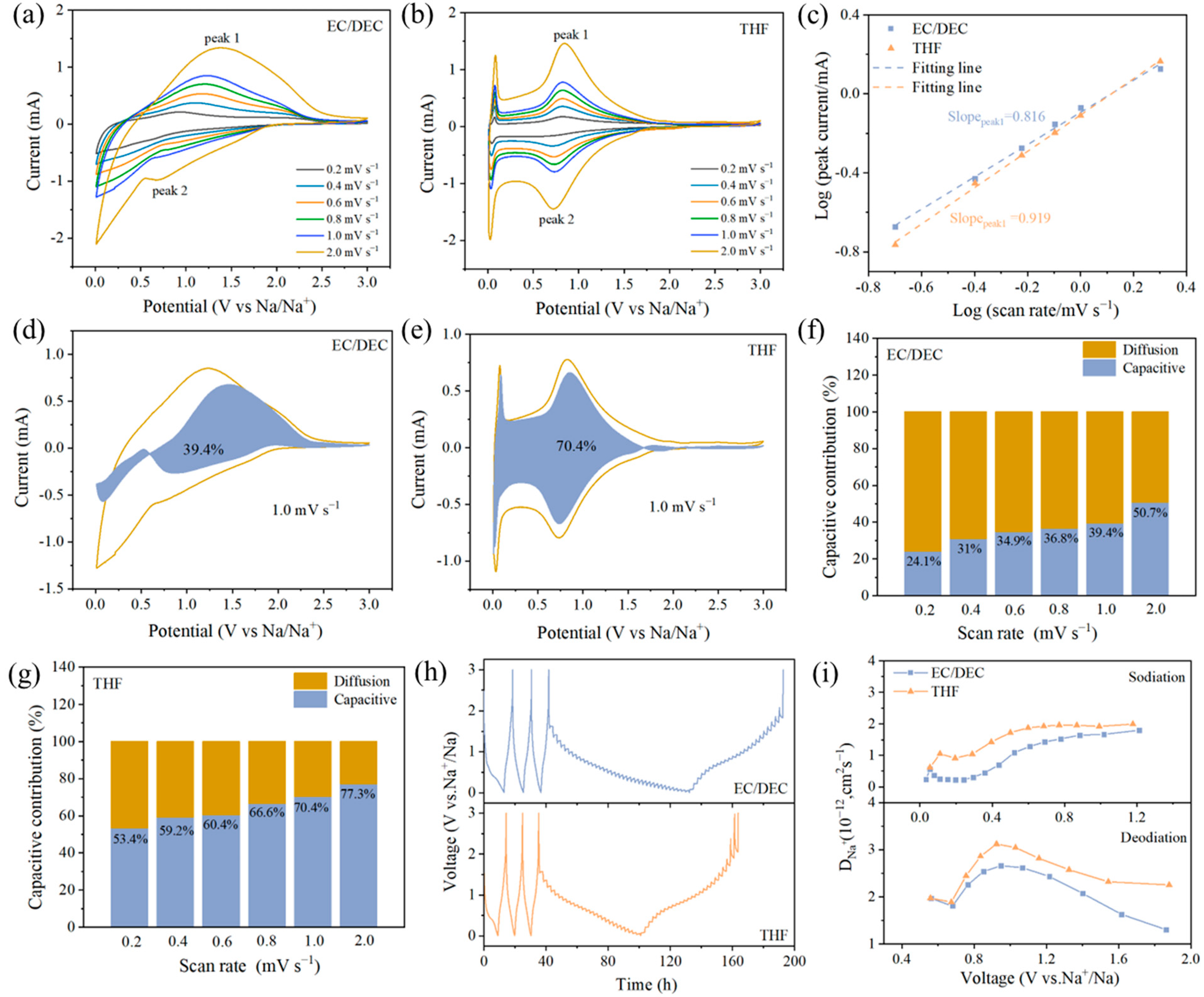
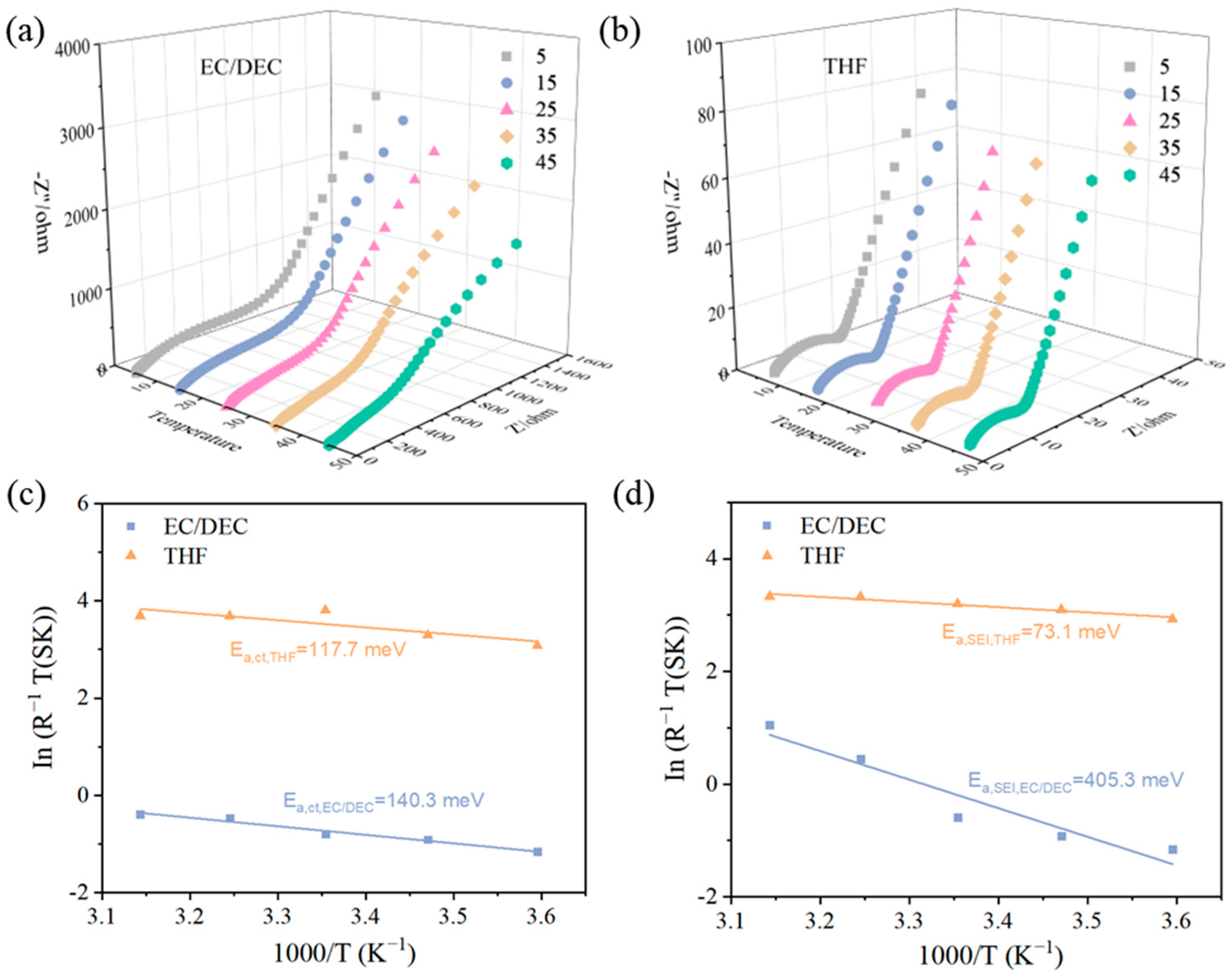
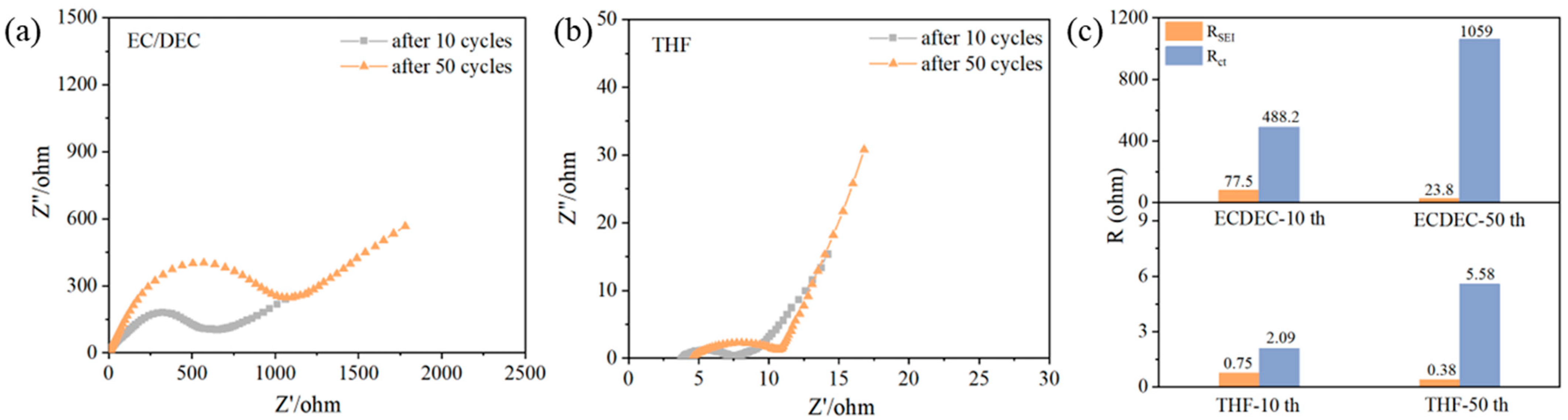
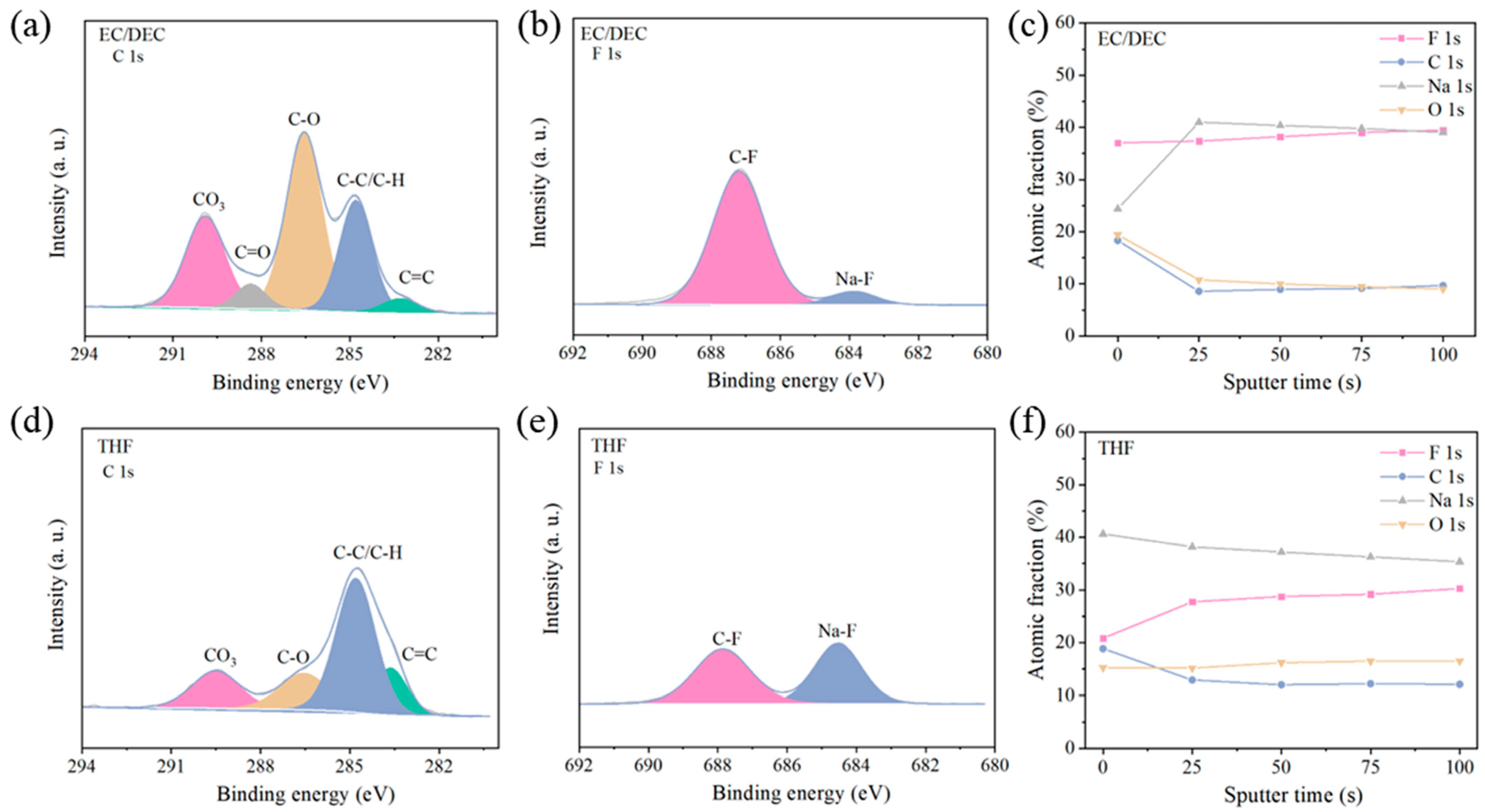
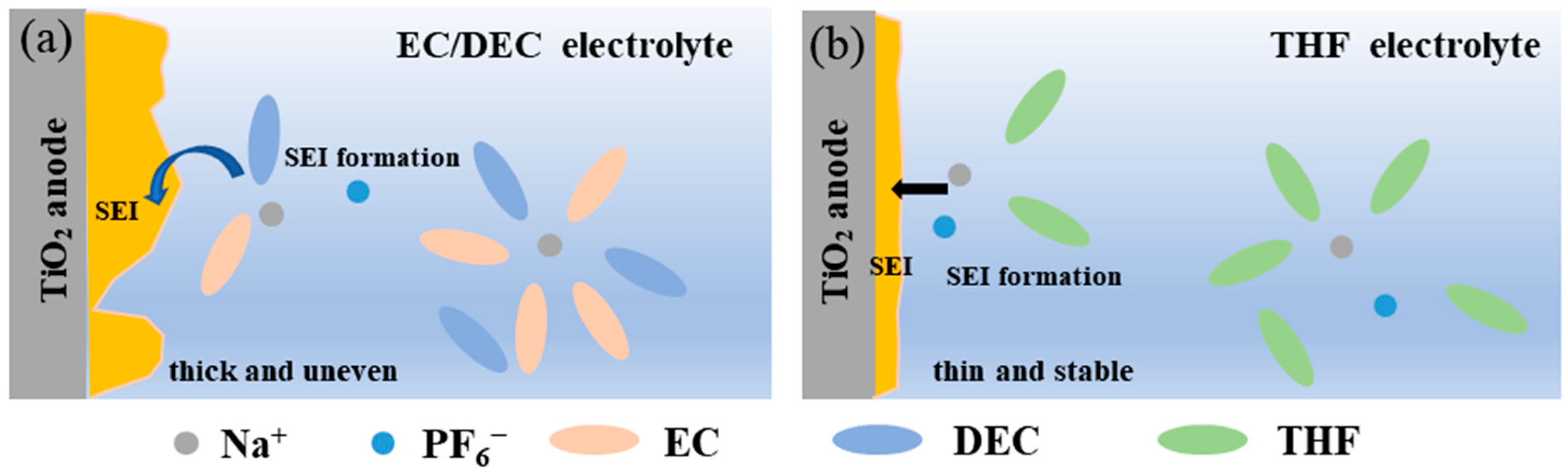
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Chen, M.; Meng, Q.; Zhang, J.; Feng, G.; Ai, X.; Cao, Y.; Chen, Z. Reconstructing Helmholtz Plane Enables Robust F-Rich Interface for Long-Life and High-Safe Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202407717. [Google Scholar] [CrossRef]
- Zhong, L.; Yue, M.; Liang, Y.; Xi, B.; An, X.; Xiao, Y.; Cheng, B.; Lei, S.; Xiong, S. In Situ Universal Construction of Thiophosphite/MXene Hybrids via Lewis Acidic Etching for Superior Sodium Storage. Adv. Funct. Mater. 2024, 2407740. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Z.; Zhang, R.; Sun, D.; Fu, L.; Tang, Y.; Li, H.; Xie, H.; Wang, H. Significantly Improving the Initial Coulombic Efficiency of TiO2 Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 40508–40518. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Lyu, J.; Tian, M.; Zhang, X.; Wang, K.; Yang, S.-W.; Zhang, Y.; Xu, G.Q. Unraveling the Synergistic Effects of Oxygen Vacancy and Amorphous Structure on TiO2 for High-Performance Lithium Storage. Small Struct. 2024, 5, 2300442. [Google Scholar] [CrossRef]
- Lv, D.; Wang, D.; Wang, N.; Liu, H.; Zhang, S.; Zhu, Y.; Song, K.; Yang, J.; Qian, Y. Nitrogen and fluorine co-doped TiO2/carbon microspheres for advanced anodes in sodium-ion batteries: High volumetric capacity, superior power density and large areal capacity. J. Energy Chem. 2022, 68, 104–112. [Google Scholar] [CrossRef]
- He, H.; Gan, Q.; Wang, H.; Xu, G.-L.; Zhang, X.; Huang, D.; Fu, F.; Tang, Y.; Amine, K.; Shao, M. Structure-dependent performance of TiO2/C as anode material for Na-ion batteries. Nano Energy 2018, 44, 217–227. [Google Scholar] [CrossRef]
- Diao, Z.; Wang, Y.; Zhao, D.; Zhang, X.; Mao, S.S.; Shen, S. Ultra-small TiO2 nanoparticles embedded in carbon nanosheets for high-performance sodium storage. Chem. Eng. J. 2021, 417, 127928. [Google Scholar] [CrossRef]
- Gan, Q.; He, H.; Zhu, Y.; Wang, Z.; Qin, N.; Gu, S.; Li, Z.; Luo, W.; Lu, Z. Defect-Assisted Selective Surface Phosphorus Doping to Enhance Rate Capability of Titanium Dioxide for Sodium Ion Batteries. ACS Nano 2019, 13, 9247–9258. [Google Scholar] [CrossRef]
- Fan, M.; Lin, Z.; Zhang, P.; Ma, X.; Wu, K.; Liu, M.; Xiong, X. Synergistic Effect of Nitrogen and Sulfur Dual-Doping Endows TiO2 with Exceptional Sodium Storage Performance. Adv. Energy Mater. 2021, 11, 2003037. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, C.; Nie, L.; Zang, S.; Chen, H.; Chen, N.; Ma, M.; Lai, Q. Electrolyte manipulation enhanced pseudo-capacitive K-storage for TiO2 anode. Appl. Surf. Sci. 2023, 611, 155617. [Google Scholar] [CrossRef]
- Tan, L.; Huang, X.; Yin, T.; Guo, Y.; Ning, T.; Mei, Y.; Zou, K.; Li, L.; Ji, X.; Zou, G. A 5 V ultrahigh energy density lithium metal capacitor enabled by the fluorinated electrolyte. Energy Storage Mater. 2024, 71, 103692. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhang, C.-H.; Guo, Y.-J.; Fan, M.; Zhao, Y.; Guo, H.; Wang, W.-P.; Tan, S.-J.; Yin, Y.-X.; Wang, F.; et al. Refined Electrolyte and Interfacial Chemistry toward Realization of High-Energy Anode-Free Rechargeable Sodium Batteries. J. Am. Chem. Soc. 2023, 145, 25643–25652. [Google Scholar] [CrossRef]
- Tang, X.; Xie, F.; Lu, Y.; Mao, H.; Chen, Z.; Pan, H.; Weng, S.; Yang, Y.; Li, X.; Guo, Z.; et al. Kinetics Manipulation for Improved Solid Electrolyte Interphase and Reversible Na Storage. ACS Energy Lett. 2024, 9, 1158–1167. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, C.; Ji, D.L.; Du, Y.Z.; Su, J.; Wang, N.A.; Yang, J.; Dou, S.X.; Qian, Y.T. Superior sodiophilicity and molecule crowding of crown ether boost the electrochemical performance of all- climate sodium- ion batteries. Proc. Natl. Acad. Sci. USA 2024, 121, e2312337121. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Liu, B.; Chen, J.; Jian, Q.; Li, Y.; Zhao, T. A Steric-Hindrance-Induced Weakly Solvating Electrolyte Boosting the Cycling Performance of a Micrometer-Sized Silicon Anode. ACS Energy Lett. 2023, 8, 3586–3594. [Google Scholar] [CrossRef]
- Jin, Y.; Le, P.M.L.; Gao, P.; Xu, Y.; Xiao, B.; Engelhard, M.H.; Cao, X.; Vo, T.D.; Hu, J.; Zhong, L.; et al. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat. Energy 2022, 7, 718–725. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Li, F.; Cheng, F.; Chen, J. Bulk Bismuth as a High-Capacity and Ultralong Cycle-Life Anode for Sodium-Ion Batteries by Coupling with Glyme-Based Electrolytes. Adv. Mater. 2017, 29, 1702212. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, H.; Wu, P.-F.; Zhou, S.-Y.; Huang, Y.-C.; Zhang, R.; Sun, D.; Tang, Y.-G.; Wang, H.-Y. Electrode–Electrolyte Interfacial Chemistry Modulation for Ultra-High Rate Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202200475. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Lim, K.; Park, K.-Y.; Yoon, G.; Seong, W.M.; Kang, K. Engineering Solid Electrolyte Interphase for Pseudocapacitive Anatase TiO2 Anodes in Sodium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802099. [Google Scholar] [CrossRef]
- Zhao, L.; Yin, J.; Lin, J.; Chen, C.; Chen, L.; Qiu, X.; Alshareef, H.N.; Zhang, W. Highly Stable ZnS Anodes for Sodium-Ion Batteries Enabled by Structure and Electrolyte Engineering. ACS Nano 2024, 18, 3763–3774. [Google Scholar] [CrossRef]
- Zou, K.; Deng, W.; Silvester, D.S.; Zou, G.; Hou, H.; Banks, C.E.; Li, L.; Hu, J.; Ji, X. Carbonyl Chemistry for Advanced Electrochemical Energy Storage Systems. ACS Nano 2024, 18, 19950–20000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Q.; Zeng, Y.; Tang, Z.; Sun, D.; Huang, D.; Tang, Y.; Wang, H. Weakly Solvating Cyclic Ether Electrolyte for High-Voltage Lithium Metal Batteries. ACS Energy Lett. 2023, 8, 1752–1761. [Google Scholar] [CrossRef]
- Yin, L.; Wang, M.; Xie, C.; Yang, C.; Han, J.; You, Y. High-Voltage Cyclic Ether-Based Electrolytes for Low-Temperature Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 9517–9523. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Yu, C.; Li, D.; Zhang, X.; Li, J.; Lu, T.; Pan, L. Improved sodium-ion storage performance of TiO2 nanotubes by Ni2+ doping. J. Mater. Chem. A 2016, 4, 11077–11085. [Google Scholar] [CrossRef]
- Feng, W.; Meng, C.; Guo, X.; Wu, B.; Sui, X.; Wang, Z. Defect-Driven Reconstruction of Na-Ion Diffusion Channels Enabling High-Performance Co-Doped TiO2 Anodes for Na-Ion Hybrid Capacitors. Adv. Energy Mater. 2024, 14, 2400558. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Zhuang, X.; Tomanec, O.; Zboril, R.; Yu, D.Y.W.; Rogach, A.L. Polypyrrole and Carbon Nanotube Co-Composited Titania Anodes with Enhanced Sodium Storage Performance in Ether-Based Electrolyte. Adv. Sustain. Syst. 2019, 3, 1800154. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Lin, D.; Wang, D.-W.; Li, B.; Lv, W.; Sun, S.; He, Y.-B.; Kang, F.; Yang, Q.-H.; et al. Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes. Nat. Commun. 2019, 10, 725. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Shi, W.; Leng, S.; Cheng, D.; Hou, H. Ultra-high initial coulombic efficiency of the TiO2 anode induced by the synergistic role of the electrolyte and binder for sodium-ion batteries. J. Mater. Chem. A 2023, 11, 17710–17717. [Google Scholar] [CrossRef]
- He, Y.; Liu, D.; Jiao, J.; Liu, Y.; He, S.; Zhang, Y.; Cheng, Q.; Fang, Y.; Mo, X.; Pan, H.; et al. Pyridinic N-Dominated Hard Carbon with Accessible Carbonyl Groups Enabling 98% Initial Coulombic Efficiency for Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2403144. [Google Scholar] [CrossRef]
- Wu, N.; Zhao, Z.; Hua, R.; Wang, X.; Zhang, Y.; Li, J.; Liu, G.; Guo, D.; Sun, G.; Liu, X.; et al. Pre-Doping of Dual-Functional Sodium to Weaken Fe–S Bond and Stabilize Interfacial Chemistry for High-Rate Reversible Sodium Storage. Adv. Energy Mater. 2024, 14, 2400371. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, N.; Liu, J.; Sun, S.; Wang, X.; Yang, J.; Yang, C.; Liu, Y. Ultralong Lifespan Zero-Cobalt/Nickel Prussian Blue Analogs Cathode Realized by Solid Solution Reaction Sodium Storage Mechanism. Adv. Funct. Mater. 2024, 2406809. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Dai, Y.; Zhang, H.; Zhang, Y.; Gu, S.; Wang, X.; Gao, T.; Zhou, G.; Xu, L. Heterostructure Interface Construction of Cobalt/Molybdenum Selenides toward Ultra-Stable Sodium-Ion Half/Full Batteries. Adv. Funct. Mater. 2024, 2406915. [Google Scholar] [CrossRef]
- Tian, S.; Chen, W.; Wang, R.; Qin, C.; Jiang, Z.-J.; Jiang, Z. Ar/NH3 Radio-Frequency Plasma Etching and N-doping to Stabilize Metallic Phase 1T-MoS2 for Fast and Durable Sodium-Ion Storage. Adv. Funct. Mater. 2024, 2408035. [Google Scholar] [CrossRef]
- Yi, X.; Li, X.; Zhong, J.; Wang, S.; Wang, Z.; Guo, H.; Wang, J.; Yan, G. Unraveling the Mechanism of Different Kinetics Performance between Ether and Carbonate Ester Electrolytes in Hard Carbon Electrode. Adv. Funct. Mater. 2022, 32, 2209523. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Sun, M.-Y.; Yu, F.-D.; Deng, L.; Xia, Y.; Jiang, Y.-S.; Que, L.-F.; Zhao, L.; Wang, Z.-B. Utilizing weakly-solvated diglyme-based electrolyte to achieve a 10,000-cycles durable Na3V2(PO4)2F3 cathode endured at −20 °C. Nano Energy 2022, 102, 107693. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, L.; Ding, H.; Xiong, Z.; Bai, L.; Xu, M.; Qi, Y. Carbon dots promoting surface defect and interphase high anion concentration for sodium-ion battery carbon anodes. Nano Energy 2024, 127, 109696. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, G.; Fang, W.; Qin, Z.; Zhang, T.; Yan, J.; Zhong, Y.; Zhang, N.; Chen, G. Transesterification Induced Multifunctional Additives Enable High-Performance Lithium Metal Batteries. Angew. Chem. Int. Ed. 2024, 63, e202403668. [Google Scholar] [CrossRef]
- Liu, M.; Wu, F.; Gong, Y.; Li, Y.; Li, Y.; Feng, X.; Li, Q.; Wu, C.; Bai, Y. Interfacial-Catalysis-Enabled Layered and Inorganic-Rich SEI on Hard Carbon Anodes in Ester Electrolytes for Sodium-Ion Batteries. Adv. Mater. 2023, 35, 2300002. [Google Scholar] [CrossRef]
- Meng, W.; Dang, Z.; Li, D.; Jiang, L. Long-Cycle-Life Sodium-Ion Battery Fabrication via a Unique Chemical Bonding Interface Mechanism. Adv. Mater. 2023, 35, 2301376. [Google Scholar] [CrossRef]
- Cheng, F.; Cao, M.; Li, Q.; Fang, C.; Han, J.; Huang, Y. Electrolyte Salts for Sodium-Ion Batteries: NaPF6 or NaClO4? ACS Nano 2023, 17, 18608–18615. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Giffin, G.A.; Castro, C.R.; Ochel, A.; Passerini, S. Unfolding the Mechanism of Sodium Insertion in Anatase TiO2 Nanoparticles. Adv. Energy Mater. 2015, 5, 1401142. [Google Scholar] [CrossRef]
- Portenkirchner, E.; Rommel, S.; Szabados, L.; Griesser, C.; Werner, D.; Stock, D.; Kunze-Liebhäuser, J. Sodiation mechanism via reversible surface film formation on metal oxides for sodium-ion batteries. Nano Sel. 2021, 2, 1533–1543. [Google Scholar] [CrossRef]
- Qin, M.; Zeng, Z.; Wu, Q.; Ma, F.; Liu, Q.; Cheng, S.; Xie, J. Microsolvating Competition in Li+ Solvation Structure Affording PC-Based Electrolyte with Fast Kinetics for Lithium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2406357. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Yun, X.; Li, J.; Yu, H.; Peng, L.; Xi, Z.; Wang, R.; Yang, L.; Xie, W.; et al. Anchored Weakly-Solvated Electrolytes for High-Voltage and Low-Temperature Lithium-ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202406596. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, R.; Sun, D.; Wang, H.; Tang, Y. Manipulating Electrolyte Interface Chemistry Enables High-Performance TiO2 Anode for Sodium-Ion Batteries. Batteries 2024, 10, 362. https://doi.org/10.3390/batteries10100362
Wang Q, Zhang R, Sun D, Wang H, Tang Y. Manipulating Electrolyte Interface Chemistry Enables High-Performance TiO2 Anode for Sodium-Ion Batteries. Batteries. 2024; 10(10):362. https://doi.org/10.3390/batteries10100362
Chicago/Turabian StyleWang, Qi, Rui Zhang, Dan Sun, Haiyan Wang, and Yougen Tang. 2024. "Manipulating Electrolyte Interface Chemistry Enables High-Performance TiO2 Anode for Sodium-Ion Batteries" Batteries 10, no. 10: 362. https://doi.org/10.3390/batteries10100362
APA StyleWang, Q., Zhang, R., Sun, D., Wang, H., & Tang, Y. (2024). Manipulating Electrolyte Interface Chemistry Enables High-Performance TiO2 Anode for Sodium-Ion Batteries. Batteries, 10(10), 362. https://doi.org/10.3390/batteries10100362




