Effects of Storage Voltage upon Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.1.1. Prussian White//Hard Carbon Chemistry (PW//HC)
2.1.2. Layered Oxide//Hard Carbon Chemistry (NMST//HC)
2.2. Electrochemical Performance
3. Results
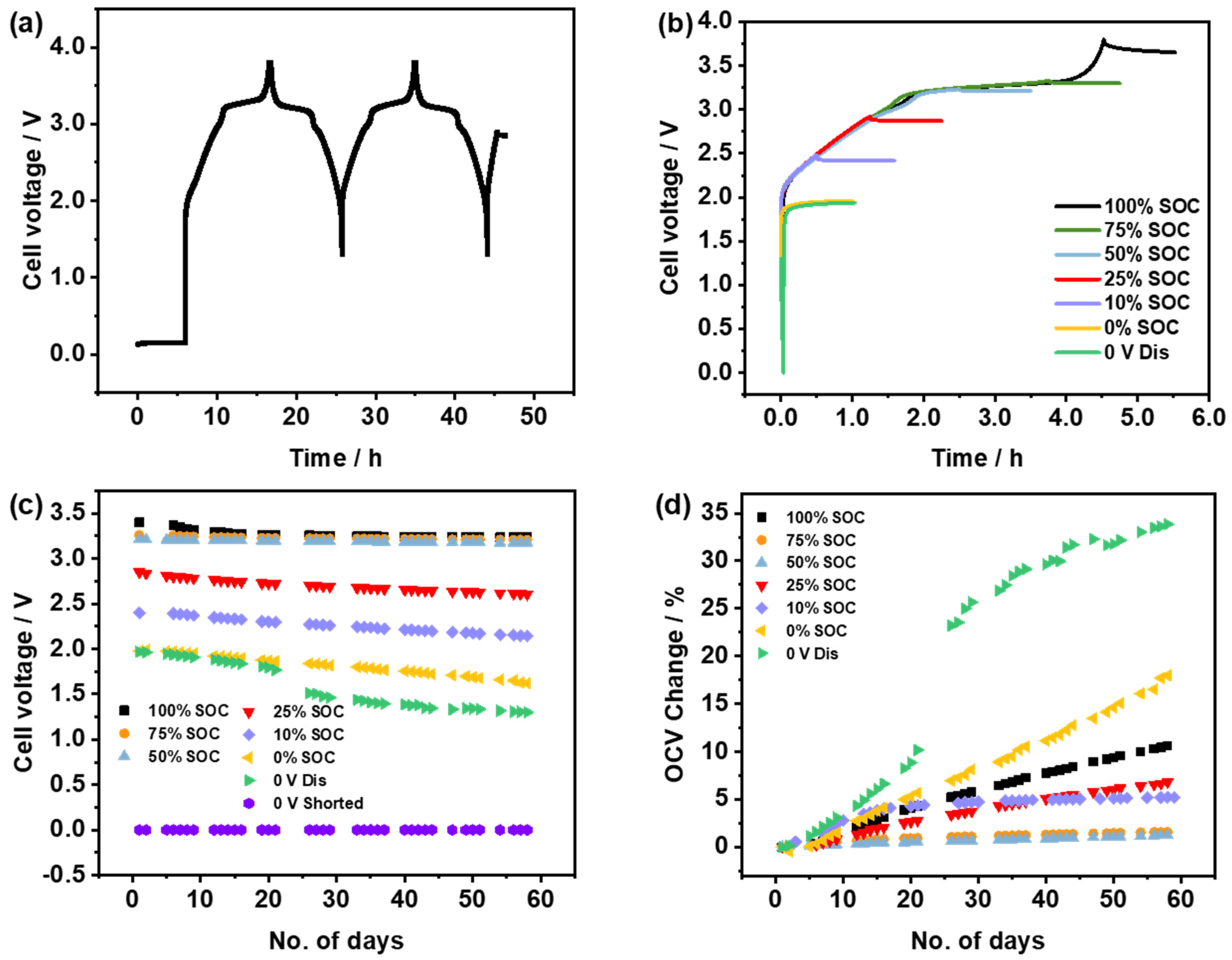
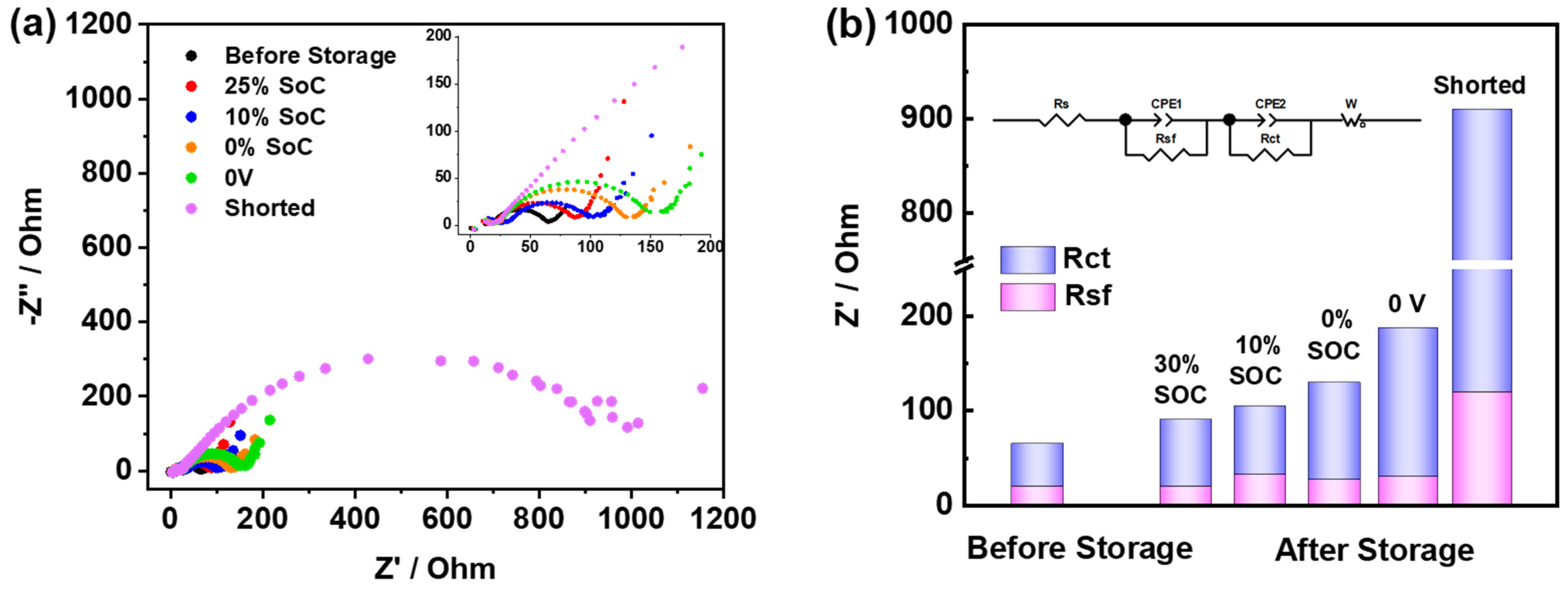
3.1. PW//HC Full Cell System
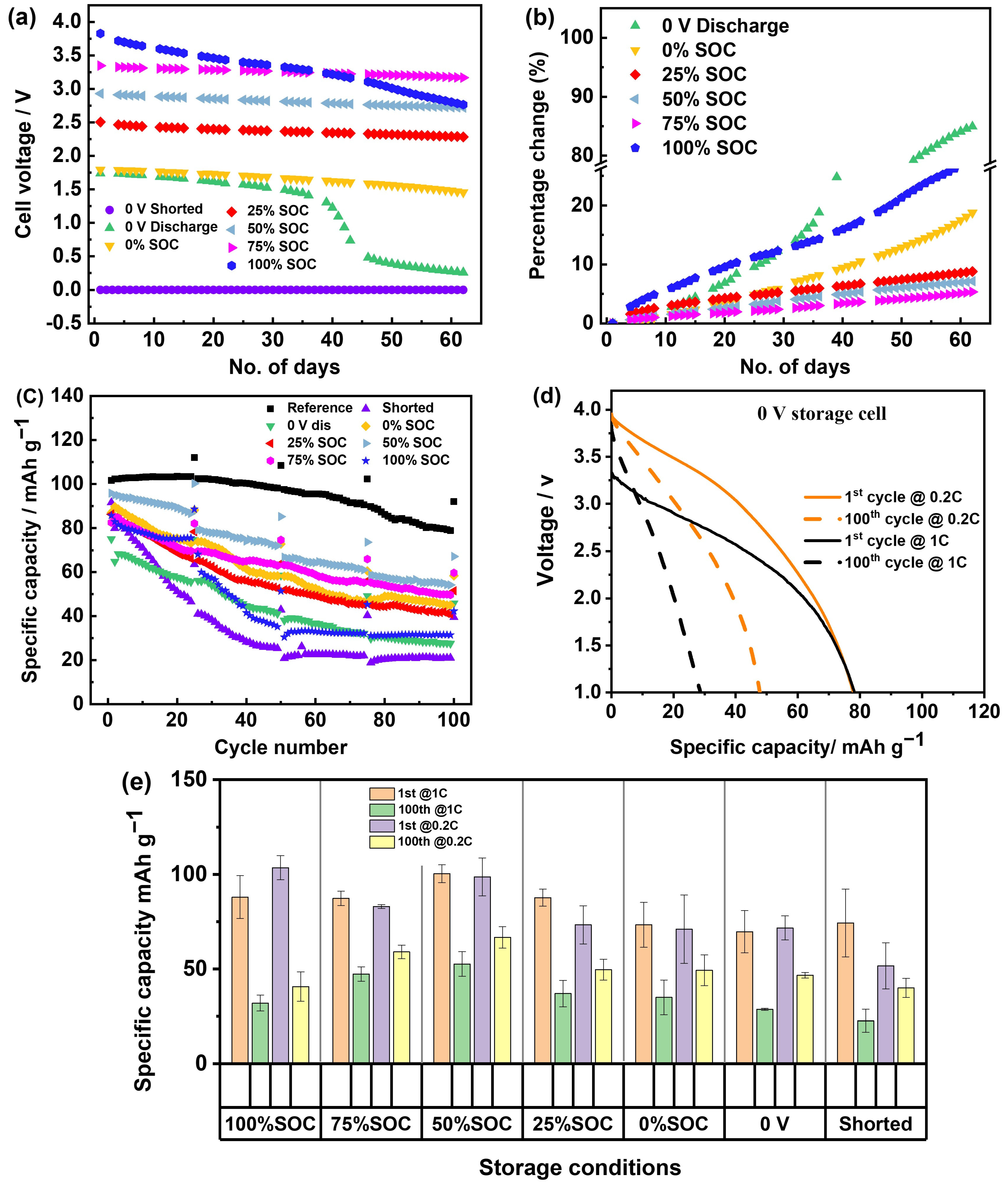
3.2. NMST//HC Full Cell System
4. Discussion
- Influence of current density: Previous studies conducted cycling tests at a charge and discharge current of 1/3 C, whereas this study was performed at 1 C. Although intermittent use of a lower current (0.2 C) during the cycle confirmed that the rapid capacity attenuation is due to increased impedance during storage, the capacity retention rate of NMST//HC chemistry under low-current conditions remains unsatisfactory.
- Influence of electrolyte: the electrolyte used in this study consists of EC:DEC in a 3:7 v/v% ratio, whereas the literature reports using an electrolyte composition of EC:DEC:PC in a 1:2:1 wt/wt ratio.
Evaluation of Reconditioning after Storage on Cell Performance
5. Conclusions
- The 0 V, 0% SOC, and 100% SOC show great self-discharge or an OCV drop. These three examples also exhibit higher impedance and the poorest cycling stability when cycled after storage.
- The 50% SOC and 75% SOC show the least OCV drop and exhibit cycling performance comparable to the reference cell (cell without storage).
- For storage conditions below 30% SOC, which is interesting due to practical considerations for transport safety, 25% SOC shows relatively less degradation and acceptable post-cycling stability.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bravo Diaz, L.; He, X.; Hu, Z.; Restuccia, F.; Marinescu, M.; Barreras, J.V.; Patel, Y.; Offer, G.; Rein, G. Review—Meta-Review of Fire Safety of Lithium-Ion Batteries: Industry Challenges and Research Contributions. J. Electrochem. Soc. 2020, 167, 090559. [Google Scholar] [CrossRef]
- Doughty, D.; Roth, E.P. A General Discussion of Li-Ion Battery Safety. Electrochem. Soc. Interface 2012, 21, 37–44. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for Lithium-Ion Battery Safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Song, J.; Vail, S.; Pan, W.; Barker, J.; Lu, Y. The Scale-up and Commercialization of Nonaqueous Na-Ion Battery Technologies. Adv. Energy Mater. 2018, 8, 1702869. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of High Energy Density Sodium-Ion Batteries: Faradion’s Journey and Outlook. J. Mater. Chem. A Mater. 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Huo, H.; Xing, Y.; Pecht, M.; Züger, B.J.; Khare, N.; Vezzini, A. Safety Requirements for Transportation of Lithium Batteries. Energies 2017, 10, 793. [Google Scholar] [CrossRef]
- IATA—Lithium Batteries Guidance Document. Available online: https://www.iata.org/en/programs/cargo/dgr/lithium-batteries/ (accessed on 21 September 2024).
- Zhao, M.; Kariuki, S.; Dewald, H.D.; Lemke, F.R.; Staniewicz, R.J.; Plichta, E.J.; Marsh, R.A. Electrochemical Stability of Copper in Lithium-Ion Battery Electrolytes. J. Electrochem. Soc. 2000, 147, 2874. [Google Scholar] [CrossRef]
- Maleki, H.; Howard, J.N. Effects of Overdischarge on Performance and Thermal Stability of a Li-Ion Cell. J. Power Sources 2006, 160, 1395–1402. [Google Scholar] [CrossRef]
- Roberts, S.; Kendrick, E. The Re-Emergence of Sodium Ion Batteries: Testing, Processing, and Manufacturability. Nanotechnol. Sci. Appl. 2018, 11, 23–33. [Google Scholar] [CrossRef]
- Rudola, A.; Sayers, R.; Wright, C.J.; Barker, J. Opportunities for Moderate-Range Electric Vehicles Using Sustainable Sodium-Ion Batteries. Nat. Energy 2023, 8, 215–218. [Google Scholar] [CrossRef]
- Tapia-Ruiz, N.; Armstrong, A.R.; Alptekin, H.; Amores, M.A.; Au, H.; Barker, J.; Boston, R.; Brant, W.R.; Brittain, J.M.; Chen, Y.; et al. 2021 Roadmap for Sodium-Ion Batteries. J. Phys. Energy 2021, 3, 031503. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, T.; Li, W.; Li, T.; Zhang, L.; Zhang, X.; Wang, Z. Engineering of Sodium-Ion Batteries: Opportunities and Challenges. Engineering 2023, 24, 172–183. [Google Scholar] [CrossRef]
- US11159027B2—Storage and/or Transportation of Sodium-Ion Cells—Google Patents. Available online: https://patents.google.com/patent/US11159027B2/en (accessed on 21 September 2024).
- Rudola, A.; Wright, C.J.; Barker, J. Reviewing the Safe Shipping of Lithium-Ion and Sodium-Ion Cells: A Materials Chemistry Perspective. Energy Mater. Adv. 2021, 2021, 9798460. [Google Scholar] [CrossRef]
- Desai, P.; Huang, J.; Foix, D.; Tarascon, J.M.; Mariyappan, S. Zero Volt Storage of Na-Ion Batteries: Performance Dependence on Cell Chemistry! J. Power Sources 2022, 551, 232177. [Google Scholar] [CrossRef]
- Robinson, J.B.; Finegan, D.P.; Heenan, T.M.M.; Smith, K.; Kendrick, E.; Brett, D.J.L.; Shearing, P.R. Microstructural Analysis of the Effects of Thermal Runaway on Li-Ion and Na-Ion Battery Electrodes. J. Electrochem. Energy Convers. Storage 2018, 15, 011010. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Gourang Patnaik, S.; Escher, I.; Ferrero, G.A.; Adelhelm, P. Electrochemical Study of Prussian White Cathodes with Glymes—Pathway to Graphite-Based Sodium-Ion Battery Full Cells. Batter. Supercaps 2022, 5, e202200043. [Google Scholar] [CrossRef]
- Pfeifer, K.; Arnold, S.; Becherer, J.; Das, C.; Maibach, J.; Ehrenberg, H.; Dsoke, S. Can Metallic Sodium Electrodes Affect the Electrochemistry of Sodium-Ion Batteries? Reactivity Issues and Perspectives. ChemSusChem 2019, 12, 3312–3319. [Google Scholar] [CrossRef]
- Song, T.; Chen, L.; Gastol, D.; Dong, B.; Marco, J.F.; Berry, F.; Slater, P.; Reed, D.; Kendrick, E. High-Voltage Stabilization of O3-Type Layered Oxide for Sodium-Ion Batteries by Simultaneous Tin Dual Modification. Chem. Mater. 2022, 34, 4153–4165. [Google Scholar] [CrossRef]
- Conder, J.; Villevieille, C. How reliable is the Na metal as a counter electrode in Na-ion half cells? Chem. Commun. 2019, 55, 1275–1278. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Lu, Y.; Hu, Y.S.; Li, J. A high-performance sodium-ion battery enhanced by macadamia shell derived hard carbon anode. Nano Energy 2017, 39, 489–498. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Liu, W.; Zhang, C.; Li, S.; Li, J. High-performance sodium-ion batteries with a hard carbon anode: Transition from the half-cell to full-cell perspective. Nanoscale 2019, 11, 22196–22205. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Chen, L.; Kishore, B.; Dancer, C.E.J.; Simmons, M.J.H.; Kendrick, E. Mechanism of Gelation in High Nickel Content Cathode Slurries for Sodium-Ion Batteries. J. Colloid Interface Sci. 2022, 627, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Kinoshita, Y.; Misaki, K.; Matsuyama, T.; Komaba, S. Electrochemical Properties of LiCoO2 Electrodes with Latex Binders on High-Voltage Exposure. J. Electrochem. Soc. 2015, 162, A538–A544. [Google Scholar] [CrossRef]
- Harris, O.C.; Lee, S.E.; Lees, C.; Tang, M. Review: Mechanisms and Consequences of Chemical Cross-Talk in Advanced Li-Ion Batteries. J. Phys. Energy 2020, 2, 032002. [Google Scholar] [CrossRef]
- Ma, L.A.; Buckel, A.; Hofmann, A.; Nyholm, L.; Younesi, R. Fundamental Understanding and Quantification of Capacity Losses Involving the Negative Electrode in Sodium-Ion Batteries. Adv. Sci. 2024, 11, 2306771. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Kishore, B.; Lakhdar, Y.; Chen, L.; Slater, P.R.; Kendrick, E. Effects of Storage Voltage upon Sodium-Ion Batteries. Batteries 2024, 10, 361. https://doi.org/10.3390/batteries10100361
Song T, Kishore B, Lakhdar Y, Chen L, Slater PR, Kendrick E. Effects of Storage Voltage upon Sodium-Ion Batteries. Batteries. 2024; 10(10):361. https://doi.org/10.3390/batteries10100361
Chicago/Turabian StyleSong, Tengfei, Brij Kishore, Yazid Lakhdar, Lin Chen, Peter R. Slater, and Emma Kendrick. 2024. "Effects of Storage Voltage upon Sodium-Ion Batteries" Batteries 10, no. 10: 361. https://doi.org/10.3390/batteries10100361
APA StyleSong, T., Kishore, B., Lakhdar, Y., Chen, L., Slater, P. R., & Kendrick, E. (2024). Effects of Storage Voltage upon Sodium-Ion Batteries. Batteries, 10(10), 361. https://doi.org/10.3390/batteries10100361







