Sustainable Synthesis of a Carbon-Supported Magnetite Nanocomposite Anode Material for Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Sample Synthesis
2.2. Sample Characterization and Electrochemical Measurement
3. Results and Discussion
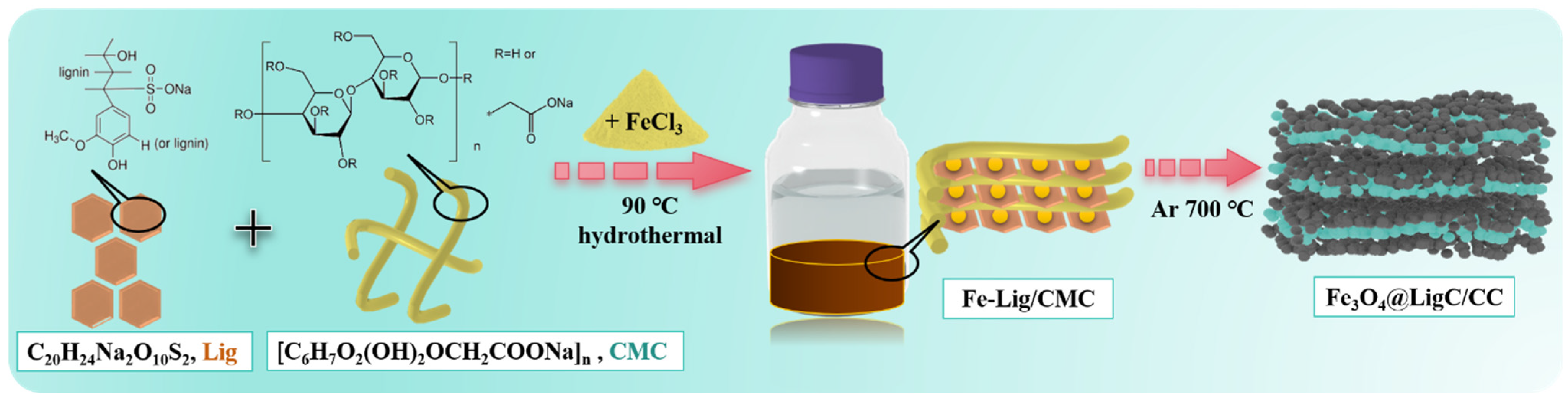
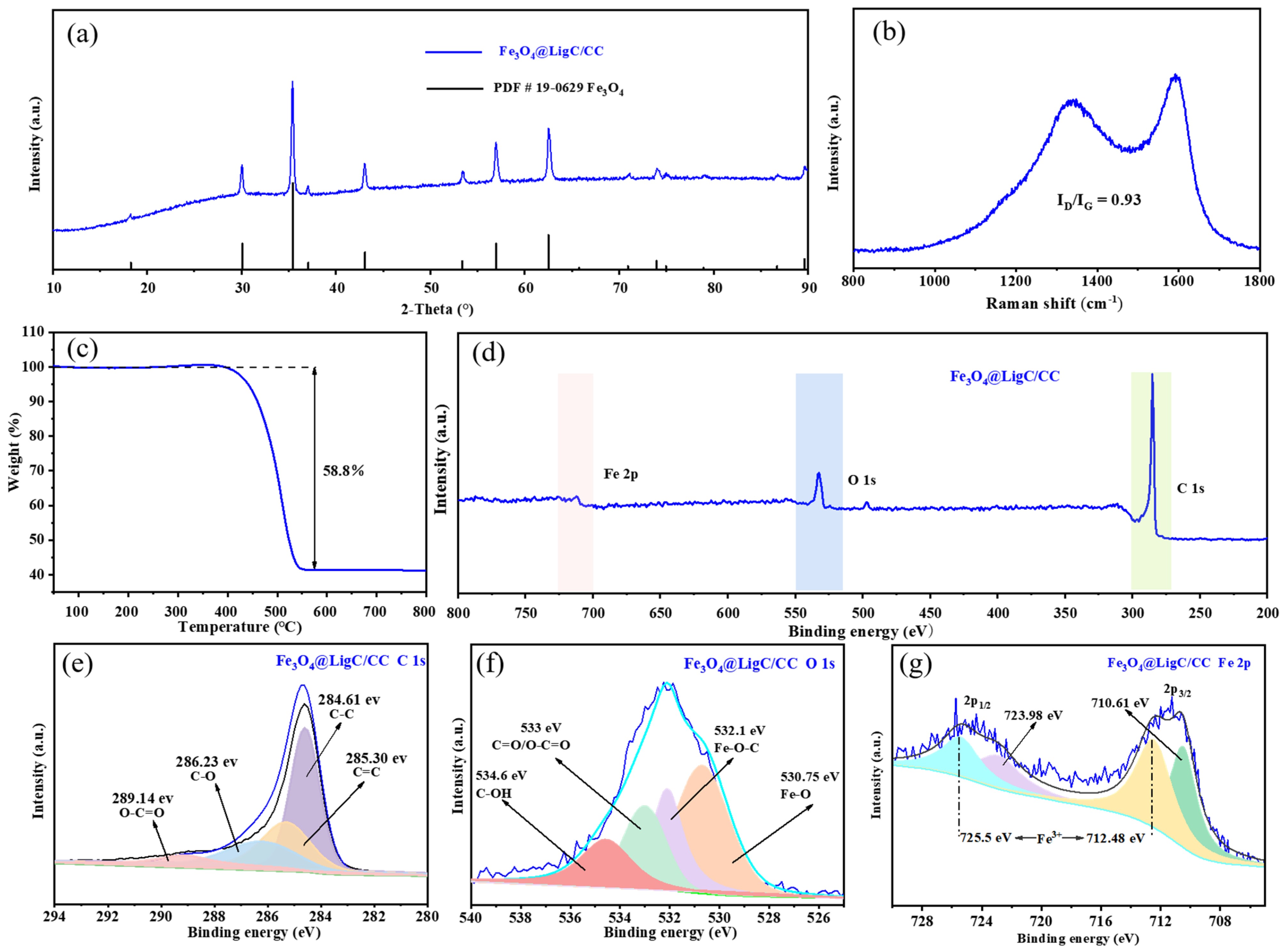
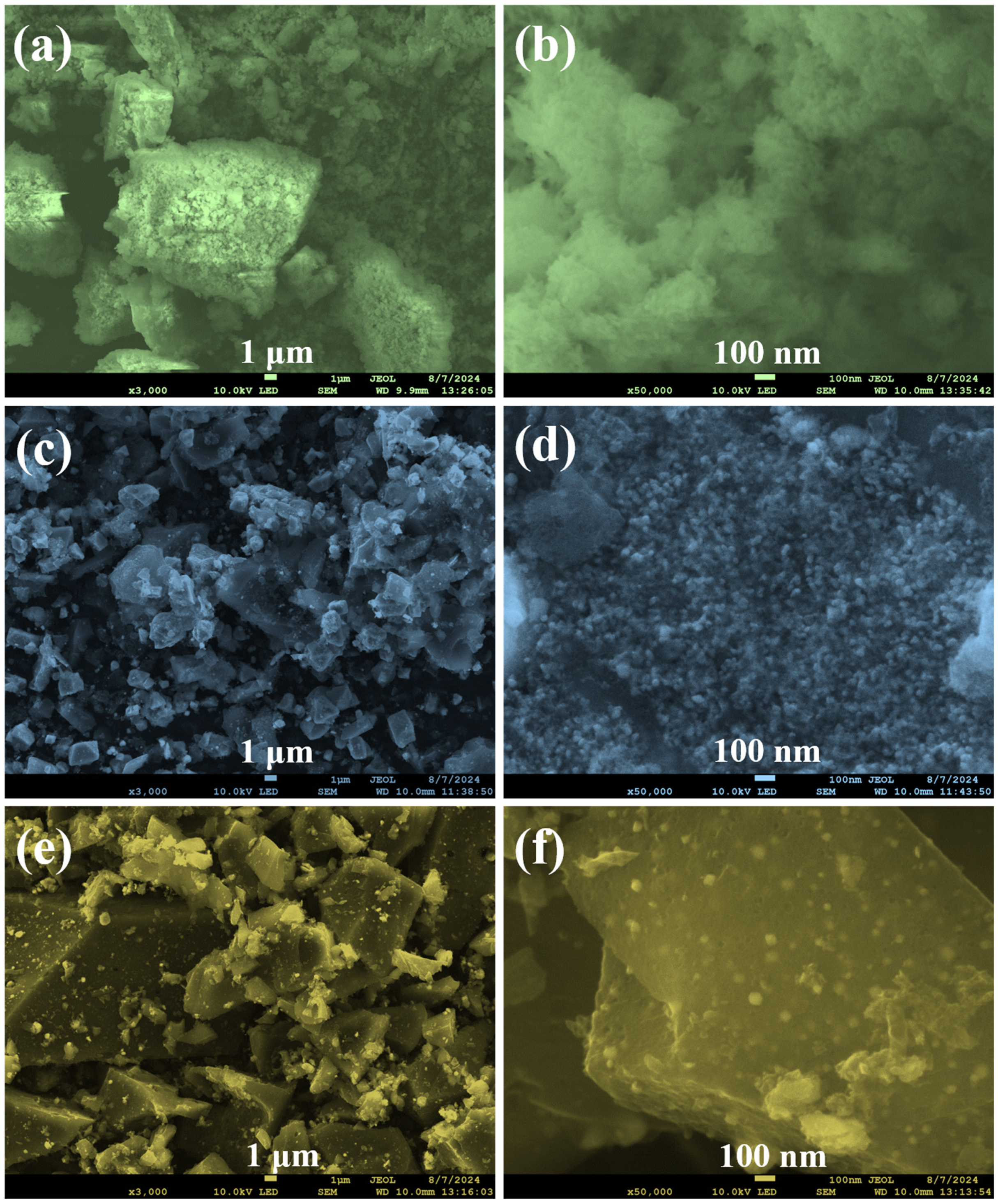
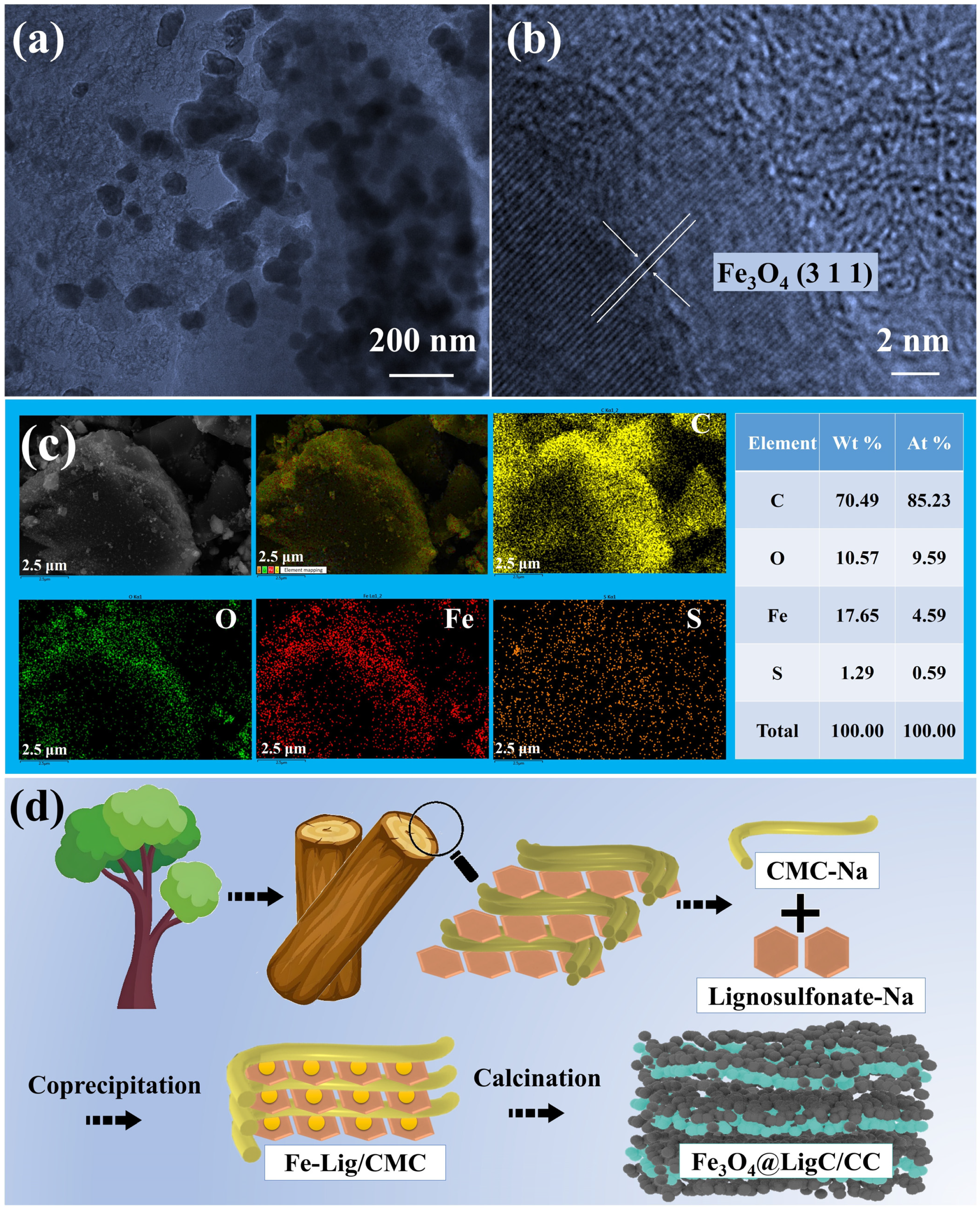
3.1. Engineer the Fe3O4@LigC/CC Nanocomposite from Natural Products
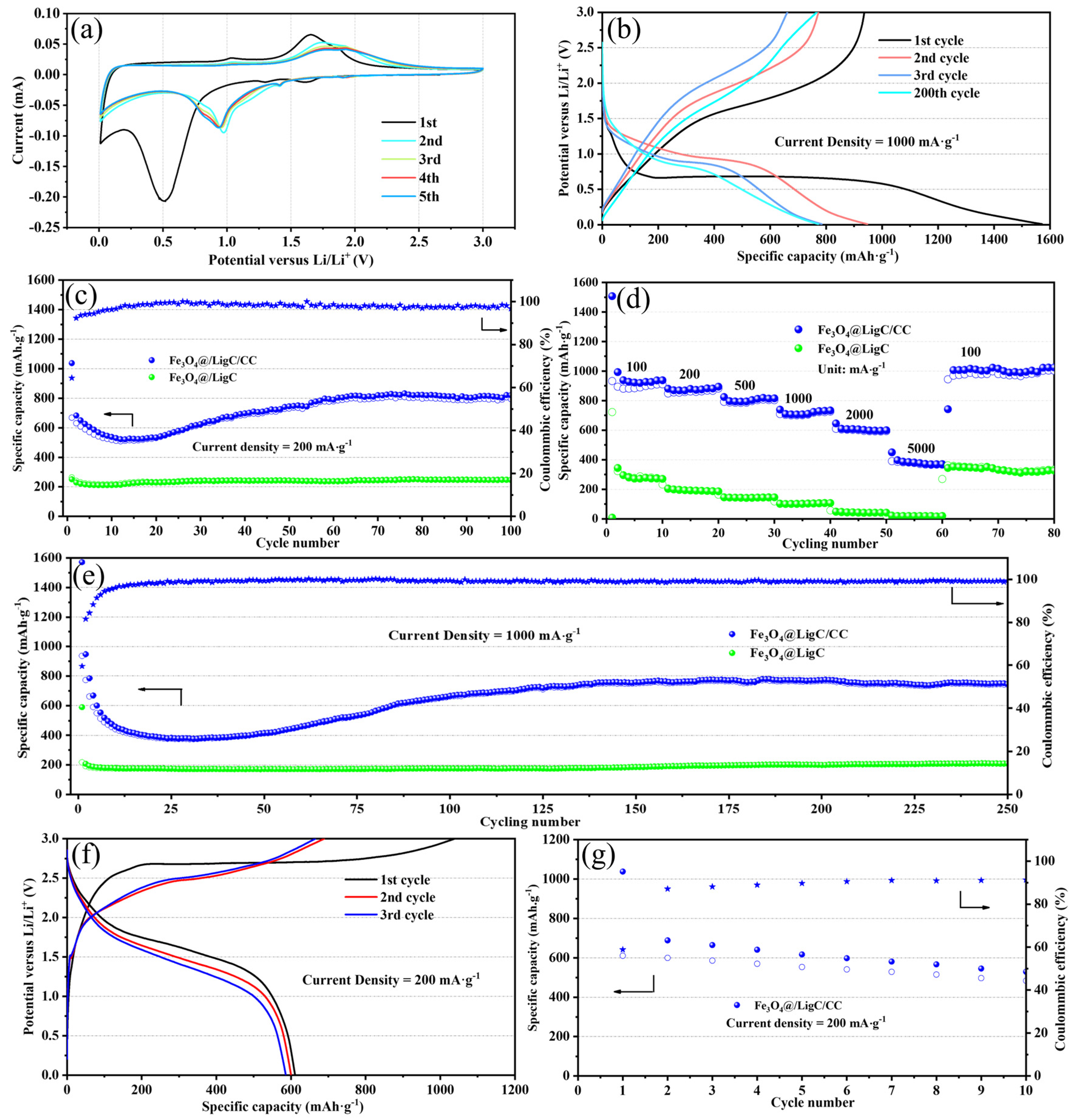
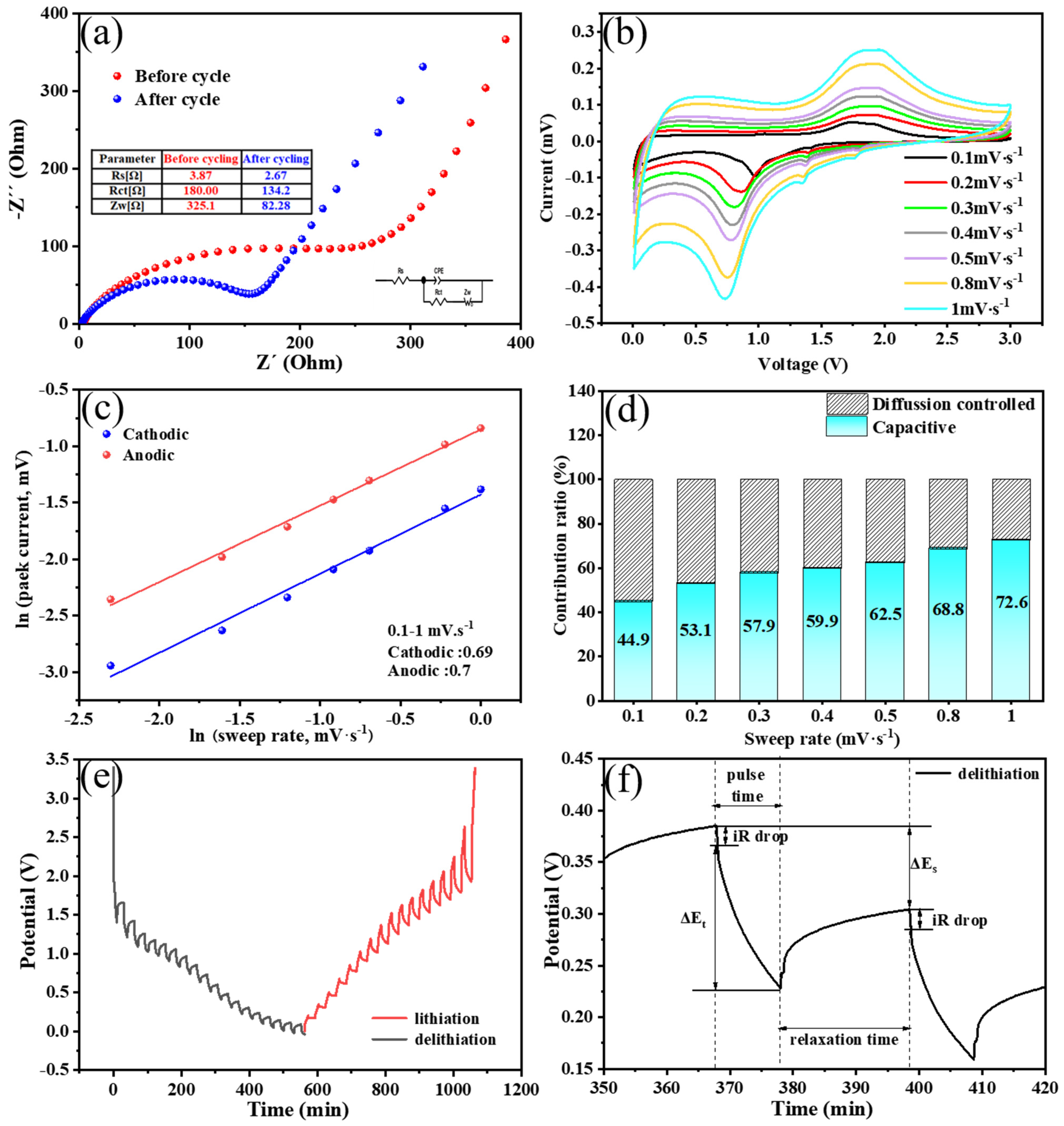
3.2. Lithium-Ion Storage Performances of the Fe3O4@LigC/CC Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Mihit, H.P.; Manikandan, P.; Vilas, G.P. Reserve lithium-ion batteries: Deciphering in situ lithiation of lithium-ion free vanadium pentoxide cathode with graphitic anode. Carbon 2023, 203, 561–570. [Google Scholar] [CrossRef]
- Kebede, A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Shia, C.; Wang, T.Y.; Liao, X.B.; Qie, B.Y.; Yang, P.F.; Chen, M.J.; Wang, X.; Srinivasane, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar] [CrossRef]
- Cavers, H.; Molaiyan, P.; Abdollahifar, M.; Lassi, U.; Kwade, A. Perspectives on Improving the Safety and Sustainability of High Voltage Lithium-Ion Batteries Through the Electrolyte and Separator Region. Adv. Energy Mater. 2022, 12, 2200147–2200179. [Google Scholar] [CrossRef]
- Chen, M.; Wang, E.; Liu, Q.; Guo, X.; Chen, W.; Chou, S.L.; Dou, S.X. Recent progress on iron- and manganese-based anodes for sodium-ion and potassium-ion batteries. Energy Storage Mater. 2019, 19, 163–178. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Jia, R.X.; Yu, L.B.; Han, Z.Q.; Liu, S.; Shang, P.P.; Deng, S.Q.; Liu, X.H.; Xu, B.H. Synthesis of a MOF-derived magnetite quantum dots on surface modulated reduced graphene oxide composite for high-rate lithium-ion storage. RSC Appl. Interfaces. 2024, 1, 233–244. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, S.Y.; Xie, X.Y.; Li, Q.X.; Ding, L.; Chen, M.L.; Tu, J.; Xing, K.Y.; Cheng, Y.H. Target-triggered Fe3O4@NPC-UCNPs assembly for photoactivatable biosensing of Aflatoxin B1. Chem. Eng. J. 2023, 407, 144028. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Qiu, L.G.; Yuan, Y.P.; Zhu, Y.J.; Jiang, X.; Xiao, J.D. Magnetic Fe3O4@C/Cu and Fe3O4@CuO core–shell composites constructed from MOF-based materials and their photocatalytic properties under visible light. Appl. Catal. B-Environ. 2014, 144, 863–869. [Google Scholar] [CrossRef]
- Purna, K.B.; Gitashree, D.; Priyakshree, B.; Benjamin, L.O.; Manash, R.D. Fe3O4 quantum dots anchored on functionalized graphene: A multimodal platform for sensing and remediation of Cr (VI). Chem. Eng. J. 2023, 474, 145797. [Google Scholar] [CrossRef]
- Wang, L.J.; Liu, F.H.; Pal, A.; Ning, Y.S.; Wang, S.; Zhao, B.Y.; Bradley, R.; Wu, W.P. Ultra-small Fe3O4 nanoparticles encapsulated in hollow porous carbon nanocapsules for high performance supercapacitors. Carbon 2021, 179, 327–336. [Google Scholar] [CrossRef]
- Dühnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. Toward Green Battery Cells: Perspective on Materials and Technologies. Small Methods 2020, 4, 2000039. [Google Scholar] [CrossRef]
- Wu, X.J.; Jiang, C.; Wang, J.; Pu, Y.; Ragauskas, A.; Li, S.; Yang, B. Lignin-derived electrochemical energy materials and systems. Biofuels. Bioprod. Biorefin. 2020, 14, 650–672. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.; Ralph, T.J.; Kennema, M.P.C.; Bruijnincx, B.M. Weckhuysen. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. Engl. 2016, 55, 8164. [Google Scholar] [CrossRef]
- Lai, X.; Jin, C.; Yi, W.; Han, X.; Feng, X.; Zheng, Y.; Ouyang, M. Mechanism, modeling, detection, and prevention of the internal short circuit in lithium-ion batteries: Recent advances and perspectives. Energy Storage Mater. 2021, 35, 470–499. [Google Scholar] [CrossRef]
- Pachfule, P.; Shinde, D.; Majumder, M.; Xu, Q. Fabrication of carbon nanorods and graphene nanoribbons from a metal–organic framework. Nat. Chem 2016, 8, 718–724. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Li, S.R.; Chen, C.H.; Yan, L.F. Self-Assembly and Embedding of Nanoparticles by In Situ Reduced Graphene for Preparation of a 3D Graphene/Nanoparticle Aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xing, H.; Zhu, Y.; Ji, X.; Liu, Z.; Wang, L. Synthesis and enhanced microwave-absorbing properties of SnO2/α-Fe2O3@RGO composites. J. Mater. Sci. Mater. Electron. 2017, 28, 13896–13904. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Liu, C.; Wang, S.; Zhou, P.; Zhou, T.; Miao, Z.; Xing, W.; Zhuo, S.; Zhou, J. Fluorinated multi-walled carbon nanotubes as cathode materials of lithium and sodium primary batteries: Effect of graphitization of carbon nanotubes. J. Mater. Chem. A 2019, 7, 7128–7137. [Google Scholar] [CrossRef]
- Li, H.H.; Saini, A.; Xu, R.Y.; Wang, N.; Lv, X.X.; Wang, Y.P.; Yang, T.; Chen, L.; Jiang, H.B. Hierarchical Fe3O4@C nanofoams derived from metal–organic frameworks for high-performance lithium storage. Rare Met. 2020, 39, 1072–1081. [Google Scholar] [CrossRef]
- Wei, Z.; Sarwar, S.; Azam, S.; Ahasan, M.R.; Voyda, M.; Zhang, X.; Wang, R. Ultrafast microwave synthesis of MoTe2@graphenecomposites accelerating polysulfide conversion and promoting Li2S nucleation for high-performance Li-S batteries. J. Colloid Interface Sci. 2023, 635, 391–405. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, G.; Xia, Y.; Wang, H. One-dimensional hybrid nanocomposite of high-density monodispersed Fe3O4 nanoparticles and carbon nanotubes for high-capacity storage of lithium and sodium. J. Mater. Chem. A 2016, 47, 18532–18542. [Google Scholar] [CrossRef]
- Cui, Y.; Feng, W.; Liu, W.; Li, J.; Zhang, Y.; Du, Y.; Li, M.; Huang, W.; Wang, H.; Liu, S. Template-assisted loading of Fe3O4 nanoparticles inside hollow carbon “rooms” to achieve high volumetric lithium storage. Nanoscale 2020, 12, 10816–10826. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.; Chen, X.; Zheng, J.; Que, L.; Yu, F.; Zhao, J.; Xie, Y.; Huang, M.; Lu, C.; et al. Tea-Derived Sustainable Materials. Adv. Funct. Mater. 2024, 34, 2310226. [Google Scholar] [CrossRef]
- Xu, B.H.; Guan, X.G.; Zhang, L.Y.; Liu, X.W.; Jiao, Z.B.; Liu, X.H.; Hu, X.; Zhao, X.S. A simple route to preparing γ-Fe2O3/RGO composite electrode materials for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 4048–4054. [Google Scholar] [CrossRef]
- Ma, C.; Shi, J.; Zhao, Y.; Song, N.J.; Wang, Y. A novel porous reduced microcrystalline graphene oxide supported Fe3O4@C nanoparticle composite as anode material with excellent lithium storage performances. Chem. Eng. J. 2017, 326, 507–517. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Liang, J.; Tang, K.; Zhu, Y.; Qian, Y. One-step thermolysis synthesis of two-dimensional ultrafine Fe3O4 particles/carbon nanonetworks for high-performance lithium-ion batteries. Nanoscale 2016, 8, 4733–4741. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.L.; Shan, L.; Zhang, R.; Bao, S.C.; Tu, M.Y.; Jia, R.X.; Yu, L.B.; Li, H.; Xu, B.H. Controllable engineering magnetite nanoparticles dispersed in a hierarchical amylose derived carbon and reduced graphene oxide framework for lithium-ion storage. J. Colloid Interface Sci. 2022, 628, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhong, K.; Yu, R.; Liu, Z.; Zhang, Y.; Wu, J.; Zhou, L.; Mai, L. Enveloping SiOx in N-doped carbon for durable lithium storage via an eco-friendly solvent-free approach. J. Mater. Chem. 2020, 8, 13285–13291. [Google Scholar] [CrossRef]
- Duan, X.; Liu, J.Q.; Lv, F.S.; Liu, T.; Cui, W.B.; Wang, J.; Wang, Q.; Yuan, S. 3D porous structure Fe3O4@SnO2/MXene composites with enhanced electrochemical performance for lithium ion battery anode. J. Energy Storage. 2024, 86, 111308. [Google Scholar] [CrossRef]
- Lv, X.X.; Zhang, Y.; Lin, W.; Yang, A.W.; Liang, J. Facile synthesis of Fe3O4 ultrathin layer coated Fe2O3 composite anode for enhanced lithium-ion storage. J. Electroanal Chem. 2023, 947, 117758. [Google Scholar] [CrossRef]
- Wu, Q.C.; Qiu, S.; Yin, M.; Li, R.; Wang, Y.; Jin, D.Q.; Yang, R.H.; Yong, D.M.; Xie, W. Hydrogenated titanium dioxide modifed core–shell structure Fe3O4@NiO for lithium-ion battery anode material. Ionics 2023, 29, 2227–2240. [Google Scholar] [CrossRef]
- Li, M.; Ma, W.; Tan, F. Fe3O4@C-500 anode derived by commercial ammonium ferric citrate for advanced lithium ion batteries. J. Power Sources 2023, 574, 233146. [Google Scholar] [CrossRef]
- Hu, W.; He, K.; Wu, S. Ordered sandwich silicon quantum dot/Fe3O4/reduced graphene oxide architectures for high-performance lithium-ion batteries. J. Alloy Compd. 2023, 943, 168947. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Q.; Hu, W. Preparation of hollow core-shell Fe3O4/nitrogen-doped carbon nanocomposites for lithium-ion batteries. Molecules 2022, 27, 396. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dai, Q.; Cui, C. Cake-like porous Fe3O4@C nanocomposite as high-performance anode for Li-ion battery. J. Electroanal. Chem. 2022, 918, 116508. [Google Scholar] [CrossRef]
- Sumit, R.S.; Jagannatham, M.; Gautam, R.; Rao Rikka, V.; Prakash, R.; Mallikarjunaiah, K.J.; Srinivas Reddy, G. A facile synthesis of raspberry-shaped Fe3O4 nanoaggregate and its magnetic and lithium-ion storage properties. Mater Sci. Eng. B Adv. 2022, 282, 115771. [Google Scholar] [CrossRef]
- Xu, J.L.; Zhang, X.; Miao, Y.X.; Wen, M.-X.; Yan, W.-Y.; Lu, P.; Wang, Z.-R.; Sun, Q. In-situ plantation of Fe3O4@C nanoparticles on reduced graphene oxide nanosheet as high-performance anode for lithium/sodium-ion batteries. Appl. Surf. Sci. 2021, 546, 149163. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, J.; Qi, J.; Li, Z.; Wang, C.; Chen, M. N-Doped Dual Carbon-Confined 3D Architecture rGO/Fe3O4/AC Nanocomposite for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces. 2018, 10, 13470–13478. [Google Scholar] [CrossRef]
- Xie, Y.; Qiu, Y.; Tian, L.; Liu, T.; Su, X. Ultrafine hollow Fe3O4 anode material modified with reduced graphene oxides for high-power lithium-ion batteries. J. Alloys Compd. 2022, 894, 162384. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, Y.; Zhao, Y.; Tian, Y.; Kurmanbayeva, I.; Bakenov, Z. Hierarchical sandwiched Fe3O4@C/Graphene composite as anode material for lithium-ion batteries. J. Electroanal. Chem. 2019, 847, 113240. [Google Scholar] [CrossRef]
- Yin, L.; Gao, Y.J.; Jeon, I.; Yang, H.; Kim, J.P.; Jeong, S.Y.; Cho, C.R. Rice-panicle-like γ-Fe2O3@C nanofibers as high-rate anodes for superior lithium-ion batteries. Chem. Eng. J. 2019, 356, 60–68. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, X.; Li, Y.J.; Song, J.Q.; Tian, Q.; Yang, L.; Sui, Z. Dispersive Fe3O4 encapsulated in porous carbon for high capacity and long-life anode of lithium-ion batteries. J. Alloys Compd. 2022, 899, 163342. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, Z.Q.; Zhao, Y.; Xie, H.W.; Xing, P.F.; Wang, D.H.; Yin, H.Y. A self-driven alloying/dealloying approach to nanostructuring micro-silicon for high-performance lithium-ion battery anodes. Energy Storage Mater. 2021, 34, 768–777. [Google Scholar] [CrossRef]
- Yuan, J.; Gan, Y.; Xu, X.; Mu, M.; He, H.; Li, X.; Zhang, X.; Liu, J. Construction of Fe7Se8@Carbon nanotubes with enhanced sodium/potassium storage. J. Colloid Interface Sci. 2022, 626, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Song, J.; Ji, Y.; Li, Y.; Lu, X.; Ren, W.; Tian, Q.; Chen, J.; Yang, L. Porous carbon assisted carbon nanotubes supporting Fe3O4 nanoparticles for improved lithium storage. Ceram. Int. 2021, 47, 26092–26099. [Google Scholar] [CrossRef]
- Wang, F.M.; Alemu, T.; Yeh, N.H.; Wang, X.C.; Lin, Y.W.; Hsu, C.C.; Chang, Y.J.; Liu, C.H.; Chuang, C.I.; Hsiao, L.H.; et al. Interface Interaction Behavior of Self-Terminated Oligomer Electrode Additives for a Ni-Rich Layer Cathode in Lithium-Ion Batteries: Voltage and Temperature Effects. ACS Appl. Mater. Interfaces 2019, 11, 39827–39840. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef]
- Bal, B.; Ozdogru, B.; Nguyen, D.T.; Li, Z.; Murugesan, V.; Çapraz, Ö.Ö. Probing the Formation of Cathode-Electrolyte Interphase on Lithium Iron Phosphate Cathodes via Operando Mechanical Measurements. ACS Appl. Mater. Interfaces 2023, 15, 42449–42459. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, P.; Chen, M.; Jin, H.; Bai, Y.; Li, S.; Ma, F.; Xu, H.; Lian, K. In Situ Synthesis of Multilayer Carbon Matrix Decorated with Copper Particles: Enhancing the Performance of Si as Anode for Li-Ion Batteries. ACS Nano 2019, 13, 3054–3062. [Google Scholar] [CrossRef]

| Sample Name | Reversible Capacity (mAh·g−1) | Cycle Number | Current Rate (mA·g−1) | Year Published |
|---|---|---|---|---|
| Fe3O4@SnO7/MXene-10 [35] | 626.1 | 900 | 1000 | 2024 |
| Fe2O3@ Fe3O4-5 [36] | 707.8 | 800 | 1000 | 2023 |
| H-TiO2/C/Fe3O4@NiO [37] | 897.47 | 200 | 200 | 2023 |
| Fe3O4/C-500 [38] | 718 | 500 | 200 | 2023 |
| Si-QDs/Fe3O4/rGO [39] | 1367.1 | 80 | 100 | 2023 |
| Fe3O4@void@N-Doped C-5 [40] | 1222 | 100 | 200 | 2022 |
| Fe3O4@C [41] | 291.7 | 300 | 1000 | 2022 |
| Fe3O4/MWCNT [42] | 662 | 100 | 50 | 2022 |
| Fe3O4/CNTS@C [43] | 612 | 200 | 1000 | 2021 |
| Fe3O4@LigC/CC | 820.6 | 100 | 200 | This work |
| 750.5 | 250 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Li, J.; Yin, H.; Jia, R.; Yu, L.; Li, H.; Xu, B. Sustainable Synthesis of a Carbon-Supported Magnetite Nanocomposite Anode Material for Lithium-Ion Batteries. Batteries 2024, 10, 357. https://doi.org/10.3390/batteries10100357
Zeng H, Li J, Yin H, Jia R, Yu L, Li H, Xu B. Sustainable Synthesis of a Carbon-Supported Magnetite Nanocomposite Anode Material for Lithium-Ion Batteries. Batteries. 2024; 10(10):357. https://doi.org/10.3390/batteries10100357
Chicago/Turabian StyleZeng, Hui, Jiahui Li, Haoyu Yin, Ruixin Jia, Longbiao Yu, Hongliang Li, and Binghui Xu. 2024. "Sustainable Synthesis of a Carbon-Supported Magnetite Nanocomposite Anode Material for Lithium-Ion Batteries" Batteries 10, no. 10: 357. https://doi.org/10.3390/batteries10100357
APA StyleZeng, H., Li, J., Yin, H., Jia, R., Yu, L., Li, H., & Xu, B. (2024). Sustainable Synthesis of a Carbon-Supported Magnetite Nanocomposite Anode Material for Lithium-Ion Batteries. Batteries, 10(10), 357. https://doi.org/10.3390/batteries10100357







