Batch Fabrication of Electrospun PAN/PU Composite Separators for Safe Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Characterization of Spinning Solution
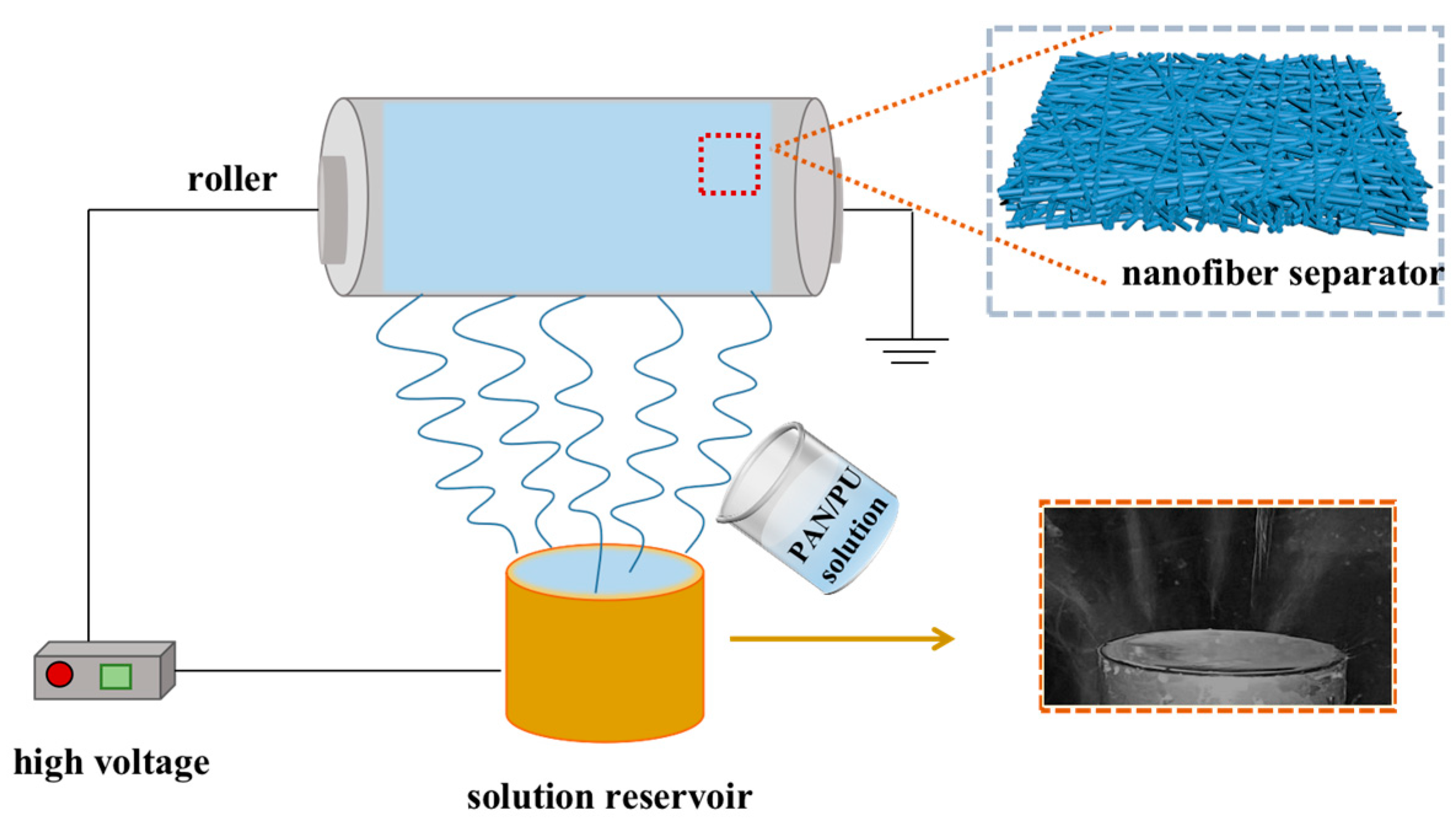
2.3. Batch Fabrication of Nanofiber Separators
2.4. Characterization of Nanofiber Separators
2.5. Battery Assembly
2.6. Electrochemical Tests
3. Results
3.1. Morphology and Mechanical Property of Separators
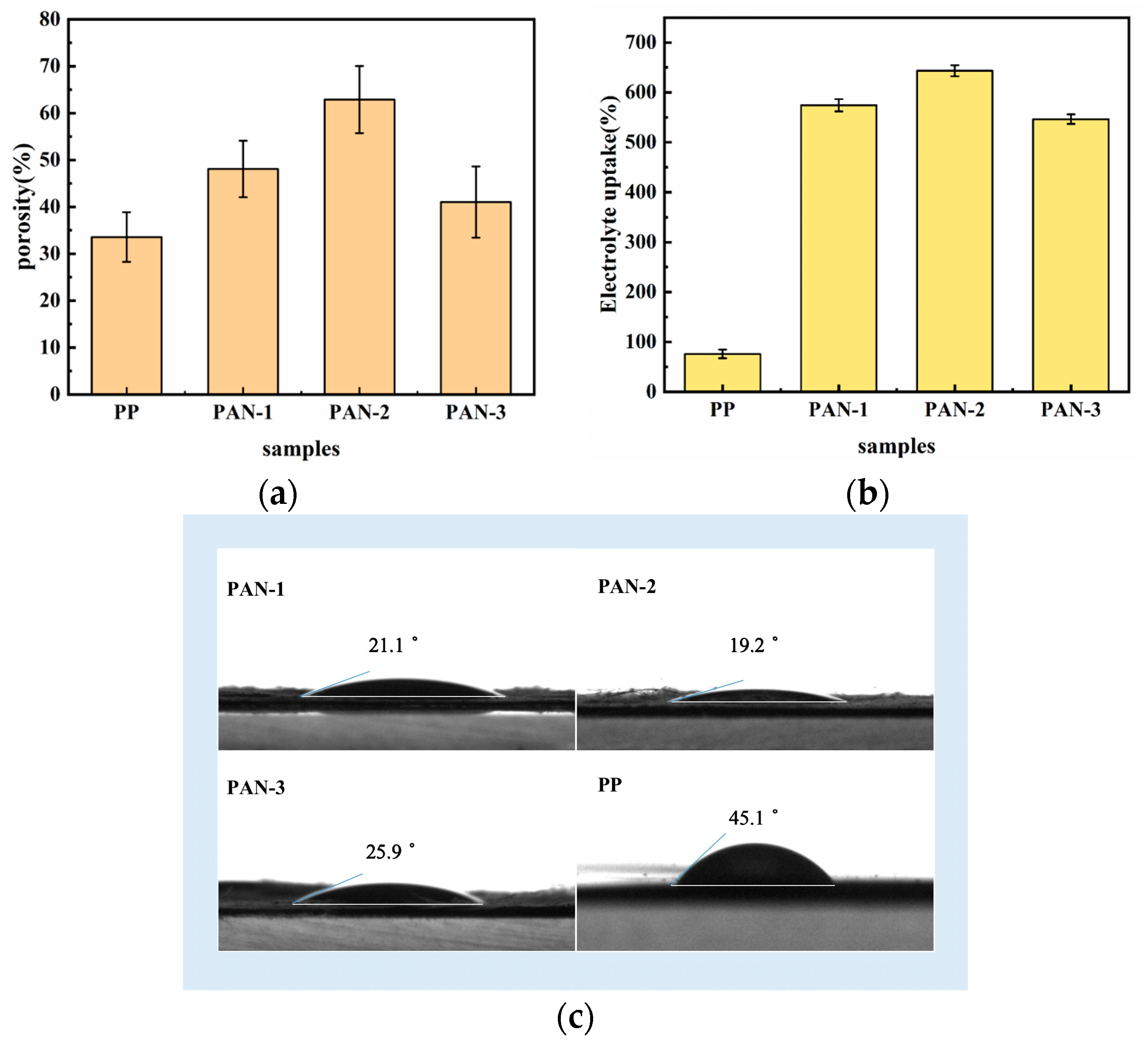
3.2. Porosity and the Electrolyte Affinity of Separators
3.3. Thermal Stability
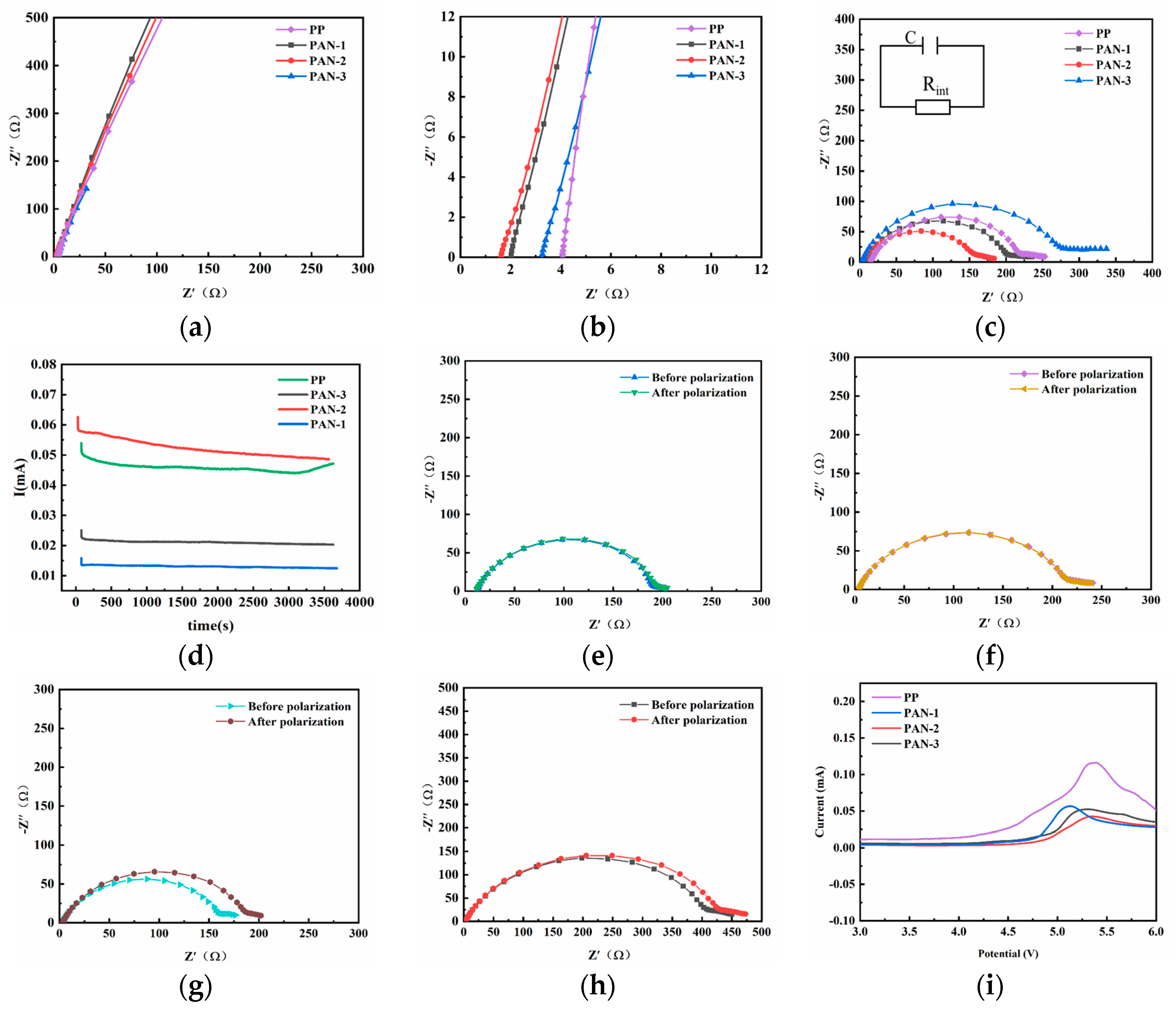
3.4. Electrochemical Performances of Nanofiber Separators
3.5. Cycling and Rate Performances of Batteries
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tomaszewska, A.; Chu, Z.Y.; Feng, X.N.; O’Kane, S.; Liu, X.H.; Chen, J.Y.; Ji, C.Z.; Endler, E.; Li, R.H.; Liu, L.S.; et al. Lithium-ion battery fast charging: A review. Etransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Liu, K.X.; Wang, Z.Y.; Shi, L.Y.; Jungsuttiwong, S.; Yuan, S. Ionic liquids for high performance lithium metal batteries. J. Energy Chem. 2021, 59, 320–333. [Google Scholar] [CrossRef]
- Xia, L.; Miao, H.; Zhang, C.F.; Chen, G.Z.; Yuan, J.L. Review-recent advances in non-aqueous liquid electrolytes containing fluorinated compounds for high energy density lithium-ion batteries. Energy Storage Mater. 2021, 38, 542–570. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Zhang, Z.M. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, S.M.; Kim, S.H.; Kim, H.S. Electrochemical and safety performances of polyimide nano fiber-based nonwoven separators for Li-ion batteries. J. Electrochem. Sci. Technol. 2015, 6, 26–33. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, G.B.; Hu, Y.Q.; Xu, J.; Wang, L.X.; Wang, H.; Cheng, C.Z. Porous sodium alginate/boehmite coating layer constructed on PP nonwoven substrate as a battery separator through polydopamine-induced water-based coating method. Chemelectrochem 2022, 9, e202200818. [Google Scholar] [CrossRef]
- Yang, L.; Sheng, L.; Gao, X.X.; Xie, X.; Bai, Y.Z.; Liu, G.J.; Dong, H.Y.; Wang, T.; Huang, X.L.; He, J.P. rGO/Li-Al-LDH composite nanosheets modified commercial polypropylene (PP) separator to suppress lithium dendrites for lithium metal battery. Electrochim. Acta 2022, 430, 141073. [Google Scholar] [CrossRef]
- Xie, Y.J.; Pan, Y.F.; Cai, P.X. Novel PVA-based porous separators prepared via freeze-drying for enhancing performance of Lithium-ion batteries. Ind. Eng. Chem. Res. 2020, 59, 15242–15254. [Google Scholar] [CrossRef]
- Wang, H.F.; Li, H.B.; Yu, L.J.; Jiang, Y.M.; Wang, K.X. Synthesis of porous Al2O3-PVDF composite separators and their application in lithium-ion batteries. J. Appl. Polym. Sci. 2013, 130, 2886–2890. [Google Scholar] [CrossRef]
- Luo, L.; Gao, Z.H.; Zheng, Z.M.; Zhang, J.M. Polymer-in-Ceramic membrane for thermally safe separator applications. ACS Omega 2022, 7, 35727–35734. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Z.; Wang, L.; Cui, H.; Liang, H.M.; Hu, Q.; Ren, D.S.; Yang, Y.; Zhang, H.; Xu, H.; He, X.M. Boosting battery safety by mitigating thermal-induced crosstalk with a bi-continuous separator. Adv. Energy Mater. 2022, 12, 2201964. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, Q.H.; Tan, Z.C. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262. [Google Scholar] [CrossRef]
- Liu, M.; Deng, N.P.; Ju, J.G.; Fan, L.L.; Wang, L.Y.; Li, Z.J.; Zhao, H.J.; Yang, G.; Kang, W.M.; Yan, J.; et al. A review: Electrospun nanofiber materials for Lithium-sulfur batteries. Adv. Funct. Mater. 2019, 29, 1905467. [Google Scholar] [CrossRef]
- Zainab, G.; Wang, X.F.; Yu, J.Y.; Zhai, Y.Y.; Babar, A.A.; Xiao, K.; Ding, B. Electrospun polyacrylonitrile/polyurethane compo-site nanofibrous separator with electrochemical performance for high power lithium ion batteries. Mater. Chem. Phys. 2016, 182, 308–314. [Google Scholar] [CrossRef]
- Mei, S.Q.; Liu, T.; Chen, L.; Wang, Y.F. Preparation and performance of a PU/PAN lithium-ion battery separator based on a centrifugal spinning method. Appl. Sci. 2023, 13, 6682. [Google Scholar] [CrossRef]
- Zhou, F.L.; Gong, R.H.; Porat, I. Needle and needleless electrospinning for nanofibers. J. Appl. Polym. Sci. 2010, 115, 2591–2598. [Google Scholar] [CrossRef]
- He, J.H.; Liu, Y.; Xu, L.; Yu, J.Y.; Sun, G. BioMimic fabrication of electrospun nanofibers with high-throughput. Chaos Soliton. Fract. 2008, 37, 643–651. [Google Scholar] [CrossRef]
- Jiang, G.J.; Johnson, L.; Xie, S. Investigations into the mechanisms of electrohydrodynamic instability in free surface electrospinning. Open Phys. 2019, 17, 313–319. [Google Scholar] [CrossRef]
- Yusuf, A.; Avvaru, V.S.; Dirican, M.; Sun, C.C.; Wang, D.Y. Low heat yielding electrospun phosphenanthrene oxide loaded polyacrylonitrile composite separators for safer high energy density Lithium-ion batteries. Appl. Mater. Today. 2020, 20, 100675. [Google Scholar] [CrossRef]
- Dong, G.X.; Li, H.J.; Wang, Y.; Jiang, W.J.; Ma, Z.S. Electrospun PAN/cellulose composite separator for high performance lith-ium-ion battery. Ionics 2021, 27, 2955–2965. [Google Scholar] [CrossRef]
- Mohanta, J.; Kwon, O.H.; Choi, J.H.; Yun, Y.M.; Kim, J.K.; Jeong, S.M. Preparation of highly porous PAN-LATP membranes as separators for lithium ion batteries. Nanomaterials 2019, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Lee, S.; Song, D.; Ryou, M.H.; Lee, Y.M.; Park, W.H. A crosslinked nonwoven separator based on an organosoluble polyimide for high-performance lithium-ion batteries. J. Ind. Eng. Chem. 2019, 72, 390–399. [Google Scholar] [CrossRef]
- Li, M.L.; Sheng, L.; Xu, R.; Yang, Y.; Bai, Y.Z.; Song, S.J.; Liu, G.J.; Wang, T.; Huang, X.L.; He, J.P. Enhanced the mechanical strength of polyimide (PI) nanofiber separator via PAALi binder for lithium ion battery. Compos. Commun. 2021, 24, 100607. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, F.; Shao, W.L.; Cui, S.Z.; Zhao, Y.M.; Zhou, Y.M.; He, J.X. Graphite oxide dopping polyimide nanofiber mem-brane via electrospinning for high performance lithium-ion batteries. Compos. Commun. 2019, 16, 150–157. [Google Scholar] [CrossRef]
- Wu, H.Y. Electrospun graphene oxide (GO)/polyvinylidene flfluoride (PVDF) nanofiber separator for lithium-ion battery. J. Optoelectron. Adv. Mater. 2022, 24, 82–87. [Google Scholar]
- Chen, Y.; Qiu, L.L.; Ma, X.Y.; Dong, L.K.; Jin, Z.F.; Xia, G.B.; Du, P.F.; Xiong, J. Electrospun cellulose polymer nanofiber mem-brane with flame resistance properties for lithium-ion batteries. Carbohyd. Polym. 2020, 234, 115907. [Google Scholar] [CrossRef]
- Li, D.T.; Gao, X.X.; Cao, M.; Sheng, L.; Yang, L.; Xie, X.; Gong, Y.; Hu, Q.Y.; Xie, Q.Q.; Wang, T.; et al. High-performance nano-TiO2@polyvinylidene fluoride composite separators prepared by electrospinning for safe lithi-um-ion battery. J. Appl. Polym. Sci. 2023, 140, e53618. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, L.L.; Ma, X.Y.; Chu, Z.D.; Zhuang, Z.S.; Dong, L.K.; Du, P.F.; Xiong, J. Electrospun PMIA and PVDF-HFP compo-site nanofibrous membranes with two different structures for improved lithium-ion battery separators. Solid State Ionics 2020, 347, 115253. [Google Scholar] [CrossRef]
- Jiang, W.W.; Han, Y.; Ding, Y.H. Sepiolite and ZIF-67 co-modified PAN/PVdF-HFP nanofiber separators for advanced Li-ion batteries. Nanotechnology 2022, 33, 425601. [Google Scholar] [CrossRef]
- Chen, D.X.; Wang, X.; Liang, J.Y.; Zhang, Z.; Chen, W.P. A Novel electrospinning polyacrylonitrile separator with dip-coating of zeolite and phenoxy resin for li-ion batteries. Membranes 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.Z.; Zhang, Z.; Wei, S.Y.; Ruan, S.L.; Shen, C.Y.; Turng, L.S. Thermosensitive polyacrylonitrile/polyethylene oxide/polyacrylonitrile membrane separators for prompt and safer thermal lithium-ion battery shutdown. J. Electrochem. Soc. 2020, 167, 020509. [Google Scholar] [CrossRef]
- Yu, Y.G.; Zhang, F.L.; Liu, Y.; Zheng, Y.S.; Xin, B.J.; Jiang, Z.L.; Peng, X.X.; Jin, S.X. Waterproof and breathable polyacrylonitrile/(polyurethane/fluorinated-silica) composite nanofiber membrane via side-by-side electrospinning. J. Mater. Res. 2020, 35, 1173–1181. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, J.; Jeon, J.; Hong, Y.; Oh, S.G.; Huh, H. Electrospun PVDF-HFP/PAN bicomponent nanofibers as separators in lithium-ion batteries with high thermal stability and electrolyte wettability. Korean J. Chem. Eng. 2023, 40, 1901–1911. [Google Scholar] [CrossRef]
- Liu, T.; Hu, X.M.; Zhang, Y.D.; He, T.; Zhou, J.P.; Qiao, J.Q. Ion transport regulated lithium metal batteries achieved by electrospun ZIF/PAN composite separator with suitable electrolyte wettability. Batteries 2023, 9, 166. [Google Scholar] [CrossRef]
- Tang, L.P.; Wu, Y.K.; He, D.; Lei, Z.Q.; Liu, N.Q.; He, Y.; De Guzman, M.R.; Chen, J. Electrospun PAN membranes toughened and strengthened by TPU/SHNT for high-performance lithium-ion batteries. J. Electroanal. Chem. 2023, 931, 117181. [Google Scholar] [CrossRef]
- Chen, Y.F.; Gao, Y.; Jian, J.J.; Lu, Y.H.; Zhang, B.Y.; Liu, H.Q.; Li, L.; Wang, X.W.; Kuang, C.X.; Zhai, Y.Y. A dual-layer mi-cro/nanostructured fibrous membrane with enhanced ionic conductivity for lithium-ion battery. Electrochim. Acta 2018, 292, 357–363. [Google Scholar] [CrossRef]
- Shao, Z.B.; Yu, L.; Xu, L.; Wang, M.D. High-throughput fabrication of quality nanofibers using a modified free surface elec-trospinning. Nanoscale Res. Lett. 2017, 12, 470. [Google Scholar] [CrossRef]
- Shao, Z.B.; Song, Y.H.; Xu, L. Fomation mechanism of highly aligned nanofibers by a modified bubble electrospinning. Therm. Sci. 2018, 22, 5–10. [Google Scholar] [CrossRef]
- Cheng, T.T.; Xu, L.; Wang, M.D. Effect of surface active agent on bubble-electrospun polyacrylonitrile nanofibers. Therm. Sci. 2019, 23, 2481–2487. [Google Scholar] [CrossRef]
- Wu, D.Z.; Xiao, Z.M.; Deng, L.; Sun, Y.; Tan, Q.L.; Dong, L.X.; Huang, S.H.; Zhu, R.; Liu, Y.F.; Zheng, W.X.; et al. Enhanced Deposition Uniformity via an Auxiliary Electrode in Massive Electrospinning. Nanomaterials 2016, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Jiang, W.; Lu, W.Z.; Mei, Y.F.; He, F.K.; Zhang, M.H.; Liu, Y.; Chen, Y.Q.; Peng, J.F.; Ding, Y.H. Anisotropic semi-aligned PAN@PVdF-HFP separator for Li-ion batteries. Nanotechnology 2020, 31, 435701. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Sun, Z.Y.; Xu, L.; Shao, Z.B. Preparation and Characterization of Highly Aligned Carbon Nano-tubes/Polyacrylonitrile Composite Nanofibers. Polymers 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, S.; Huh, H. PU-RGO Composite; Effect of Chain Extender’s Structure on Properties. J. Nanosci. Nanotechnol. 2017, 17, 7480–7484. [Google Scholar] [CrossRef]
- Bai, Z.K.; Xu, W.L.; Xu, J.; Liu, X.; Yang, H.J.; Xiao, S.L.; Liang, G.J.; Chen, L.B. Microstructure and mechanical properties of polyurethane fibrous membrane. Fiber. Polym. 2012, 13, 1239–1248. [Google Scholar] [CrossRef]
- Xiao, W.; Cheng, D.; Huang, L.; Song, J.; Yang, Z.X.; Qiao, Q.D. An integrated separator/anode assembly based on electrospin-ning technique for advanced lithium-ion batteries. Electrochim. Acta 2021, 389, 138776. [Google Scholar] [CrossRef]
- Jiang, F.J.; Nie, Y.; Yin, L.; Feng, Y.; Yu, Q.C.; Zhong, C.Y. Core-shell-structured nanofibrous membrane as advanced separator for lithium-ion batteries. J. Membr. Sci. 2016, 510, 1–9. [Google Scholar] [CrossRef]
- He, T.S.; Fu, Y.R.; Meng, X.L.; Yu, X.D.; Wang, X.L. A novel strategy for the high performance supercapacitor based on polyac-rylonitrile-derived porous nanofibers as electrode and separator in ionic liquid electrolyte. Electrochim. Acta 2018, 282, 97–104. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Liu, Q.; Wang, A.X.; Li, B.H. A sandwich-structure composite membrane as separator with high wettability and thermal properties for advanced lithium-ion batteries. Int. J. Electrochem. Sci. 2019, 14, 7088–7103. [Google Scholar] [CrossRef]
- Xie, Y.; Xiang, H.F.; Shi, P.C.; Guo, J.P.; Wang, H.H. Enhanced separator wettability by LiTFSI and its application for lithium metal batteries. J. Membr. Sci. 2017, 524, 315–320. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, L.L.; Yang, C.L.; Li, Z.H.; Xiao, Q.Z.; Lei, G.T.; Ding, Y.H. A hard-template process to prepare three-dimensionally macroporous polymer electrolyte for lithium-ion batteries. Electrochim. Acta 2014, 121, 328–336. [Google Scholar] [CrossRef]


| Spinning Solutions of Samples | Spinning Solution Property | |
|---|---|---|
| Viscosity (mPa·s) | Conductivity (μS·cm−1) | |
| PAN | 1789 ± 5 | 856 ± 2 |
| PAN-1 | 1179 ± 2 | 735 ± 12 |
| PAN-2 | 844 ± 7 | 678 ± 8 |
| PAN-3 | 730 ± 3 | 622 ± 2 |
| S(%) | T(°C) | 25 | 140 | 160 | 180 |
|---|---|---|---|---|---|
| Separator | |||||
| PP | 0 | 97.89 ± 0.42 | 100 | 100 | |
| PAN-1 | 0 | 0 | 1.05 ± 2.89 | 3.16 ± 1.45 | |
| PAN-2 | 0 | 0 | 2.63 ± 1.58 | 4.21 ± 3.93 | |
| PAN-3 | 0 | 5.26 ± 3.16 | 7.89 ± 4.53 | 10.52 ± 5.50 | |
| Sample | Thickness (mm) | Rb (Ω) | tLi+ | |
|---|---|---|---|---|
| PP | 0.025 ± 0.002 | 4.0 | 0.24 | 0.136 |
| PAN-1 | 0.084 ± 0.008 | 2.1 | 1.56 | 0.215 |
| PAN-2 | 0.083 ± 0.007 | 1.7 | 1.90 | 0.265 |
| PAN-3 | 0.080 ± 0.005 | 3.2 | 0.98 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Xu, L. Batch Fabrication of Electrospun PAN/PU Composite Separators for Safe Lithium-Ion Batteries. Batteries 2024, 10, 6. https://doi.org/10.3390/batteries10010006
Ding W, Xu L. Batch Fabrication of Electrospun PAN/PU Composite Separators for Safe Lithium-Ion Batteries. Batteries. 2024; 10(1):6. https://doi.org/10.3390/batteries10010006
Chicago/Turabian StyleDing, Wenfei, and Lan Xu. 2024. "Batch Fabrication of Electrospun PAN/PU Composite Separators for Safe Lithium-Ion Batteries" Batteries 10, no. 1: 6. https://doi.org/10.3390/batteries10010006
APA StyleDing, W., & Xu, L. (2024). Batch Fabrication of Electrospun PAN/PU Composite Separators for Safe Lithium-Ion Batteries. Batteries, 10(1), 6. https://doi.org/10.3390/batteries10010006








