Recycling of Valuable Metals from the Priority Lithium Extraction Residue Obtained through Hydrogen Reduction of Spent Lithium Batteries
Abstract
1. Introduction
2. Experimental Section
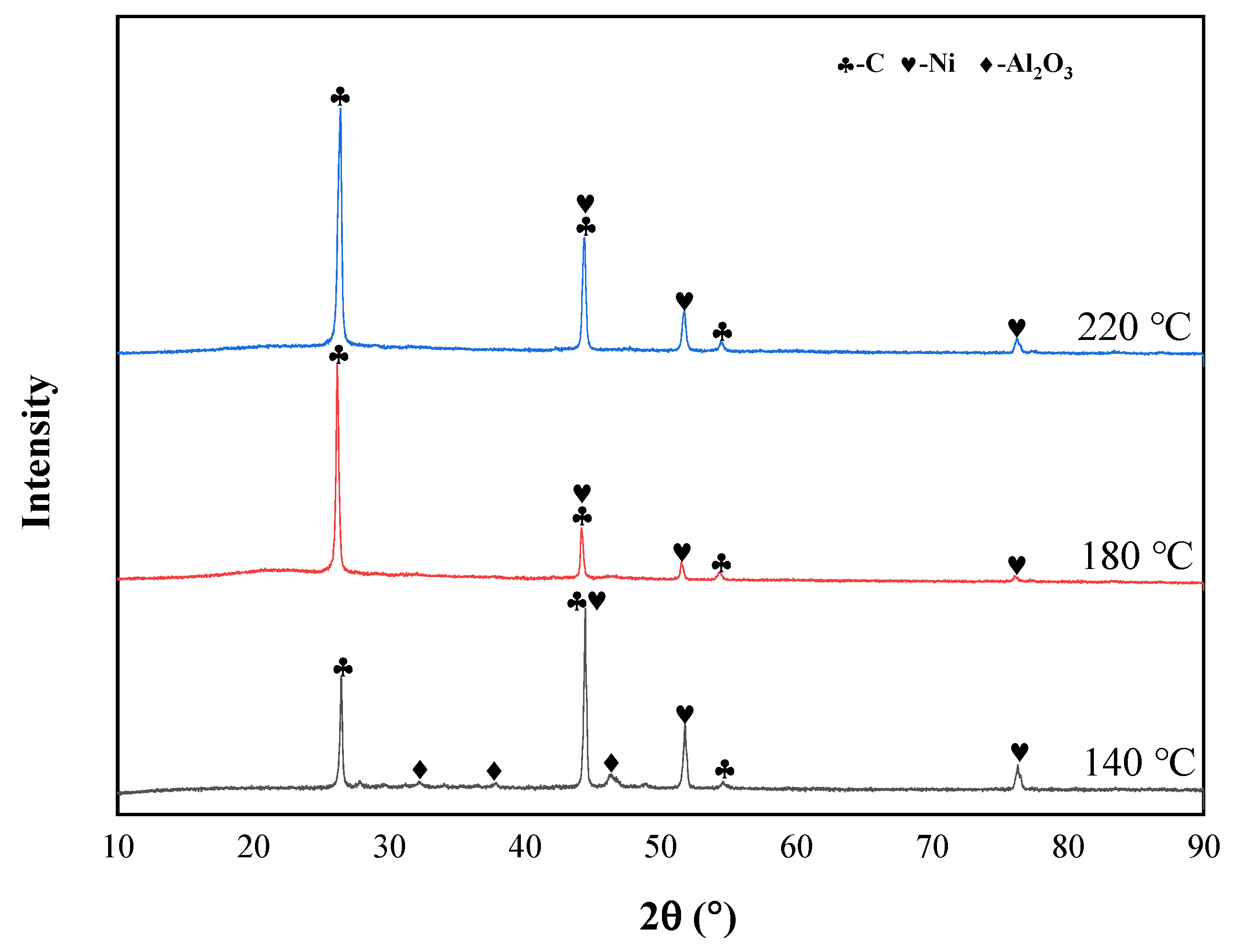
2.1. Materials and Characterization
2.2. Experimental Procedures
3. Results and Discussion
3.1. Sulfation Roasting
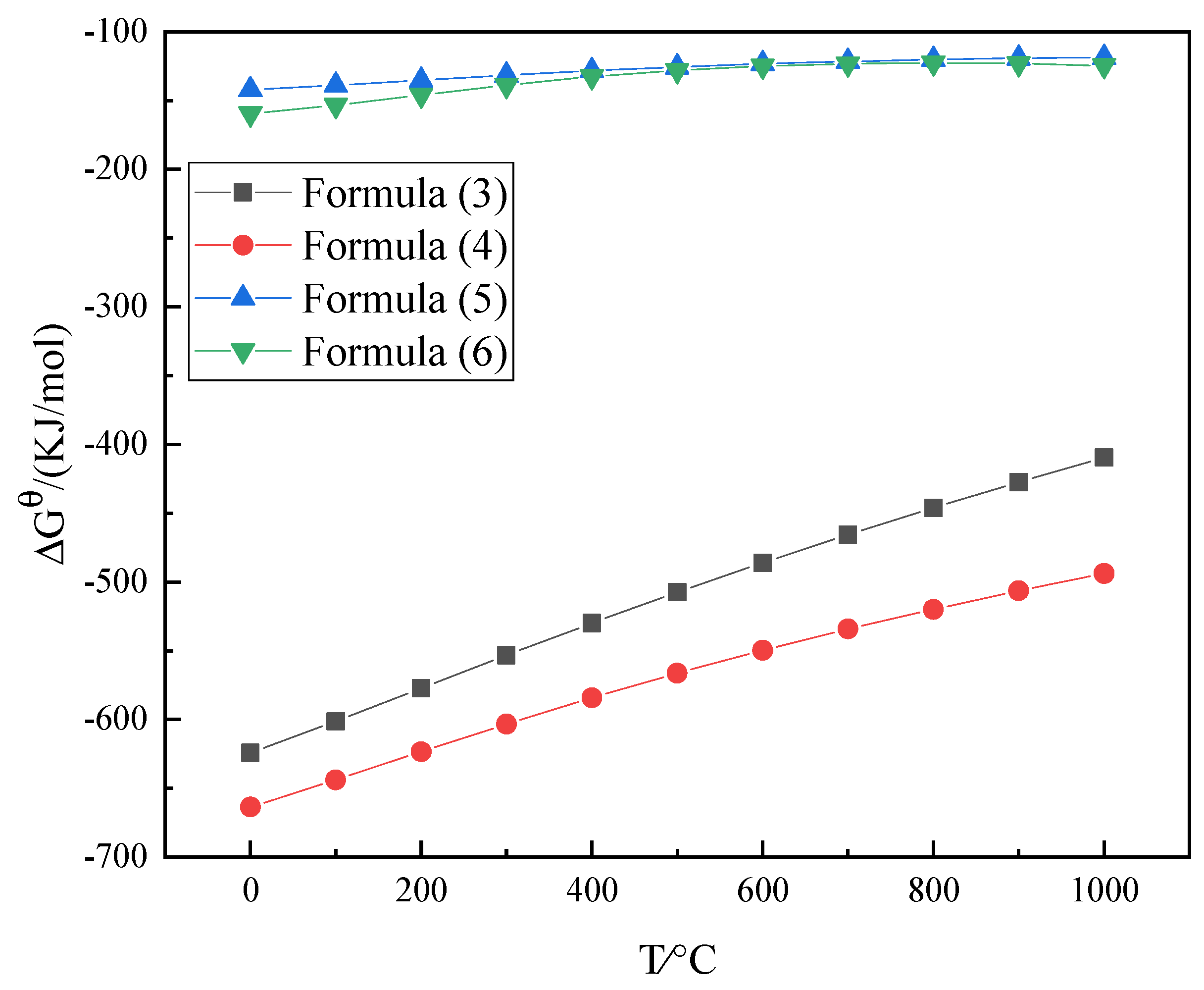
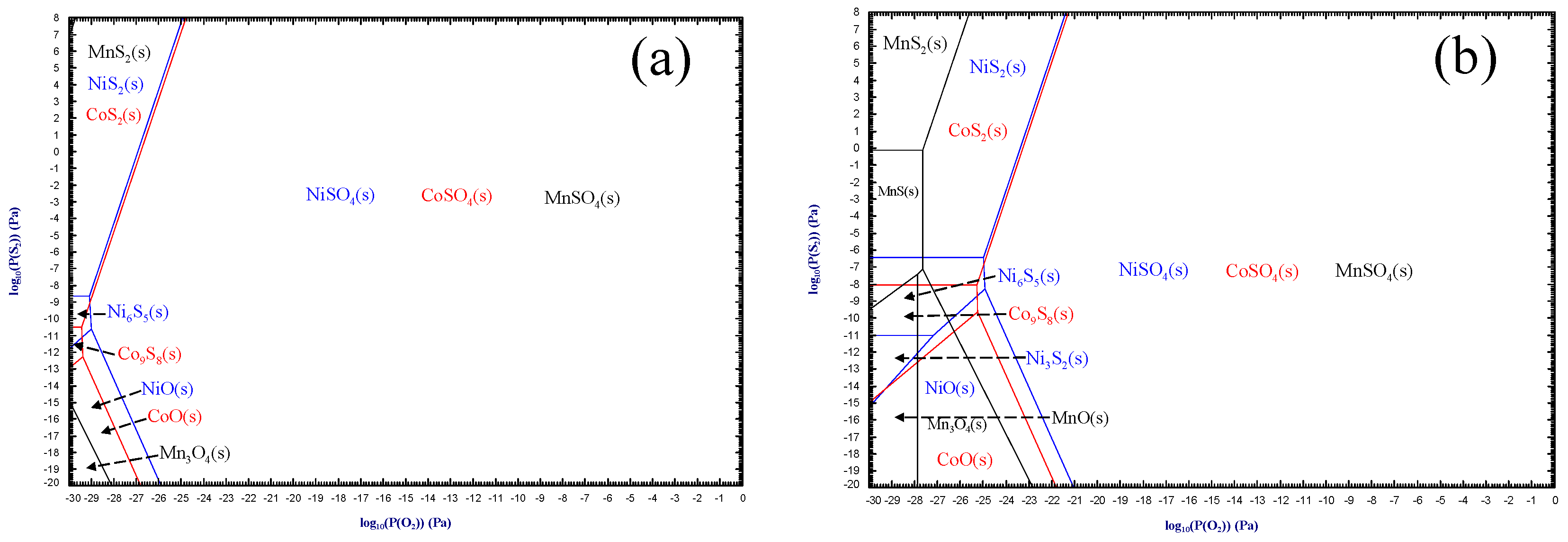
3.1.1. Experimental Principles
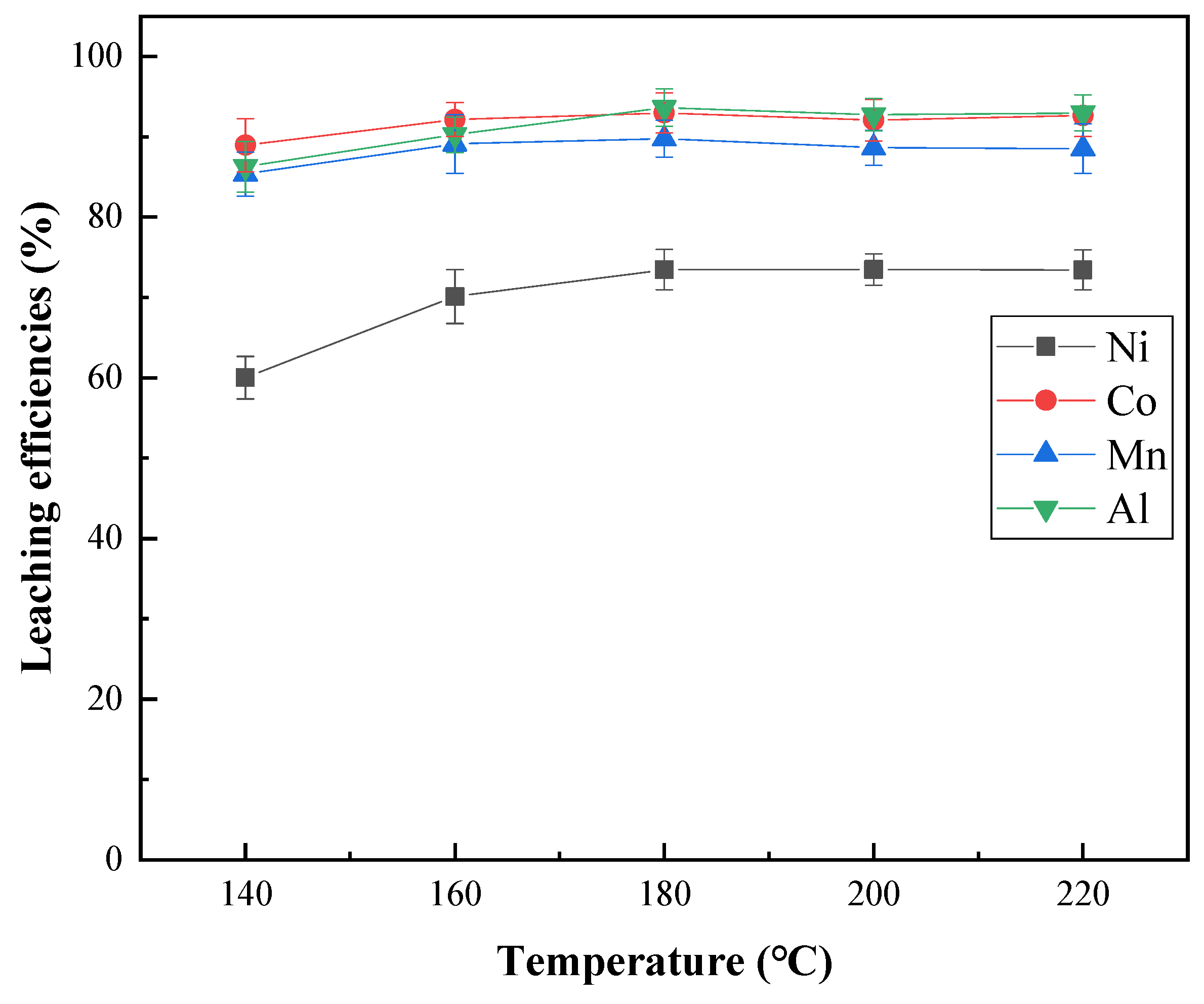
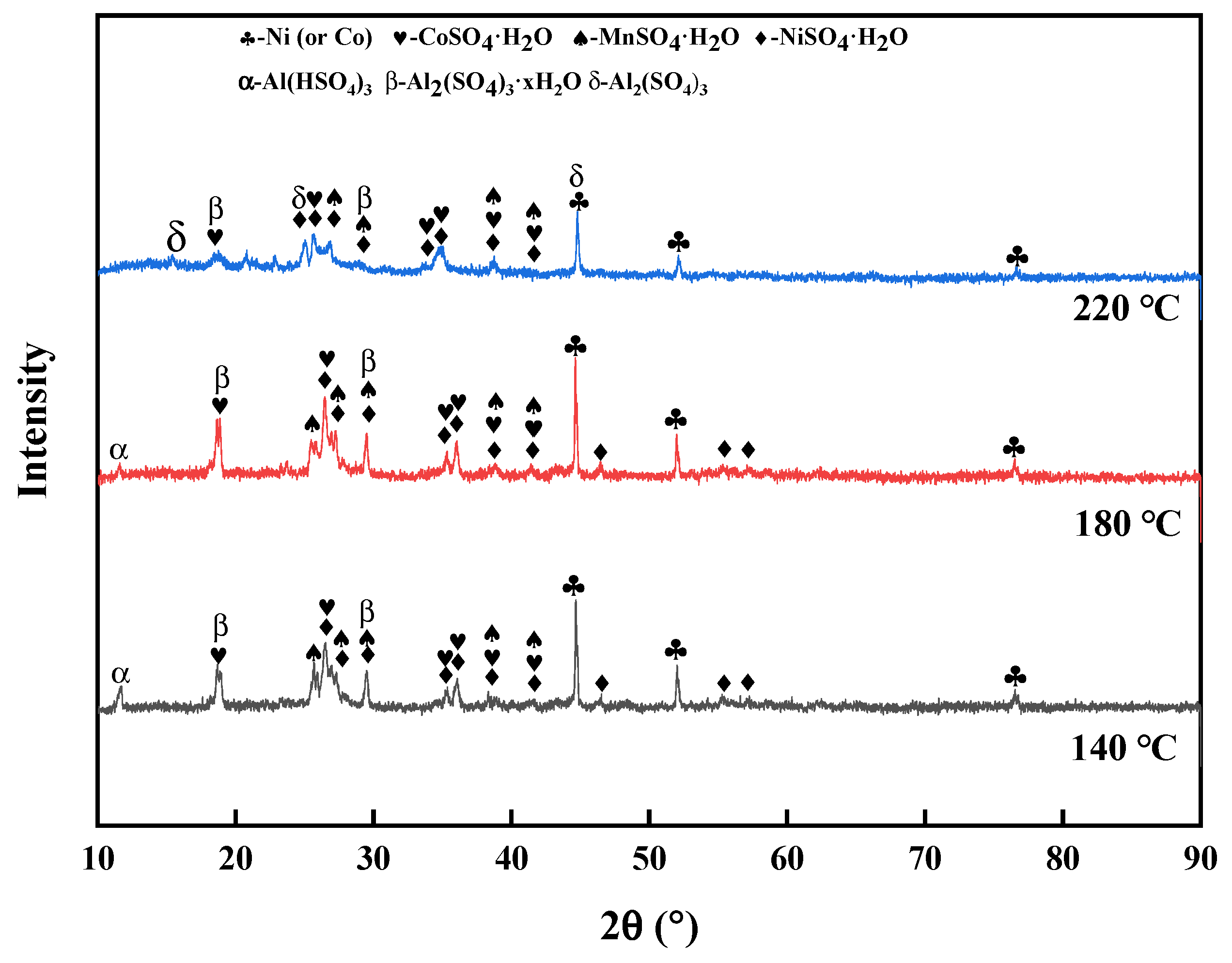
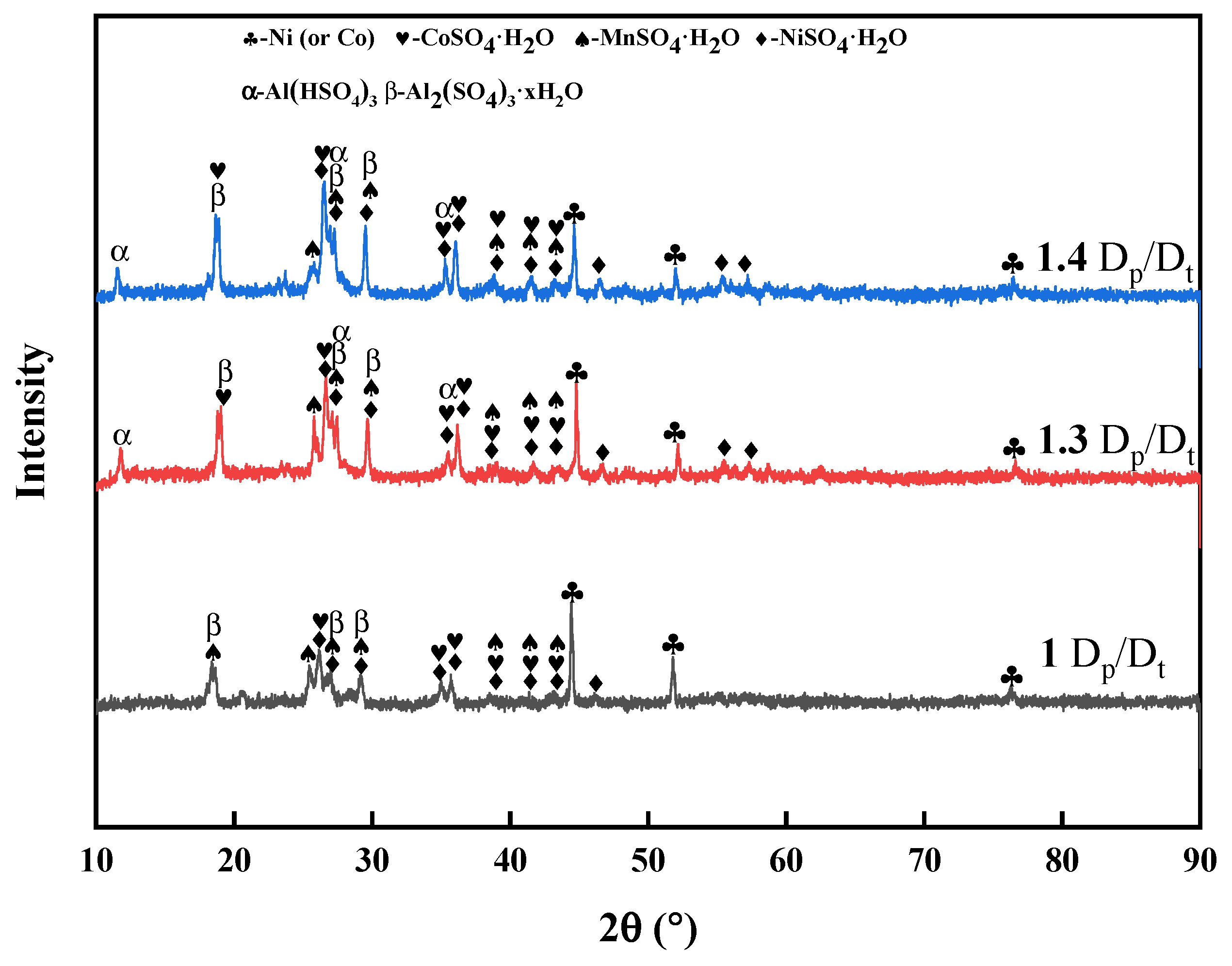
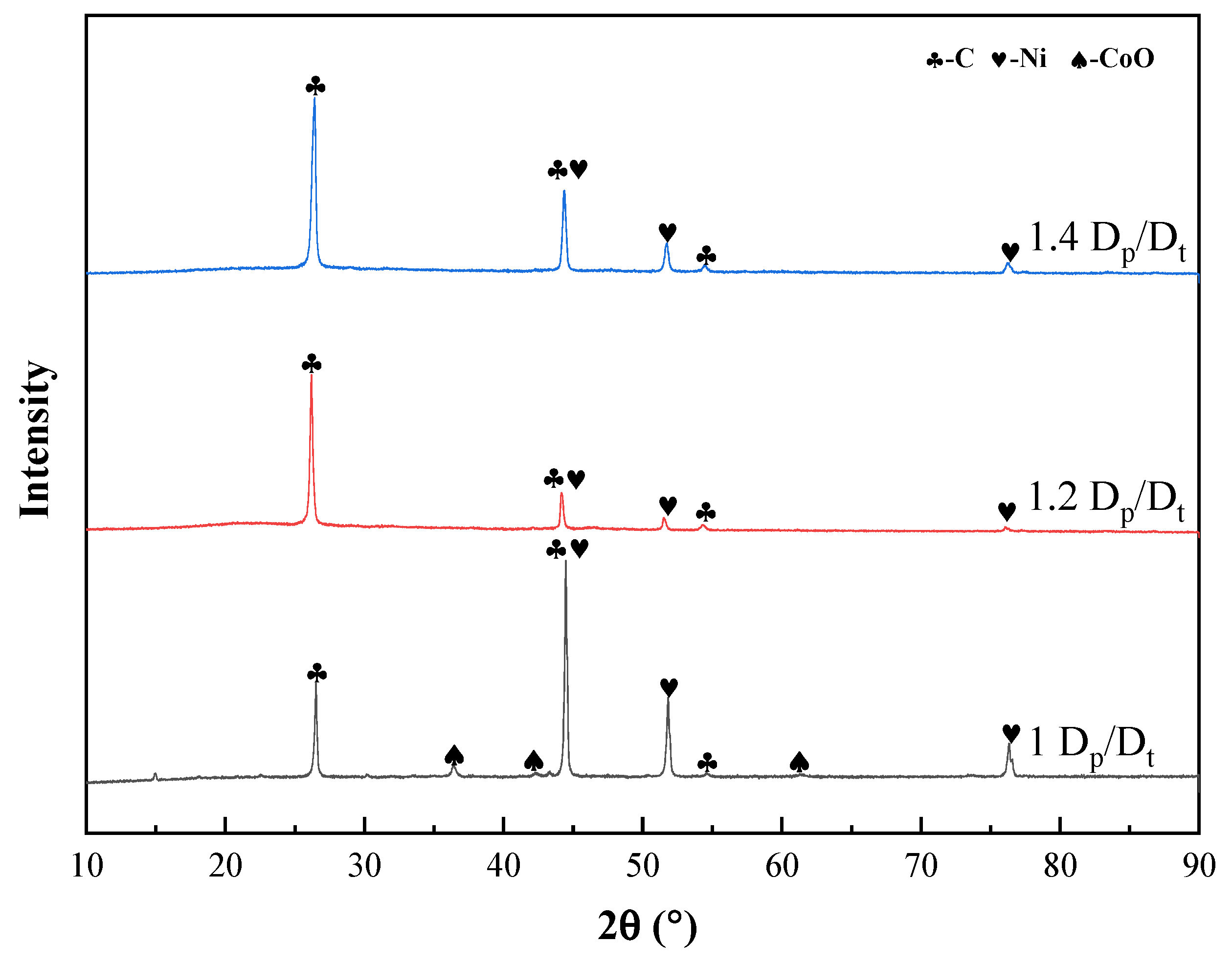
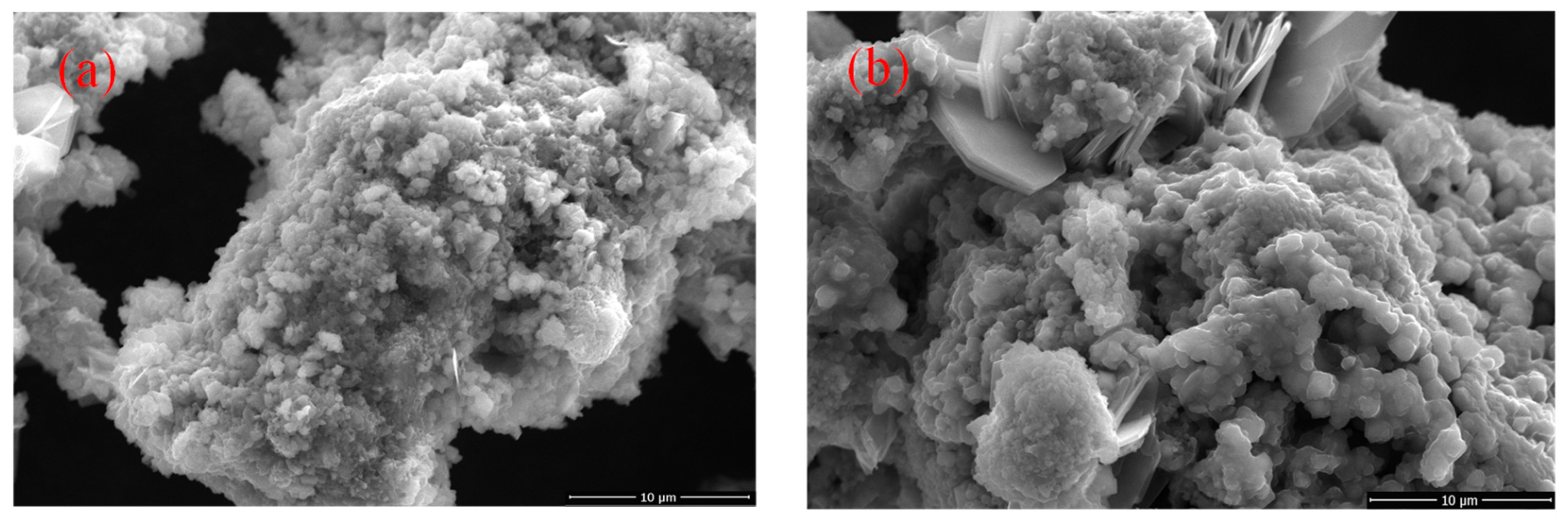
3.1.2. Effect of the Roasting Temperature
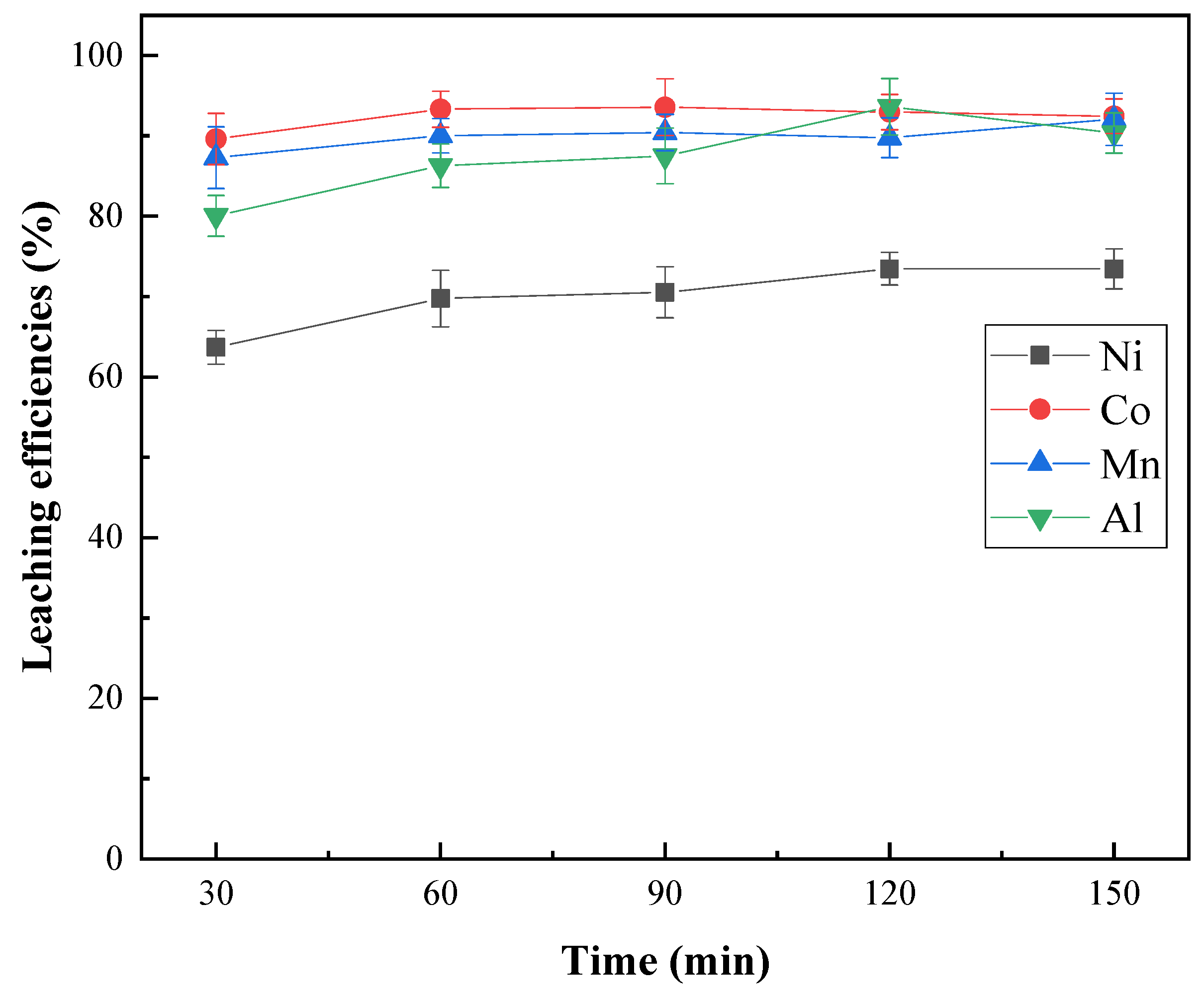
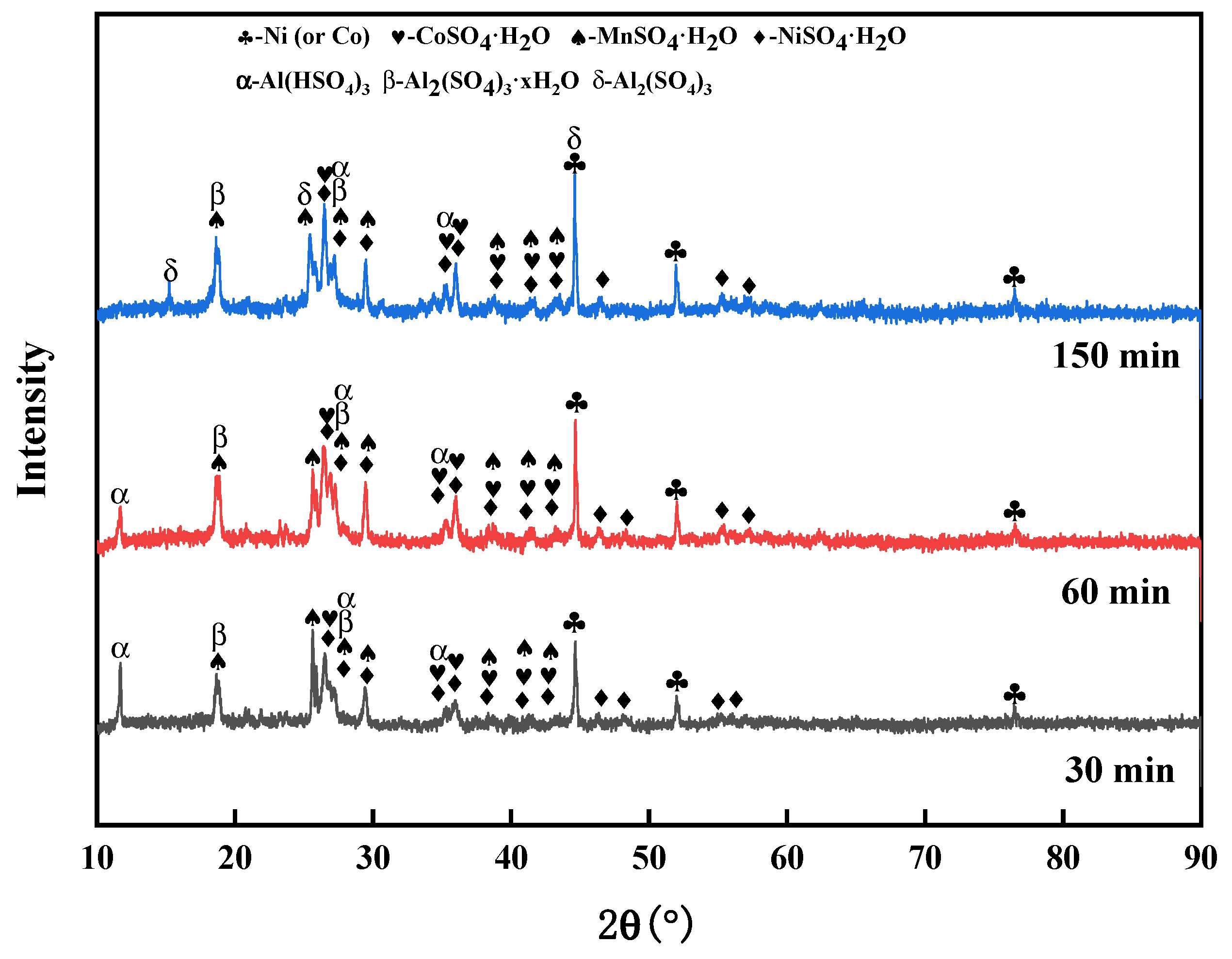
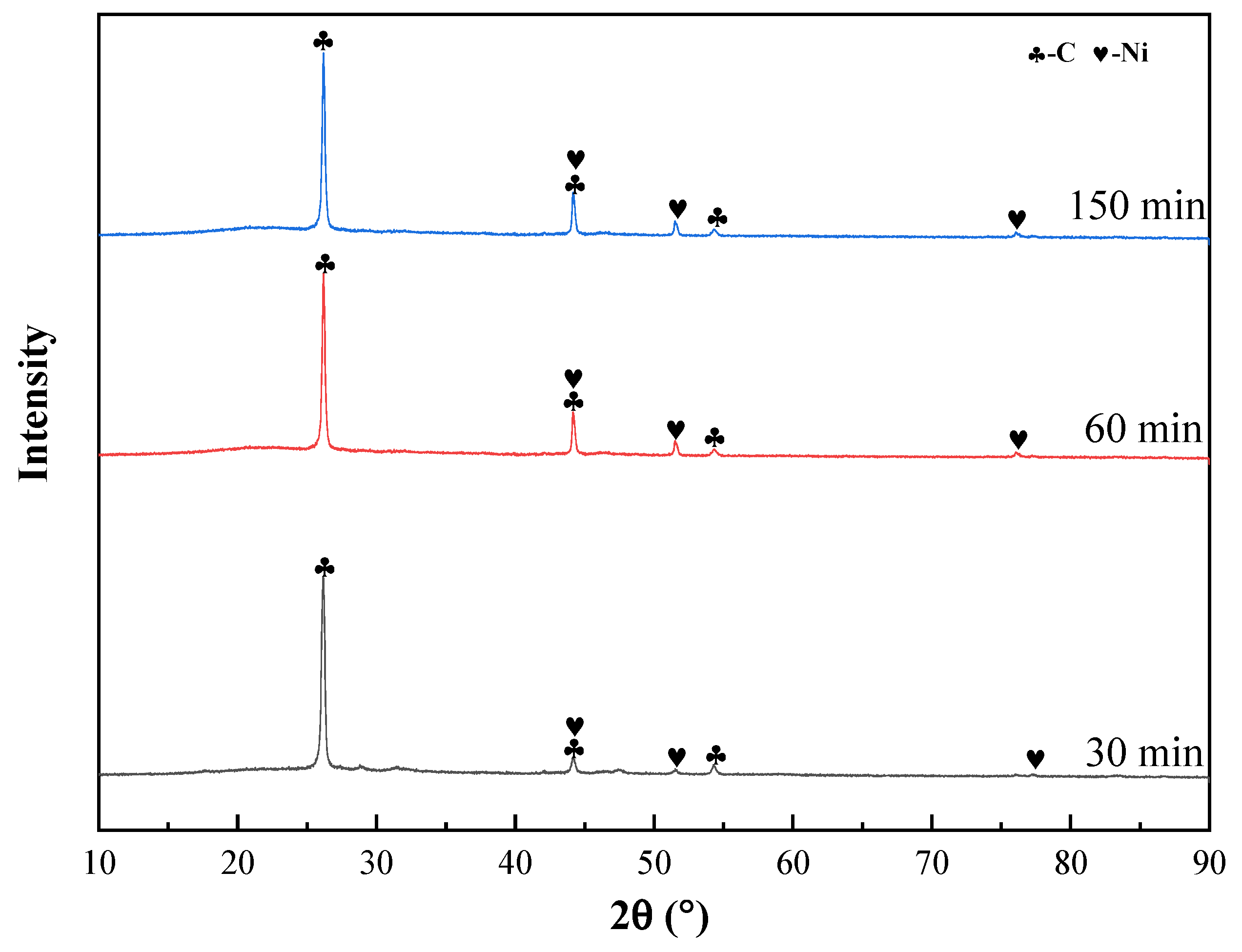
3.1.3. Effect of Roasting Time
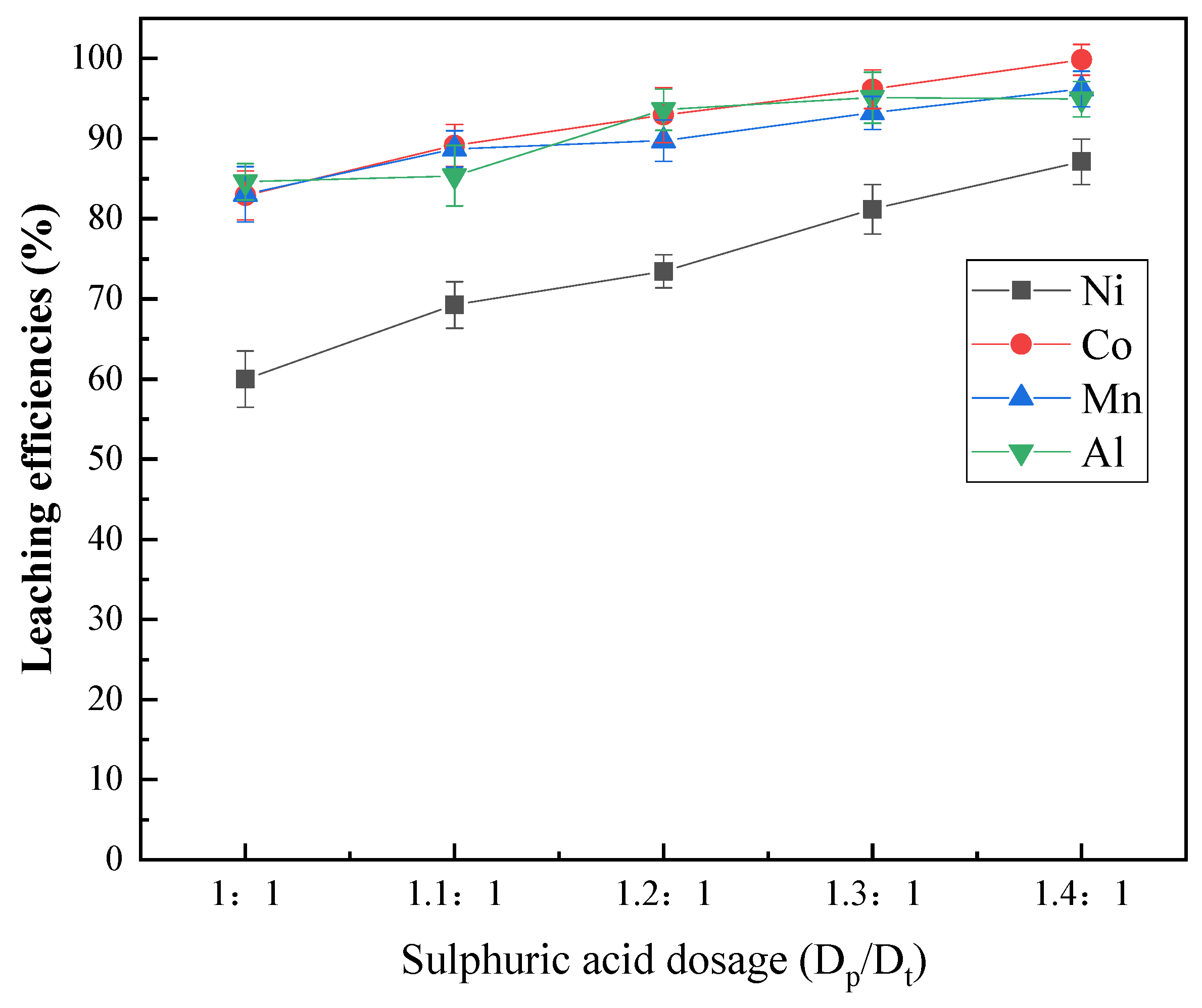
3.1.4. Effect of the Sulfuric Acid Amount
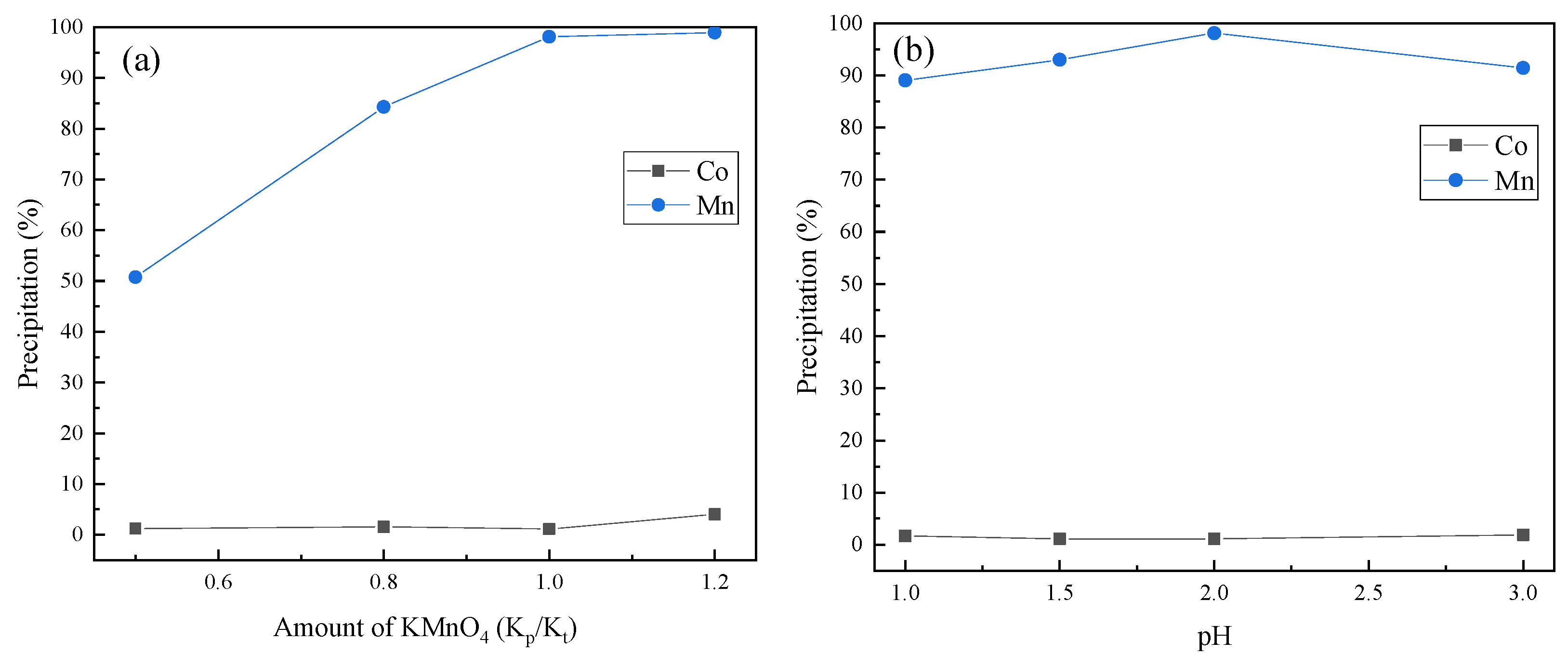
3.2. Selective Separation of Mn
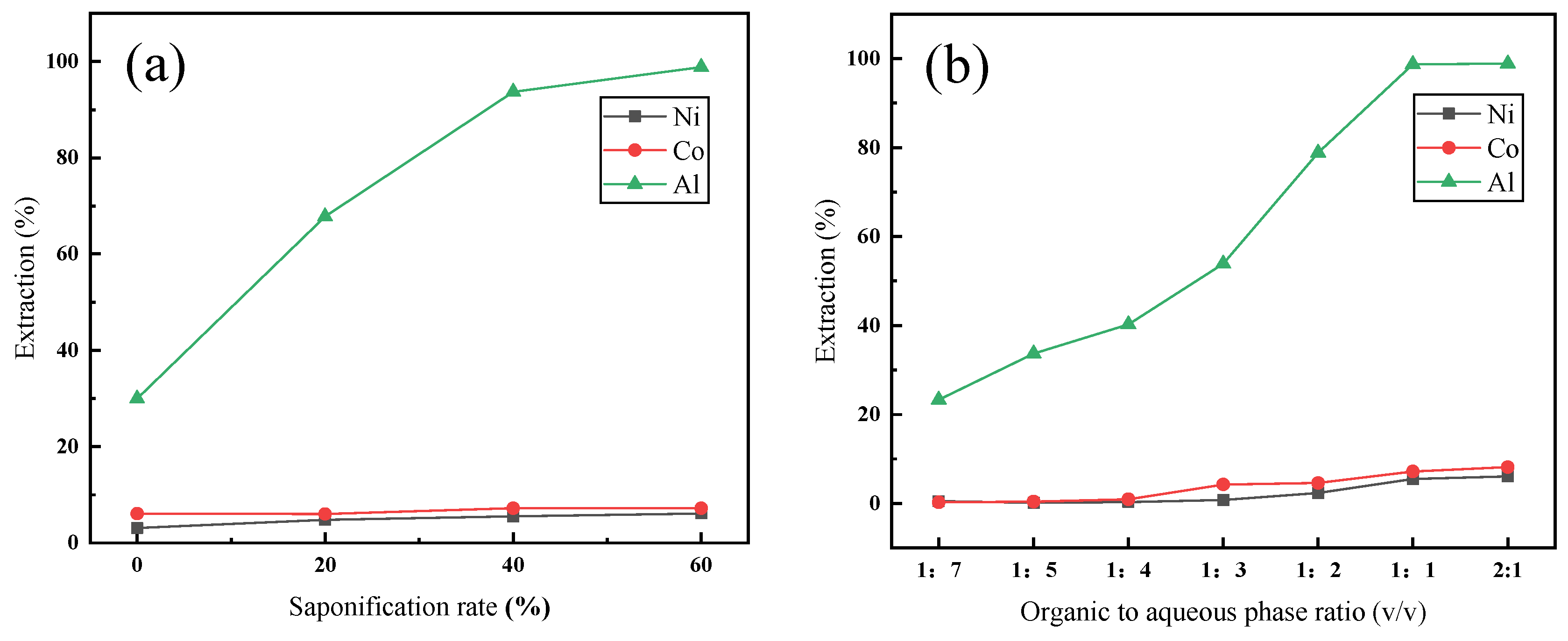
3.3. Removal of Al from the Water Leaching Solution
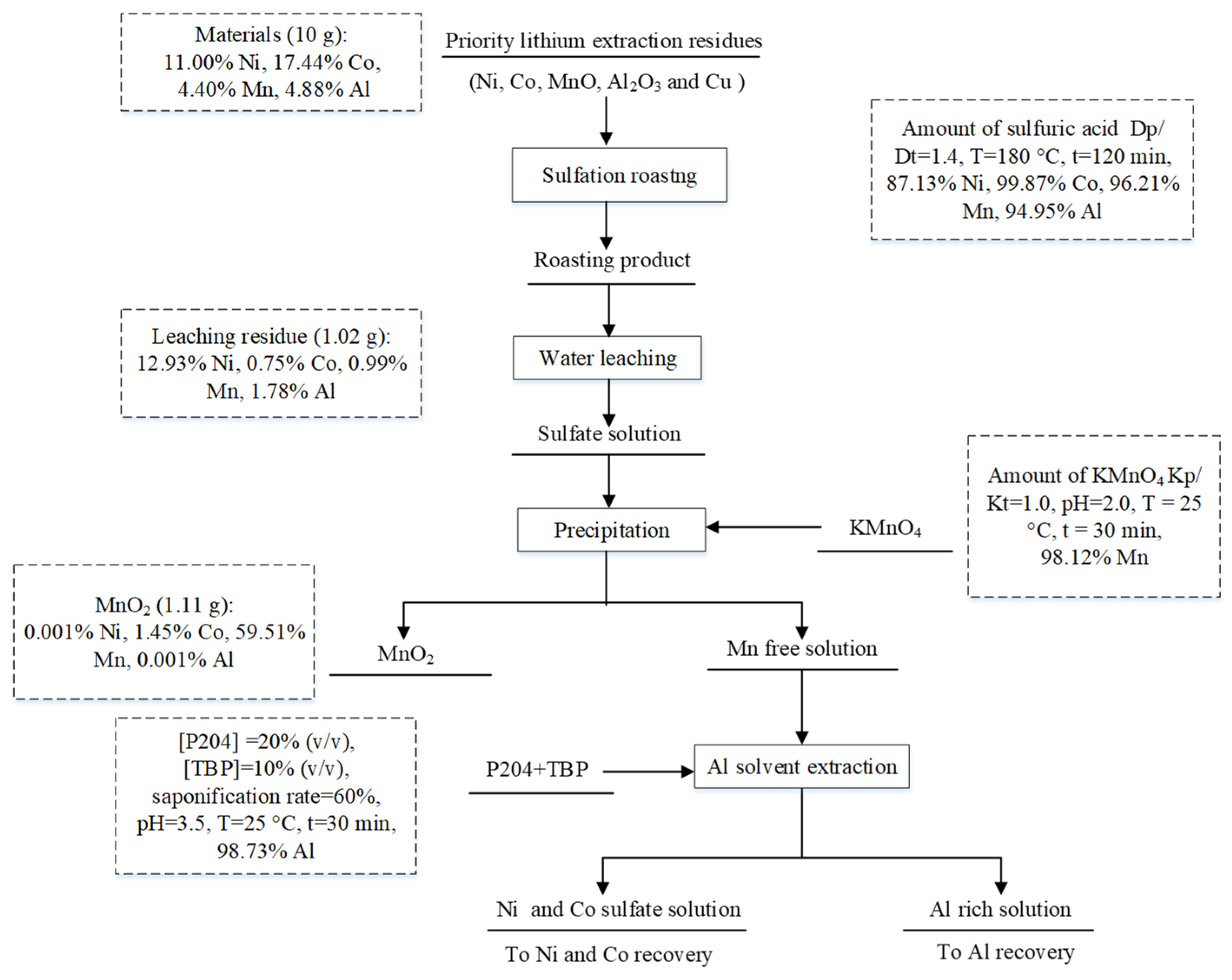
3.4. Flowsheet Development
4. Conclusions
- (1)
- A sulfation roasting–water leaching process enabled the efficient extraction of Ni, Co and Mn from priority lithium residues obtained from the hydrogen reduction of spent lithium ternary batteries. More than 87% Ni, 99% Co and 96% Mn were extracted at 180 °C and 120 min with 1.4 times the theoretical amount of sulfuric acid.
- (2)
- More than 98% of the Mn was selectively separated and precipitated under the optimal conditions. Al was also separated through extraction with P204 from the leaching solution. More than 98% of the Al was extracted with 20% (v/v) P204 + 10% (v/v) TBP at an A/O ratio of 1:1 within 30 min at 30 °C.
- (3)
- This optimized and efficient process for the extraction of valuable metals from priority extraction lithium residues increased the feasibility of hydrogen reduction treatment for waste lithium batteries and enabled industrialization of the new process. The proposed new process not only achieves efficient recovery of valuable metals such as lithium, nickel, cobalt, manganese, etc., but also has advantages such as high efficiency, low energy consumption and environmental protection.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gastol, M.; Marshall, J.; Cooper, E.; Mitchell, C.; Burnett, D.; Song, T.; Sommerville, R.; Middleton, B.; Crozier, M.; Smith, R.; et al. Reclaimed and Up-Cycled Cathodes for Lithium-Ion Batteries. Glob. Chall. 2022, 6, 2200046. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.Q.; Ji, J.P.; Ma, X.M. Environmental and economic evaluation of remanufacturing lithium-ion batteries from electric vehicles. Waste Manag. 2020, 102, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global Lithium Availability A Constraint for Electric Vehicles? J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Li, F.H.; Yang, J.Y. Research progress on recovery of cathode materials from spent lithium batteries. Mod. Chem. Ind. 2021, 41, 90–94. [Google Scholar]
- Sonoc, A.; Jeswiet, J. A Review of Lithium Supply and Demand and a Preliminary Investigation of a Room Temperature Method to Recycle Lithium Ion Batteries to Recover Lithium and Other Materials. Procedia CIRP 2014, 15, 289–293. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Sonoc, A.; Jeswiet, J.; Soo, V.K. Opportunities to Improve Recycling of Automotive Lithium Ion Batteries. Procedia CIRP 2015, 29, 752–757. [Google Scholar] [CrossRef]
- Du, G.C.; Zheng, L.L.; Zhang, Z.C.; Feng, Y.; Wang, D.; Dai, Z.Q. Overview of research on thermal safety of lithium-ion batteries. Energy Storage Sci. Technol. 2019, 8, 500–505. [Google Scholar]
- Li, J.G.; Zhao, R.S.; He, X.M.; Liu, H.C. Preparation of LiCoO2 cathode materials from spent lithium-ion batteries. Ionics 2009, 15, 111–113. [Google Scholar] [CrossRef]
- Yang, J.; Qin, J.; Li, F.C.; Jiang, L.X.; Lai, Y.Q.; Liu, F.Y.; Jia, M. Review of hydrometallurgical processes for recycling spent lithium-ion batteries. J. Cent. South Univ. (Sci. Technol.). 2020, 51, 3261–3278. [Google Scholar]
- Liu, G.Q.; Wang, F. Status of Power Lithium Ion Battery Recycle Technology. China Resour. Compr. Util. 2018, 36, 88–92. [Google Scholar]
- Wang, F.; Zhang, B.S.; Liu, G.Q.; Xie, X. Progress in Recycling Technology of Waste Power Battery Resources. China Resour. Compr. Util. 2018, 36, 106–111. [Google Scholar]
- Ren, G.; Xiao, S.; Xie, M.; Pan, B.; Fan, Y.; Wang, F.; Xia, X. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Smelting Reduction Process Based on MnO-SiO2-Al2O3 Slag System. In Advances in Molten Slags, Fluxes, and Salts: Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts 2016, Washington, DC, USA, 22–25 May 2016; Springer: Berlin/Heidelberg, Germany, 2016; pp. 211–218. [Google Scholar]
- Heydarian, A.; Mousavi, S.M.; Vakilchap, F.; Baniasadi, M. Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J. Power Sources 2018, 378, 19–30. [Google Scholar] [CrossRef]
- Yao, Y.L.; Zhu, M.Y.; Zhao, Z.; Tong, B.H.; Fan, Y.Q.; Hua, Z.S. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Gu, F.; Guo, J.F.; Yao, X.; Summers, P.A.; Widijatmoko, S.D.; Hall, P. An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China. J. Clean. Prod. 2017, 161, 765–780. [Google Scholar] [CrossRef]
- Tong, Z.; Ren, X.B.; Ni, M.Q.; Bu, X.N.; Dong, L.S. Review of Ultrasound-Assisted Recycling and Utilization of Cathode Materials from Spent Lithium-Ion Batteries: State-of-the-Art and Outlook. Energy Fuels 2023, 37, 14574–14588. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Chen, H. Separation of Li and Co from the active mass of spent Li-ion batteries by selective sulfat-ing roasting with sodium bisulfate and water leaching. Miner. Eng. 2018, 126, 28–35. [Google Scholar] [CrossRef]
- Peng, C.; Liu, F.; Wang, Z. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 2019, 415, 179–188. [Google Scholar] [CrossRef]
- Zhang, J.L.; Hu, J.T.; Zhang, W.J. Efficient and economical recovery of lithium, cobalt, nickel, manganese from cathode scrap of spent lithium-ion batteries. J. Clean. Prod. 2018, 204, 437–446. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.H.; Ma, E. Selective Recovery of Lithium from Spent Lithium-ion Batteries Synergized by Carbon and Sulfur Elements. Acta Chim. Sin. 2021, 79, 1073–1081. [Google Scholar] [CrossRef]
- Xu, P.; Liu, C.W.; Zhang, X.H. Synergic Mechanisms on Carbon and Sulfur during the Selective Recovery of Valuable Metals from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 2271–2279. [Google Scholar] [CrossRef]
- Liu, F.; Peng, C.; Ma, Q.; Wang, J.; Zhou, S.; Chen, Z.; Wilson, B.P.; Lundström, M. Selective lithium recovery and integrated preparation of high-purity lithium hydroxide products from spent lithium-ion batteries. Sep. Purif. Technol. 2021, 259, 118181. [Google Scholar] [CrossRef]
- Zheng, H.S.; Dong, T.; Sha, Y.F.; Jiang, D.F.; Zhang, H.T.; Zhang, S.J. Selective Extraction of Lithium from Spent Lithium Batteries by Functional Ionic Liquid. ACS Sustain. Chem. Eng. 2021, 9, 7022–7029. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K.Q. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pan, Z.F.; Su, X.Y.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Peng, C.; Chang, C.; Wang, Z.; Wilson, B.P.; Liu, F.; Lundstrm, M. Correction to: Recovery of High-Purity MnO2 from the Acid Leaching Solution of Spent Li-Ion Batteries. JOM J. Miner. Met. Mater. Soc. 2020, 72, 978. [Google Scholar] [CrossRef]
- Huang, Y.; Han, G.; Liu, J.; Chai, W.; Wang, W.; Yang, S.; Su, S. A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leaching-flotation-precipitation process. J. Power Sources 2016, 325, 555–564. [Google Scholar] [CrossRef]
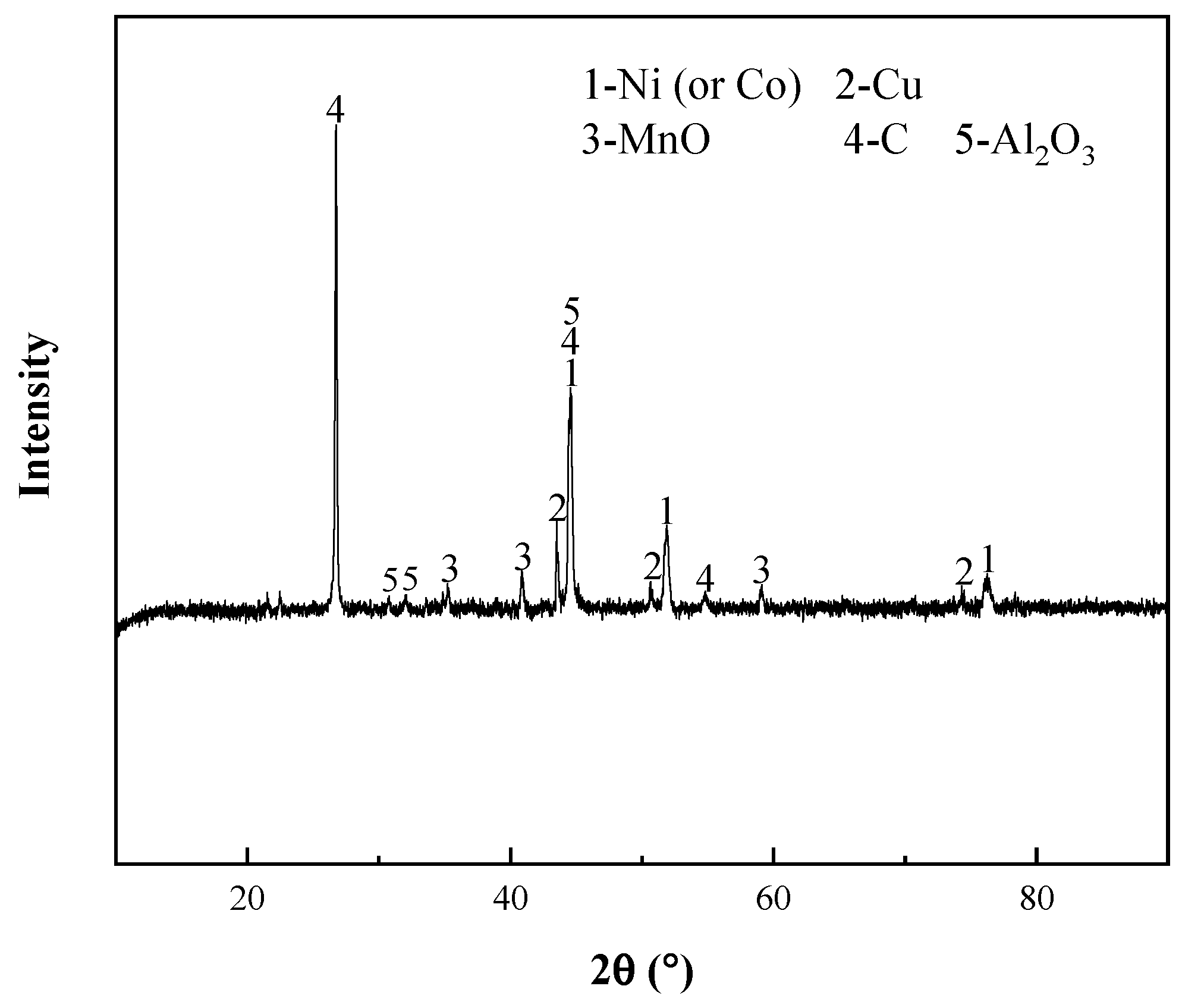
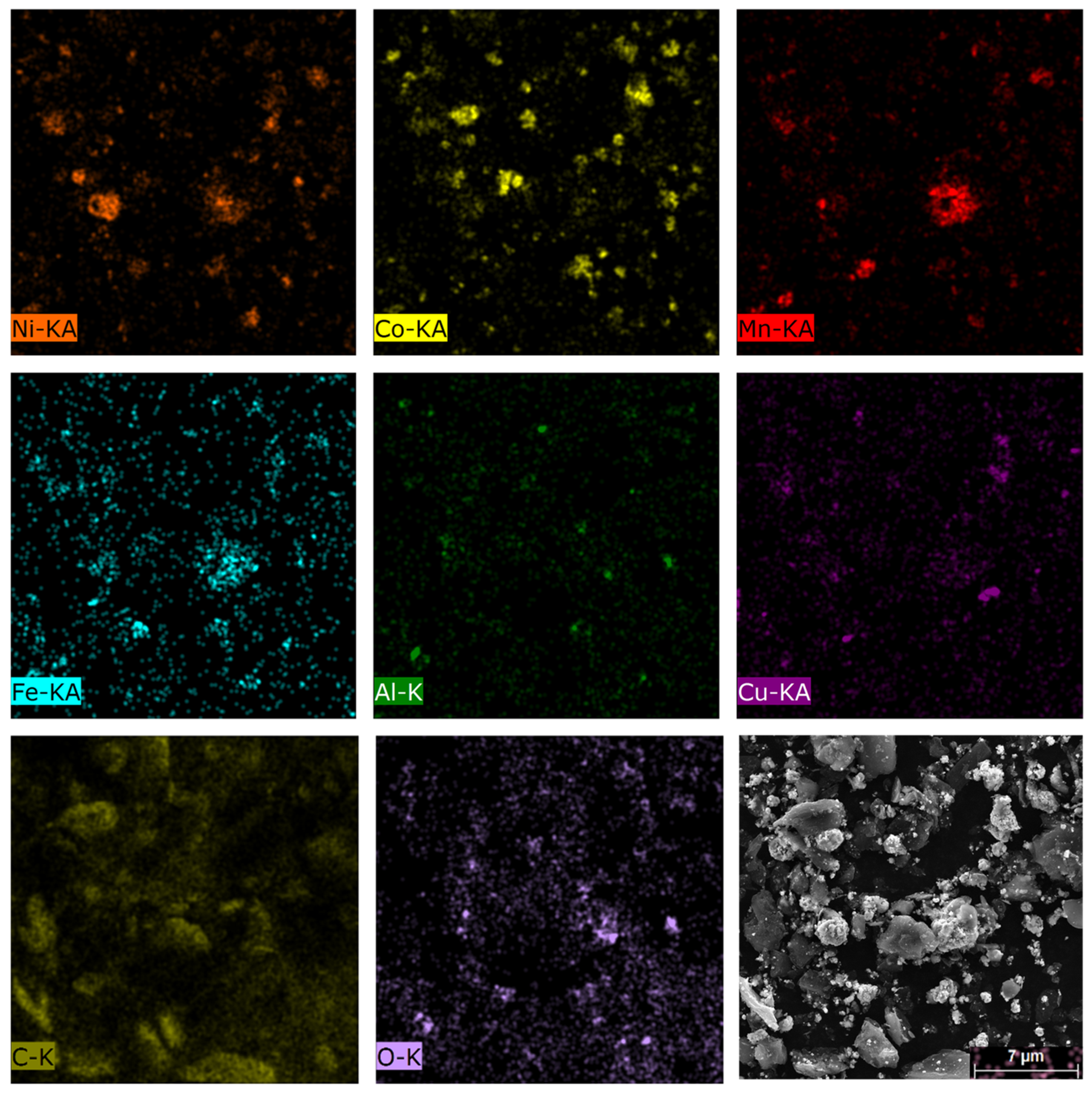
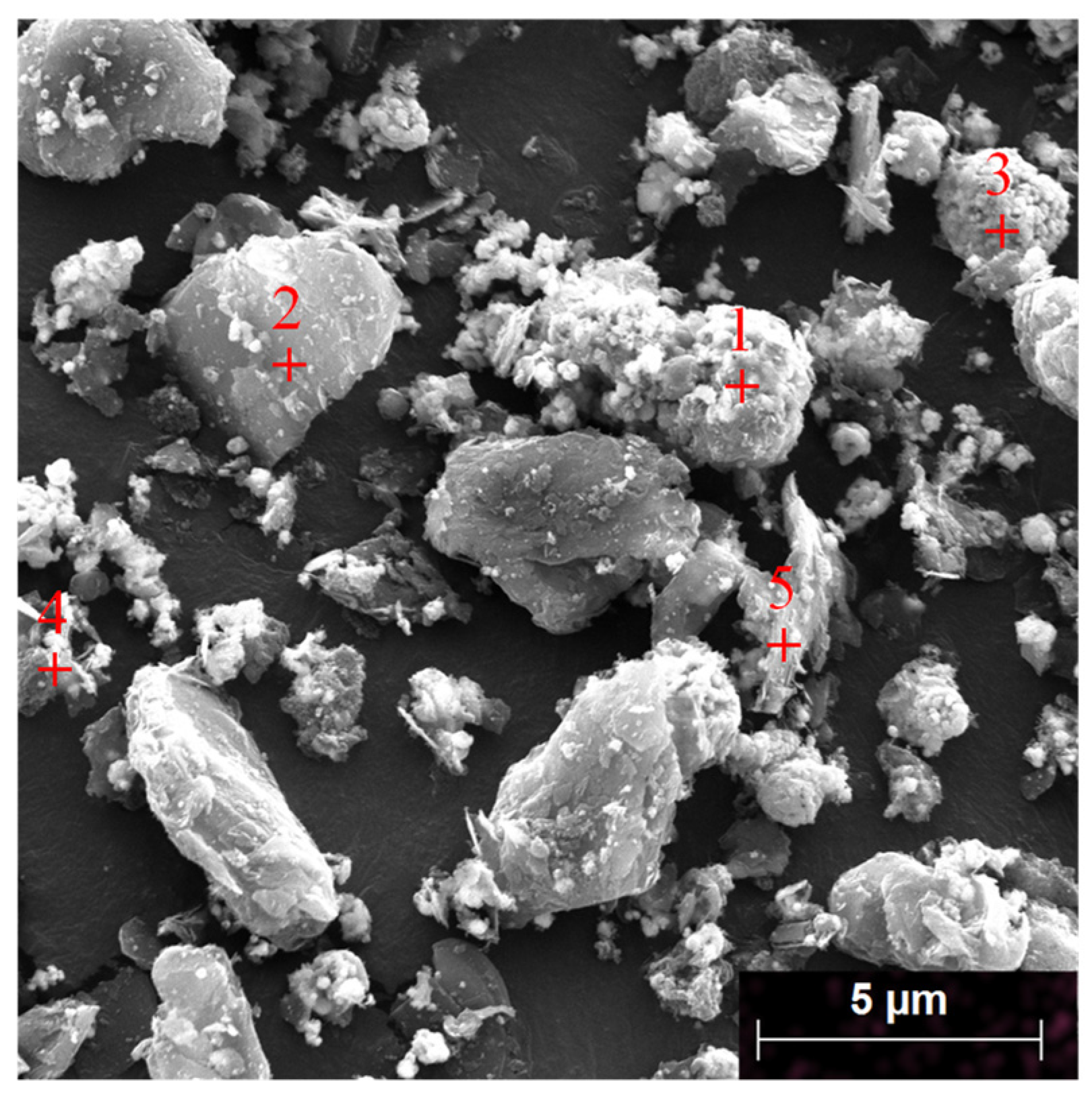
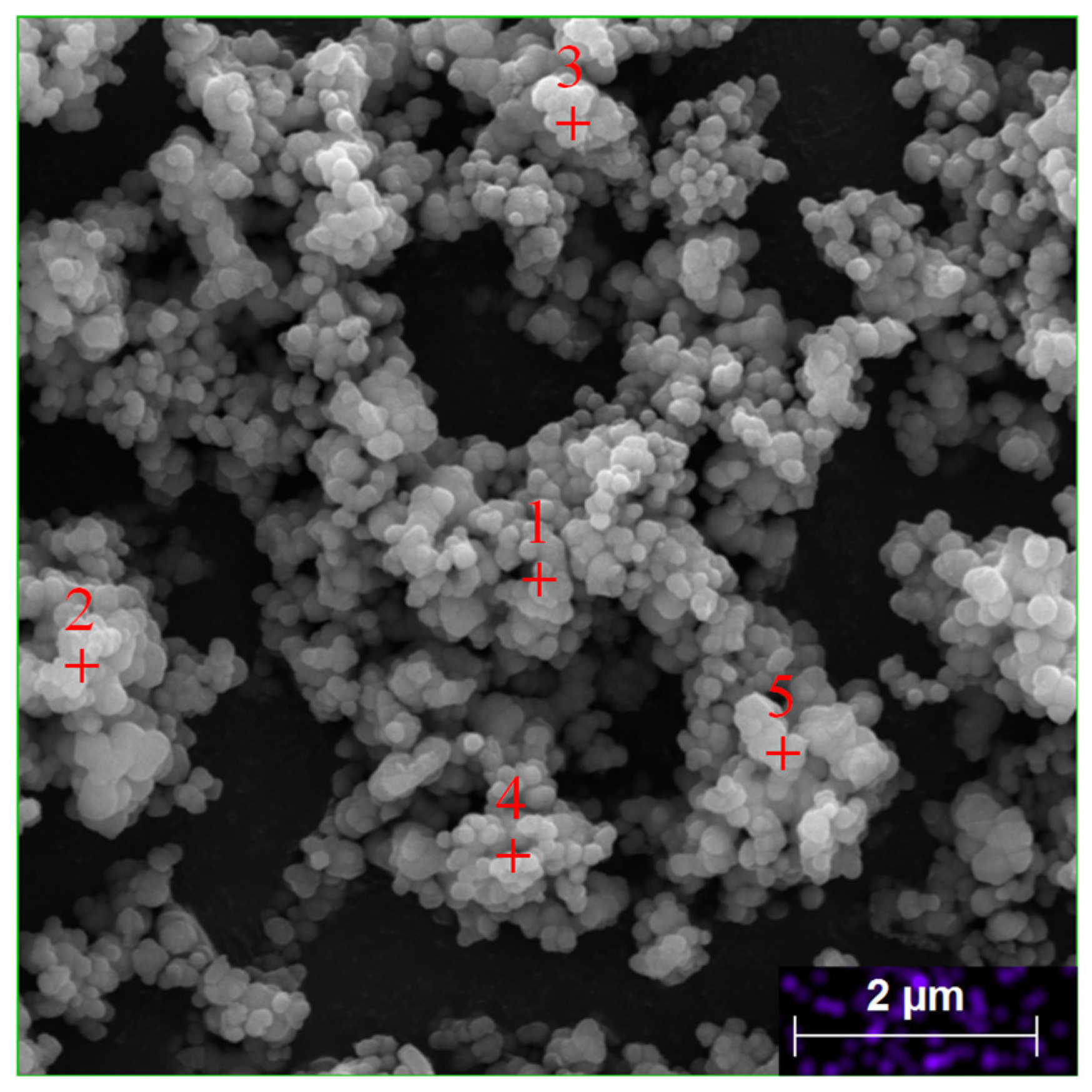
| Elements | Ni | Co | Mn | Al | Cu | Fe |
|---|---|---|---|---|---|---|
| wt.% | 11.00 | 17.44 | 4.40 | 4.88 | 11.29 | 0.58 |
| Spectrum | O | Mn | Co | Ni | Al | Fe | Cu |
|---|---|---|---|---|---|---|---|
| 1 | 50.66 | 45.91 | 1.47 | 0.27 | 0.20 | 0.38 | 1.24 |
| 2 | 7.68 | 3.47 | 84.74 | 1.34 | 0.32 | 0.15 | 2.30 |
| 3 | 7.37 | 4.07 | 4.26 | 81.28 | 0.27 | 0.14 | 2.61 |
| 4 | 7.77 | 2.00 | 84.01 | 1.76 | 0.00 | 0.00 | 4.47 |
| 5 | 36.45 | 7.95 | 30.10 | 16.71 | 2.33 | 0.00 | 6.45 |
| Spectrum | O | Mn | Co | Ni | Al | K | Fe | Cu | S |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 65.53 | 30.80 | 1.89 | 0.00 | 0.20 | 0.19 | 0.55 | 0.60 | 0.25 |
| 2 | 72.54 | 24.34 | 1.51 | 0.02 | 0.42 | 0.26 | 0.60 | 0.13 | 0.18 |
| 3 | 72.43 | 24.87 | 1.34 | 0.00 | 0.22 | 0.16 | 0.50 | 0.23 | 0.25 |
| 4 | 71.11 | 26.05 | 1.22 | 0.01 | 0.20 | 0.28 | 0.37 | 0.50 | 0.25 |
| 5 | 70.05 | 26.78 | 0.94 | 0.35 | 0.37 | 0.15 | 0.32 | 0.82 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, F.; Chen, F.; Chen, Z.; Zeng, H.; Zhang, T.; Shen, C. Recycling of Valuable Metals from the Priority Lithium Extraction Residue Obtained through Hydrogen Reduction of Spent Lithium Batteries. Batteries 2024, 10, 28. https://doi.org/10.3390/batteries10010028
Guo Y, Liu F, Chen F, Chen Z, Zeng H, Zhang T, Shen C. Recycling of Valuable Metals from the Priority Lithium Extraction Residue Obtained through Hydrogen Reduction of Spent Lithium Batteries. Batteries. 2024; 10(1):28. https://doi.org/10.3390/batteries10010028
Chicago/Turabian StyleGuo, Yong, Fupeng Liu, Feixiong Chen, Zaoming Chen, Hong Zeng, Tao Zhang, and Changquan Shen. 2024. "Recycling of Valuable Metals from the Priority Lithium Extraction Residue Obtained through Hydrogen Reduction of Spent Lithium Batteries" Batteries 10, no. 1: 28. https://doi.org/10.3390/batteries10010028
APA StyleGuo, Y., Liu, F., Chen, F., Chen, Z., Zeng, H., Zhang, T., & Shen, C. (2024). Recycling of Valuable Metals from the Priority Lithium Extraction Residue Obtained through Hydrogen Reduction of Spent Lithium Batteries. Batteries, 10(1), 28. https://doi.org/10.3390/batteries10010028








