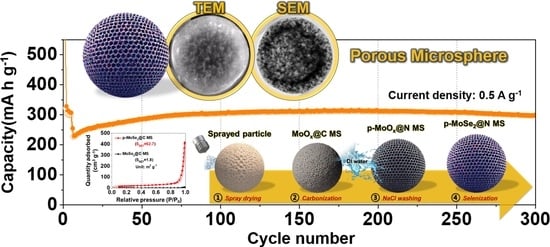
Facile Fabrication of Porous MoSe2/Carbon Microspheres via the Aerosol Process as Anode Materials in Potassium-Ion Batteries
Abstract
1. Introduction
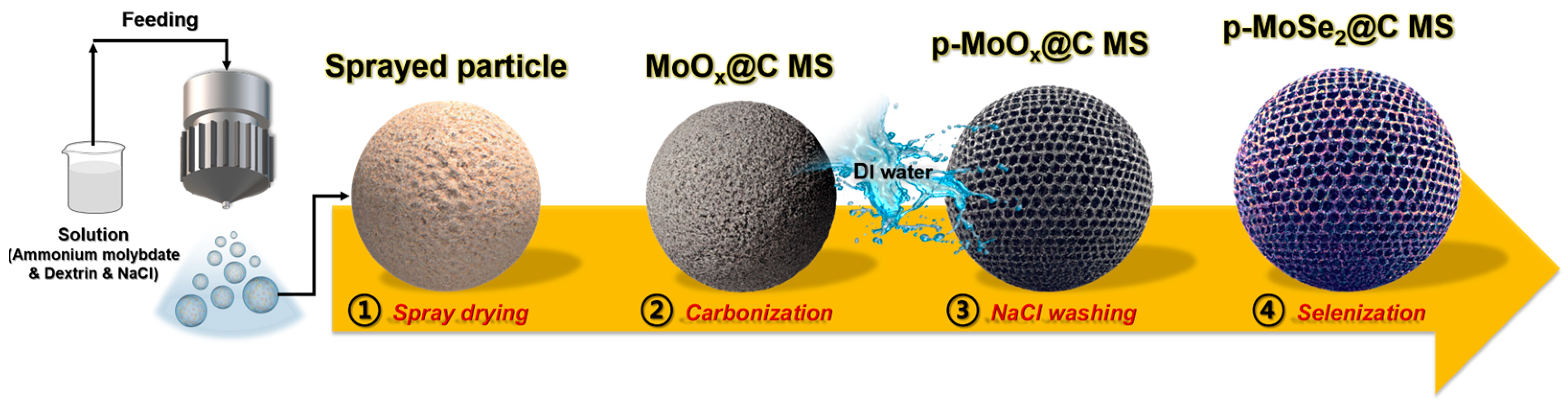
2. Experimental Procedures
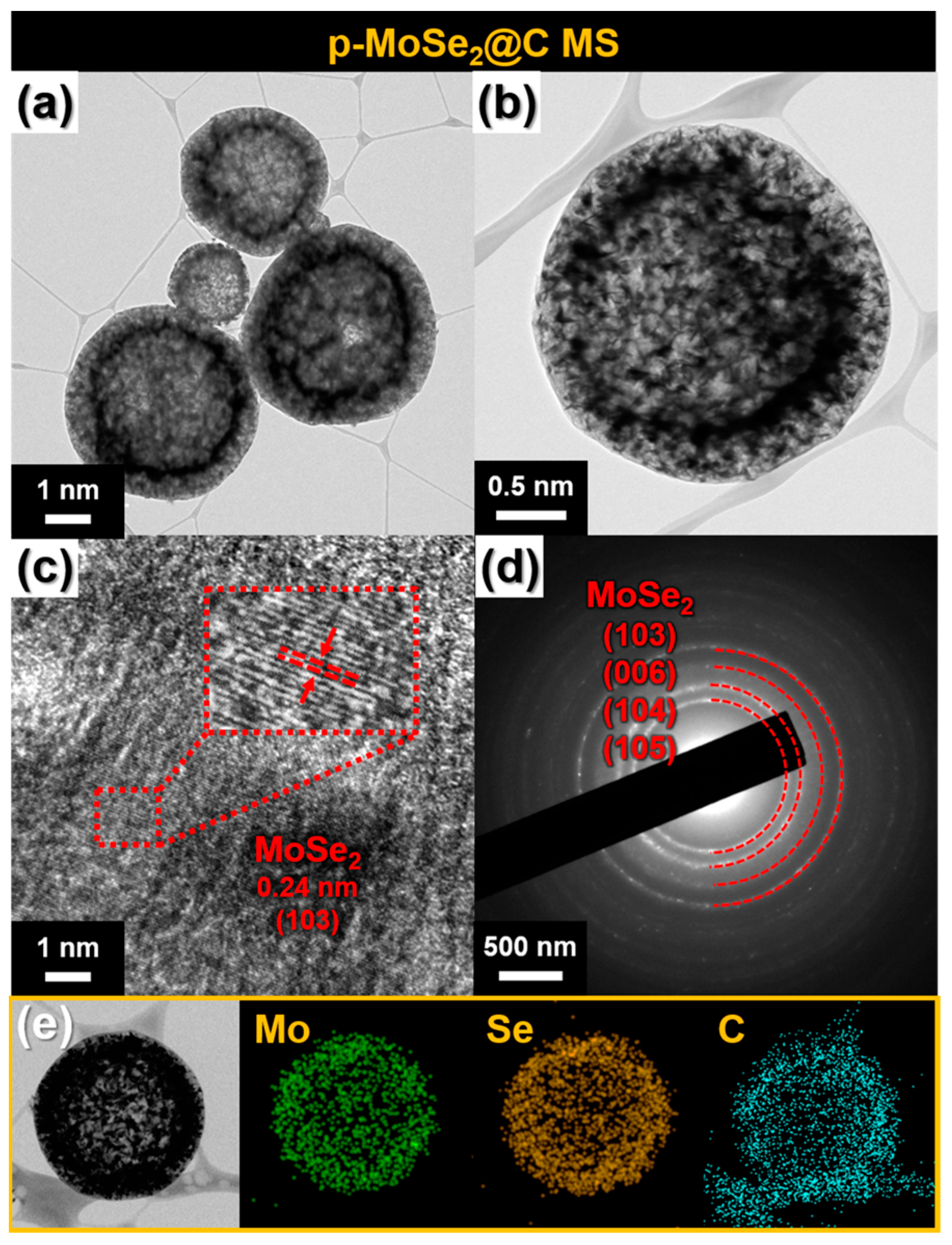
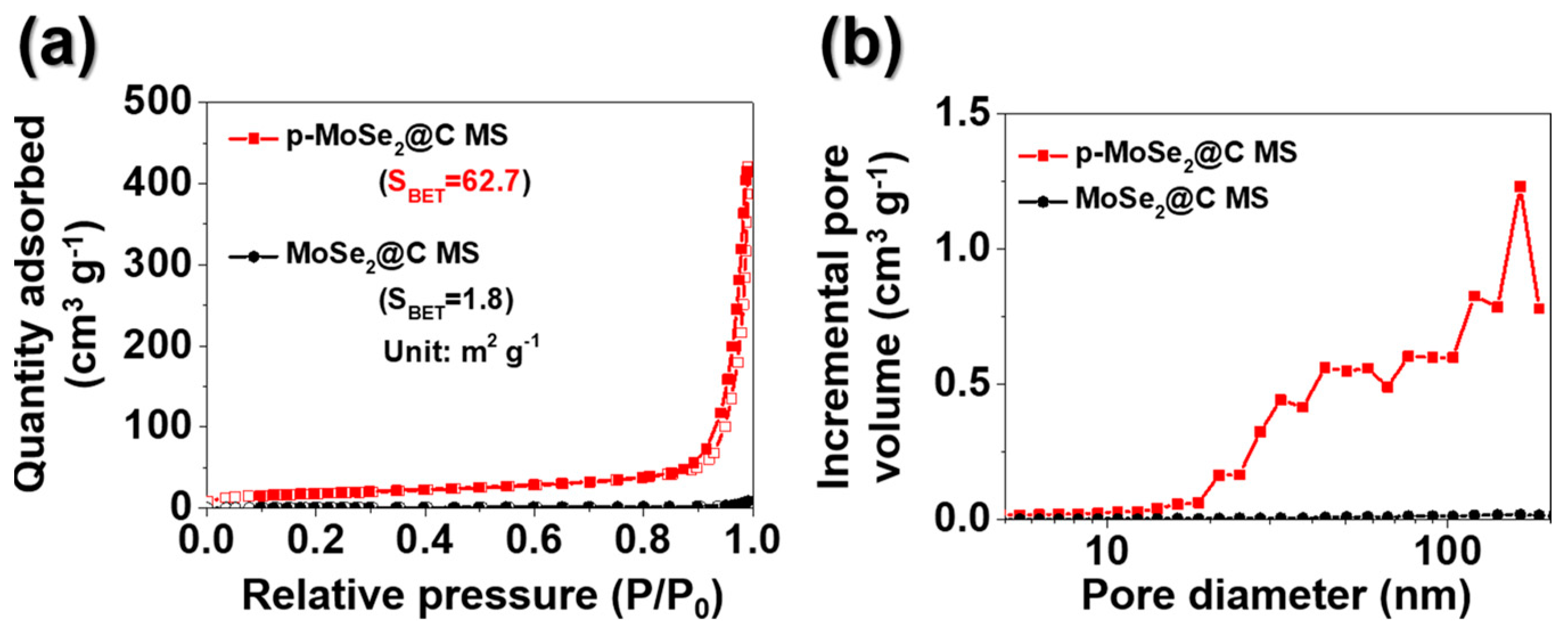
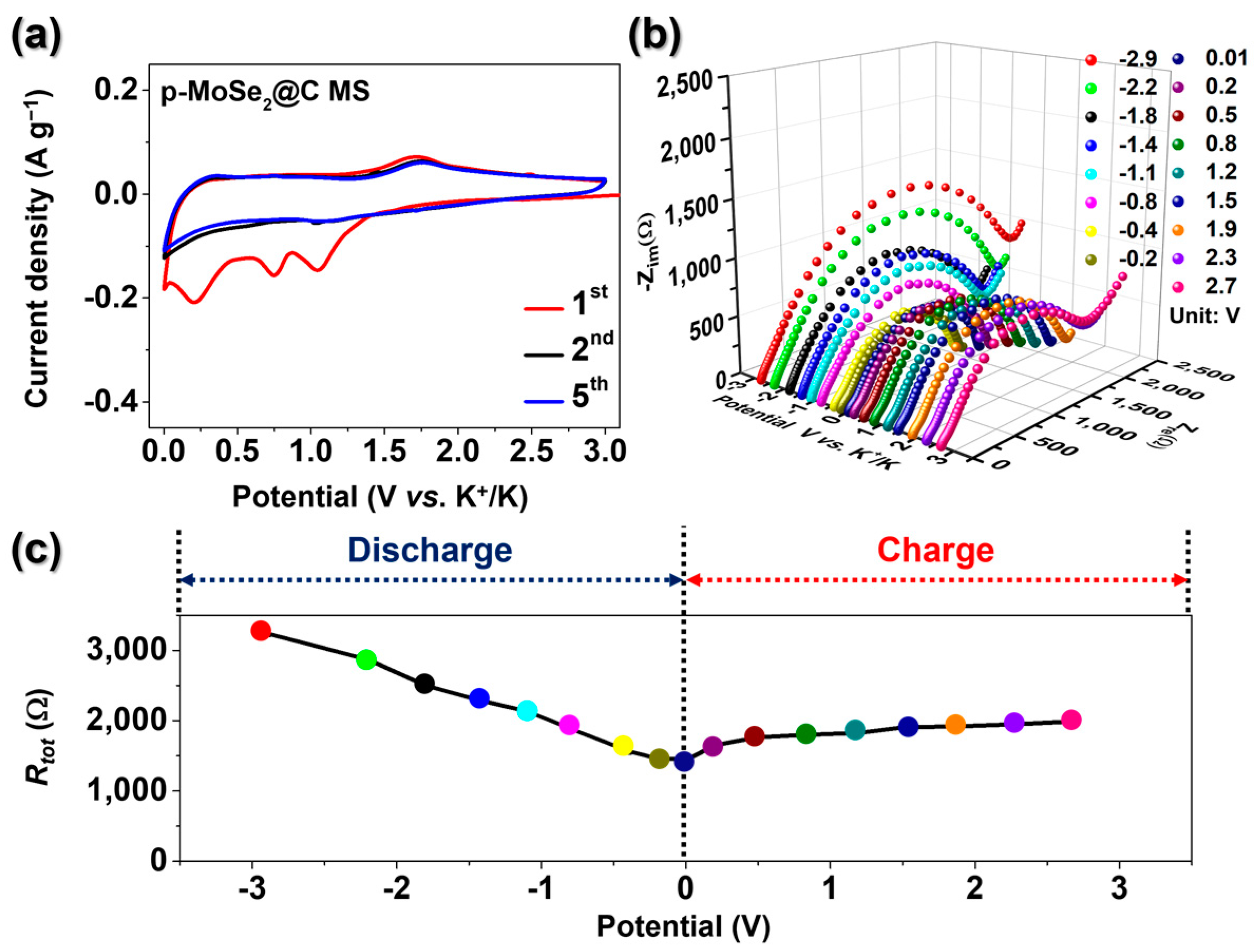
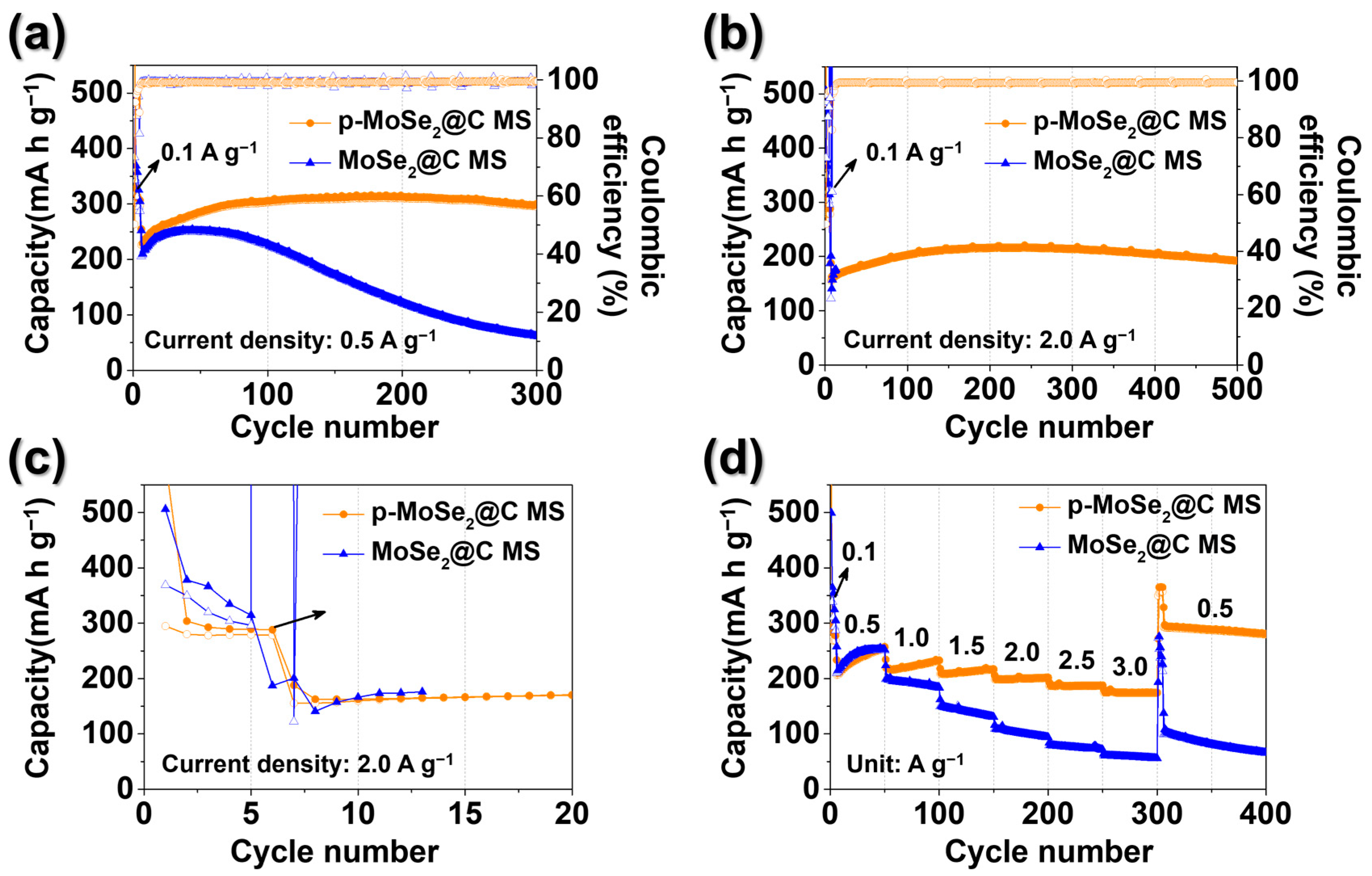
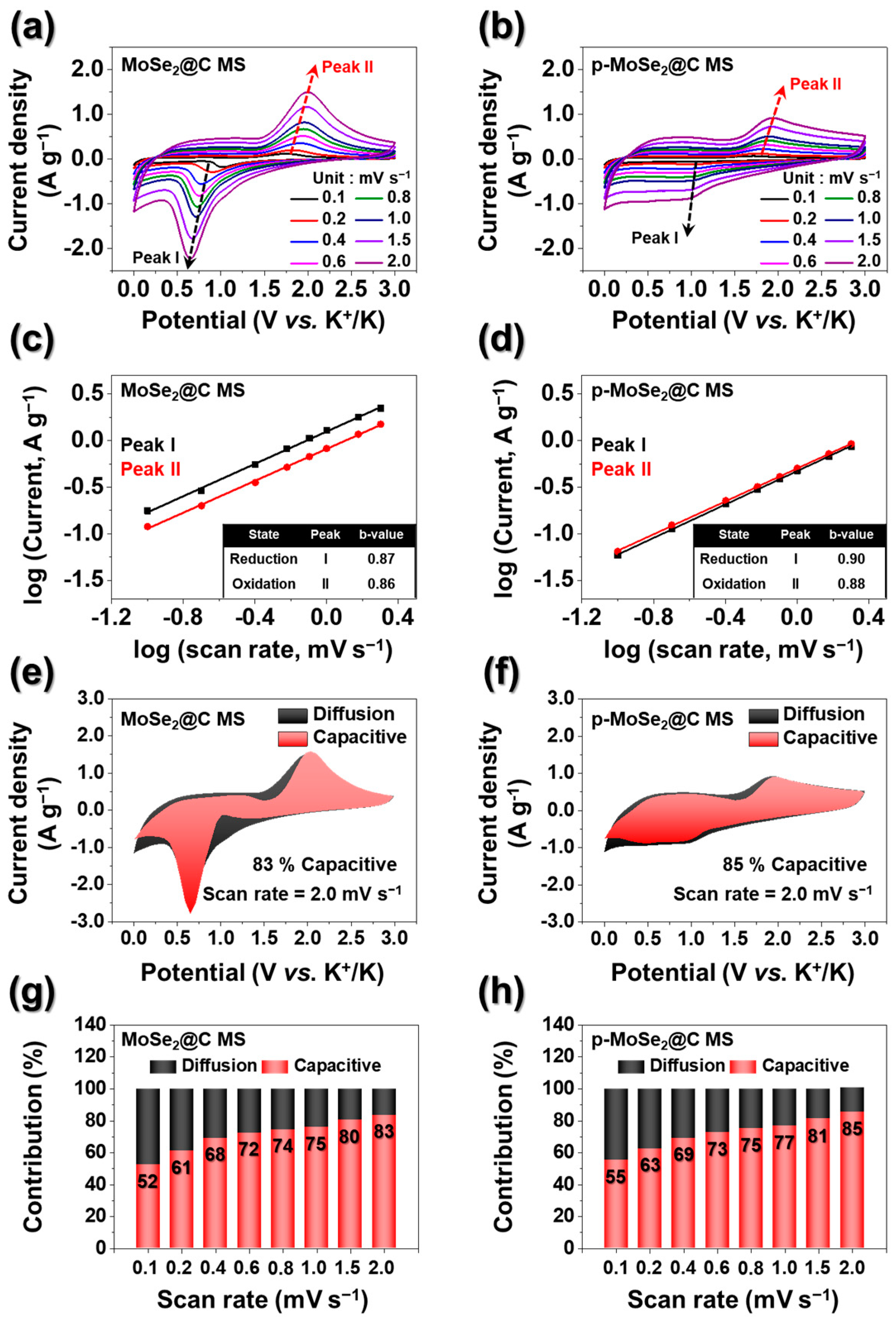
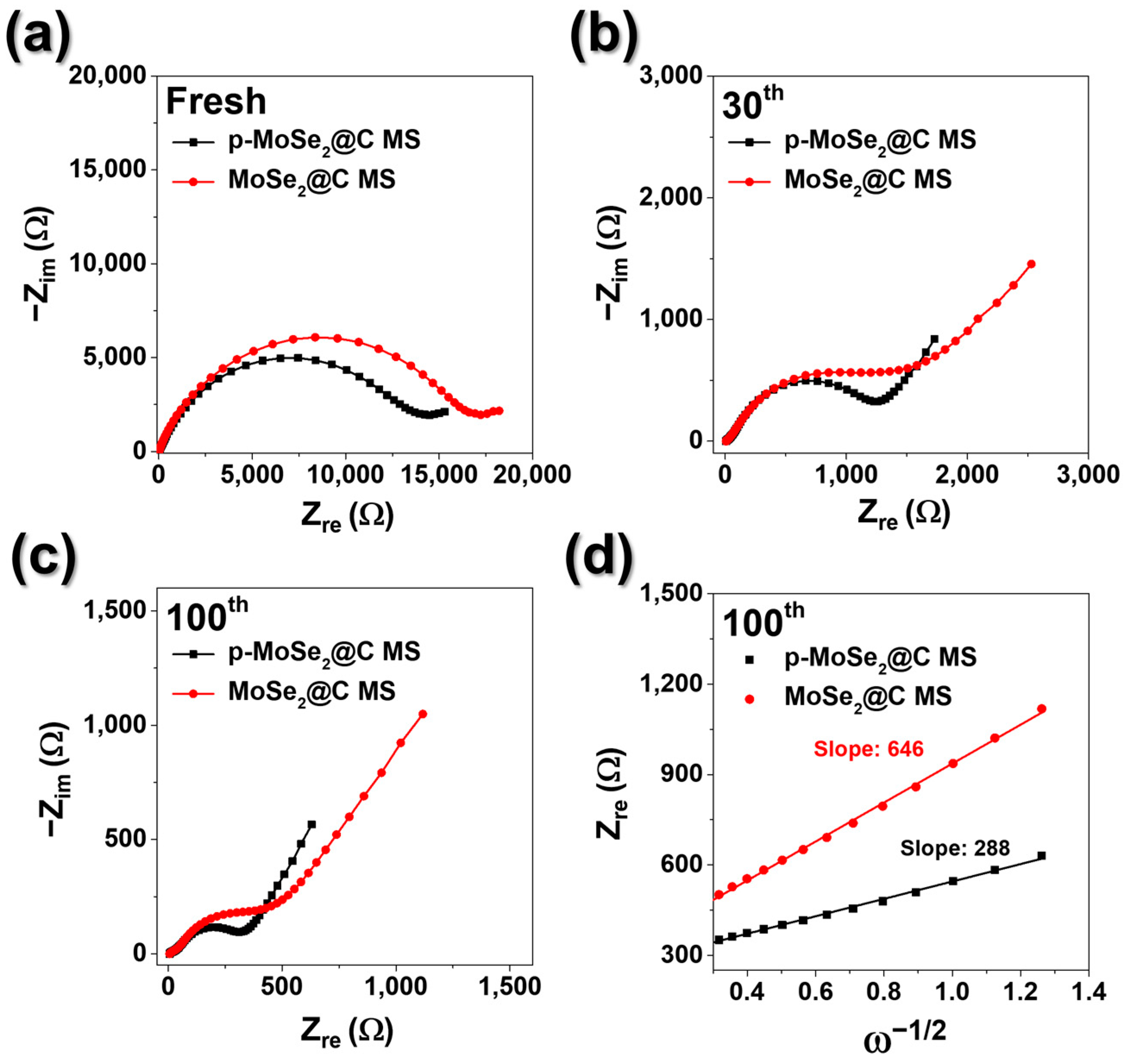
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Lu, Y.-C. A Retrospective on Lithium-Ion Batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Deng, H.; He, P.; Zhou, H. A 500 Wh/kg Lithium-Metal Cell Based on Anionic Redox. Joule 2020, 4, 1445–1458. [Google Scholar] [CrossRef]
- Tarascon, J.-M. Is Lithium the New Gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, Y.; Wang, Z.; Shao, G. First Principle Material Genome Approach for All Solid-State Batteries. Energy Environ. Mater. 2019, 2, 234–250. [Google Scholar] [CrossRef]
- Ji, B.; Yao, W.; Zheng, Y.; Kidkhunthod, P.; Zhou, X.; Tunmee, S.; Sattayaporn, S.; Cheng, H.-M.; He, H.; Tang, Y. A Fluoroxalate Cathode Material for Potassium-Ion Batteries with Ultra-Long Cyclability. Nat. Commun. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-H.; Zhang, Q.; Yang, X.; Zhao, E.-Y.; Sun, T.; Zhang, X.-B.; Wang, S.; Yu, X.-Q.; Yan, J.-M.; Jiang, Q. Reconstructed Orthorhombic V2O5 Polyhedra for Fast Ion Diffusion in K-Ion Batteries. Chem 2019, 5, 168–179. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Gao, Y.; Wei, Z.; Zhang, M.; Wang, C.; Zeng, Y.; Zou, B.; Chen, G.; Du, F. Fast Potassium Storage in Hierarchical Ca0.5Ti2(PO4)3@C Microspheres Enabling High-Performance Potassium-Ion Capacitors. Adv. Funct. Mater. 2018, 28, 1802684. [Google Scholar] [CrossRef]
- Lei, K.; Li, F.; Mu, C.; Wang, J.; Zhao, Q.; Chen, C.; Chen, J. High K-Storage Performance Based on the Synergy of Dipotassium Terephthalate and Ether-Based Electrolytes. Energy Environ. Sci. 2017, 10, 552–557. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Deng, H.; Wang, L.; Li, S.; Zhang, M.; Wang, T.; Zhou, J.; Chen, M.; Chen, S.; Cao, J.; Zhang, Q.; et al. Radial Pores in Nitrogen/Oxygen Dual-Doped Carbon Nanospheres Anode Boost High-Power and Ultrastable Potassium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2107246. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Wang, J.; Zhang, Q.; Liu, Z.; Zhang, E.; Liu, Q.; Yu, X.; Lu, B. MoSe2/N-Doped Carbon as Anodes for Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801477. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Liu, W.-R. Few-Layered MoSe2 Ultrathin Nanosheets as Anode Materials for Lithium Ion Batteries. J. Alloys Compd. 2020, 813, 152074. [Google Scholar] [CrossRef]
- Zheng, C.; Wu, J.; Li, Y.; Liu, X.; Zeng, L.; Wei, M. High-Performance Lithium-Ion-Based Dual-Ion Batteries Enabled by Few-Layer MoSe2/Nitrogen-Doped Carbon. ACS Sustain. Chem. Eng. 2020, 8, 5514–5523. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, H.; Guan, C.; Cheng, C. Ultrathin MoS2 Nanosheets@Metal Organic Framework-Derived N-Doped Carbon Nanowall Arrays as Sodium Ion Battery Anode with Superior Cycling Life and Rate Capability. Adv. Funct. Mater. 2017, 27, 1702116. [Google Scholar] [CrossRef]
- Morant-Giner, M.; Sanchis-Gual, R.; Romero, J.; Alberola, A.; García-Cruz, L.; Agouram, S.; Galbiati, M.; Padial, N.M.; Waerenborgh, J.C.; Martí-Gastaldo, C.; et al. Prussian Blue@MoS2 Layer Composites as Highly Efficient Cathodes for Sodium- and Potassium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1706125. [Google Scholar] [CrossRef]
- Yi, Y.; Sun, Z.; Li, C.; Tian, Z.; Lu, C.; Shao, Y.; Li, J.; Sun, J.; Liu, Z. Designing 3D Biomorphic Nitrogen-Doped MoSe2/Graphene Composites toward High-Performance Potassium-Ion Capacitors. Adv. Funct. Mater. 2020, 30, 1903878. [Google Scholar] [CrossRef]
- Tao, J.; Yan, Z.; Wang, D.; Zhong, W.; Yang, Y.; Li, J.; Lin, Y.; Huang, Z. Rational Designing of MoSe2 Nanosheets in Carbon Framework for High-Performance Potassium-Ion Batteries. Chem. Eng. J. 2022, 448, 137658. [Google Scholar] [CrossRef]
- Su, C.; Ru, Q.; Gao, Y.; Shi, Z.; Zheng, M.; Chen, F.; Chi-Chung Ling, F.; Wei, L. Biowaste-Sustained MoSe2 Composite as an Efficient Anode for Sodium/Potassium Storage Applications. J. Alloys Compd. 2021, 850, 156770. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, Z.; Liang, H.; Li, S.; Wang, H.; Gao, F.; Sang, X.; Li, H. Nanosized MoSe2@Carbon Matrix: A Stable Host Material for the Highly Reversible Storage of Potassium and Aluminum Ions. ACS Appl. Mater. Interfaces 2019, 11, 44333–44341. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, Y.; Sun, Y.; Rong, J.; Li, H.; Niu, L. Advanced Anode Materials of Potassium Ion Batteries: From Zero Dimension to Three Dimensions. Nano-Micro Lett. 2020, 13, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Zhai, Y.; Wang, N.; Zhang, Y.; Lu, Z.; Xue, P.; Bai, L.; Guo, M.; Huang, D.; Bai, Z. Ultrathin MoSe2 Nanosheets Confined in N-Doped Macroporous Carbon Frame for Enhanced Potassium Ion Storage. ChemistrySelect 2020, 5, 2412–2418. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, L.; Wang, Y.; Qin, M.; Wu, R.; Huang, Z.; Yang, H.-J.; Li, Y.; Zhou, T.; Hu, J. Rational Design of MoSe2 Nanosheet-Coated MOF-Derived N-Doped Porous Carbon Polyhedron for Potassium Storage. J. Colloid Interface Sci. 2021, 600, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Wu, X.; Wang, Y.; Zheng, S.; Sun, D.; Song, Y.; Chen, M. Nitrogen-Doped Hollow Carbon Nanospheres towards the Application of Potassium Ion Storage. J. Mater. Chem. A 2019, 7, 19305–19315. [Google Scholar] [CrossRef]
- Xia, G.; Wang, C.; Jiang, P.; Lu, J.; Diao, J.; Chen, Q. Nitrogen/Oxygen Co-Doped Mesoporous Carbon Octahedrons for High-Performance Potassium-Ion Batteries. J. Mater. Chem. A 2019, 7, 12317–12324. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Liang, X.; Liu, Y.; Chen, Q.; Chen, M. Inhibiting Structural Degeneration of MoSe2 Anode with Dual-Layer Protection for Highly Robust Na-Ion Battery. Mater. Chem. Phys. 2022, 278, 125681. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kempaiah Devaraju, M.; Nakayasu, Y.; Tamura, N.; Sasaki, Y.; Tomai, T.; Honma, I. Exfoliated MoS2 and MoSe2 Nanosheets by a Supercritical Fluid Process for a Hybrid Mg–Li-Ion Battery. ACS Omega 2017, 2, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yu, Y.; Wang, L.; Zhang, X.; Wang, Y.; Shao, Z.; Jie, J. Ultrafast, Broadband Photodetector Based on MoSe2/Silicon Heterojunction with Vertically Standing Layered Structure Using Graphene as Transparent Electrode. Adv. Sci. 2016, 3, 1600018. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. Single-Layer MoS2/Graphene Dispersed in Amorphous Carbon: Towards High Electrochemical Performances in Rechargeable Lithium Ion Batteries. J. Mater. Chem. 2011, 21, 17175–17184. [Google Scholar] [CrossRef]
- Xu, D.; Chen, L.; Su, X.; Jiang, H.; Lian, C.; Liu, H.; Chen, L.; Hu, Y.; Jiang, H.; Li, C. Heterogeneous MoSe2/Nitrogen-Doped-Carbon Nanoarrays: Engineering Atomic Interface for Potassium-Ion Storage. Adv. Funct. Mater. 2022, 32, 2110223. [Google Scholar] [CrossRef]
- Shen, Q.; Jiang, P.; He, H.; Chen, C.; Liu, Y.; Zhang, M. Encapsulation of MoSe2 in Carbon Fibers as Anodes for Potassium Ion Batteries and Nonaqueous Battery–Supercapacitor Hybrid Devices. Nanoscale 2019, 11, 13511–13520. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Li, S.; Yang, L.; Ji, X. Tailoring Rod-Like FeSe2 Coated with Nitrogen-Doped Carbon for High-Performance Sodium Storage. Adv. Funct. Mater. 2018, 28, 1801765. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.; Xie, D.; Lin, Z.; Zheng, J.; Zheng, F.; Li, Y.; Liu, Y.; Yang, C.; Liu, M. Chemically Activated Hollow Carbon Nanospheres as a High-Performance Anode Material for Potassium Ion Batteries. J. Mater. Chem. A 2018, 6, 24317–24323. [Google Scholar] [CrossRef]
- Atangana Etogo, C.; Huang, H.; Hong, H.; Liu, G.; Zhang, L. Metal–Organic-Frameworks-Engaged Formation of Co0.85Se@C Nanoboxes Embedded in Carbon Nanofibers Film for Enhanced Potassium-Ion Storage. Energy Storage Mater. 2020, 24, 167–176. [Google Scholar] [CrossRef]
- Liu, P.; Han, J.; Zhu, K.; Dong, Z.; Jiao, L. Heterostructure SnSe2/ZnSe@PDA Nanobox for Stable and Highly Efficient Sodium-Ion Storage. Adv. Energy Mater. 2020, 10, 2000741. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, Q.; Liu, H.; Xu, B.; Zhang, S.; Zhou, T.; Mao, J.; Pang, W.K.; Guo, Z.; Li, A.; et al. Graphitic Carbon Nanocage as a Stable and High Power Anode for Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801149. [Google Scholar] [CrossRef]
- Zhang, W.; Ming, J.; Zhao, W.; Dong, X.; Hedhili, M.N.; Costa, P.M.F.J.; Alshareef, H.N. Graphitic Nanocarbon with Engineered Defects for High-Performance Potassium-Ion Battery Anodes. Adv. Funct. Mater. 2019, 29, 1903641. [Google Scholar] [CrossRef]
- Shen, W.; Wang, C.; Xu, Q.; Liu, H.; Wang, Y. Nitrogen-Doping-Induced Defects of a Carbon Coating Layer Facilitate Na-Storage in Electrode Materials. Adv. Energy Mater. 2015, 5, 1400982. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Investigation of the Effect of Fluoroethylene Carbonate Additive on Electrochemical Performance of Sb-Based Anode for Sodium-Ion Batteries. Electrochim. Acta 2016, 190, 402–408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, D.Y.; Park, S.-K. Facile Fabrication of Porous MoSe2/Carbon Microspheres via the Aerosol Process as Anode Materials in Potassium-Ion Batteries. Batteries 2024, 10, 25. https://doi.org/10.3390/batteries10010025
Jo DY, Park S-K. Facile Fabrication of Porous MoSe2/Carbon Microspheres via the Aerosol Process as Anode Materials in Potassium-Ion Batteries. Batteries. 2024; 10(1):25. https://doi.org/10.3390/batteries10010025
Chicago/Turabian StyleJo, Du Yeol, and Seung-Keun Park. 2024. "Facile Fabrication of Porous MoSe2/Carbon Microspheres via the Aerosol Process as Anode Materials in Potassium-Ion Batteries" Batteries 10, no. 1: 25. https://doi.org/10.3390/batteries10010025
APA StyleJo, D. Y., & Park, S.-K. (2024). Facile Fabrication of Porous MoSe2/Carbon Microspheres via the Aerosol Process as Anode Materials in Potassium-Ion Batteries. Batteries, 10(1), 25. https://doi.org/10.3390/batteries10010025