Recent Advances in Electrospun Nanostructured Electrodes in Zinc-Ion Batteries
Abstract
1. Introduction
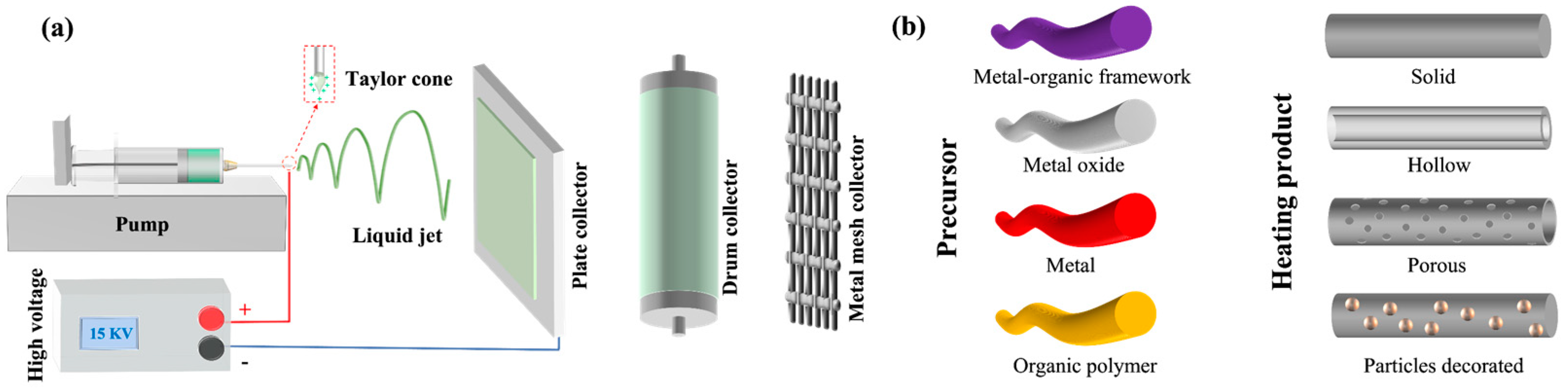
2. Electrospinning Technology
2.1. Electrospinning Principle
2.2. Factors and Parameters
2.3. Collection Devices
2.4. Thermal Treatment
3. Electrospun Cathode Materials
3.1. Vanadium-Based Materials
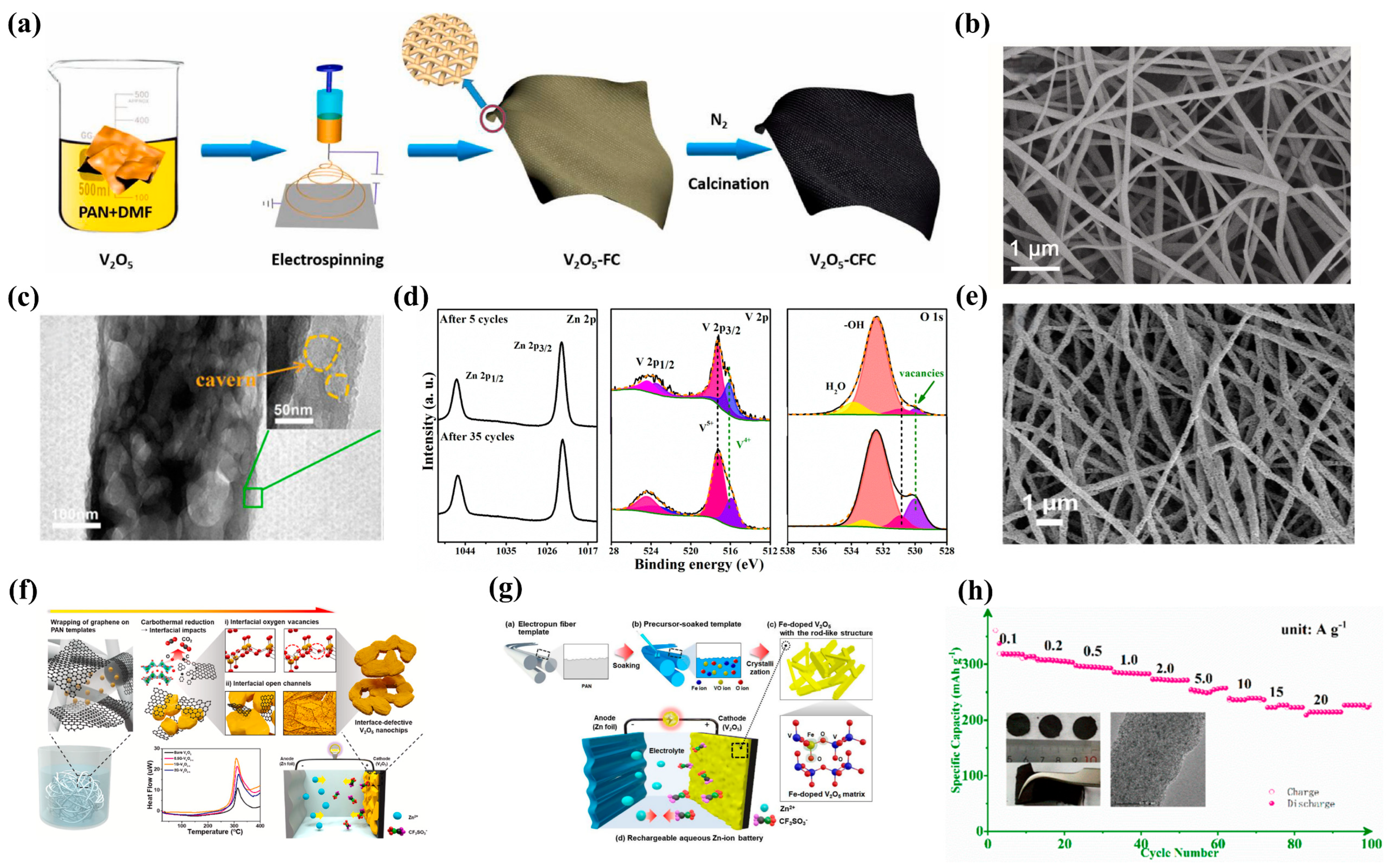
3.2. Manganese-Based Materials
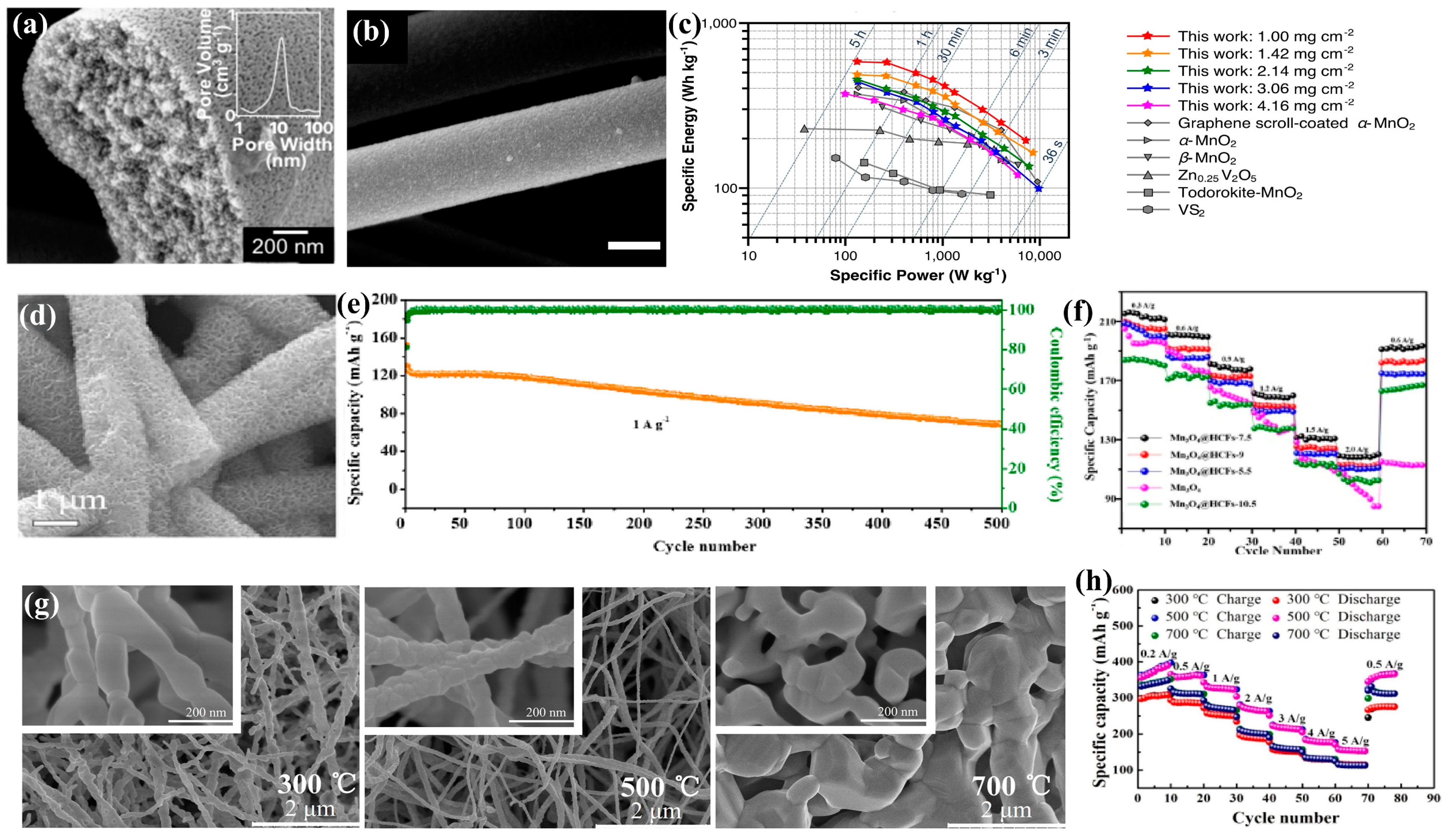
3.3. Organic Compounds
3.4. MOF-Derived Materials
4. Electrospun Anode Materials
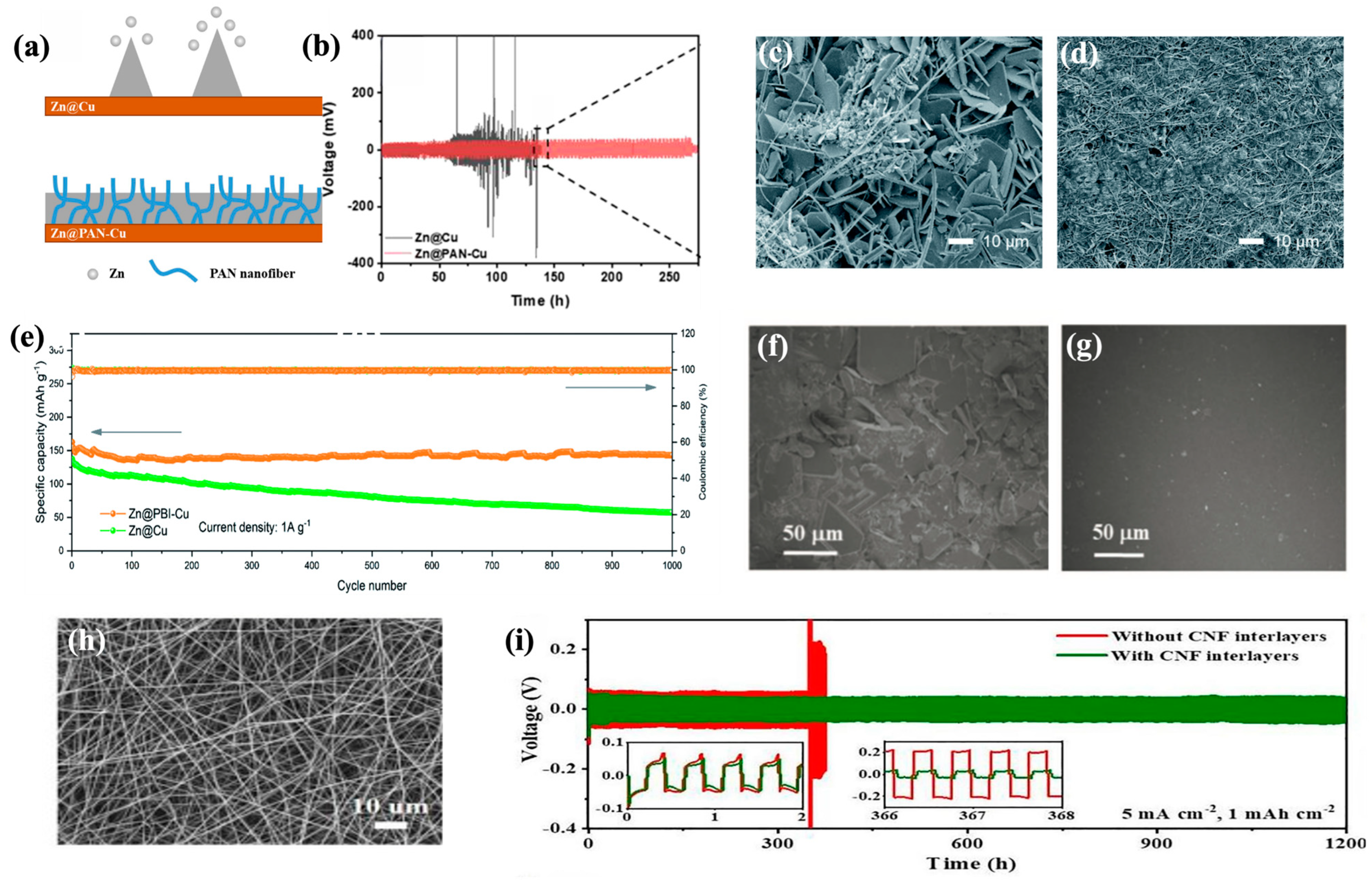
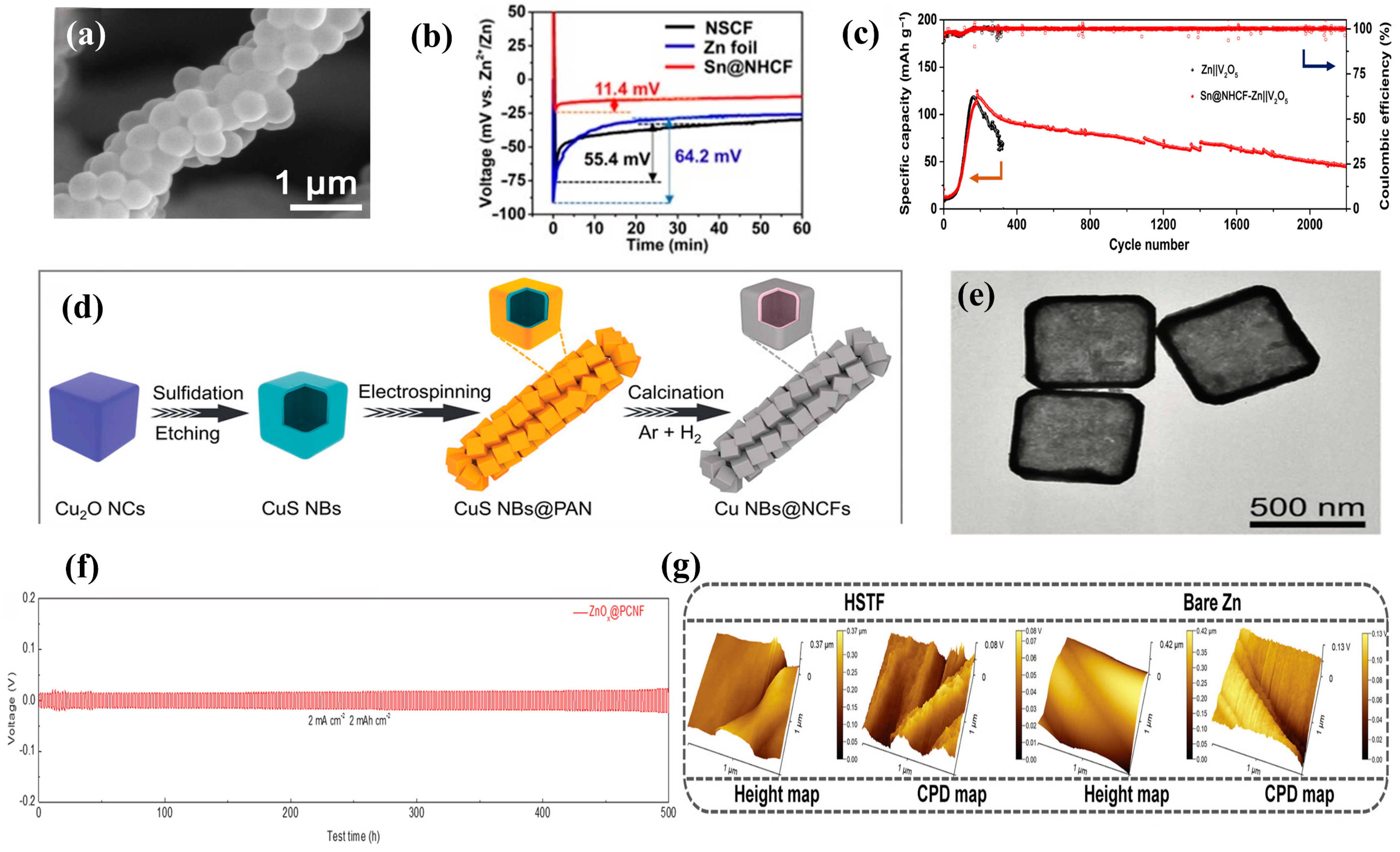
4.1. Functional Interlayers
4.2. Functional Substrates
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, X.; Liu, L.; Zhang, L.; Schmidt, O.G. Introducing rolled-up nanotechnology for advanced energy storage devices. Adv. Energy Mater. 2016, 6, 1600797. [Google Scholar] [CrossRef]
- Yang, J.; Yin, B.; Sun, Y.; Pan, H.; Sun, W.; Jia, B.; Zhang, S.; Ma, T. Zinc anode for mild aqueous zinc-ion batteries: Challenges, strategies, and perspectives. Nano-Micro Lett. 2022, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Chen, W.; Rasmussen, K.D.; Zhu, X.; Lundhaug, M.; Müller, D.B.; Tan, J.; Keiding, J.K.; Liu, L.; Dai, T.; et al. Battery technology and recycling alone will not save the electric mobility transition from future cobalt shortages. Nat. Commun. 2022, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, H.; Cheng, X.-B.; Yan, C.; Huang, J.-Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Storage Mater. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. Burgeoning prospects of spent lithium-ion batteries in multifarious applications. Adv. Energy Mater. 2018, 8, 1802303. [Google Scholar] [CrossRef]
- Kim, T.-H.; Park, J.-S.; Chang, S.K.; Choi, S.; Ryu, J.H.; Song, H.-K. The current move of lithium ion batteries towards the next phase. Adv. Energy Mater. 2012, 2, 860–872. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X.; Li, C.; Wang, K.; Sun, X.; Ma, Y. Recent advances in prelithiation materials and approaches for lithium-ion batteries and capacitors. Energy Storage Mater. 2020, 32, 497–516. [Google Scholar] [CrossRef]
- Yu, P.; Zeng, Y.; Zhang, H.; Yu, M.; Tong, Y.; Lu, X. Flexible Zn-ion batteries: Recent progresses and challenges. Small 2019, 15, e1804760. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Guo, J.; Lai, F.; Zhao, F.; Jiao, Y.; Brett, D.J.L.; Liu, T.; He, G.; Parkin, I.P. Insights on flexible zinc-ion batteries from lab research to commercialization. Adv. Mater. 2021, 33, e2007548. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Lv, Y.; Xiao, Y.; Ma, L.; Zhi, C.; Chen, S. Recent advances in electrolytes for “beyond aqueous” zinc-ion batteries. Adv. Mater. 2022, 34, 2106409. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ruan, P.; Mao, C.; Chang, Y.; Wang, L.; Dai, L.; Zhou, P.; Lu, B.; Zhou, J.; He, Z. Metal-organic frameworks functionalized separators for robust aqueous zinc-ion batteries. Nano-Micro Lett. 2022, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liang, J.; Zheng, J.; Huang, Z.; Zhang, X.; Zhu, G.; Wang, Z.; Liang, H.; Zhang, Y.-Z. Recent progress in advanced flexible zinc ion battery design. Appl. Phys. Rev. 2022, 9, 021304. [Google Scholar] [CrossRef]
- Chu, Y.; Ren, L.; Hu, Z.; Huang, C.; Luo, J. An in-depth understanding of improvement strategies and corresponding characterizations towards Zn anode in aqueous Zn-ions batteries. Green Energy Environ. 2023, 8, 1006–1042. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Wu, X.; Liang, H.; Zhang, W. Status and opportunities of zinc ion hybrid capacitors: Focus on carbon materialss, current collectors, and separators. Nano-Micro Lett. 2023, 15, 78. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Xie, X.; Lu, B.; He, Z.; Liang, S.; Zhou, J. Anode materials for aqueous zinc ion batteries: Mechanisms, properties, and perspectives. ACS Nano 2020, 14, 16321–16347. [Google Scholar] [CrossRef]
- Aizudin, M.; Fu, W.; Pottammel, R.P.; Dai, Z.; Wang, H.; Rui, X.; Zhu, J.; Li, C.C.; Wu, X.-L.; Ang, E.H. Recent advancements of graphene-based materials for zinc-based batteries: Beyond lithium-ion batteries. Small 2023, 2305217. [Google Scholar] [CrossRef]
- Zhou, L.F.; Du, T.; Li, J.Y.; Wang, Y.S.; Gong, H.; Yang, Q.R.; Chen, H.; Luo, W.B.; Wang, J.Z. A strategy for anode modification for future zinc-based battery application. Mater. Horiz. 2022, 9, 2722–2751. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, W.; Huang, Q.; Guan, C.; Zong, W.; Dai, Y.; Du, Z.; Zhang, Z.; Li, J.; Guo, F.; et al. Trace amounts of triple-functional additives enable reversible aqueous zinc-ion batteries from a comprehensive perspective. Nano-Micro Lett. 2023, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Dong, Y. Recent progress of carbon nanomaterials for high-performance cathodes and anodes in aqueous zinc ion batteries. Energy Storage Mater. 2021, 41, 715–737. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiong, F.; Meng, J.; Wang, X.; Niu, C.; An, Q.; Mai, L. Vanadium-based nanomaterials: A promising family for emerging metal-ion batteries. Adv. Funct. Mater. 2020, 30, 1904398. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, C.; Tang, C.; Tang, W.; Lan, B.; Fu, X.; Dong, S.; Luo, P. The Current Developments and Perspectives of V2O5 as Cathode for Rechargeable Aqueous Zinc-Ion Batteries. Energy Technol. 2020, 9, 2000789. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat 2019, 2, 237–260. [Google Scholar] [CrossRef]
- Shi, L.-N.; Li, X.-Z.; Cui, L.-T.; Wang, P.-F.; Xie, Y.; Yi, T.-F. Recent progresses and perspectives of VN-based materials in the application of electrochemical energy storage. J. Ind. Eng. Chem. 2022, 114, 52–76. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Kang, W.; Deng, N.; Wang, X.; Kang, X.; Yan, Z.; Pan, Y.; Ni, J. Synthesis of MoS2-based nanostructures and their applications in rechargeable ion batteries, catalysts and gas sensors: A review. RSC Adv. 2022, 12, 19512–19527. [Google Scholar] [CrossRef]
- Tie, Z.; Niu, Z. Design strategies for high-performance aqueous Zn/organic batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 21293–21303. [Google Scholar] [CrossRef]
- Han, C.; Zhu, J.; Zhi, C.; Li, H. The rise of aqueous rechargeable batteries with organic electrode materials. J. Mater. Chem. A 2020, 8, 15479–15512. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Lin, M.F.; Chen, J.; Eh, A.L.S.; Xu, Q. A π–π Stacked High-Performance Organic Anode for Durable Rocking-Chair Zinc-Ion Battery. Batteries 2023, 9, 318. [Google Scholar] [CrossRef]
- Wang, J.; Kirlikovali, K.O.; Kim, S.Y.; Kim, D.-W.; Varma, R.S.; Jang, H.W.; Farha, O.K.; Shokouhimehr, M. Metal organic framework-based nanostructure materials: Applications for non-lithium ion battery electrodes. CrystEngComm 2022, 24, 2925–2947. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Du, C.; Ma, X.; Cao, C. Advances and challenges in metal–organic framework derived porous materials for batteries and electrocatalysis. J. Mater. Chem. A 2020, 8, 24895–24919. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Jiang, Z.; Chen, J.; Wei, C.; Wu, W.; Li, S.; Xu, Q. Cation-Anion Redox Active Organic Complex for High Performance Aqueous Zinc Ion Battery. Energy Environ. Mater. 2022, e12507. [Google Scholar] [CrossRef]
- Shi, F.; Chen, C.; Xu, Z.-L. Recent advances on electrospun nanofiber materials for post-lithium ion batteries. Adv. Fiber Mater. 2021, 3, 275–301. [Google Scholar] [CrossRef]
- Yao, H.; Yu, H.; Zheng, Y.; Li, N.W.; Li, S.; Luan, D.; Lou, X.W.; Yu, L. Pre-intercalaction of ammonium ions in layered δ-MnO2 nanosheets for high-performance aqueous zinc-ion batteries. Angew.Chem. Int. Ed. 2023, 62, e202315257. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, T.; Bergh, W.V.D.; Stefik, M.; Huang, K. A high performing Zn-ion battery cathode enabled by in situ transformation of V2O5 atomic layers. Angew. Chem. 2020, 132, 17152–17159. [Google Scholar] [CrossRef]
- Zong, Q.; Du, W.; Liu, C.; Yang, H.; Zhang, Q.; Zhou, Z.; Atif, M.; Alsalhi, M.; Cao, G. Enhanced reversible zinc ion intercalation in deficient ammonium vanadated for high-performance aqueous zinc-ion battery. Nano-Micro Lett. 2021, 13, 116. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Yan, J.; Guan, C.; Wang, J. Electrospun nanofibers for new generation flexible energy storage. Energy Environ. Mater. 2021, 4, 502–521. [Google Scholar] [CrossRef]
- Ilango, P.R.; Savariraj, A.D.; Huang, H.; Li, L.; Hu, G.; Wang, H.; Hou, X.; Kim, B.C.; Ramakrishna, S.; Peng, S. Electrospun Flexible Nanofibres for Batteries: Design and Application. Electrochem. Energy Rev. 2023, 6, 12. [Google Scholar] [CrossRef]
- Zhao, H.; Lam, W.-Y.A.; Ao, K.L.; Xian, Y.; Ren, Y.; Si, L.; Wei, Z.; Wang, J.; Daoud, W.A. Application Progress and Practical Evaluations of Nanofiber Nonwoven Fabrics for Flexible/wearable Batteries. J. Electrochem. Soc. 2022, 169, 120518. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Kim, Y.; Yarin, A.L.; Swihart, M.T.; Yoon, S.S. Progress and potential of electrospinning-derived substrate-free and binder-free lithium-ion battery electrodes. Chem. Eng. J. 2022, 430, 132876. [Google Scholar] [CrossRef]
- He, H.; Lian, J.; Chen, C.; Xiong, Q.; Li, C.C.; Zhang, M. Enabling multi-chemisorption sites on carbon nanofibers cathodes by an in-situ exfoliation strategy for high-performance Zn-ion hybrid capacitors. Nano-Micro Lett. 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Ni, J.; Li, L. Electrospinning for flexible sodium-ion batteries. Energy Storage Mater. 2022, 45, 704–719. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, Y.; Shen, Q.; Chen, C.; Qiu, F.; Li, P.; Jiao, L.; Qu, X. Recent advances in electrospun electrode materials for sodium-ion batteries. J. Energy Chem. 2021, 54, 225–241. [Google Scholar] [CrossRef]
- Xia, C.; Zhou, Y.; He, C.; Douka, A.I.; Guo, W.; Qi, K.; Xia, B.Y. Recent advances on electrospun nanomaterials for zinc–air batteries. Small Sci. 2021, 1, 2100010. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: Designed architectures and electrochemical performance. Adv. Energy Mater. 2017, 7, 1601301. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shen, Y.; Dong, K.; Shen, L.; Alzalab, A.A.A. Research progress, models and simulation of electrospinning technology: A review. J. Mater. Sci. 2022, 57, 58–104. [Google Scholar] [CrossRef]
- Medeiros, G.B.; Lima, F.A.; de Almeida, D.S.; Guerra, V.G.; Aguiar, M.L. Modification and functionalization of fibers formed by electrospinning: A review. Membranes 2022, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, 89–106. [Google Scholar] [CrossRef] [PubMed]
- He, J.-H. On the height of Taylor cone in electrospinning. Results. Phys. 2020, 17, 103096. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Wang, S.; Wu, H.; Narasimhan, V.K.; Kong, D.; Ryoung Lee, H.; Cui, Y. Performance enhancement of metal nanowire transparent conducting electrodes by mesoscale metal wires. Nat. Commun. 2013, 4, 2522. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ni, X.; Wu, Q.; Yuan, C.; Li, C.; Li, D.; Chen, H.; Lv, Y.; Ju, A. Carbon-Based Fibers: Fabrication, Characterization and Application. Adv. Fiber Mater. 2022, 4, 631–682. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, S.; Zhou, F.; Das, P.; Sun, C.; Zheng, S.; Wu, Z.-S. 2D Amorphous V2O5/Graphene Heterostructures for High-Safety Aqueous Zn-Ion Batteries with Unprecedented Capacity and Ultrahigh Rate Capability. Adv. Energy Mater. 2020, 10, 2000081. [Google Scholar] [CrossRef]
- Deng, S.; Yuan, Z.; Tie, Z.; Wang, C.; Song, L.; Niu, Z. Electrochemically Induced Metal–Organic-Framework-Derived Amorphous V2O5 for Superior Rate Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 22002–22006. [Google Scholar] [CrossRef]
- Li, Z.; Ganapathy, S.; Xu, Y.; Zhou, Z.; Sarilar, M.; Wagemaker, M. Mechanistic Insight into the Electrochemical Performance of Zn/VO2 Batteries with an Aqueous ZnSO4 Electrolyte. Adv. Energy Mater. 2019, 9, 1900237. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Liu, H.; Wu, X.; Zhi, C. Tetragonal VO2 hollow nanospheres as robust cathode material for aqueous zinc ion batteries. Mater. Today Energy 2020, 17, 100431. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Liang, S.; Wu, Z.; Fang, G.; Cao, X.; Wang, C.; Lin, T.; Pan, A.; Zhou, J. Transition metal ion-preintercalated V2O5 as high-performance aqueous zinc-ion battery cathode with broad temperature adaptability. Nano Energy 2019, 61, 617–625. [Google Scholar] [CrossRef]
- Ren, X.; Liu, H.; Wang, N.; Hu, L.; Sun, M.; Bo, L.; Li, Z.; Jia, C. Dual Pre-Insertion Strategy to Achieve High-Performance Vanadium Oxide toward Advanced Cylindrical Zinc Ion Batteries. ACS Sustain. Chem. Eng. 2023, 11, 16965–16974. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, R.; Sang, Z.; Tan, S.; Liang, J.; Wang, L.; Hou, F. Anion and Cation Co-Modified Vanadium Oxide for Cathode Material of Aqueous Zinc-Ion Battery. Batteries 2023, 9, 352. [Google Scholar] [CrossRef]
- Song, Y.; Jing, L.; Wang, R.; Cui, J.; Li, M.; Zhang, Y. Vanadium oxide nanospheres encapsulated in N-doped carbon nanofibers with morphology and defect dual-engineering toward advanced aqueous zinc-ion batteries. J. Energy Chem. 2024, 89, 599–609. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, Z.H.; Sun, F.F.; Zhang, Y.; Li, H.; Liu, Y.; Ma, T. Oxygen-Vacancy-Reinforced Vanadium Oxide/Graphene Heterojunction for Accelerated Zinc Storage with Long Life Span. Small 2023, 2306275. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Yan, C.; He, W.; Xu, L.; Jiang, Z.; Zheng, A.; Wu, H.; Chen, M.; Diao, G. Flexible electrode material of V2O5 carbon fiber cloth for enhanced zinc ion storage performance in flexible zinc-ion battery. J. Power Source 2022, 533, 231358. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Deng, C. In Situ Encapsulating Metal Oxides into Core–Shell Hierarchical Hybrid Fibers for Flexible Zinc-Ion Batteries toward High Durability and Ultrafast Capability for Wearable Applications. Acs Appl. Mater. Inter. 2019, 11, 35796–35808. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, J.; Liu, S.; Hou, H.; Zou, G.; Deng, W.; Ji, X. Dual defects boosting zinc ion storage of hierarchical vanadium oxide fibers. Chem. Eng. J. 2021, 404, 126536. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, H.; Cheng, F.; Chen, J. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J. Energy Chem. 2019, 38, 20–25. [Google Scholar] [CrossRef]
- Yoo, G.; Ryu, G.H.; Koo, B.-R.; An, G.-H. Interfacial defect engineering via combusted graphene in V2O5 nanochips to develop high-rate and stable zinc-ion batteries. Ceram. Int. 2021, 47, 31817–31825. [Google Scholar] [CrossRef]
- Yoo, G.; Koo, B.-R.; An, H.-R.; Huang, C.; An, G.-H. Enhanced and stabilized charge transport boosting by Fe-doping effect of V2O5 nanorod for rechargeable Zn-ion battery. J. Ind. Eng. Chem. 2021, 99, 344–351. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, L.; Sun, K.; Huang, K.; Wei, T.; Wang, C. Electrospinning Preparation of a High-Rate Self-Supported Cathode for Rechargeable Aqueous Zinc-Ion Batteries. Energy Fuel 2022, 36, 13278–13285. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Niu, Y.; Liu, C.; Chen, H.; Ren, X.; Liu, Z.; Lau, W.-M.; Zhou, D. Electrospun V2O3@Carbon Nanofibers as a Flexible and Binder-Free Cathode for Highly Stable Aqueous Zn-Ion Full Batteries. ACS Appl. Energy Mater. 2022, 5, 3525–3535. [Google Scholar] [CrossRef]
- Tan, H.; Feng, Y.; Rui, X.; Yu, Y.; Huang, S. Metal chalcogenides: Paving the way for high-performance sodium/potassium-ion batteries. Small Methods 2020, 4, 1900563. [Google Scholar] [CrossRef]
- Yang, W.; Chen, D.; She, Y.; Zeng, M.; Lin, X.; Ang, E.H.; Yan, C.; Qin, Y.; Rui, X. Rational design of vanadium chalcogenides for sodium-ion batteries. J. Power Source 2020, 478, 228769. [Google Scholar] [CrossRef]
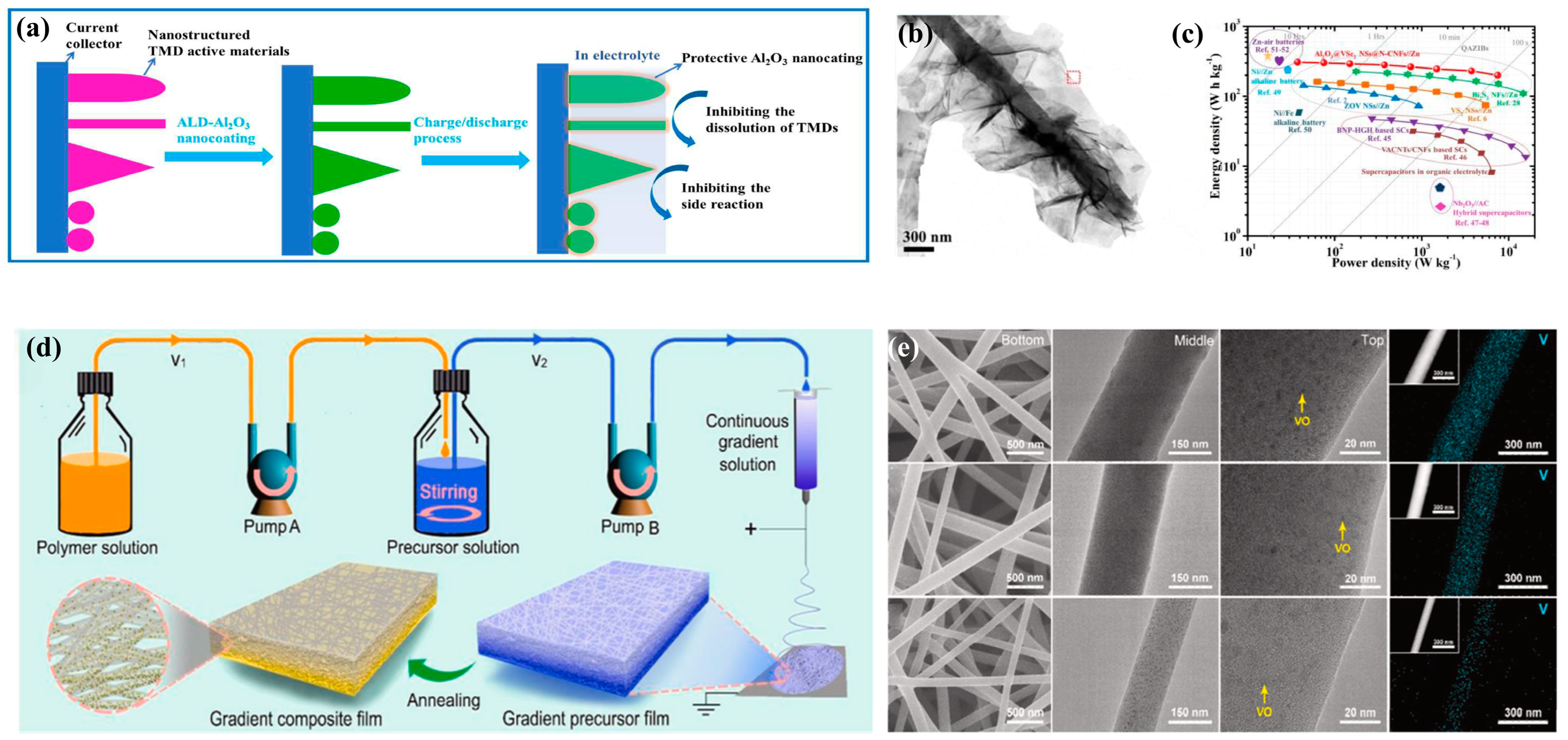
- Yang, J.; Yang, H.; Ye, C.; Li, T.; Chen, G.; Qiu, Y. Conformal surface-nanocoating strategy to boost high-performance film cathodes for flexible zinc-ion batteries as an amphibious soft robot. Energy Storage Mater. 2022, 46, 472–481. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Zhu, J.; Niu, Z. Rational design of continuous gradient composite films for high-performance zinc-ion batteries. Energy Storage Mater. 2022, 51, 382–390. [Google Scholar] [CrossRef]
- Nolis, G.M.; Adil, A.; Yoo, H.D.; Hu, L.; Bayliss, R.D.; Lapidus, S.H.; Berkland, L.; Phillips, P.J.; Freeland, J.W.; Kim, C.; et al. Electrochemical Reduction of a Spinel-Type Manganese Oxide Cathode in Aqueous Electrolytes with Ca2+ or Zn2+. J. Phys. Chem. C 2018, 122, 4182–4188. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H.; Li, W.; Tian, Y.; Zhang, Y.; Wu, H.; Yang, L.; Zou, G.; Hou, H.; Ji, X. H+-Insertion Boosted α-MnO2 for an Aqueous Zn-Ion Battery. Small 2020, 16, 1905842. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, J.; Zhang, X.; Insin, N.; Wang, S.; Han, J.; Zhao, Y.; Qin, J.; Huang, Y. Inhibition of Manganese Dissolution in Mn2O3 Cathode with Controllable Ni2+ Incorporation for High-Performance Zinc Ion Battery. Adv. Funct. Mater. 2021, 31, 2009412. [Google Scholar] [CrossRef]
- Tan, Q.; Li, X.; Zhang, B.; Chen, X.; Tian, Y.; Wan, H.; Zhang, L.; Miao, L.; Wang, C.; Gan, Y.; et al. Valence Engineering via In Situ Carbon Reduction on Octahedron Sites Mn3O4 for Ultra-Long Cycle Life Aqueous Zn-Ion Battery. Adv. Energy Mater. 2020, 10, 2001050. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, W.; Pan, F.; Liu, G. Block Copolymer-Derived Porous Carbon Fibers Enable High MnO2 Loading and Fast Charging in Aqueous Zinc-Ion. Batter. Supercaps 2022, 5, e2021003801. [Google Scholar] [CrossRef]
- Fang, L.; Wang, X.; Shi, W.; Le, Z.; Wang, H.; Nie, P.; Xu, T.; Chang, L. Carbon nanofibers enabling manganese oxide cathode superior low temperature performance for aqueous zinc-ion batteries. J. Electroanal. Chem. 2023, 940, 117488. [Google Scholar] [CrossRef]
- Tang, F.; Wu, X.; Shen, Y.; Xiang, Y.; Wu, X.; Xiong, L.; Wu, X. The intercalation cathode materials of heterostructure MnS/MnO with dual ions defect embedded in N-doped carbon fibers for aqueous zinc ion batteries. Energy Storage Mater. 2022, 52, 180–188. [Google Scholar] [CrossRef]
- Tang, F.; He, T.; Zhang, H.; Wu, X.; Li, Y.; Long, F.; Xiang, Y.; Zhu, L.; Wu, J.; Wu, X. The MnO@N-doped carbon composite derived from electrospinning as cathode material for aqueous zinc ion battery. J. Electroanal. Chem. 2020, 873, 114368. [Google Scholar] [CrossRef]
- Yang, J.; Yao, G.; Li, Z.; Zhang, Y.; Wei, L.; Niu, H.; Chen, Q.; Zheng, F. Highly Flexible K-Intercalated MnO2/Carbon Membrane for High-Performance Aqueous Zinc-Ion Battery Cathode. Small 2023, 19, e2205544. [Google Scholar] [CrossRef]
- Long, J.; Yang, Z.; Yang, F.; Cuan, J.; Wu, J. Electrospun core-shell Mn3O4/carbon fibers as high-performance cathode materials for aqueous zinc-ion batteries. Electrochim. Acta 2020, 344, 136155. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, Y.; Guo, S.; Liu, Y.; Li, W.; Liu, Q.; Mo, F.; Yu, S.; Huang, Y.; Wei, J. Electrospun manganese sesquioxide as cathode for aqueous zinc ion battery with high-rate performance and long cycle life. Mater. Lett. 2022, 327, 132920. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Wu, F.; Chen, R.; Li, L. High-Performance Aqueous Zinc Batteries Based on Organic/Organic Cathodes Integrating Multiredox Centers. Adv. Mater. 2021, 33, 2106469. [Google Scholar] [CrossRef]
- Kim, C.; Ahn, B.Y.; Wei, T.S.; Jo, Y.; Jeong, S.; Choi, Y.; Kim, I.D.; Lewis, J.A. High-power aqueous zinc-ion batteries for customized electronic devices. ACS Nano 2018, 12, 11838–11846. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.; Shao, C.; Han, C.; Liu, Y.; Li, X.; Ma, X.; Chen, F.; Liu, Y. Synchronous-ultrahigh conductive-reactive N-atoms doping strategy of carbon nanofibers networks for high-performance flexible energy storage. Energy Storage Mater. 2022, 44, 250–262. [Google Scholar] [CrossRef]
- Cai, Z.; Guo, J.; Yang, H.; Xu, Y. Electrochemical properties of electrospun poly (5-cyanoindole) submicron-fibrous electrode for zinc/polymer secondary battery. J. Power Source 2015, 279, 114–122. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, H.; Lu, X.-F.; Tong, Y.-X.; Li, G.-R. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction. Adv. Sci. 2018, 5, 1700515. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cai, D.; Si, J.; Zhan, H.; Wang, Q. MOF-derived NiCo2S4 and carbon hybrid hollow spheres compactly concatenated by electrospun carbon nanofibers as self-standing electrodes for aqueous alkaline Zn batteries. J. Mater. Chem. A 2022, 10, 4100–4109. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; Li, Y.; Ren, X.; Zhang, P.; Sun, L.; Yang, H.Y. In Situ Grown Hierarchical Electrospun Nanofiber Skeletons with Embedded Vanadium Nitride Nanograins for Ultra-Fast and Super-Long Cycle Life Aqueous Zn-Ion Batteries. Adv. Energy Mater. 2022, 13, 2202826. [Google Scholar] [CrossRef]
- Ding, L.; Gao, J.; Yan, T.; Cheng, C.; Chang, L.Y.; Zhang, N.; Feng, X.; Zhang, L. Boosting the Cycling Stability of Aqueous Zinc-Ion Batteries through Nanofibrous Coating of a Bead-like MnOx Cathode. ACS Appl. Mater. Inter. 2022, 14, 17570–17577. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S.; Li, J.; Luo, X.; Liu, Z.; Zhang, T.; Cao, X.; Long, M.; Lu, B.; Pan, A.; et al. Surface-preferred crystal plane for a stable and reversible zinc anode. Adv. Mater. 2021, 33, 2100187. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Huang, X.; Wang, Q.; Sun, D.; Tang, Y.; Ji, X.; Wang, H. Revealing the role of crystal orientation of protective layers for stable zinc anode. Nat. Commun. 2020, 11, 3961. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Fu, L.; Wu, S.; Tang, Y.; Ji, X.; Wang, H. The three-dimensional dendrite-free zinc anode on a copper mesh with a zinc-oriented polyacrylamide electrolyte additive. Angew. Chem. Int. Ed. 2019, 58, 15841–15847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Jiang, Y.; Jin, C.; Huang, W.; Ding, X.; Huang, Y. Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 2016, 25, 211–217. [Google Scholar] [CrossRef]
- Kumar, S.; Yoon, H.; Park, H.; Park, G.; Suh, S.; Kim, H.-J. A dendrite-free anode for stable aqueous rechargeable zinc-ion batteries. J. Ind. Eng. Chem. 2022, 108, 321–327. [Google Scholar] [CrossRef]
- Park, G.; Park, H.; Seol, W.; Suh, S.; Jo, J.Y.; Kumar, S.; Kim, H.J. Inhibition of Zinc Dendrites Realized by a β-P(VDF-TrFE) Nanofiber Layer in Aqueous Zn-Ion Batteries. Membranes 2022, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Jian, Q.; Wan, Y.; Sun, J.; Wu, M.; Zhao, T. A dendrite-free zinc anode for rechargeable aqueous batteries. J. Mater. Chem. A 2020, 8, 20175–20184. [Google Scholar] [CrossRef]
- Li, H.; Han, C.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Wang, Z.; Liu, Z.; Tang, Z.; et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci. 2018, 11, 941–951. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Hong, X.; Zhou, R.; Hou, Z.; Zhang, B. Elastomer–Alginate Interface for High-Power and High-Energy Zn Metal Anodes. Adv. Energy Mater. 2022, 12, 2200318. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Mi, H.; Sun, L.; Ma, D.; Li, H.; He, C.; Zhang, P. Functionalized carbon nanofiber interlayer towards dendrite-free, Zn-ion batteries. Chem. Eng. J. 2021, 425, 131862. [Google Scholar] [CrossRef]
- Wan, F.; Hao, Z.; Wang, S.; Ni, Y.; Zhu, J.; Tie, Z.; Bi, S.; Niu, Z.; Chen, J. A Universal Compensation Strategy to Anchor Polar Organic Molecules in Bilayered Hydrated Vanadates for Promoting Aqueous Zinc-Ion Storage. Adv. Mater. 2021, 33, e2102701. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Du, H.; Liu, Y.; Xiang, Y.; Xiong, L.; Wu, X.; Wu, X. Copper nanoparticle-modified carbon nanofiber for seeded zinc deposition enables stable Zn metal anode. Acs Sustain. Chem. Eng. 2022, 10, 12630–12641. [Google Scholar] [CrossRef]
- Baek, S.H.; Cho, Y.J.; Park, J.M.; Xiong, P.; Yeon, J.S.; Rana, H.H.; Park, J.H.; Jang, G.; Lee, S.J.; Park, H.S. Electrospun conductive carbon nanofiber hosts for stable zinc metal anode. Int. J. Energy Res. 2022, 46, 7201–7214. [Google Scholar] [CrossRef]
- Yu, H.; Zeng, Y.; Li, N.W.; Luan, D.; Yu, L.; Lou, X.W. Confining Sn nanoparticles in interconnected N-doped hollow carbon spheres as hierarchical zincophilic fibers for dendrite-free Zn metal anodes. Sci. Adv. 2022, 8, eabm5766. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Yang, P.; Yan, W.; Zhao, J.-W.; Fan, H.J. 3D zincophilic micro-scaffold enables stable Zn deposition. Energy Storage Mater. 2022, 51, 259–265. [Google Scholar] [CrossRef]
- Zeng, Y.; Sun, P.X.; Pei, Z.; Jin, Q.; Zhang, X.; Yu, L.; Lou, X.W.D. Nitrogen-Doped Carbon Fibers Embedded with Zincophilic Cu Nanoboxes for Stable Zn-Metal Anodes. Adv. Mater. 2022, 34, e2200342. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yin, P.; Xu, A.N.; Jin, B.W.; Li, Z.H.; Shao, M.F. Fluorine enhanced nucleophilicity of TiO2 nanorod arrays: A general approach for dendrite-free anodes towards high-performance metal batteries. Nano Energy 2022, 93, 106837. [Google Scholar] [CrossRef]
- Xue, P.; Sun, C.; Li, H.P.; Liang, J.J.; Lai, C. Superlithiophilic Amorphous SiO2–TiO2 Distributed into Porous Carbon Skeleton Enabling Uniform Lithium Deposition for Stable Lithium Metal Batteries. Adv. Sci. 2019, 6, 1900943. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wang, Z.; Zhao, W.; Qin, C.; Yu, H.; Wang, X.; Bakenov, Z.; Zhang, Y. Defective ZnOx@porous carbon nanofiber network inducing dendrite-free zinc plating as zinc metal anode for high-performance aqueous rechargeable Zn/Na4Mn9O18 battery based on hybrid electrolyte. J. Power Source 2022, 518, 230761. [Google Scholar] [CrossRef]
- Xue, P.; Guo, C.; Wang, N.; Zhu, K.; Jing, S.; Kong, S.; Zhang, X.; Li, L.; Li, H.; Feng, Y.; et al. Synergistic Manipulation of Zn2+ Ion Flux and Nucleation Induction Effect Enabled by 3D Hollow SiO2/TiO2/Carbon Fiber for Long-Lifespan and Dendrite-Free Zn–Metal Composite Anodes. Adv. Funct. Mater. 2021, 31, 2106417. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wei, C.; Gao, L.; Lin, M.-F.; Eh, A.L.-S.; Chen, J.; Li, S. Recent Advances in Electrospun Nanostructured Electrodes in Zinc-Ion Batteries. Batteries 2024, 10, 22. https://doi.org/10.3390/batteries10010022
Zhang L, Wei C, Gao L, Lin M-F, Eh AL-S, Chen J, Li S. Recent Advances in Electrospun Nanostructured Electrodes in Zinc-Ion Batteries. Batteries. 2024; 10(1):22. https://doi.org/10.3390/batteries10010022
Chicago/Turabian StyleZhang, Lilin, Cong Wei, Lin Gao, Meng-Fang Lin, Alice Lee-Sie Eh, Jingwei Chen, and Shaohui Li. 2024. "Recent Advances in Electrospun Nanostructured Electrodes in Zinc-Ion Batteries" Batteries 10, no. 1: 22. https://doi.org/10.3390/batteries10010022
APA StyleZhang, L., Wei, C., Gao, L., Lin, M.-F., Eh, A. L.-S., Chen, J., & Li, S. (2024). Recent Advances in Electrospun Nanostructured Electrodes in Zinc-Ion Batteries. Batteries, 10(1), 22. https://doi.org/10.3390/batteries10010022






