Two-Step Synthesis of ZnS-NiS2 Composite with Rough Nanosphere Morphology for High-Performance Asymmetric Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnS Nanosheets
2.2. Synthesis of ZnS-NiS2 Composites
2.3. Synthesis of ZnS-NiS2-G Composites
2.4. Materials Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hua, Y.; Krishna, B.V.; Wang, T.; Ankinapalli, O.R.; Yu, J.S. Green synthesis and electrochemical properties of A3(PO4)2 (A = Mn, Zn, and Co) hydrates for supercapacitors with long-term cycling stability. J. Power Sources 2022, 552, 232245. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Hu, Z.; Li, S.; Ding, F.; Xiao, X.; Si, P.; Ulstrup, J. Hetero-structured NiS2/CoS2 nanospheres embedded on N/S co-doped carbon nanocages with ultra-thin nanosheets for hybrid supercapacitors. Electrochim. Acta 2022, 424, 140604. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Chen, W.; Zhang, C.; Rong, K.; Gao, X.; Fan, W. Reliable coaxial wet spinning strategy to fabricate flexible MnO2-based fiber supercapacitors. J. Alloys Compd. 2023, 935, 168110. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.; Liu, P.; Zhong, Y.L.; Chong, K.F. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2020, 409, 128216. [Google Scholar] [CrossRef]
- Ng, W.; Yang, Y.; van der Veen, K.; Rothenberg, G.; Yan, N. Enhancing the performance of 3D porous N-doped carbon in oxygen reduction reaction and supercapacitor via boosting the meso-macropore interconnectivity using the “exsolved” dual-template. Carbon 2018, 129, 293–300. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Hu, N.; Liao, J.; Zong, N.; Wei, J.; Li, M.; Wang, L.; Xu, R.; Yang, L. Facile synthesis of neuronal nickel–cobalt-manganese sulfide for asymmetric supercapacitors with excellent energy density. J. Electroanal. Chem. 2023, 932, 117262. [Google Scholar] [CrossRef]
- Bahnasawy, N.; Sayed, D.M.; Allam, N.K. Engineered self-standing monolithic Cu-Zn-Ni oxide nanocubes as battery materials for durable all-solid-state hybrid supercapacitor devices. J. Energy Storage 2023, 63, 106997. [Google Scholar] [CrossRef]
- El-Gendy, D.; Allam, N.; El Sawy, E. Novel high energy density electrodes based on functionalized/exfoliated molybdenum oxide nanoflakes for high-performance supercapacitors. Mater. Today Chem. 2023, 29, 101414. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, Q.; Lu, L.; Zou, Y.; Xu, F.; Sun, L.; Cai, D.; Xiang, C. Hollow core–shell CuCo2O4@MoNi-layered double hydroxides as an electrode material for supercapacitors. J. Energy Storage 2023, 61, 6106691. [Google Scholar] [CrossRef]
- Attia, S.Y.; Bedir, A.G.; Barakat, Y.F.; Mohamed, S.G. A two-dimensional nickel-doped bismuth-layered double hydroxide structure as a bifunctional efficient electrode material for symmetric supercapacitors. Sustain. Mater. Technol. 2023, 36, e00595. [Google Scholar] [CrossRef]
- Khan, M.W.; Shaheen, M.; Siddique, S.; Aftab, S.; Alzaid, M.; Iqbal, M.J. Evaluation of d-block metal sulfides as electrode materials for battery-supercapacitor energy storage devices. J. Energy Storage 2022, 55, 105418. [Google Scholar] [CrossRef]
- Dong, J.; Li, S.; Ding, Y. Anchoring nickel-cobalt sulfide nanoparticles on carbon aerogel derived from waste watermelon rind for high-performance asymmetric supercapacitors. J. Alloys Compd. 2020, 845, 155701. [Google Scholar] [CrossRef]
- Hussain, I.; Mohapatra, D.; Dhakal, G.; Lamiel, C.; Sayed, M.S.; Sahoo, S.; Mohamed, S.G.; Kim, J.S.; Lee, Y.R.; Shim, J.-J. Uniform growth of ZnS nanoflakes for high-performance supercapacitor applications. J. Energy Storage 2021, 36, 102408. [Google Scholar] [CrossRef]
- Xie, X.; Wu, D.; Feng, X.; Ni, C.; Sun, X.; Kimura, H.; Du, W. A novel (α-β)NiS/Ni3S4-rGO electrode material for supercapacitors. Colloid Interface Sci. Commun. 2021, 43, 100453. [Google Scholar] [CrossRef]
- Wang, H.; Tian, L.; Zhao, X.; Ali, M.; Yin, K.; Xing, Z. In situ growth MoS2/NiS composites on Ni foam as electrode materials for supercapacitors. Mater. Today Commun. 2023, 34, 105041. [Google Scholar] [CrossRef]
- Dar, M.A.; Dinagaran, S.; Govindarajan, D.; Ahamed, S.R.; Habib, F.; Siva, C.; Moholkar, A.V.; Ahmad, Z.; Yatoo, M.A. Snx-0MnxS nanomaterial based electrodes for future-generation supercapacitor and data storage devices. J. Alloys Compd. 2023, 958, 170523. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Yang, B.; Shi, H.; Wang, K.; Han, L.; Wang, S.; Wang, Y. Targeted synthesis of NiS and NiS2 nanoparticles for high−performance hybrid supercapacitor via a facile green solid−phase synthesis route. J. Energy Storage 2020, 32, 101852. [Google Scholar] [CrossRef]
- Fischereder, A.; Martinez-Ricci, M.L.; Wolosiuk, A.; Haas, W.; Hofer, F.; Trimmel, G.; Soler-Illia, G.J.A.A. Mesoporous ZnS Thin Films Prepared by a Nanocasting Route. Chem. Mater. 2012, 24, 1837–1845. [Google Scholar] [CrossRef]
- Olasanmi, O.O.; Anthony, M. Variation of ZnS deposition time on chemically prepared Cd1-xZnxS ternary compound from CdS/ZnS bilayers. Results Opt. 2023, 11, 100419. [Google Scholar] [CrossRef]
- Yan, J.; Wang, S.; Chen, Y.; Yuan, M.; Huang, Y.; Lian, J.; Qiu, J.; Bao, J.; Xie, M.; Xu, H.; et al. Construction of a z-scheme CdIn2S4/ZnS heterojunction for the enhanced photocatalytic hydrogen evolution. J. Alloys Compd. 2023, 948, 169692. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, J.; Cao, T.; Lei, Z.; Ma, Y.; Liu, H.; Zhu, L.; Feng, X.; Wei, W.; Zhang, H. A facile method synthesizing marshmallow ZnS grown on Ti3C2 MXene for high-performance asymmetric supercapacitors. J. Energy Storage 2022, 50, 104652. [Google Scholar] [CrossRef]
- Palanisamy, P.; Thangavel, K.; Murugesan, S.; Marappan, S.; Chavali, M.; Siril, P.F.; Perumal, D.V. Investigating the synergistic effect of hybridized WO3-ZnS nanocomposite prepared by microwave-assisted wet chemical method for supercapacitor application. J. Electroanal. Chem. 2018, 833, 93–104. [Google Scholar] [CrossRef]
- Yu, F.; Tiong, V.T.; Pang, L.; Zhou, R.; Wang, X.; Waclawik, E.R.; Ostrikov, K.; Wang, H. Flower-like Cu5Sn2S7/ZnS nanocomposite for high performance supercapacitor. Chin. Chem. Lett. 2019, 30, 1115–1120. [Google Scholar] [CrossRef]
- Teli, A.M.; Beknalkar, S.A.; Mane, S.M.; Chaudhary, L.S.; Patil, D.S.; Pawar, S.A.; Efstathiadis, H.; Shin, J.C. Facile hydrothermal deposition of Copper-Nickel sulfide nanostructures on nickel foam for enhanced electrochemical performance and kinetics of charge storage. Appl. Surf. Sci. 2021, 571, 151336. [Google Scholar] [CrossRef]
- Ji, Z.; Chen, L.; Liu, K.; Ma, D.; Zhang, S.; Zhu, G.; Shen, X.; Song, P.; Premlatha, S. Nickel sulfide and cobalt sulfide nanoparticles deposited on ultrathin carbon two-dimensional nanosheets for hybrid supercapacitors. Appl. Surf. Sci. 2021, 574, 151727. [Google Scholar] [CrossRef]
- Madhavi, J.; Prasad, V. ZnS and ZnS/CdS core-shell Nano particles: Synthesis, properties and Perspectives. Surfaces Interfaces 2020, 21, 100757. [Google Scholar] [CrossRef]
- Yan, J.; Wang, S.; Chen, Y.; Yuan, M.; Huang, Y.; Lian, J.; Qiu, J.; Bao, J.; Xie, M.; Xu, H.; et al. Smart in situ construction of NiS/MoS2 composite nanosheets with ultrahigh specific capacity for high-performance asymmetric supercapacitor. J. Alloys Compd. 2019, 811, 151915. [Google Scholar] [CrossRef]
- Wang, B.; Williams, G.R.; Chang, Z.; Jiang, M.; Liu, J.; Lei, X.; Sun, X. Hierarchical NiAl layered double hydroxide/multiwalled carbon nanotube/nickel foam electrodes with excellent pseudocapacitive properties. ACS Appl. Mater. Interfaces 2014, 6, 16304–16311. [Google Scholar] [CrossRef]
- Arif, M.; Shah, M.Z.U.; Ahmad, S.A.; Hussain, I.; Song, P.; Sajjad, M.; Huang, T.; Yi, J.; Shah, A. Exploring the potential of a ZnS/CNFs cloudy-like architecture for high-performance asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2023, 34, 1340. [Google Scholar] [CrossRef]
- Barazandeh, M.; Kazemi, S.H. High-performance freestanding supercapacitor electrode based on polypyrrole coated nickel cobalt sulfide nanostructures. Sci. Rep. 2022, 12, 4628. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, X.; Gao, Q.; Zhang, X.; Rong, K.; Cao, M.; Han, L.; Fan, W. Diversified constructions and electrochemical cycling stability of metal oxide fiber supercapacitors. J. Mater. Res. Technol. 2023, 24, 909–917. [Google Scholar] [CrossRef]
- Salah, N.; Shehab, M.; El Nady, J.; Ebrahim, S.; El-Maghraby, E.; Sakr, A.-H. Polyaniline/ZnS quantum dots nanocomposite as supercapacitor electrode. Electrochim. Acta 2023, 449, 142174. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Zhang, Z.; Liu, H.; Ling, S.; Liu, X.; Lian, C.; Xu, J. Anisotropic ZnS Nanoclusters/Ordered Macro-Microporous Carbon Superstructure for Fibrous Supercapacitor toward Commercial-Level Energy Density. Adv. Funct. Mater. 2023, 33, 2300329. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Yin, H.; Hou, S.; Lin, H.; Zhou, J.; Zhuo, S. Investigation on the role of different conductive polymers in supercapacitors based on a zinc sulfide/reduced graphene oxide/conductive polymer ternary composite electrode. RSC Adv. 2020, 10, 3122–3129. [Google Scholar] [CrossRef]
- Si, L.; Xia, Q.; Liu, K.; Guo, W.; Shinde, N.; Wang, L.; Hu, Q.; Zhou, A. Hydrothermal synthesis of layered NiS2/Ti3C2Tx composite electrode for supercapacitors. Mater. Chem. Phys. 2022, 291, 126733. [Google Scholar] [CrossRef]
- Hu, J.; Sun, L.; Xie, F.; Qu, Y.; Tan, H.; Zhang, Y. An urchin-like Co-doped NiS2/C nanorod array with enriched sulfur vacancies for asymmetric supercapacitors. J. Mater. Chem. A 2023, 11, 8380–8391. [Google Scholar] [CrossRef]
- Beemarao, M.; Periyannan, P.; Ravichandran, K. High-performance supercapacitor electrode obtained by directly bonding 2D materials: Hierarchal NiS2 on reduced graphene oxide. J. Mater. Sci. Mater. Electron. 2023, 34, 1423. [Google Scholar] [CrossRef]
- Rasappan, A.S.; Thangamuthu, V.; Natarajan, M.; Velauthapillai, D. Kirkendall effect induced NiFe: WS2 core-shell nanocubes for Dye-sensitized solar cell and battery-type Supercapacitor applications. J. Energy Storage 2023, 63, 106964. [Google Scholar] [CrossRef]
- Diao, Y.; Lu, Y.; Yang, H.; Wang, H.; Chen, H.; D’Arcy, J.M. Direct conversion of Fe2O3 to 3D nanofibrillar PEDOT microsupercapacitors. Adv. Funct. Mater. 2020, 30, 202003394. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A.; Gallo, M.J.H.; Otero-Irurueta, G.; Mikhalev, S.; Staiti, P.; Lufrano, F. Energy storage of supercapacitor electrodes on carbon cloth enhanced by graphene oxide aerogel reducing conditions. J. Energy Storage 2020, 32, 101839. [Google Scholar] [CrossRef]
- Shen, X.; Wei, X.; Wang, T.; Li, S.; Li, H. Polypyrrole embedded in nickel-cobalt sulfide nanosheets grown on nickel particles passivated silicon nanowire arrays for high-performance supercapacitors. Chem. Eng. J. 2023, 461, 141745. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.; Shim, J.-J.; Chong, K.F. Cobalt-doped tungsten suboxides for supercapacitor applications. Chem. Eng. J. 2023, 473, 145341. [Google Scholar] [CrossRef]
- Pandey, P.; Bhowmick, S.; Qureshi, M. Magnetically Triggered Interplay of Capacitive and Diffusion Contributions for Boosted Supercapacitor Performance. ACS Appl. Mater. Interfaces 2023, 15, 39435–39447. [Google Scholar] [CrossRef]
- Li, X.F.; Liu, J.; Banis, M.N.; Lushington, A.; Li, R.Y.; Cai, M.; Sun, X.L. Atomic layer deposition of solid-state electrolyte coated cathode materials with superior high-voltage cycling behavior for lithium ion battery application. Energy Environ. Sci. 2014, 7, 768–778. [Google Scholar] [CrossRef]
- Runfa, L.; Chen, X.; Hongliang, C.; Wei, Y.; Yuanfang, Z.; Siyu, C.; Wenrui, J.; Qi, Z.; Yi, E.; Meng, J.; et al. Facile synthesis of Ni3Se4/Ni0.6Zn0.4O/ZnO nanoparticle as high-performance electrode materials for electrochemical energy storage device. Nanotechnology 2023, 34, 185401. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, L.; Zhao, C.; Yu, H.; Zhao, B.; Gu, X.; Wang, J.; Meng, L.; Gao, X. Preparation of GO-based Cr-Zn bimetallic layered porous sulfide by ZIF template method for high performance supercapacitors. J. Mol. Struct. 2023, 1275, 134643. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.-Z.; Dubbink, D.; Elshof, J.E.T. Inkjet printing of δ-MnO2 nanosheets for flexible solid-state micro-supercapacitor. Nano Energy 2018, 49, 481–488. [Google Scholar] [CrossRef]
- Dong, Y.; Yue, X.; Liu, Y.; Zheng, Q.; Cao, Z.; Lin, D. Hierarchical core–shell-structured bimetallic nickel–cobalt phosphide nanoarrays coated with nickel sulfide for high-performance hybrid supercapacitors. J. Colloid Interface Sci. 2022, 628, 222–232. [Google Scholar] [CrossRef]
- Fang, S.; Chen, Y.; Wang, S.; Xu, J.; Xia, Y.; Yang, F.; Wang, Y.; Lao, J.; Xiang, C.; Xu, F.; et al. Modified CNTs interfacial anchoring and particle-controlled synthesis of amorphous cobalt-nickel-boron alloy bifunctional materials for NaBH4 hydrolysis and supercapacitor energy storage. J. Alloys Compd. 2023, 936, 167990. [Google Scholar] [CrossRef]
- Sudhakaran, M.S.P.; Raju, R.; Youk, J.H. Polypyrrole-derived N-doped CNT nanocomposites decorated with CoNi alloy nanoparticles for high-performance supercapacitor electrodes. Appl. Surf. Sci. 2023, 619, 156796. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Shang, M.; Zhang, M.; Zhao, X.; Liu, S.; Liu, X.; Liu, S.; Yi, X. N, O co-doped carbon aerogel derived from sodium alginate/melamine composite for all-solid-state supercapacitor. Appl. Surf. Sci. 2023, 608, 155109. [Google Scholar] [CrossRef]
- Chen, Y.; Muthukumar, K.; Leban, L., II.; Li, J. Microwave-assisted high-yield exfoliation of vanadium pentoxide nanoribbons for supercapacitor applications. Electrochim. Acta 2020, 330, 135200. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Alfantazi, A.; Lee, Y.R.; Lee, M.; Shim, J.J. Chemically synthesized copper sulfide nanoflakes on reduced graphene oxide for asymmetric supercapacitors. J. Ind. Eng. Chem. 2021, 101, 423–429. [Google Scholar] [CrossRef]
- Xu, H.; Lei, Z.; Xu, M.; Zhu, J.; Song, X.; Jin, X. Free-standing reduced graphene oxide/carboxymethylcellulose-polyaniline (RGO/CMC-PANI) hybrid film electrode for high-performance asymmetric supercapacitor device. Int. J. Biol. Macromol. 2023, 236, 123934. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yao, M.; Chen, Y.; Tang, X.; Hu, W.; Pillai, S.C. Facile fabrication of flower-like γ-Fe2O3@PPy from iron rust for high-performing asymmetric supercapacitors. J. Alloys Compd. 2022, 922, 166055. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, J.; Qin, Y.; Wang, Y.; Xu, S.; Li, R. Rational construction of Ni–Co–S nanosheets encapsulated NiS2 micro-blocks on carbon cloth as high-performance supercapacitor electrode. Mater. Lett. 2020, 262, 127021. [Google Scholar] [CrossRef]

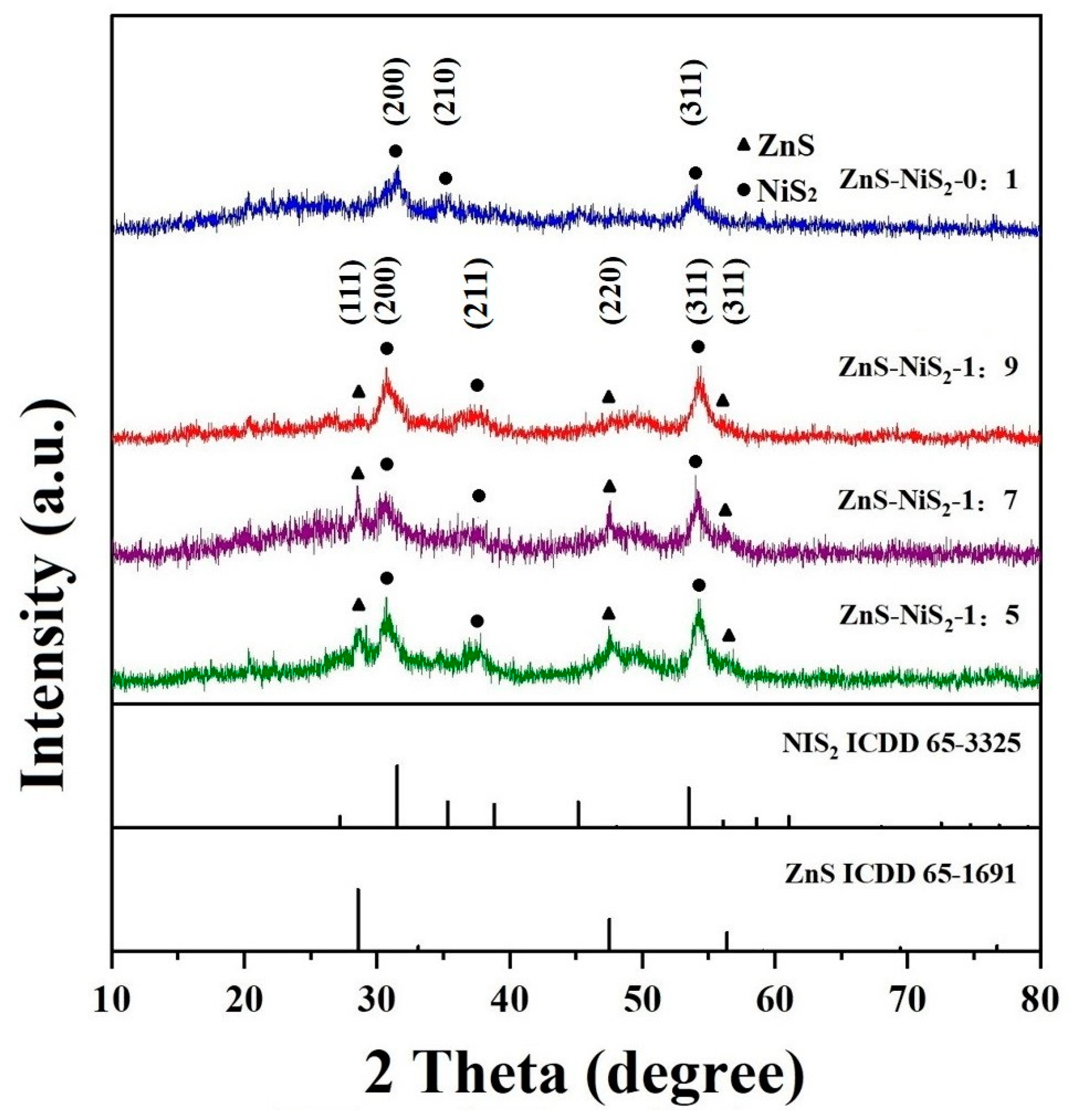
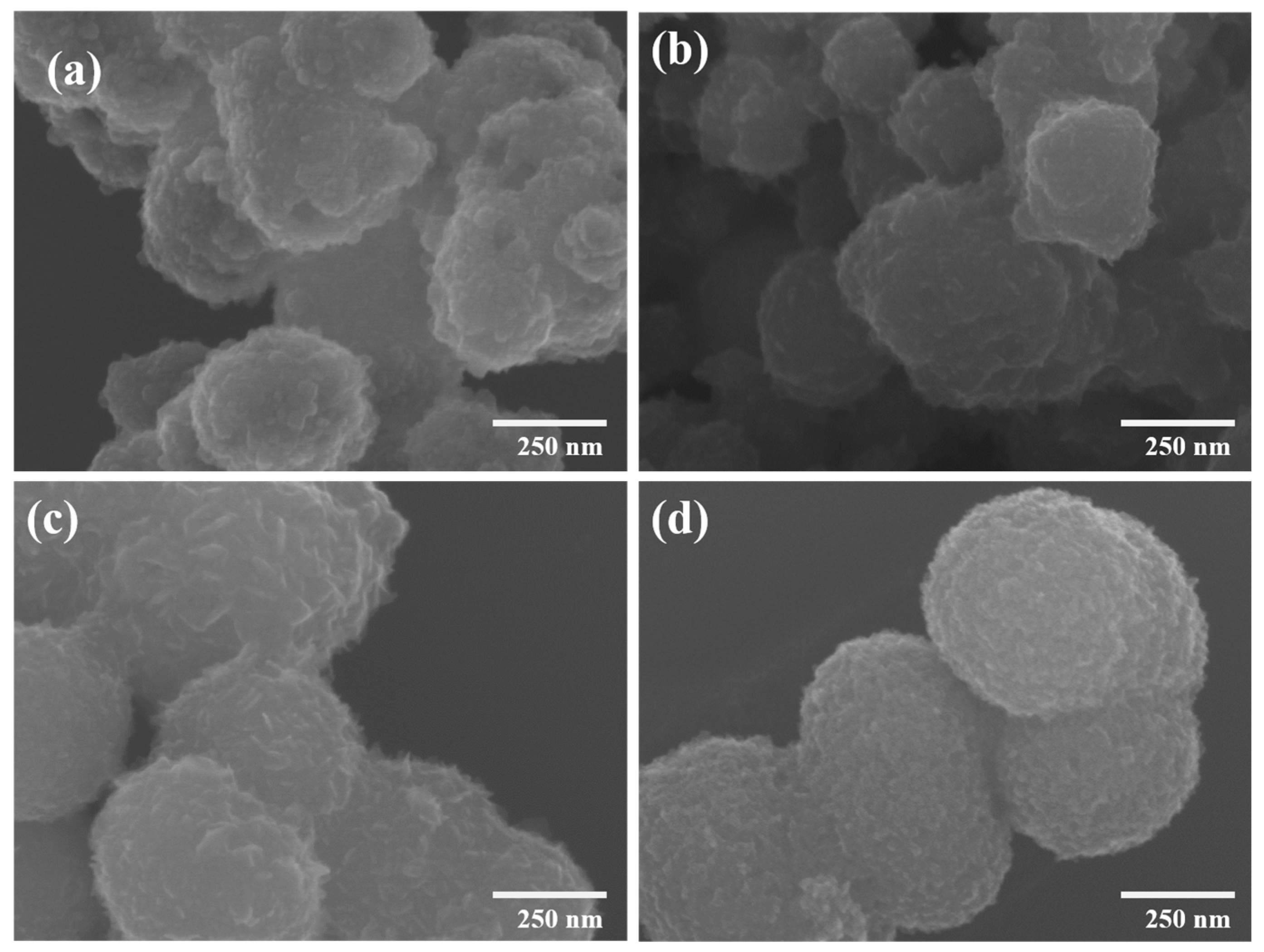

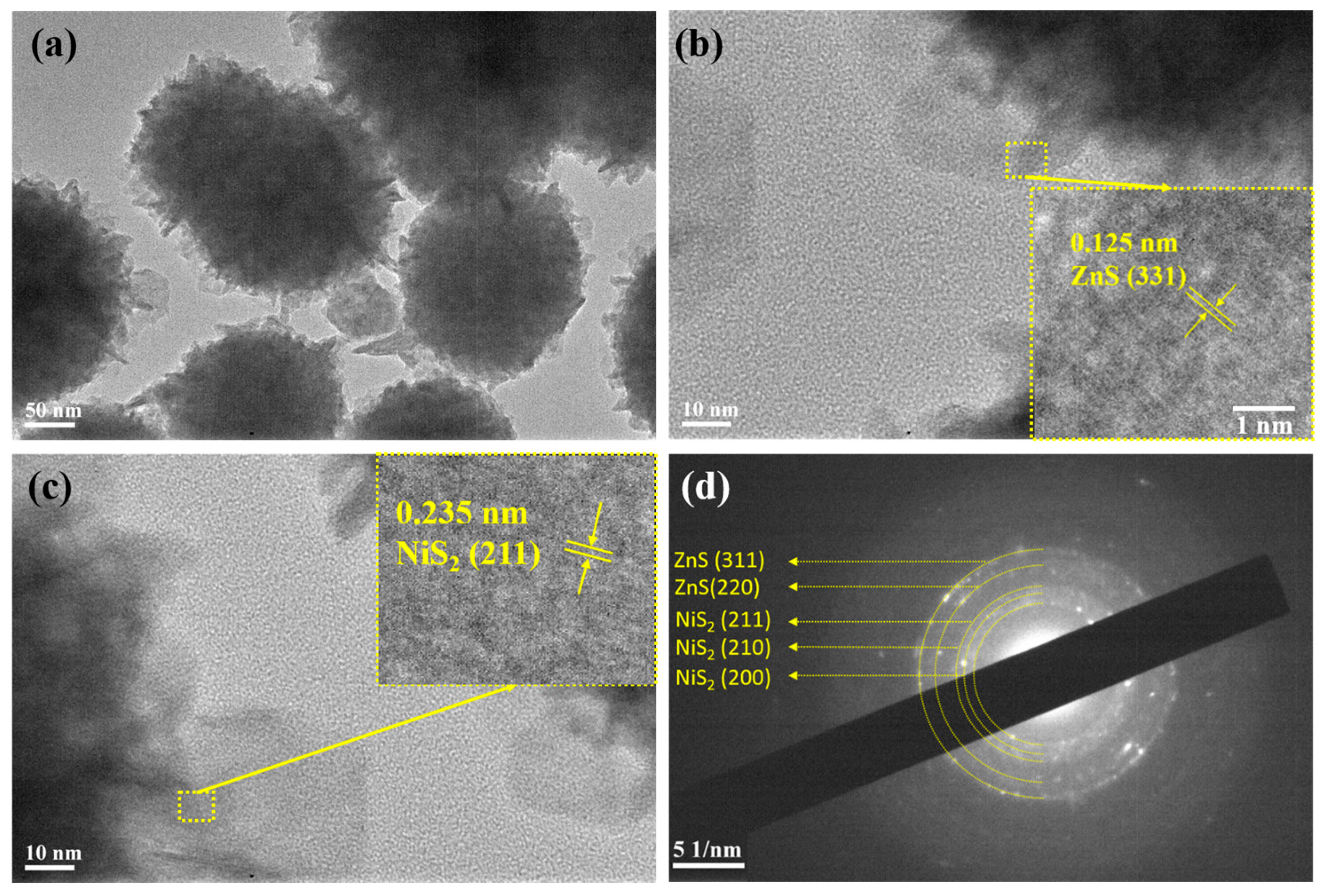




| Type of Material | Electrolyte | Specific Capacity (F g−1) | Current Density | Cycle Stability | Ref. |
|---|---|---|---|---|---|
| PANI/ZnS QDs | 1 M H2SO4 | 893.75 | 0.5 A g−1 | 59.7%@1000 | [32] |
| ZnS/SOM-C | 6 M KOH | 1158 | 1 A g−1 | 71%@8000 | [33] |
| ZnS/RGO/PANI | 6 M KOH | 1045.3 | 1 A g−1 | 160%@1000 | [34] |
| NiS2/Ti3C2Tx | 1 M KOH | 518.4 | 1 A g−1 | 77.27%@9000 | [35] |
| Co-NiS2/C | 3 M KOH | 1080 | 1 A g−1 | 89.2%@8000 | [36] |
| NiS2@C-rGO | 1 M H2SO4 | 1297 | 1 A g−1 | 95.3%@10,000 | [37] |
| Pure pyrite NiS2 | 6 M KOH | 1072.6 | 2 A g−1 | 78.1%@1000 | [17] |
| ZnS-NiS2 | 3 M KOH | 1467.9 | 1 A g−1 | 94.9%@5000 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Abdullah, M.; Chen, X.; E, Y.; Tan, L.; Yan, W.; Liu, Y.; Jiang, W. Two-Step Synthesis of ZnS-NiS2 Composite with Rough Nanosphere Morphology for High-Performance Asymmetric Supercapacitors. Batteries 2024, 10, 16. https://doi.org/10.3390/batteries10010016
Jiang M, Abdullah M, Chen X, E Y, Tan L, Yan W, Liu Y, Jiang W. Two-Step Synthesis of ZnS-NiS2 Composite with Rough Nanosphere Morphology for High-Performance Asymmetric Supercapacitors. Batteries. 2024; 10(1):16. https://doi.org/10.3390/batteries10010016
Chicago/Turabian StyleJiang, Meng, Muhammad Abdullah, Xin Chen, Yi E, Liyi Tan, Wei Yan, Yang Liu, and Wenrui Jiang. 2024. "Two-Step Synthesis of ZnS-NiS2 Composite with Rough Nanosphere Morphology for High-Performance Asymmetric Supercapacitors" Batteries 10, no. 1: 16. https://doi.org/10.3390/batteries10010016
APA StyleJiang, M., Abdullah, M., Chen, X., E, Y., Tan, L., Yan, W., Liu, Y., & Jiang, W. (2024). Two-Step Synthesis of ZnS-NiS2 Composite with Rough Nanosphere Morphology for High-Performance Asymmetric Supercapacitors. Batteries, 10(1), 16. https://doi.org/10.3390/batteries10010016







