3.1. Magnetic Behavior during the Re-Calcination after Optimized CH4 Gas Heat Treatment
In our previous paper [
20], the phase transformation in a GTR process for preparing nanocrystalline Sr-hexaferrite powder using CH
4 heat treatment was studied. The process included gaseous heat treatment and subsequent re-calcination. The phases formed at different calcination temperatures obtained by XRD patterns [
20] along with their percentages are listed in
Table 1. The crystallite sizes of these phases are also shown in
Table S1.
The properties of initial Sr-hexaferrite powder sample prepared by the conventional method were investigated and are shown in
Figures S1–S3.
Figure S3 shows the magnetization curve of the initial Sr-hexaferrite powder. The magnetic properties resulting from this hysteresis curve are 60.88 emu g
−1 saturation magnetization, 35.30 emu g
−1 remanence, and 3.5 kOe coercivity.
During the gas heat treatment, the samples contain non-magnetic phases such as carbon or iron carbide, which have a negative effect on the magnetic properties. Therefore, the investigation of magnetic properties was started by investigating the magnetic behavior of the powdered sample of Sr-hexaferrite, which has been re-formed by methane gas under the best conditions (temperature: 950 °C, gas flow rate:15 cc min
−1 and time: 30 min) [
20]. The hysteresis curve of the mentioned sample is shown in
Figure 1.
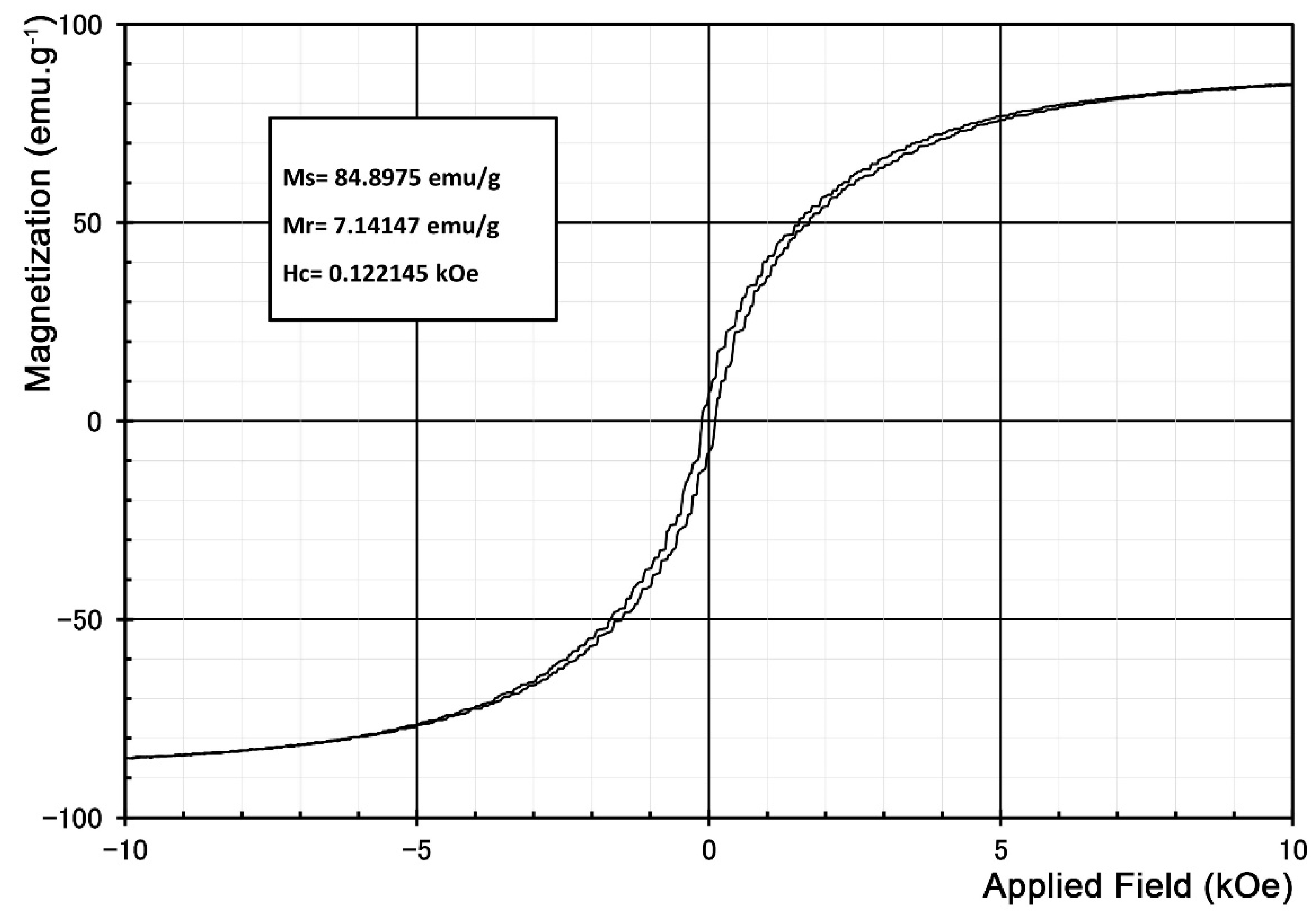
Figure 1 shows the soft magnetic properties of the powder. According to the XRD pattern of this sample (
Figure S4), the predominance of the pure iron phase in the composition of this sample could be the reason for such magnetic behavior.
The magnetization curve of the sample reduced under the best conditions and then re-calcined at 200 °C is shown in
Figure 2 (also the individual hysteresis curve of this sample is shown in
Figure S5). According to
Table 1, one hour of calcination at 200 °C does not make any particular phase transformation in this sample. However, it causes a significant increase in the saturation magnetization and a very low decrease in the remanence and coercivity. These changes can be attributed to the effects of diffusive phenomena, such as relaxation and stress relief caused by heat treatment (annealing).
The magnetization curve of the sample reduced under the best conditions and then re-calcined at 500 °C is shown in
Figure 2 (the individual hysteresis curve of this sample is shown in
Figure S6). At this temperature, the saturation magnetization has decreased (about 65% reduction), the remanence has increased slightly, and the coercivity is still very low. Therefore, this material can be described as a poor soft magnetic material.
Such behavior can be expected after the oxidation of pure iron and the formation of oxides such as hematite. Almost all iron is re-oxidized and converted to hematite up to the calcination temperature of 500 °C.
Figure 3 shows FE-SEM image and EDS analysis of a reduced Sr-hexaferrite powder sample calcined at 500°C. Points (a) and (b) on this image show the EDS analysis on fine aggregate particles and on a relatively large and flat particle, respectively.
The EDS analysis of point (a) is definitely for iron oxides, while the analysis point (b) shows a composition quite close to the Sr-rich phase, Sr7Fe10O22.
The magnetization curve of the sample reduced under the best conditions and then re-calcined at 700 °C is shown in
Figure 2 (the individual hysteresis curve of this sample is shown in
Figure S7). According to
Figure 2, magnetic properties are still changing from soft to hard. Although this sample has a very low saturation magnetization, there has been a significant increase in the remanence and coercivity. According to the molar percentage of phases in this sample (
Table 1), the composition of this sample is almost hematite. A small percentage of Sr-hexaferrite is also present in the sample, and no pure iron or magnetite is seen. Therefore, the magnetic behavior of this sample (reduction of the saturation magnetization and remanence) is more affected by the presence of hematite. The increase in coercivity can also be attributed to the structural anisotropy of the Sr-hexaferrite phase. According to
Figure 2, a kink can be seen in the hysteresis loop. Almessiere et al. [
11] observed a similar distortion kink during investigation of hard/soft composite of SrBaSc/NiFe. They identified the weak exchange coupling between the phases to be the reason for the kink in the hysteresis loop as the weak exchange causes the separate switch of the magnetic moments of the hard and soft phases towards the applied field. In a hard/soft ferrite composite, three interactions of hard/soft exchange, soft/soft dipole and hard/hard dipole are integrated; if dipole/dipole interactions are dominant, weak magnetic properties will be obtained [
21]. They [
11] were able to overcome the dipole/dipole interactions by changing the concentration of the composite composition and obtain smooth hysteresis loops.
Figure 3 and
Figure S13 show an FE-SEM image of a reduced Sr-hexaferrite powder sample calcined at 700 °C. Large cracked particles can still be observed. Points (a) and (b) on this image show the EDS analysis on the aggregation region of interconnected small particles and on relatively large cracked particles, respectively. The EDS analyzes confirm that the small, interconnected particles are oxidized irons, and the larger cracked particles are Sr-rich phase.
The magnetization curve of the sample reduced under the best conditions and then re-calcined at 800 °C is shown in
Figure 2 (the individual hysteresis curve of this sample is shown in
Figure S8). By increasing the temperature from 700 °C to 800 °C, the saturation magnetization has increased from 17.31 to 30.86 emu g
−1, which indicates an increase of about 78%. The remanence has also increased from 9.05 to 19.44 emu g
−1 (~115%), and the coercivity from 2.47 to 4.13 kOe (~67%), respectively. Hence, it can be said that all the magnetic properties in the curve of
Figure S8 (800 °C) have almost doubled compared to the curve of
Figure S7 (700 °C). It can also be said that this is the first sample that can be considered a hard magnetic material. In particular, the coercivity field in this sample is significant and higher than the initial sample prepared by the conventional method. According to
Table 1, the main difference between the sample prepared at 800 °C and the sample prepared at 700 °C is the double increase of the Sr-hexaferrite phase percentage. However, the amount of Sr-hexaferrite phase is still very low compared to the amount of hematite in the composition. Hence, it is concluded that the significant change in the magnetic properties of the sample requires more amount of Sr-hexaferrite phase.
The magnetization curve of the sample reduced under the best conditions and then re-calcined at 900 °C is shown in
Figure 2 (also the individual hysteresis curve of this sample is shown in
Figure S9). According to
Figure 2, by increasing the temperature from 800 °C to 900 °C, the saturation magnetization and remanence have decreased from 30.86 emu g
−1 to 26.08 emu g
−1 (~15% reduction) and 19.44 emu g
−1 to 16.36 emu g
−1 (~16% reduction), respectively. Despite the inconsiderable decrease in saturation magnetization and remanence, the hysteresis curve at 900 °C has a higher coercivity (4.62 kOe) in comparison with the coercivity of 4.13 kOe at 800 °C.
Generally, the samples prepared at 800 °C and 900 °C have a higher coercivity than the initial Sr-hexaferrite sample. The investigation of phase percentage [
20] shows that the Sr-hexaferrite phase in the sample prepared at 900 °C is much more (about twice as much) than the sample prepared at 800 °C, which is the reason for an increase in the coercivity.
The magnetization curve of the sample reduced under the best conditions and then re-calcined at 1000 °C is shown in
Figure 2 (also the individual hysteresis curve of this sample is shown in
Figure S10). The shape of the hysteresis loop perfectly shows the properties of a hard magnetic material. The highest saturation magnetization and remanence among the samples that have been investigated so far, as well as the high coercivity, make this sample very favorable for hard magnetic applications. Comparing the magnetic properties of this sample with the initial Sr-hexaferrite sample shows that about 10% decrease in saturation magnetization, 4% decrease in remanence and about 17% increase in coercivity have been achieved. The area of hysteresis loop in the second quadrant also had its highest value in this sample. However, according to the X-ray diffraction patterns in our previous work [
20], this sample is still not a single-phase Sr-hexaferrite and contains other phases, such as hematite.
Figure S14 shows FE-SEM images of a reduced Sr-hexaferrite powder sample calcined at 1000 °C for one hour.
Figure 3 also shows the EDS analysis of this sample. EDS analysis shows the composition of Sr-hexaferrite and confirms the formation of the desired product at the end of this stage. According to the TEM image of this sample (
Figure S15), the Sr-hexaferrite particle size is less than 50 nm.
The magnetization curve of the sample reduced under the best conditions and then re-calcined at 1100 °C is shown in
Figure 2 (the individual hysteresis curve of this sample is shown in
Figure S11). At this high temperature, Sr-hexaferrite grows rapidly, and the magnetic properties are closer to the values of the initial Sr-hexaferrite. In addition, a sudden change in the slope of the second quadrant of the hysteresis loop was observed, indicating the existence of two different phases. Probably, the non-uniform growth of particles can cause a non-uniform particle size distribution, and due to the difference in the magnetic behavior of small and large particles, will result in a multiphasic behavior.
Finally, the magnetization curve of the sample reduced under the best conditions and then calcined at 1200 °C is shown in
Figure 2 (also the individual hysteresis curve of this sample is shown in
Figure S12). According to
Figure 2, the coercivity is decreased, and the behavior of the curve in the second quadrant is still similar to the two-phase behavior.
According to
Figure 2, it can be concluded that the sample has the lowest saturation magnetization at 700 °C, and below this temperature, the saturation magnetization increases with a soft behavior; at a higher temperature, the saturation magnetization increases but with a hard behavior. Therefore, 700 °C can be considered as the transition temperature from soft to hard magnetic behavior. After 700 °C, there is a direct relationship between the calcination temperature and the saturation magnetization as well as the remanence. The saturation magnetization approaches its maximum at temperatures of 1100 and 1200 °C.
The area of the hysteresis loop in the second quadrant has the highest value for the sample calcined at 1000 °C. According to
Figure 2, the temperature range of 900 to 1000 °C seems to be the turning point of the uniform behavior in the second quadrant of hysteresis loop, and at other temperatures, this behavior is similar to the two or multi-phase behavior.
3.2. Magnetic Properties Change Procedure during the Re-Calcination after Optimized CH4 Gas Heat Treatment
The saturation magnetization of samples reduced under the best conditions and then calcined at different temperatures was summarized and listed in
Table 2.
Figure 4 also shows the curve of these changes.
According to
Figure 4, the saturation magnetization of the sample obtained from the reduction process increases after calcination at 200 °C. This increase in the saturation magnetization can be attributed to the effect of relaxation and stress relief during heat treatment because there is no phase transformation in the samples. By increasing the calcination temperature to 500 and then 700 °C, the saturation magnetization of the sample decreases, which is caused by the gradual transformation of the pure iron phase into hematite during the oxidation process.
The magnetic behavior changes at 700 °C. Above 700 °C, the saturation magnetization of the sample has an increasing trend as the calcination temperature increases and reaches its maximum above 1000 °C. The observed behavior after 700 °C is due to the gradual formation of the Sr-hexaferrite phase. The saturation magnetization of samples increases with as the Sr-hexaferrite phase percentage increases, and after 1000 °C, when almost all of the sample turns into Sr-hexaferrite, the increasing trend of saturation magnetization stops.
The remanence of samples reduced under the best conditions and then calcined at different temperatures is summarized and listed in
Table 2.
Figure 4 shows the curve of these changes.
According to
Table 2 and
Figure 4, at high temperatures (after 500 °C), the remanence has an increasing trend with increasing calcination temperature. In fact, when the first particles of Sr-hexaferrite (at 700 °C) are formed, the remanence, which is the result of the asymmetric structure of the Sr-hexaferrite phase, starts to increase. However, the maximum value of the remanence is obtained at 1100 °C, and the decrease of remanence after 1100 °C can be attributed to the increase of particle size.
The coercivity of samples was summarized and listed in
Table 2.
Figure 4 also shows the curve of these changes.
According to
Figure 4, the coercivity is from close to zero up to 500 °C; after this temperature, it increases and reaches its maximum at 800–1000 °C, then decreases at higher temperatures. No hard magnetic phase was formed in the sample up to 700 °C; therefore, the coercivity did not increase significantly. The increase of coercivity up to 900 °C is proportional to the increase of Sr-hexaferrite phase in the sample, confirming the direct effect of Sr-hexaferrite in increasing the coercivity. However, after this temperature, a decreasing trend in the coercivity was observed, which is proportional to the calcination temperature. In fact, 900 °C is the transition point for the coercivity. According to the phase percentages in the sample (
Table 1), it was observed that the amount of Sr-hexaferrite at 900 °C is about half in comparison with 1200 °C. Therefore, it may be possible to attribute the rise and fall of the coercivity to the stages of nucleation and growth of the Sr-hexaferrite phase. This means that, as long as the nucleation of Sr-hexaferrite fine particles continues, the coercivity increases in proportion to the number of new nuclei, and as a result, the percentage of the Sr-hexaferrite phase in the sample increases. Then, the growth of these nuclei will reduce the coercivity due to the increase in the particle size and the change in the demagnetization mechanism of the particles.
Another important parameter in determining the magnetic behavior of materials is M
r/M
s ratio. The ratio M
r/M
s of samples were summarized and listed in
Table 2.
Figure 4 also shows the curve of these changes.
Figure 4 shows that the ratio M
r/M
s increases rapidly after 500 °C, reaches its maximum at 800–1100 °C, and then decreases again. Two factors can play a role in the ratio M
r/M
s: the values of M
r and M
s, and the distance between these two quantities. For example, the distance between these two quantities is the lowest at 700 °C, and it would see that the ratio M
r/M
s should have its highest value in this condition; instead, the ratio M
r/M
s had its maximum at 800 to 1100 °C. This occurred because of the higher value of both M
r and M
s compared to their values at 700 °C. As
Figure 2 shows, however, only the samples calcined at 900 and 1000 °C have uniform curves with no change in slope.
Figure 5 shows the area under the second quadrant of hysteresis curves of reduced and consequently calcined samples at different temperatures for comparison. Although the ratio M
r/M
s was almost equal in both temperatures of 900 °C and 1000 °C, the hysteresis curves indicate that the most significant area under the second quadrant belongs to the sample calcined at 1000 °C. Therefore, the temperature 1000 °C was selected as the optimum temperature for re-calcination in the GTR process to have the best magnetic properties.
The area under each curve (in the second quadrant) was determined by counting pixels with the help of a graphical software and then normalized and expressed as a percentage for ease of comparison. The results are presented in
Table 3 and compared in the
Figure 6.
3.3. Analysis of Exchange Coupling Effect in Increasing the Magnetic Properties of the Optimum Sample
The results of magnetic behavior investigations of samples during the GTR process showed that, unexpectedly, the best magnetic properties were not obtained for the final product of single-phase Sr-hexaferrite powder. The sample calcined at 1000 °C, which still contains some hematite phase, showed better magnetic properties. It was observed that this product had a uniform magnetization curve along with the best magnetic properties. Due to the unexpectedness of the result, this sample was again prepared and analyzed under the same conditions, and completely similar results were obtained. Therefore, the possibility of forming an exchange coupling between Sr-hexaferrite and hematite in this sample is the most probable reason for such a phenomenon.
The most straightforward criterion for the exchange coupling phenomenon is the shape of magnetization curve. The composite obtained from two materials with different magnetic properties can produce different states of magnetic behavior depending on the interaction between these two materials. When the two constituent materials cannot interact effectively with each other, the shape of the magnetization curve will be distorted due to the behavior of two different phases. For example, during demagnetization, the phase with soft behavior will quickly decrease the magnetization, and the curve will be more affected by the hard phase behavior. When the two phases cause the demagnetization behavior of the resulting composite to be reversible in the second quadrant by stopping the reverse field, the soft phase returns to the initial magnetization value. However, when the behavior of the nanocomposite magnetization curve is the same as the magnetic behavior of a single-phase material, the phenomenon of exchange coupling occurs. Nanocomposite systems with such behavior are called rigid magnetic nanocomposites [
10,
22].
Up to 800 °C, there is less of a hard phase, and it is not possible for exchange coupling between two phases to occur. On the other hand, from 1100 °C and above, the ratio of the two phases is not enough to cause a strong exchange interaction, and due to high temperatures, the growth of some hard phase particles causes a drop in their hard properties. Fine and coarse hard particles together cause non-rigid two-phase behavior. If the content of the soft phase is low, not all hindered spins of the hard phase can rotate towards the magnetic field. Hence, an additional amount of soft phase is needed to overcome the magnetocrystalline anisotropy of the hard ferrite phase. When the amount of soft phase is sufficient, its combination with the hard phase forms an interfacial region. In this region, the soft phase has low magnetocrystalline anisotropy, and its magnetic moments can softly orient towards the magnetic field. By creating a torque, the hindered spins of the hard phase switch to the magnetic field, and exchange coupling occurs [
11,
23].
Finally, according to the ratio of ferrite and hematite (
Table 1) the shape of the magnetization curve at 1000 °C can indicate the exchange interaction between these two phases. The ratio M
r/M
s can also be a measure for exchange coupling. In composites with dual magnetic behavior, at M
r/M
s > 0.5, the composite’s behavior is close to the ideal rigid behavior [
24,
25]. In this research, it was observed that above 700 °C, the magnetic behavior tends to the ideal rigid behavior, but the maximum value of ratio M
r/M
s occurs between 800 and 1100 °C. Therefore, it can be concluded that the sample calcined at 1000 °C has the necessary conditions for exchange coupling.