Abstract
A mononuclear Ni(II) complex, [Ni(HL1)2], (1) and a novel tetranuclear heterometal Mn-Ni complex, [Mn3Ni(L1)4Cl2(EtOH)2], (2) [H2L1 = N-(2-hydroxymethylphenyl)salicylideneimine], have been synthesized and characterized via X-ray crystal structure analyses, infrared spectra, and elemental analyses. The structure analyses revealed that the tridentate ligand, H2L1, coordinates in a facial mode for Ni and a mer mode for Mn, respectively. Complex 2 includes Mn(II)Mn(III)2Ni(II) tetranuclear metal core bridged by μ-phenoxo and μ-alkoxo oxygens. Magnetic measurements for 2 indicate that weak ferromagnetic interactions (JMn(III)Ni(II) = 2.23, JMn(III)Mn(II) = 0.46, JMn(II)Ni(II) = 1.78, and JMn(III)Mn(III) = 0.58 cm−1) dominate in the tetranuclear core. Additionally, in alternating current (AC) magnetic measurements, frequency-dependent out-of-phase responses were observed.
1. Introduction
The field of molecular magnetism in discrete polynuclear complexes has seen significant research over the last few decades [1,2,3,4,5,6]. This research covers a wide range of objectives, from a fundamental understanding of the correlation between spin-exchange interactions and molecular structure [1,2,7,8,9,10] to the development of functional magnetic materials like single-molecule magnets (SMMs) [3,4,5,6,11,12]. While the study of homometallic complexes is well-established, the exploration of heterometallic complexes remains relatively limited. This is due to the complexities associated with synthesizing new compounds, even though such complexes can significantly impact the spin ground state, magnetic anisotropy, and magnetic exchange interactions, leading to the development of low-dimensional magnets, SMMs and SCMs (single-chain magnets) [5,13,14,15,16,17].
Three primary synthetic strategies have been employed for the synthesis of heterometallic complexes: (i) one-pot reactions, such as hydrothermal synthesis [18]; (ii) the design of ligands with multiple coordination sites that selectively bind specific metal ions [13,19]; and (iii) the reaction of complex ligands or bridging metal complexes with other metal ions [20,21]. While method (i) is suitable for synthesizing metal–organic frameworks (MOFs), it can be challenging to produce discrete complexes. Method (ii) offers opportunities for molecular design but can encounter obstacles in ligand synthesis. On the other hand, method (iii) holds an advantage as it allows for a relatively straightforward stepwise synthetic approach. In this method, tri- or tetradentate Schiff base ligands with bridgeable oxygen atoms at their terminals, similar to the Salen-type ligands in {[Cu(Mesalen)]2VO)}(ClO4)2 (Mesalen = N,N′-bis(2-hydroxyacetophenone)ethylenediamine) [22], have often been used.
The tridentate ligand N-(2-hydroxymethylphenyl)salicylideneimine (H2L1), which possesses bridgeable oxygen atoms (phenolic and alcoholic) at both terminals, has been employed to synthesize various polynuclear complexes with distinct bridging structures, such as tetranuclear Ni(II) complexes [23,24] and trinuclear Mn(III) complexes [25]. In our research, we have focused on leveraging the unique properties of the phenolic and alcoholic oxygen atoms at each end of this ligand. Through this feature, we have successfully synthesized heterometallic Mn(III)2Ni(II)2 complexes [24,26] and mixed-valence Co(II)2Co(III)2 complexes [27] and reported their crystal structures and unique magnetic properties. While these complexes were synthesized via one-pot reactions, we achieved a controlled structure due to the distinct properties of the bridging oxygen sites in the ligand. However, to attain even finer control over the structures, it is desirable to develop synthetic methods involving the isolation of mononuclear and/or binuclear complexes as complex ligands for reactions with other metal ions. In this study, we report the synthesis of a mononuclear Ni(II) complex using H2L1, and the structure and magnetic properties of a novel heterometallic mixed-valence Mn(II)Mn(III)2Ni(II) tetranuclear complex, which was obtained by utilizing the mononuclear Ni complex as a complex ligand.
2. Materials and Methods
2.1. Preparation
All chemicals were purchased and used as received, unless otherwise noted. Methanol and ethanol were purified by distillation over magnesium turnings. The ligand H2L1 was obtained by the literature method [28]. The IR spectrum of H2L1 is shown in Figure S1.
2.1.1. [Ni(HL1)2] (1)
A methanol solution (10 mL) containing nickel(II) acetate tetrahydrate (0.124 g, 0.5 mmol) and H2L1 (0.224 g, 1.0 mmol) was stirred with the addition of triethylamine (0.010 g, 0.10 mmol) for 90 min. The resulting pale yellowish-green solution was filtered and allowed to stand at room temperature. After 4 days, green single crystals suitable for X-ray analysis were obtained. The IR and PXRD spectra are shown in Figures S2 and S3, respectively. [Ni(HL1)2]·0.5MeOH; yield: 88 mg, 34.0%. Anal. Calc. for C28.5H26N2NiO4.5: C, 64.93; H, 4.97; N, 5.31%. Found: C, 65.18; H, 4.81; N, 5.31%. IR data [/cm−1]: 3041 (w), 2912 (w), 1612 (s), 1595 (s), 1517 (m), 1448 (s), 1269 (m), 1178 (s), 1149 (s), 1001 (m), 916 (m), 849 (m), 748 (s), 725 (m), and 509 (m).
2.1.2. [Mn3Ni(HL1)4Cl2(EtOH)2] (2)
To an ethanol/dichloromethane mixed solution (1:3 in volume, 6 mL) of 1 (0.256 g, 0.5 mmol), an ethanol solution (1 mL) of manganese(II) chloride tetrahydrate (0.049 g, 0.25 mmol) was added. The resulting dark-brown solution was stirred for 90 min and then filtered. After allowing the filtrate to stand at room temperature for 2 days, dark brown single crystals suitable for X-ray analysis were obtained. The IR and PXRD spectra are shown in Figures S4 and S5, respectively. [Mn3Ni(L1)4Cl2(EtOH)2]; yield: 17 mg, 15.8%. Anal. Calc. for C60H56Cl2Mn3NiN4O10: C, 55.97; H, 4.38; N, 4.35%. Found: C, 55.86; H, 4.47; N, 4.36%. IR data [/cm−1]: 3198 (s), 2930 (w), 1605 (s), 1574 (m), 1533 (s), 1435 (s), 1315 (s), 1182 (s), 1149 (s), 1016 (s), 983 (m), 860 (m), 748 (s), 634 (s), and 550 (s).
2.2. Measurements
Elemental analyses for C, H, and N were obtained at the Elemental Analysis Service Center at Kyushu University. Fluorescent X-ray analysis was obtained on a Shimadzu energy dispersive X-ray spectrometer Rayny EDX-700HS (Shimadzu Co., Ltd., Kyoto, Japan). PXRD measurements were conducted on a Shimadzu X-ray diffractometer XRD-7000 (Shimadzu Co., Ltd., Kyoto, Japan). Infrared spectra were recorded on a Bruker VERTEX70-S FT-IR Spectrometer on the ATR (Attenuated Total Reflection) method. Reflection spectra were recorded on a PERKIN ELMER Lambda900Z UV/VIS/NIR Spectrometer (PerkinElmer, Inc., Waltham, MA, USA) and Ocean Optics USB2000+ Fiber Optic Spectrometer (Ocean Optics, Inc., Dunedin, FL, USA). The magnetic susceptibilities were measured on a Quantum Design MPMS-XL5R SQUID susceptometer (Quantum Design, Inc., San Diego, CA, USA) under an applied magnetic field of 0.1 T at room temperature for 1 and in the temperature range 2–300 K for 2 and 3. The susceptibilities were corrected for the diamagnetism of the constituent atoms using Pascal’s constant [29]. The DC-magnetic data were fitted using the PHI program 3.1.6 [30].
2.3. Single Crystal X-ray Diffraction
Single crystals suitable for X-ray analysis were obtained by slow evaporation of methanol for 1 and ethanol for 2, respectively. Diffraction data were measured on a Rigaku Vari-Max Saturn CCD 724 diffractometer (Rigaku Corp., Tokyo, Japan) with graphite monochromated Mo Ka radiation (λ = 0.71069 Å) at the Analytical Research Center for Experimental Sciences, Saga University. Data were collected and processed using CrystalClear-SM 2.0 r16 [31]. The crystal was kept at 113 K during data collection. Multi-scan correction for absorption was applied. The crystal data and experimental parameters are summarized in Table 1. The structures were solved by direct methods (ShelXT-2016/6) and expanded using Fourier techniques [32]. Non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed geometrically in calculated positions and refined with a riding model. The final cycle of full-matrix least-squares refinement on F2 using ShelXL-2016/6 [33] was based on observed reflections and variable parameters and converged with unweighted and weighted agreement factors of R and Rw. Olex2-1.5 [34] was used as an interface to the ShelX program package. The structure drawing of the molecules was performed using Mercury-2022.1.0 [35]. The Hirshfeld surface was generated using Crystal Explorer 3.1 [36].

Table 1.
Crystallographic data and refinement parameters.
3. Results and Discussion
3.1. Synthetic Outcomes and Characterization
The elemental analysis results for 1 were consistent with the calculated composition of [Ni(HL1)2]·0.5MeOH. According to the molecular formula obtained from the single-crystal X-ray structural analysis (SCXRD), two methanols were initially included. However, crystalline decomposition was observed during the vacuum drying process, indicating that the loss and evaporation of methanol were responsible for this observation. The powder X-ray diffraction (PXRD) pattern of the dried sample does not match well with the simulated pattern obtained from SCXRD, further suggesting the loss of the crystal solvents (Figure S3). So far, crystal structures of Ni(II) complexes with H2L1 have only been reported for tetranuclear complexes, [{Ni(L1)(EtOH)}4] [23] and [{Ni2(L1)(HL1)Cl}2] [24], which have a cubane-like structure. This can be attributed to the strong bridging capability of deprotonated alkoxo-oxygen donors. In this study, our goal was to synthesize a mononuclear Ni(II) complex by carefully controlling the pH conditions, employing nickel(II) acetate as the metal source to prevent the dissociation of protons from benzyl alcohol. Remarkably, we successfully isolated complex 1 as a mononuclear Ni(II) complex (Scheme 1). The FT-IR spectrum revealed intense and characteristic bands corresponding to the azomethine (C=N), phenolic (C–O), and alcoholic (C–O) stretching vibrations of free H2L1, which were observed at 1615, 1279, and 1030 cm−1, respectively. Upon complexation in 1, these bands were downshifted to 1612, 1178–1149, and 1001 cm−1, respectively. The heterometal complex 2 was obtained by reacting the nickel complex 1 with manganese(II) chloride in ethanol. Initially, our aim was to produce a linear Ni(II)-Mn(II)-Ni(II) complex; therefore, 1 was reacted with Mn(II) in a 2:1 ratio. However, based on EDX measurements, the composition of 2 was determined to be in a 3:1:2 ratio of Mn, Ni, and Cl, respectively. Taking into account the EDX results and charge balance considerations, the oxidation states of the four metal ions in 2 are presumed to be Mn(II)Mn(III)2Ni(II). Analytical data of 2 showed good agreement with its molecular formula obtained by single-crystal X-ray analysis. In previous studies, we have reported several heterometallic tetranuclear Mn(III)–Ni(II) complexes with H2L1 and its homologs [24,26]. Complex 2 is a novel complex that exhibits characteristics of a mixed-valence electronic state in addition to being a heterometallic complex. In the FT-IR spectrum, a strong and sharp (O–H) band was observed at 3196 cm−1. This characteristic band likely originates from coordinated ethanol molecules, indicating the presence of strong intramolecular hydrogen bonds. The three IR bands stemming from the tridentate ligand were shifted to 1604 [(C=N)], 1182—1149 [(C–O)phenolic], and 1016—983 [(C–O)alcoholic] cm−1, respectively.
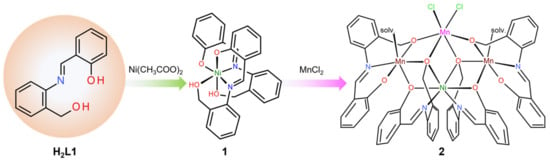
Scheme 1.
Synthetic route of complexes.
3.2. Structural Studies
3.2.1. Complex 1: [Ni(HL1)2]·2MeOH
The molecular structure and packing diagram of 1 are shown in Figure 1a and Figure S6, respectively. The selected bond lengths and angles are listed in Table 2. The coordination environment surrounding the Ni(II) ion adopts an octahedral geometry, with a N2O4 donor set originating from the tridentate ligand (HL1)−. Previously reported mononuclear complexes with (HL1)−, such as [Co(HL1)2]NO3·2H2O, has a mer-coordination mode [37]. However, due to the larger ionic radius of Ni(II) in comparison to trivalent transition metal ions, the tridentate ligands in this complex coordinate to the Ni atom in a fac mode. All three types of coordination atoms from the two ligands adopt a cis configuration. Consequently, complex 1 exhibits chirality around Ni.
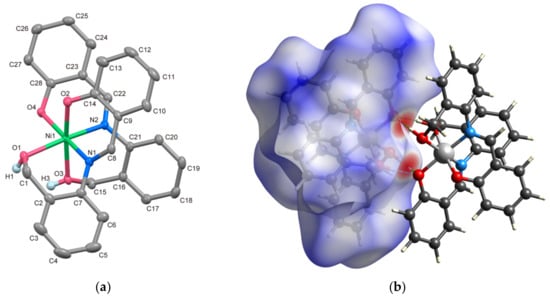
Figure 1.
(a) X-ray crystal structure of 1 showing thermal ellipsoids drawn at a 50% probability level. Hydrogen atoms, except for benzyl alcohols, have been omitted for clarity. (b) Enantiomeric pair formed by intermolecular hydrogen bonds with the Hirshfeld surface.

Table 2.
Selected bond distances and angles of 1.
The bond lengths of Ni–Ophenolic and Ni–N are closely comparable, falling within the range of 2.0113(15)–2.0531(17) Å. In contrast, the Ni1–O1 and Ni1–O3 distances, which involve protonated O atoms, are 2.1064(15) and 2.1103(16) Å, respectively, approximately 0.1 Å longer than the other coordination bonds (refer to Table 2). According to SHAPE analysis [38] score of 1, OC-6 = 0.501, indicating minimal distortion of the octahedral structure surrounding Ni. The benzyl alcohol moiety and the deprotonated phenolate group engage in intermolecular hydrogen bonds, specifically O1–H1D⋯O4i = 2.686(2) and O–H3D⋯O4i = 2.641(2) Å [symmetry code: (i) 1 − x, 1 − y, 1 − z], respectively. This interaction results in the formation of an enantiomeric pair, as illustrated in Figure 1b. The red-colored regions on the Hirshfeld surface clearly indicate the presence of these intermolecular hydrogen bonds.
3.2.2. Complex 2: [Mn3Ni(L1)4Cl2(EtOH)2]
Complex 2 is a heterometallic tetranuclear complex featuring a double cubane-like metal core structure comprising three Mn and one Ni ions. It crystallizes in the orthorhombic polar space group Aea2. The molecular structure and packing diagram of 2 are illustrated in Figure 2a and Figure S7, respectively. The selected bond lengths and angles are provided in Table 3.
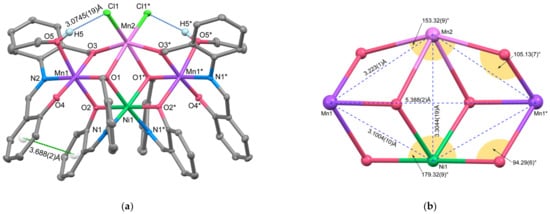
Figure 2.
(a) X-ray crystal structure of complex 2 showing thermal ellipsoids drawn at a 50% probability level. Hydrogen atoms, except for hydroxy group of the coordinated ethanol, have been omitted for clarity. Symmetry code (*): 1 − x, 1 − y, +z. (b) Metal core structure comprised of 4 metal ions and 6 bridging oxygen atoms.

Table 3.
Selected bond distances and angles of 2.
This molecule exhibits C2 symmetry with the axis along the line connecting Ni1 and Mn2. Ni1 forms an octahedral [Ni(L1)2]2− unit, coordinated by two (L1)2− ligands in a fac mode. However, unlike complex 1 where all three kinds of coordinating atoms were in cis positions, in this Ni unit, the phenolic oxygens adopt trans positions, introducing a rotational symmetry axis that was absent in complex 1. The coordination bond distances around Ni are Ni1–O1 = 2.0937(18), Ni1–O2 = 2.0454(18), and Ni1–N1 = 2.022(2) Å, respectively. The Ni1–O1 distance, corresponding to the benzyl alcohol oxygen, is approximately 0.15 Å shorter in complex 2 compared to 1 due to the dissociation of the alcohol proton. This benzyl alcohol oxygen, O1, acts as a μ3-alkoxo bridge, connecting Ni1, Mn1, and Mn2. Mn1 is linked to Ni1 through this μ3-alkoxo oxygen (O1) and a μ2-phenoxo oxygen (O2). It forms an octahedral arrangement with another tridentate ligand’s donor set (O3, N2, O4) and the alcohol oxygen (O5) from the coordinating ethanol. The coordination bond distances within the square plane formed by the tridentate ligand and the μ3-oxygen atom are 1.8761(17), 2.0431(19), 1.8607(17), and 1.9756(16) Å. In contrast, the Mn1–O2 distance for the μ2-bridging oxygen is 2.1820(17) Å, and the Mn1–O5 distance for the coordinating ethanol moiety is 2.2587(18) Å, indicating elongation. This suggests that Mn1 is a Mn(III) ion with the O2–Mn1–O5 axis corresponding to the Jahn–Teller axis. On the other hand, Mn2 lacks chelating coordination from the tridentate ligand and exhibits a highly distorted six-coordinate structure formed by two μ3-bridging alcoholic oxygens, two μ2-bridging alcoholic oxygens, and two monodentate chloride ions. The bond distances around Mn2 are Mn2–O1 = 2.3145(18), Mn2–O3 = 2.1765(18), and Mn1–Cl1 = 2.4560(10) Å, which are longer than the bond distances around Mn1, indicating that Mn2 is in the Mn(II) oxidation state. To further confirm the oxidation state assignments of Mn1 and Mn2, bond valence sum (BVS) calculations were conducted for each manganese atom [39,40]. The calculated BVS values of 3.01 and 1.95 for Mn1 and Mn2, respectively, support the valence assignment based on the bond lengths [41]. To the best of our knowledge, only one Mn3Ni complex has been reported [42], and in that complex, all Mn ions are in the trivalent state. Therefore, complex 2 represents the first report of a complex in which three Mn ions are in a mixed valence state.
The four metal ions are located on the same plane, forming a rhombus shape, as depicted in Figure 2b. The intramolecular metal-to-metal distances are as follows: Mn1⋯Ni1 = 3.1004(10), Mn1⋯Mn2 = 3.223(1), Ni1⋯Mn2 = 3.3044(19) Å, and Mn1⋯Mn1* = 5.388(2) Å (symmetry code: (*) 1 − x, 1 − y, +z), respectively. Due to the slight difference in length between the Mn1⋯Ni and Mn1⋯Mn2 sides, the rhombus shape is distorted towards Mn2. Comparing the bond angles around Ni1 and Mn2, the O2–N1–O2* angle corresponding to the longer side of the double-cubane framework is 179.32(9)°, while the O3–Mn2–O3* angle is 153.32(9)°, indicating significant distortion at Mn2. Consequently, the defective double-cubane metal core, comprising four metal ions and six bridging oxygen atoms, exhibits a distorted structure with Mn2 protruding from the double-cubane framework. This distortion can be attributed to the larger ionic radius of Mn2 and the steric repulsion of the coordinated two chloride ions. The SHAPE calculation results (OCT-6) further confirm the substantial distortion at Mn2, with values of 0.594 for Ni1, 0.964 for Mn1, and 2.459 for Mn2. Complex 2 exhibits hydrogen bonds between the O–H proton of the coordinated ethanol and the chloride ion, with a distance of O5–H5⋯Cl1 = 3.0745(19) Å. Additionally, weak π-π stacking interactions occur between the two phenyl rings defined by C9–C14 and C24–C28, with a centroid-to-centroid distance of 3.688(2) Å. These interactions are believed to stabilize the molecular structure.
3.3. Electronic Spectra
The electronic spectra of the present complexes were recorded using the diffuse reflectance technique. The obtained spectra are displayed in Figure 3, and the respective wavenumbers of the absorption bands are listed in Table 4. In the reflection spectrum of 1, three distinct d-d transition bands were observed at approximately 9.92 × 103, 16.6 × 103, and ~22 × 103 (shoulder) cm−1. These transitions can be attributed to 3A2g → 3T2g, 3A2g → 3T1g, and 3A2g → 3T1g(P) transitions originating from the octahedral Ni(II) center [43]. Additionally, there’s an intense band at around 26.0 × 103 cm−1, which is attributed to ligand-to-metal charge transfer (LMCT) originating from either the phenolic or alcoholic oxygen in the ligand to the Ni(II) center. For complex 2, in addition to the d-d bands of Ni(II), three distinct d-d absorption bands are predicted. These bands arise from the octahedral Mn(III) ion with Jahn–Teller distortion and are assigned as 5B1g → 5A1g, 5B1g → 5B2g, and 5B1g → 5Eg transitions [44]. In fact, more than six indistinct absorption bands were observed as shoulders in the reflectance spectrum of 2. Since the coordination environment of Ni(II) is not significantly different between 1 and 2, the three absorption bands at ~10 × 103, ~16 × 103, and ~22 × 103 cm−1 can be assigned to Ni(II) d-d transitions. The other three bands observed at around ~13 × 103, ~19 × 103, and ~20 × 103 cm−1 are likely associated with Mn(III) d-d transitions. The absorption peak at 26 × 103 cm−1 is assigned to LMCT from the ligands to the metal ions.
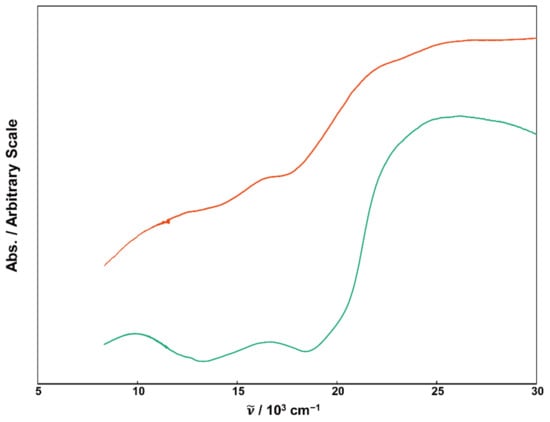
Figure 3.
Reflectance spectra of powder samples for 1 (green), 2 (orange).

Table 4.
Spectral data for d-d bands (/103 cm−1) of 1 and 2.
3.4. Magnetic Properties
Magnetic susceptibility measurements for 1 and 2 were performed with a SQUID (superconducting quantum interference device) magnetometer. The effective magnetic moment of 1 at 300 K is 1.26 cm3 mol−1 K (3.17 μB), which falls within the expected range for mononuclear octahedral Ni(II) complexes.
For complex 2, the temperature dependence of magnetic susceptibility was measured in the temperature range from 2 to 300 K. Figure 4a displays the temperature dependence of direct-current (DC) molar magnetic susceptibilities (χM) and χMT vs. T plots of 2. At 300 K, the χMT value measures 11.18 cm3 mol−1 K, slightly smaller than the expected spin-only value of 11.38 cm3 mol−1 K for a magnetically uncoupled system comprising two high-spin Mn(III) ions (S = 2), one Mn(II) ion (S = 5/2), and one Ni(II) ion (S = 1). As temperature decreases, χMT values increase, reaching a maximum of 24.19 cm3 mol−1 K at 8.0 K before decreasing rapidly to 15.27 cm3 mol−1 K at 2.0 K.
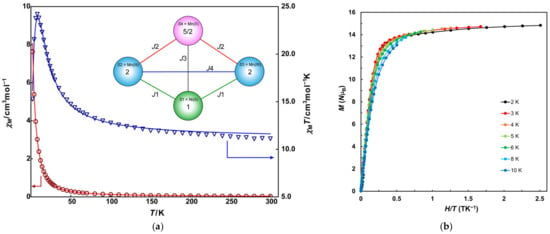
Figure 4.
(a) Temperature dependence of χM (red circles) and χMT (blue triangles) vs. T plots of 2. Solid lines are drawn with the best-fitted parameter values described in the text. Inset figure is magnetic coupling scheme. (b) M vs. H/T plots for 2. Solid lines are guides for the eye.
This magnetic behavior suggests the presence of ferromagnetic coupling between the metal ions. The decline at low temperatures might arise from zero-field splitting (ZFS) or intermolecular antiferromagnetic interaction. The behavior was analyzed using the PHI program based on the magnetic coupling scheme shown in the inset of Figure 4a and spin Hamiltonian (Equation (1)).
H = −2J1(S1S2 + S1S3) − 2J2(S2S4 + S3S4) − 2J3S1S4 − 2J4S2S3
The obtained parameters are as follows: J1 = 2.23 cm−1, J2 = 0.46 cm−1, J3 = 1.78 cm−1, J4 = 0.58 cm−1, gMn(III) = 2.00, gMn(II) = 1.98, gNi = 2.00, zJ′ = −0.0158 cm−1. Comparing these four J values reveals that the interactions between Ni and Mn and J1 and J3 are the main contributions to the magnetic behavior of 3. The average Mn–O–Ni angles, 96.76° for Mn(III)–O–Ni(II) and 97.09° for Mn(II)–O–Ni(II), fall within the 95–98° range known as the crossover angle, indicating ferromagnetic interaction [45,46].
Mn(III) and Ni(II) ions with distorted octahedral geometries often exhibit large ZFS and negative anisotropic D parameters. Although the D parameter may influence the decrease in the χT behavior, its value could not be satisfactorily analyzed with the χT vs. T plots. Magnetization (M) measurements were performed for a more accurate determination of the D parameter. Isothermal magnetization data were collected between 2 and 10 K at applied fields ranging from 0.001 to 5 T. The magnetization approaches saturation at 2 K, with a value of 14.8 NμB, slightly below the expected value of 15 NμB when g ~ 2.0 for S = 15/2 ground state. The M vs. H/T plots for 2, 3, and 4 K largely overlap on a single curve, while deviations are observed above 5 K, as shown in Figure 4b. These results suggest that the magnetic anisotropy D in 2 is notably small. Finally, the D value could not be precisely determined even through analysis of magnetization data (Figure S8).
Frequency-dependent alternating-current (AC) magnetization measurements were performed at frequencies of 1, 10, 100, and 1000 Hz (Figure 5a,b). The measurements without static DC field exhibited subtle out-of-phase (χ″M) signals only. Using an applied magnetic field of 2000 Oe, no alteration was observed in χ’M. However, a more prominent frequency dependence was observed in χ″M below 10 K, indicating field-induced slow magnetic relaxation in magnetization. The energy barrier (Ea) and preexponential factor (τ0) could be roughly estimated using the Debye model through Equation (2) [47].
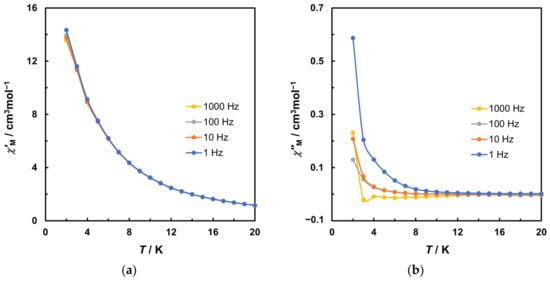
Figure 5.
Variable temperature AC susceptibility data in a 3 Oe AC field oscillating at 1, 10, 100, and 1000 Hz with 2000 Oe DC static field for 2. (a) Plot of in-phase (χ’M) signal. (b) Plot of out-of-phase (χ″M) signal. Solid lines are guides for the eye.
The calculated results provide Ea values ranging from 6.13 to 9.45 cm−1 and τ0 values between 9.44 × 10−7 and 7.46 × 10−4 s for 2 under 2000 Oe DC field (Figure 6), corresponding to relatively slow magnetic relaxation, similar to metal complexes reported as weak SMMs [2,48].
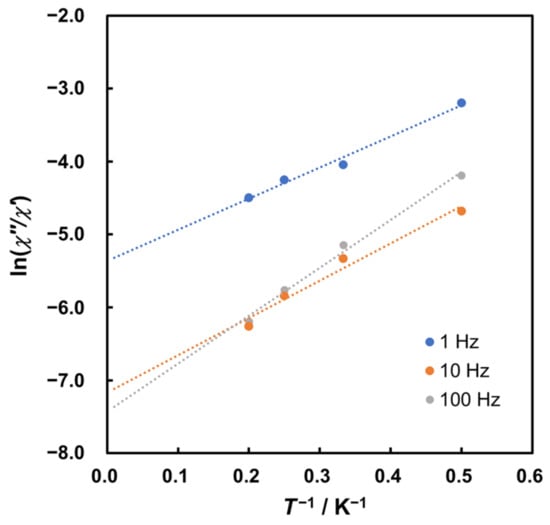
Figure 6.
Plots of ln(χ″/χ′) versus 1/T for 2 under 2000 Oe DC field. Dotted lines represent linear approximation.
4. Conclusions
The new mononuclear complex, [Ni(HL1)2] (1), was synthesized via simple reactions between metal sources and the Schiff base ligand. The novel heteronuclear complex, [Mn3Ni(L1)4Cl2(EtOH)2] (2), was obtained through a stepwise synthesis using 1 as a complex ligand. The crystal structures of these complexes were elucidated through single-crystal X-ray analysis. Complex 1 represents the first example of a mononuclear complex with fac-coordinated H2L1 ligands. The tetranuclear complex 2 features a unique Mn(II)Mn(III)2Ni(II) metal core connected by two μ3-alkoxo, two μ2-alkoxo, and two μ2-phenoxo bridges. Investigations into the magnetic properties of 2 through DC magnetic measurements revealed the presence of dominant ferromagnetic interactions among all metal ions, resulting in an S = 15/2 ground state. Additionally, 2 exhibits frequency-dependent out-of-phase signals below 10 K in AC studies, indicating relatively slow magnetic relaxation in magnetization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/magnetochemistry9110225/s1, Figure S1. IR spectrum of H2L1 (ATR). Figure S2. IR spectrum of 1 (ATR). Figure S3. The PXRD pattern (blue) and the simulation (orange) for 1. Figure S4. IR spectrum of 2 (ATR). Figure S5. The PXRD pattern (blue) and the simulation (orange) for 2. Figure S6. Molecular packing diagram of 1. Figure S7. Molecular packing diagram of 2. Figure S8. The plots of M–H for 2. The line represents the fitting results by PHI. Table S1. Crystal data and structure refinement for 1. Table S2. Bond lengths for 1. Table S3. Bond angles for 1. Table S4. Hydrogen bonds for 1. Table S5. Crystal data and structure refinement for 2. Table S6. Bond lengths for 2. Table S7. Bond angles for 2. Table S8. Hydrogen bonds for 2.
Author Contributions
Conceptualization, M.K.; Data Curation, Y.Y.; Formal Analysis, M.F., K.E., K.M., K.Y., H.Y., Y.I., N.Y. and M.O.; Funding Acquisition, M.K.; Investigation, M.F. and K.E.; Project Administration, M.K.; Software, M.K.; Supervision, M.K.; Visualization, M.K.; Writing—Original Draft, M.K.; Writing—Review and Editing, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by JSPS KAKENHI Grant Number JP26410075 from the Japan Society for the Promotion of Science.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the author upon reasonable request.
Acknowledgments
This research was performed using the equipment (IR and UV-NIR spectroscopy, fluorescent X-ray analysis, single crystal X-ray analysis, a part of DC-SQUID measurement) of Analytical Research Center for Experimental Science, Saga University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kamilya, S.; Mehta, S.; Semwal, M.; Lescouëzec, R.; Li, Y.; Pechousek, J.; Reddy, V.R.; Rivière, E.; Rouzières, M.; Mondal, A. ON/OFF Photo(switching) along with reversible spin-state change and single-crystal-to-single-crystal transformation in a mixed-valence Fe(II)Fe(III) molecular system. Inorg. Chem. 2023, 62, 8794–8802. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, L.H.G.; Rabelo, R.; Valdo, A.K.; Martins, F.T.; Moliner, N.; Ferrando-Soria, J.; Julve, M.; Lloret, F.; Cano, J.; Cangussu, D. Trinuclear Cobalt(II) Triple Helicate with a Multidentate Bithiazolebis(oxamate) Ligand as a Supramolecular Nanomagnet. Inorg. Chem. 2022, 61, 5696–5700. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-F.; Wang, Z.; Chen, X.-L.; Kurmoo, M.; Zeng, M.-H. Ligand effect on the single-molecule magnetism of tetranuclear Co(II) cubane. Inorg. Chem. 2017, 56, 15178–15186. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Aromi, G.; Teat, S.J.; Ribas-Ariño, J.; Gamez, P.; Reedijk, J. Hydrogen bond assisted co-crystallization of a bimetallic MnIII2NiII2 cluster and a NiII2 cluster unit: Synthesis, structure, spectroscopy and magnetism. Dalton Trans. 2010, 39, 4986–4990. [Google Scholar] [CrossRef] [PubMed]
- Oshio, H.; Hoshino, N.; Ito, T.; Nakano, M. Single-molecule of ferrous cubes: Structurally controlled magnetic anisotropy. J. Am. Chem. Soc. 2004, 126, 8805–8812. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Marino, N.; Armentano, D.; Mastropietro, T.F.; Julve, M.; Munno, G.D.; Martínez-Lillo, J. Cubane-type CuII4 and MnII2MnIII2 complexes based on pyridoxine: A versatile ligand for metal assembling. Inorg. Chem. 2013, 52, 11934–11943. [Google Scholar] [CrossRef]
- Hoshikawa, R.; Mitsuhashi, R.; Asato, E.; Liu, J.; Sakiyama, H. Structures of Dimer-of-Dimers Type Defect Cubane Tetranuclear Copper(II) Complexes with Novel Dinucleating Ligands. Molecules 2022, 27, 576–589. [Google Scholar] [CrossRef]
- Wang, H.-S.; Song, Y. Conversion of tetranuclear Ni complexes from a defect dicubane core to a [Ni4O4] cubane-like core via addition of 2-hydroxymethylpyridine: Synthesis, crystal structures, and magnetic properties. Inorg. Chem. Commun. 2013, 35, 86–88. [Google Scholar] [CrossRef]
- Berg, N.; Rajeshkumar, T.; Taylor, S.M.; Brechin, E.K.; Rajaraman, G.; Jones, L.F. What controls the magnetic interaction in bis-μ-alkoxo MnIII dimers? A combined experimental and theoretical exploration. Chem. Eur. J. 2012, 18, 5906–5918. [Google Scholar] [CrossRef]
- Yao, B.; Singh, M.K.; Deng, Y.-F.; Zhang, Y.-Z. A dicobalt(II) single-molecule magnet via a well-designed dual-capping tetrazine radical ligand. Inorg. Chem. 2021, 60, 18698–18705. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Piligkos, S.; Brechin, E.K. Ground state spin-switching via targeted structural distortion: Twisted single-molecule magnets from derivatised salicylaldoximes. Dalton Trans. 2008, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Colacio, E.; Ruiz-Sanchez, J.; White, F.J.; Brechin, E.K. Strategy for the rational design of asymmetric triply bridged dinuclear 3d-4f single-molecule magnets. Inorg. Chem. 2011, 50, 7268–7273. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.S.; Bazhenova, T.A.; Manakin, Y.V.; Yagubskii, E.B. Pentagonal-bipyramidal 4d and 5d complexes with unquenched orbital angular momentum as a unique platform for advanced single-molecule magnets: Current state and perspectives. Dalton Trans. 2023, 52, 509–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Avendañoa, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar] [CrossRef] [PubMed]
- Vostrikova, K.E. Application of the heptacyanidorhenate(IV) as a metalloligand in the design of molecular magnets. Magnetochemistry 2022, 8, 189. [Google Scholar] [CrossRef]
- Kobylarczyk, J.; Pakulski, P.; Potępa, I.; Podgajny, R. Manipulation of the cyanido-bridged Fe2W2 rhombus in the crystalline state: Co-crystallization, desolvation and thermal treatment. Polyhedron 2022, 224, 116028. [Google Scholar] [CrossRef]
- Zhao, X.-Q.; Zhao, B.; Wei, S.; Cheng, P. Synthesis, structures, and luminescent and magnetic properties of Ln-Ag heterometal-organic frameworks. Inorg. Chem. 2009, 48, 11048–11057. [Google Scholar] [CrossRef]
- Dopp, C.M.; Golwankar, R.R.; Kelsey, S.R.; Douglas, J.T.; Erickson, A.N.; Oliver, A.G.; Day, C.S.; Day, V.W.; Blakemore, J.D. Vanadyl as a spectroscopic probe of tunable ligand donor strength in bimetallic complexes. Inorg. Chem. 2023, 62, 9827–9843. [Google Scholar] [CrossRef]
- Honda, H.; Yoshimura, T.; Hirotsu, M.; Kawamoto, T.; Konno, T. Synthesis, characterization, and chiral behavior of S-bridged CoIIIPtIICoIII trinuclear complexes composed of bis(thiolato)-type octahedral units cis(S)-[Co(aet)2(en)]+ and/or trans(N)-[Co(D-pen-N,O,S)2]− (aet = 2-aminoethanethiolate, D-pen = D-penicillaminate). Inorg. Chem. 2002, 41, 2229–2237. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Wang, J.; Bai, F.; Ma, Y.; Li, L.; Wang, Q.; Zhao, B.; Cheng, P. The different magnetic relaxation behaviors in [Fe(CN)6]3− or [Co(CN)6]3− bridged 3d-4f heterometallic compounds. CrystEngComm 2020, 22, 2998–3004. [Google Scholar] [CrossRef]
- Bencini, A.; Benelli, C.; Dei, A.; Gatteschi, D. EPR spectra of and exchange interactions in trinuclear complexes. 3. Synthesis, crystal structure, and magnetic properties of the oxovanadium(IV) adduct of a tetradentate Schiff base copper(II) complex. Inorg. Chem. 1985, 24, 695–699. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Wang, H.-Y.; Shi, D.-Q.; Tian, J.-L. Synthesis and crystal structure of a tetranuclear cubane-like nickel(II) complex with 2-hydroxymethyl-N-salicylideneaniline. Chin. J. Chem. 2006, 24, 518–520. [Google Scholar] [CrossRef]
- Koikawa, M.; Ohba, M.; Tokii, T. Syntheses, structures, and magnetic properties of tetranuclear NiII4 and NiII2MnIII2 complexes with ONO tridentate ligands. Polyhedron 2005, 24, 2257–2262. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Wagner, R.; Weyhermüller, T. An oximate-free ferromagnetically coupled triangular [MnIII3(μ3-O)]7+ core. Eur. J. Inorg. Chem. 2010, 2010, 1339–1342. [Google Scholar] [CrossRef]
- Suemitsu, Y.; Matsunaga, R.; Toyofuku, T.; Yamada, Y.; Mikuriya, M.; Tokii, T.; Koikawa, M. Tetranuclear hetero-metal [MnIII2NiII2] complexes involving defective double-cubane structure: Synthesis, crystal structures, and magnetic properties. Magnetochemistry 2019, 5, 14–24. [Google Scholar] [CrossRef]
- Yoshitake, M.; Nishihashi, M.; Ogata, Y.; Yoneda, K.; Yamada, Y.; Sakiyama, H.; Mishima, A.; Ohba, M.; Koikawa, M. Syntheses, structures, and magnetic properties of cubane-based cobalt and nickel complexes with ONO-tridentate ligands. Polyhedron 2017, 136, 136–142. [Google Scholar] [CrossRef]
- Nath, M.; Yadav, R. Synthesis, spectral and thermal studies of Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) complexes of Schiff bases derived from o-aminobenzyl alcohol. Synth. React. Inorg. Met.-Org. Chem. 1995, 25, 1529–1547. [Google Scholar] [CrossRef]
- Selwood, P.W. Magnetochemistry; Interscience Publishers: New York, NY, USA, 1956; pp. 78–91. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Rigaku Corporation. CrystalClear: Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 1998. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomano, V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Muto, M.; Nakagawa, N.; Koikawa, M.; Tokii, T. Bis{2-[2-(hydroxymethyl)phenyliminomethyl]phenolato}cobalt(III) nitrate monohydrate. Acta Crystallogr. 2007, E63, m64–m66. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE; Version 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model, 2nd ed.; Oxford University Press: Oxford, UK, 2002; ISBN 9780198742951. [Google Scholar]
- Gagne, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. 2015, B71, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D. Accumulated Table of Bond Valence Parameters. 2020. Available online: https://www.iucr.org/__data/assets/file/0011/150779/bvparm2020.cif (accessed on 25 September 2023).
- Feng, P.L.; Beedle, C.C.; Wernsdorfer, W.; Koo, C.; Nakano, M.; Hill, S.; Hendrickson, D.N. Heterometallic cubane single-molecule magnets. Inorg. Chem. 2007, 46, 8126–8128. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, M.; Vadivelu, P.; Suresh, E.; Palaniandavar, M. Mixed ligand nickel(II) complexes as catalysts for alkane hydroxylation using m-chloroperbenzoic acid as oxidant. Inorg. Chim. Acta 2013, 407, 98–107. [Google Scholar] [CrossRef]
- Ghosh, S.; Bagchi, S.; Das, M.; Kamilya, S.; Mondal, A. Stepwise spin-state switching in a manganese(III) complex. Dalton Trans. 2020, 49, 14776–14780. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publications: New York, NY, USA, 1993; pp. 145–210. ISBN 1-56081-566-3. [Google Scholar]
- Sasmal, S.; Roy, S.; Carrella, L.; Jana, A.; Rentschler, E.; Mohanta, S. Structures and magnetic properties of bis(μ-phenoxido), bis(μ-phenoxido)-μ-carboxylato and bis(μ-phenoxido)bis(μ-carboxylato) FeIIINiII compounds—Magnetostructural correlations. Eur. J. Inorg. Chem. 2015, 2015, 680–689. [Google Scholar] [CrossRef]
- Bartolomé, J.; Filoti, G.; Kuncser, V.; Schinteie, G.; Mereacre, V.; Anson, C.E.; Powell, A.K.; Prodius, D.; Turta, C. Magnetostructural correlations in the tetranuclear series of {Fe3LnO2} butterfly core clusters: Magnetic and Mössbauer spectroscopic study. Phys. Rev. 2009, B80, 014430–014446. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Bag, R.; Satpathi, S.; Pati, S.K.; Butcher, R.J.; Tang, J.; Goswami, S. Structure and magnetism of LnIII2 (Ln = Gd, Tb, Dy, and Ho) assemblies constructed from a bis(hydrazone) compartmental ligand: Slow magnetic relaxation in the DyIII2 analogue. Cryst. Growth. Des. 2023, 33, 7459–7471. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).