Variability of the Conductance Changes Associated with the Change in the Spin State in Molecular Spin Crossover Complexes
Abstract
:1. Introduction
2. Variability of Conductance Change
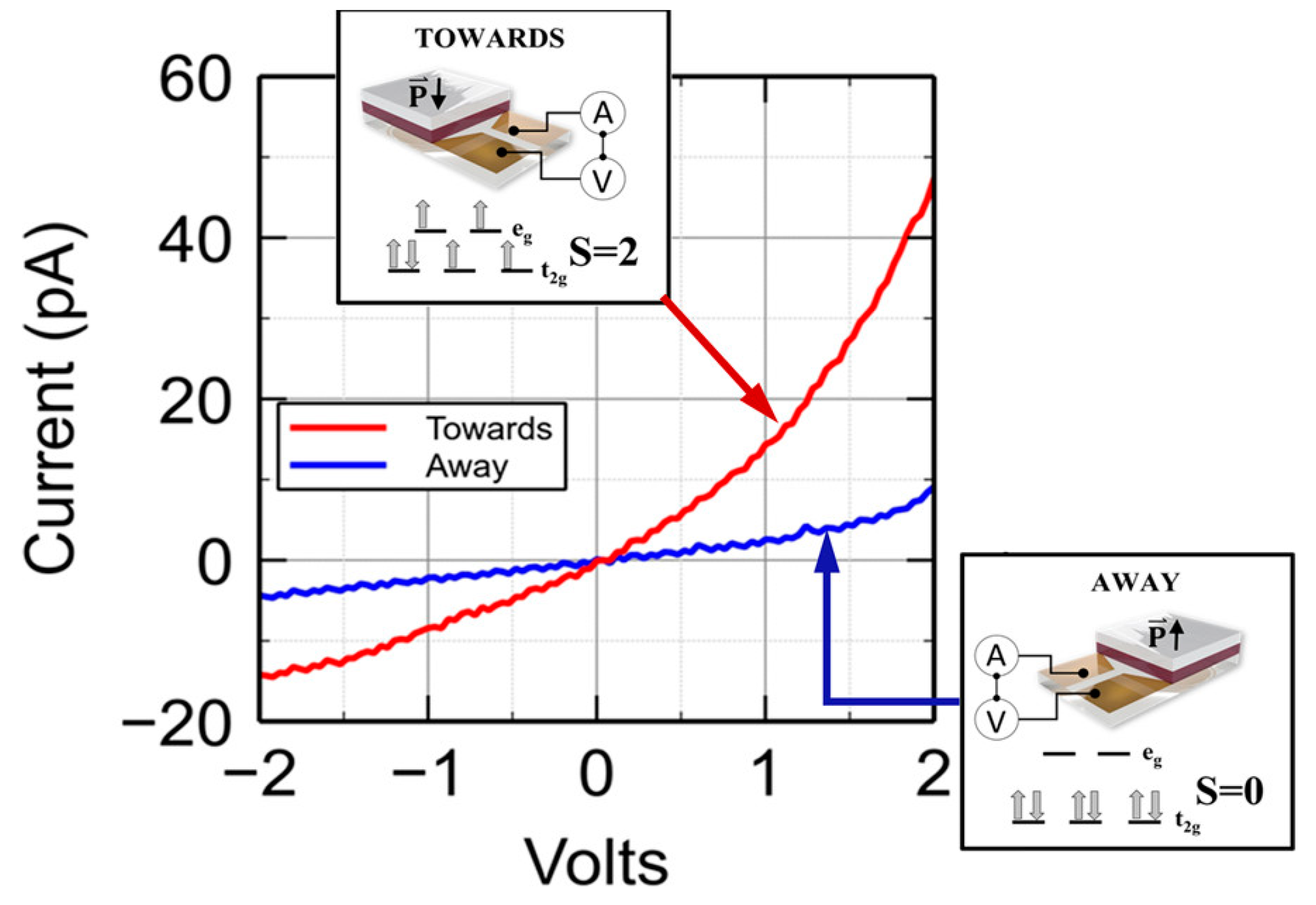
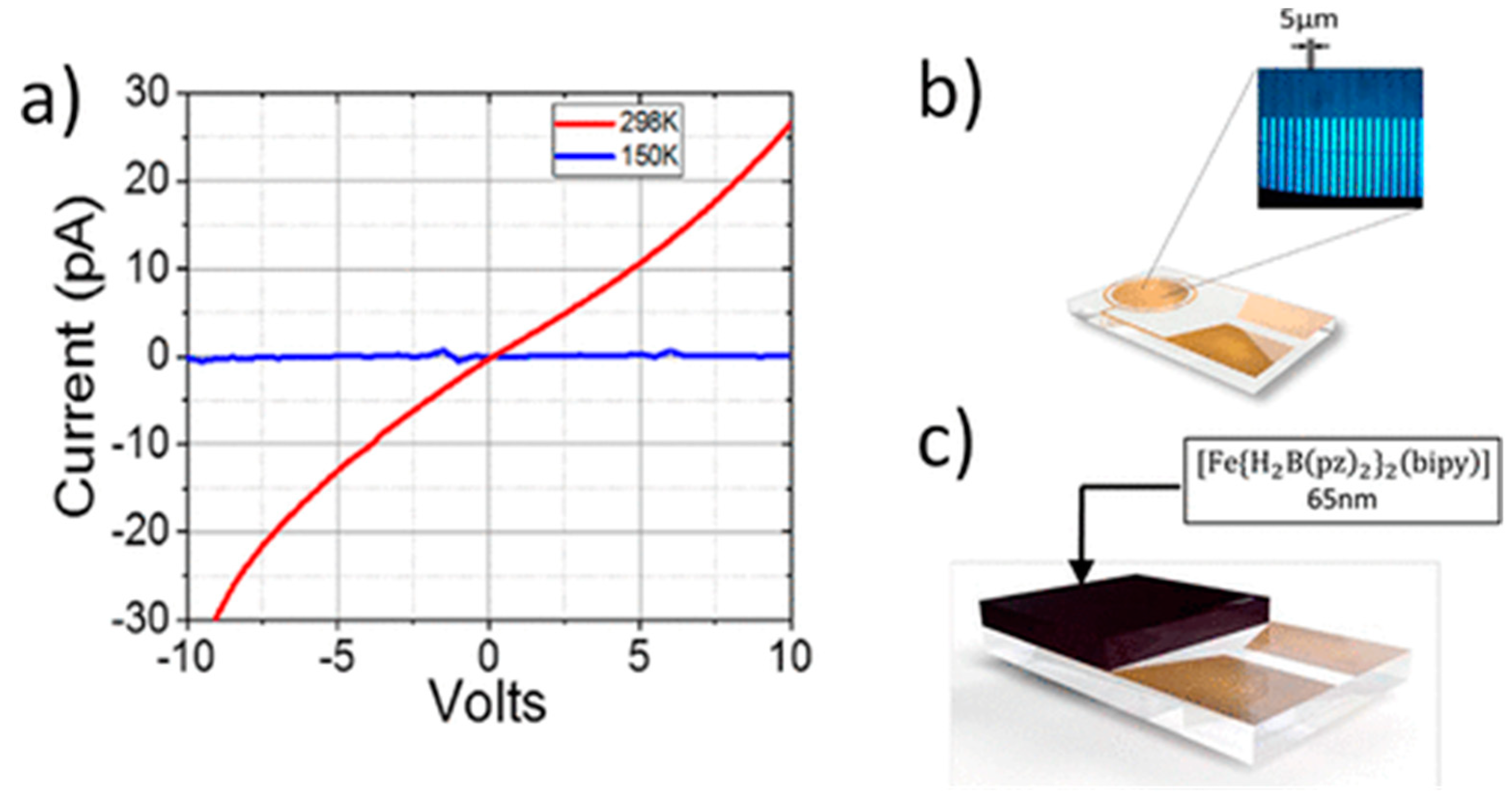
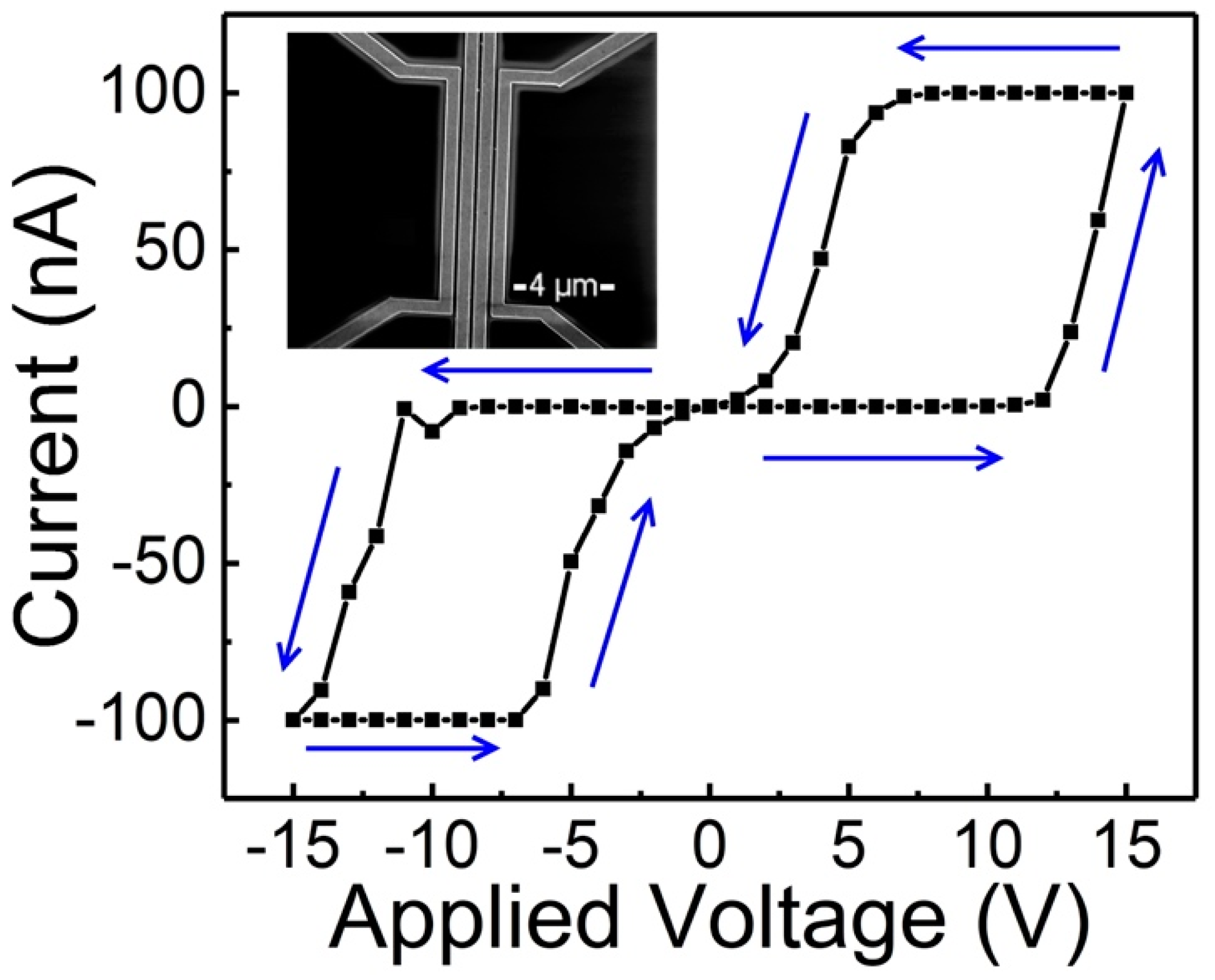
2.1. Thermal- versus Voltage-Controlled Switching
2.2. The Effect of the Device Structure and Underlying Substrate
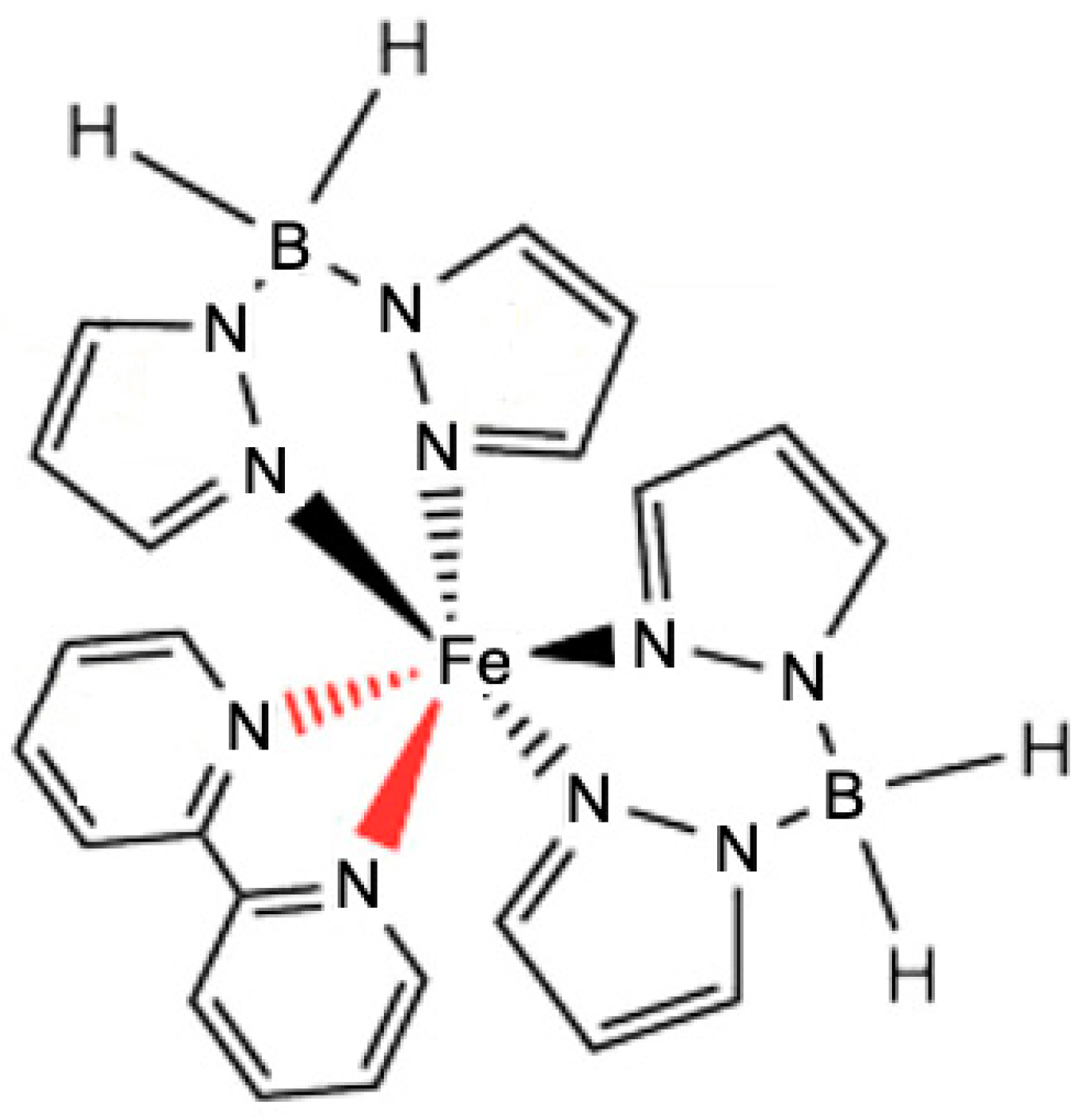
3. Influence of Additives
4. The Conductance Change Mechanism
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds I; Springer: Berlin/Heidelberg, Germany, 2004; Volume 233, ISBN 978-3-540-40394-4. [Google Scholar]
- Létard, J.-F.; Guionneau, P.; Goux-Capes, L. Towards Spin Crossover Applications. In Spin Crossover in Transition Metal Compounds III; Spinger: Berlin/Heidelberg, Germany, 2004; Volume 235, pp. 221–249. ISBN 978-3-540-40395-1. [Google Scholar]
- Gütlich, P. Spin Crossover—Quo Vadis? Eur. J. Inorg. Chem. 2013, 2013, 581–591. [Google Scholar] [CrossRef]
- Halcrow, M.A. (Ed.) Spin-Crossover Materials: Properties and Applications; John Wiley & Sons: Chichester, UK, 2013; ISBN 978-1-118-51931-8. [Google Scholar]
- Senthil Kumar, K.; Ruben, M. Emerging Trends in Spin Crossover (SCO) Based Functional Materials and Devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular Spin Crossover Phenomenon: Recent Achievements and Prospects. Chem. Soc. Rev. 2011, 40, 3313. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, T.K.; Hao, G.; Mosey, A.; Dale, A.S.; Jiang, X.; Yost, A.J.; Sapkota, K.R.; Wang, G.T.; Zhang, J.; N’Diaye, A.T.; et al. Nonvolatile Voltage Controlled Molecular Spin-State Switching for Memory Applications. Magnetochemistry 2021, 7, 37. [Google Scholar] [CrossRef]
- Ekanayaka, T.K.; Maity, K.; Doudin, B.; Dowben, P.A. Dynamics of Spin Crossover Molecular Complexes. Nanomaterials 2022, 12, 1742. [Google Scholar] [CrossRef]
- Chorazy, S.; Charytanowicz, T.; Pinkowicz, D.; Wang, J.; Nakabayashi, K.; Klimke, S.; Renz, F.; Ohkoshi, S.-I.; Sieklucka, B. Octacyanidorhenate (V) Ion as an Efficient Linker for Hysteretic Two-Step Iron (II) Spin Crossover Switchable by Temperature, Light, and Pressure. Angew. Chem. Int. Ed. 2020, 59, 15741–15749. [Google Scholar] [CrossRef] [PubMed]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C. Thermal, Pressure and Light Switchable Spin-Crossover Materials. Dalton Trans. 2005, 12, 2062–2079. [Google Scholar] [CrossRef]
- Galet, A.; Gaspar, A.B.; Agusti, G.; Muñoz, M.C.; Levchenko, G.; Real, J.A. Pressure effect investigations on the spin crossover systems {Fe[H2B(pz)2]2(bipy)} and {Fe[H2B(pz)2]2(phen)}. Eur. J. Inorg. Chem. Short Commun. 2006, 2006, 3571–3573. [Google Scholar] [CrossRef]
- Orlov, Y.S.; Nikolaev, S.V.; Ovchinnikov, S.G. Magnetic Properties and Spin Crossover in Transition Metal Oxides with D5 Ions at High Pressures. J. Exp. Theor. Phys. 2019, 129, 1062–1069. [Google Scholar] [CrossRef]
- Rotaru, A.; Gural’skiy, I.A.; Molnár, G.; Salmon, L.; Demont, P.; Bousseksou, A. Spin State Dependence of Electrical Conductivity of Spin Crossover Materials. Chem. Commun. 2012, 48, 4163–4165. [Google Scholar] [CrossRef]
- Mosey, A.; Dale, A.S.; Hao, G.; N’Diaye, A.; Dowben, P.A.; Cheng, R. Quantitative Study of the Energy Changes in Voltage-Controlled Spin Crossover Molecular Thin Films. J. Phys. Chem. Lett. 2020, 11, 8231–8237, Correct in J. Phys. Chem. Lett. 2021, 12, 2463. [Google Scholar] [CrossRef]
- Ruiz, E. Charge Transport Properties of Spin Crossover Systems. Phys. Chem. Chem. Phys. 2014, 16, 14–22. [Google Scholar] [CrossRef]
- Mishra, E.; Ekanayaka, T.K.; McElveen, K.A.; Lai, R.Y.; Dowben, P.A. Evidence for Long Drift Carrier Lifetimes in [Fe(Htrz)2(trz)](BF4)] plus Polyaniline Composites. Org. Electron. 2022, 105, 106516. [Google Scholar] [CrossRef]
- Hao, G.; Mosey, A.; Jiang, X.; Yost, A.J.; Sapkota, K.R.; Wang, G.T.; Zhang, X.; Zhang, J.; N’Diaye, A.T.; Cheng, R.; et al. Nonvolatile Voltage Controlled Molecular Spin State Switching. Appl. Phys. Lett. 2019, 114, 032901. [Google Scholar] [CrossRef]
- Miyamachi, T.; Gruber, M.; Davesne, V.; Bowen, M.; Boukari, S.; Joly, L.; Scheurer, F.; Rogez, G.; Yamada, T.K.; Ohresser, P.; et al. Robust Spin Crossover and Memristance across a Single Molecule. Nat. Commun. 2012, 3, 938. [Google Scholar] [CrossRef] [PubMed]
- Gopakumar, T.G.; Matino, F.; Naggert, H.; Bannwarth, A.; Tuczek, F.; Berndt, R. Electron-Induced Spin Crossover of Single Molecules in a Bilayer on Gold. Angew. Chem. Int. Ed. 2012, 51, 6262–6266. [Google Scholar] [CrossRef] [PubMed]
- Faulmann, C.; Jacob, K.; Dorbes, S.; Lampert, S.; Malfant, I.; Doublet, M.-L.; Valade, L.; Real, J.A. Electrical Conductivity and Spin Crossover: A New Achievement with a Metal Bis Dithiolene Complex. Inorg. Chem. 2007, 46, 8548–8559. [Google Scholar] [CrossRef]
- Frisenda, R.; Harzmann, G.D.; Celis Gil, J.A.; Thijssen, J.M.; Mayor, M.; van der Zant, H.S.J. Stretching-Induced Conductance Increase in a Spin-Crossover Molecule. Nano Lett. 2016, 16, 4733–4737. [Google Scholar] [CrossRef]
- Shvachko, Y.N.; Starichenko, D.V.; Korolyov, A.V.; Yagubskii, E.B.; Kotov, A.I.; Buravov, L.I.; Lyssenko, K.A.; Zverev, V.N.; Simonov, S.V.; Zorina, L.V.; et al. The Conducting Spin-Crossover Compound Combining Fe(II) Cation Complex with TCNQ in a Fractional Reduction State. Inorg. Chem. 2016, 55, 9121–9130. [Google Scholar] [CrossRef]
- Rubio-Giménez, V.; Tatay, S.; Martí-Gastaldo, C. Electrical Conductivity and Magnetic Bistability in Metal–Organic Frameworks and Coordination Polymers: Charge Transport and Spin Crossover at the Nanoscale. Chem. Soc. Rev. 2020, 49, 5601–5638. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.-Y.; Ishikawa, R.; Yamashita, M. Spin Crossover and Valence Tautomerism Conductors. Coord. Chem. Rev. 2021, 435, 213819. [Google Scholar] [CrossRef]
- Takahashi, K.; Cui, H.-B.; Okano, Y.; Kobayashi, H.; Einaga, Y.; Sato, O. Electrical Conductivity Modulation Coupled to a High-Spin−Low-Spin Conversion in the Molecular System [FeIII(Qsal)2][Ni(Dmit)2]3·CH3CN·H2O. Inorg. Chem. 2006, 45, 5739–5741. [Google Scholar] [CrossRef] [PubMed]
- Lefter, C.; Rat, S.; Costa, J.S.; Manrique-Juárez, M.D.; Quintero, C.M.; Salmon, L.; Séguy, I.; Leichle, T.; Nicu, L.; Demont, P.; et al. Current Switching Coupled to Molecular Spin-States in Large-Area Junctions. Adv. Mater. 2016, 28, 7508–7514. [Google Scholar] [CrossRef] [PubMed]
- Shalabaeva, V.; Ridier, K.; Rat, S.; Manrique-Juarez, M.D.; Salmon, L.; Séguy, I.; Rotaru, A.; Molnár, G.; Bousseksou, A. Room Temperature Current Modulation in Large Area Electronic Junctions of Spin Crossover Thin Films. Appl. Phys. Lett. 2018, 112, 013301. [Google Scholar] [CrossRef]
- Poggini, L.; Gonidec, M.; Canjeevaram Balasubramanyam, R.K.; Squillantini, L.; Pecastaings, G.; Caneschi, A.; Rosa, P. Temperature-Induced Transport Changes in Molecular Junctions Based on a Spin Crossover Complex. J. Mater. Chem. C 2019, 7, 5343–5347. [Google Scholar] [CrossRef]
- Poggini, L.; Gonidec, M.; González-Estefan, J.H.; Pecastaings, G.; Gobaut, B.; Rosa, P. Vertical Tunnel Junction Embedding a Spin Crossover Molecular Film. Adv. Electron. Mater. 2018, 4, 1800204. [Google Scholar] [CrossRef]
- Schleicher, F.; Studniarek, M.; Kumar, K.S.; Urbain, E.; Katcko, K.; Chen, J.; Frauhammer, T.; Hervé, M.; Halisdemir, U.; Kandpal, L.M.; et al. Linking Electronic Transport through a Spin Crossover Thin Film to the Molecular Spin State Using X-Ray Absorption Spectroscopy Operando Techniques. ACS Appl. Mater. Interfaces 2018, 10, 31580–31585. [Google Scholar] [CrossRef]
- Rotaru, A.; Dugay, J.; Tan, R.P.; Guralskiy, I.A.; Salmon, L.; Demont, P.; Carrey, J.; Molnár, G.; Respaud, M.; Bousseksou, A. Nano-Electromanipulation of Spin Crossover Nanorods: Towards Switchable Nanoelectronic Devices. Adv. Mater. 2013, 25, 1745–1749. [Google Scholar] [CrossRef]
- Dugay, J.; Giménez-Marqués, M.; Kozlova, T.; Zandbergen, H.W.; Coronado, E.; van der Zant, H.S.J. Spin Switching in Electronic Devices Based on 2D Assemblies of Spin-Crossover Nanoparticles. Adv. Mater. 2015, 27, 1288–1293. [Google Scholar] [CrossRef]
- Torres-Cavanillas, R.; Sanchis-Gual, R.; Dugay, J.; Coronado-Puchau, M.; Giménez-Marqués, M.; Coronado, E. Design of Bistable Gold@Spin-Crossover Core–Shell Nanoparticles Showing Large Electrical Responses for the Spin Switching. Adv. Mater. 2019, 31, 1900039. [Google Scholar] [CrossRef]
- Prins, F.; Monrabal-Capilla, M.; Osorio, E.A.; Coronado, E.; van der Zant, H.S.J. Room-Temperature Electrical Addressing of a Bistable Spin-Crossover Molecular System. Adv. Mater. 2011, 23, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Dugay, J.; Aarts, M.; Giménez-Marqués, M.; Kozlova, T.; Zandbergen, H.W.; Coronado, E.; van der Zant, H.S.J. Phase Transitions in Spin-Crossover Thin Films Probed by Graphene Transport Measurements. Nano Lett. 2017, 17, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Holovchenko, A.; Dugay, J.; Giménez-Marqués, M.; Torres-Cavanillas, R.; Coronado, E.; van der Zant, H.S.J. Near Room-Temperature Memory Devices Based on Hybrid Spin-Crossover SiO2 Nanoparticles Coupled to Single-Layer Graphene Nanoelectrodes. Adv. Mater. 2016, 28, 7228–7233. [Google Scholar] [CrossRef]
- Zhang, Y.; Séguy, I.; Ridier, K.; Shalabaeva, V.; Piedrahita-Bello, M.; Rotaru, A.; Salmon, L.; Molnár, G.; Bousseksou, A. Resistance Switching in Large-Area Vertical Junctions of the Molecular Spin Crossover Complex [Fe(HB(Tz)3)2]: ON/OFF Ratios and Device Stability. J. Phys. Condens. Matter 2020, 32, 214010. [Google Scholar] [CrossRef] [PubMed]
- Etrillard, C.; Faramarzi, V.; Dayen, J.-F.; Letard, J.-F.; Doudin, B. Photoconduction in [Fe(Htrz)2(trz)](BF4)].H2O Nanocrystals. Chem. Commun. 2011, 47, 9663. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Meng, Y.; Ni, Z.-P.; Tong, M.-L. Synergistic Electrical Bistability in a Conductive Spin Crossover Heterostructure. J. Mater. Chem. C 2015, 3, 945–949. [Google Scholar] [CrossRef]
- Tanaka, D.; Aketa, N.; Tanaka, H.; Horike, S.; Fukumori, M.; Tamaki, T.; Inose, T.; Akai, T.; Toyama, H.; Sakata, O.; et al. Facile Preparation of Hybrid Thin Films Composed of Spin-Crossover Nanoparticles and Carbon Nanotubes for Electrical Memory Devices. Dalton Trans. 2019, 48, 7074–7079. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Domanov, O.; Schafler, E.; Vejpravova, J.; Shiozawa, H. Synthesis and Size-Dependent Spin Crossover of Coordination Polymer [Fe(Htrz)2(trz)](BF4)]. J. Mater. Chem. C 2021, 9, 1077–1084. [Google Scholar] [CrossRef]
- Lefter, C.; Gural’skiy, I.A.; Peng, H.; Molnár, G.; Salmon, L.; Rotaru, A.; Bousseksou, A.; Demont, P. Dielectric and Charge Transport Properties of the Spin Crossover Complex [Fe(Htrz)2(trz)](BF4)]. Phys. Status Solidi—Rapid Res. Lett. (RRL) 2014, 8, 191–193. [Google Scholar] [CrossRef]
- Ekanayaka, T.K.; Üngör, Ö.; Hu, Y.; Mishra, E.; Phillips, J.P.; Dale, A.S.; Yazdani, S.; Wang, P.; McElveen, K.A.; Zaz, M.Z.; et al. Perturbing the Spin State and Conduction of Fe (II) Spin Crossover Complexes with TCNQ. Mater. Chem. Phys. 2023, 296, 127276. [Google Scholar] [CrossRef]
- Amin, N.A.A.M.; Said, S.M.; Salleh, M.F.M.; Afifi, A.M.; Ibrahim, N.M.J.N.; Hasnan, M.M.I.M.; Tahir, M.; Hashim, N.Z.I. Review of Fe-Based Spin Crossover Metal Complexes in Multiscale Device Architectures. Inorganica Chim. Acta 2023, 544, 121168. [Google Scholar] [CrossRef]
- Nieto-Castro, D.; Garcés-Pineda, F.A.; Moneo-Corcuera, A.; Sánchez-Molina, I.; Galán-Mascarós, J.R. Mechanochemical Processing of Highly Conducting Organic/Inorganic Composites Exhibiting Spin Crossover–Induced Memory Effect in Their Transport Properties. Adv. Funct. Mater. 2021, 31, 2102469. [Google Scholar] [CrossRef]
- Üngör, Ö.; Choi, E.S.; Shatruk, M. Optimization of Crystal Packing in Semiconducting Spin-Crossover Materials with Fractionally Charged TCNQδ− Anions (0 < δ < 1). Chem. Sci. 2021, 12, 10765–10779. [Google Scholar] [CrossRef]
- Shvachko, Y.N.; Starichenko, D.V.; Korolyov, A.V.; Kotov, A.I.; Buravov, L.I.; Zverev, V.N.; Simonov, S.V.; Zorina, L.V.; Yagubskii, E.B. The highly conducting spincrossover compound combining Fe(III) cation complex with TCNQ in a fractional reduction state. Synthesis, structure, electric and magnetic properties. Magnetochemistry 2017, 3, 9. [Google Scholar] [CrossRef]
- Phan, H.; Benjamin, S.M.; Steven, E.; Brooks, J.S.; Shatruk, M. Photomagnetic Response in Highly Conductive Iron(II) Spin-Crossover Complexes with TCNQ Radicals. Angew. Chem. 2015, 127, 837–841. [Google Scholar] [CrossRef]
- Yazdani, S.; Collier, K.; Yang, G.; Phillips, J.; Dale, A.; Mosey, A.; Grocki, S.; Zhang, J.; Shanahan, A.E.; Cheng, R.; et al. Optical Characterization of Isothermal Spin State Switching in an Fe (II) Spin Crossover Molecular and Polymer Ferroelectric Bilayer. J. Phys. Condens. Matter 2023, 35, 365401. [Google Scholar] [CrossRef]
- Costa, P.; Hao, G.; N’Diaye, A.T.; Routaboul, L.; Braunstein, P.; Zhang, X.; Zhang, J.; Doudin, B.; Enders, A.; Dowben, P.A. Perturbing the Spin Crossover Transition Activation Energies in [Fe(H2B(pz)2)2(bipy)] with Zwitterionic Additions. J. Phys. Condens. Matter 2018, 30, 305503. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.S.; Hao, G.; N’Diaye, A.T.; Routaboul, L.; Braunstein, P.; Zhang, X.; Zhang, J.; Ekanayaka, T.K.; Shi, Q.-Y.; Schlegel, V.; et al. Manipulation of the Molecular Spin Crossover Transition of [Fe(H2B(pz)2)2(bipy)] by Addition of Polar Molecules. J. Phys. Condens. Matter 2020, 32, 034001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Costa, P.S.; Hooper, J.; Miller, D.P.; N’Diaye, A.T.; Beniwal, S.; Jiang, X.; Yin, Y.; Rosa, P.; Routaboul, L.; et al. Locking and Unlocking the Molecular Spin Crossover Transition. Adv. Mater. 2017, 29, 1702257. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, S.; Chastanet, G.; Daro, N.; Palamarciuc, T.; Rosa, P.; Létard, J.-F.; Liu, J.; Sterbinsky, G.E.; Arena, D.A.; et al. Complexities in the Molecular Spin Crossover Transition. J. Phys. Chem. C 2015, 119, 16293–16302. [Google Scholar] [CrossRef]
- Zhang, X.; Palamarciuc, T.; Rosa, P.; Létard, J.-F.; Doudin, B.; Zhang, Z.; Wang, J.; Dowben, P.A. Electronic Structure of a Spin Crossover Molecular Adsorbate. J. Phys. Chem. C 2012, 116, 23291–23296. [Google Scholar] [CrossRef]
- Moliner, N.; Salmon, L.; Capes, L.; Muñoz, M.C.; Létard, J.-F.; Bousseksou, A.; Tuchagues, J.-P.; McGarvey, J.J.; Dennis, A.C.; Castro, M.; et al. Thermal and Optical Switching of Molecular Spin States in the {[FeL[H2B(pz)2]2} Spin-Crossover System (L = Bpy, Phen). J. Phys. Chem. B 2002, 106, 4276–4283. [Google Scholar] [CrossRef]
- Zhang, X.; Palamarciuc, T.; Létard, J.-F.; Rosa, P.; Lozada, E.V.; Torres, F.; Rosa, L.G.; Doudin, B.; Dowben, P.A. The Spin State of a Molecular Adsorbate Driven by the Ferroelectric Substrate Polarization. Chem. Commun. 2014, 50, 2255. [Google Scholar] [CrossRef]
- Yazdani, S.; Phillips, J.; Ekanayaka, T.K.; Cheng, R.; Dowben, P.A. The Influence of the Substrate on the Functionality of Spin Crossover Molecular Materials. Molecules 2023, 28, 3735. [Google Scholar] [CrossRef]
- Ekanayaka, T.K.; Kurz, H.; Dale, A.S.; Hao, G.; Mosey, A.; Mishra, E.; N’Diaye, A.T.; Cheng, R.; Weber, B.; Dowben, P.A. Probing the Unpaired Fe Spins across the Spin Crossover of a Coordination Polymer. Mater. Adv. 2021, 2, 760–768. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Demont, P.; Menegotto, J. Observation of a Thermal Hysteresis Loop in the Dielectric Constant of Spin Crossover Complexes: Towards Molecular Memory Devices. J. Mater. Chem. 2003, 13, 2069–2071. [Google Scholar] [CrossRef]
- Pavlik, J.; Linares, J. Microscopic Models of Spin Crossover. Comptes Rendus Chim. 2018, 21, 1170–1178. [Google Scholar] [CrossRef]
- Hao, G.; Dale, A.S.; N’Diaye, A.T.; Chopdekar, R.V.; Koch, R.J.; Jiang, X.; Mellinger, C.; Zhang, J.; Cheng, R.; Xu, X.; et al. Intermolecular Interaction and Cooperativity in an Fe (II) Spin Crossover Molecular Thin Film System. J. Phys. Condens. Matter 2022, 34, 295201. [Google Scholar] [CrossRef]
- Konishi, Y.; Tokoro, H.; Nishino, M.; Miyashita, S. Monte Carlo Simulation of Pressure-Induced Phase Transitions in Spin-Crossover Materials. Phys. Rev. Lett. 2008, 100, 067206. [Google Scholar] [CrossRef]
- Ishikawa, R.; Ueno, S.; Nifuku, S.; Horii, Y.; Iguchi, H.; Miyazaki, Y.; Nakano, M.; Hayami, S.; Kumagai, S.; Katoh, K.; et al. Simultaneous Spin-Crossover Transition and Conductivity Switching in a Dinuclear Iron(II) Coordination Compound Based on 7,7′,8,8′-Tetracyano- p -quinodimethane. Chem.–A Eur. J. 2020, 26, 1278–1285. [Google Scholar] [CrossRef]
- Stoleriu, L.; Nishino, M.; Miyashita, S.; Stancu, A.; Hauser, A.; Enachescu, C. Cluster Evolution in Molecular Three-Dimensional Spin-Crossover Systems. Phys. Rev. B 2017, 96, 064115. [Google Scholar] [CrossRef]
- Dankhoff, K.; Lochenie, C.; Weber, B. Iron (II) Spin Crossover Complexes with 4,4′-Dipyridylethyne—Crystal Structures and Spin Crossover with Hysteresis. Molecules 2020, 25, 581. [Google Scholar] [CrossRef] [PubMed]
- Bauer, W.; Lochenie, C.; Weber, B. Synthesis and Characterization of 1D Iron (II) Spin Crossover Coordination Polymers with Hysteresis. Dalton Trans. 2014, 43, 1990–1999. [Google Scholar] [CrossRef]
- Bauer, W.; Schlamp, S.; Weber, B. A Ladder Type Iron (II) Coordination Polymer with Cooperative Spin Transition. Chem. Commun. 2012, 48, 10222. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z. Molecular Packing: Another Key Point for the Performance of Organic and Polymeric Optoelectronic Materials. Acc. Chem. Res. 2020, 53, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, J. Substituent Effects on Packing Entropy and Film Morphologies in the Nucleation of Functionalized Pentacenes on SiO2 Substrate: Molecular Dynamics Simulations. J. Chem. Phys. 2012, 137, 074708. [Google Scholar] [CrossRef]
- Gentili, D.; Sonar, P.; Liscio, F.; Cramer, T.; Ferlauto, L.; Leonardi, F.; Milita, S.; Dodabalapur, A.; Cavallini, M. Logic-Gate Devices Based on Printed Polymer Semiconducting Nanostripes. Nano Lett. 2013, 13, 3643–3647. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Tavares, A.C.B.; Trang Do, T.; Patil, B.B.; Sonar, P.; Hümmelgen, I.A. Experimental and Modeling Study of Low-Voltage Field-Effect Transistors Fabricated with Molecularly Aligned Copolymer Floating Films. Flex. Print. Electron. 2018, 3, 015006. [Google Scholar] [CrossRef]
- Aleeva, Y.; Pignataro, B. Recent Advances in Upscalable Wet Methods and Ink Formulations for Printed Electronics. J. Mater. Chem. C 2014, 2, 6436–6453. [Google Scholar] [CrossRef]
- Craze, A.R.; Marjo, C.E.; Li, F. A Complementary Characterisation Technique for Spin Crossover Materials; the Application of X-Ray Photoelectron Spectroscopy for Future Device Applications. Dalton Trans. 2022, 51, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, K.; Iasco, O.; Bellec, A.; Kartsev, A.; Li, D.; Lagoute, J.; Chacon, C.; Girard, Y.; Rousset, S.; Miserque, F.; et al. Molecular-Scale Dynamics of Light-Induced Spin Cross-over in a Two-Dimensional Layer. Nat. Commun. 2016, 7, 12212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; N’Diaye, A.T.; Jiang, X.; Zhang, X.; Yin, Y.; Chen, X.; Hong, X.; Xu, X.; Dowben, P.A. Indications of Magnetic Coupling Effects in Spin Cross-over Molecular Thin Films. Chem. Commun. 2018, 54, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Beniwal, S.; Zhang, X.; Mu, S.; Naim, A.; Rosa, P.; Chastanet, G.; Létard, J.-F.; Liu, J.; Sterbinsky, G.E.; Arena, D.A.; et al. Surface-Induced Spin State Locking of the [Fe(H2B(pz)2)2(bipy)] Spin Crossover Complex. J. Phys. Condens. Matter 2016, 28, 206002. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; N’Diaye, A.T.; Ekanayaka, T.K.; Dale, A.S.; Jiang, X.; Mishra, E.; Mellinger, C.; Yazdani, S.; Freeland, J.W.; Zhang, J.; et al. Magnetic Field Perturbations to a Soft X-Ray-Activated Fe (II) Molecular Spin State Transition. Magnetochemistry 2021, 7, 135. [Google Scholar] [CrossRef]
- Warner, B.; Oberg, J.C.; Gill, T.G.; El Hallak, F.; Hirjibehedin, C.F.; Serri, M.; Heutz, S.; Arrio, M.-A.; Sainctavit, P.; Mannini, M.; et al. Temperature- and Light-Induced Spin Crossover Observed by X-Ray Spectroscopy on Isolated Fe (II) Complexes on Gold. J. Phys. Chem. Lett. 2013, 4, 1546–1552. [Google Scholar] [CrossRef]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy Level Alignment and Interfacial Electronic Structures at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 1999, 22, 605–625. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-Level Alignment at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Dowben, P.A. The Metallicity of Thin Films and Overlayers. Surf. Sci. Rept. 2000, 40, 151–247. [Google Scholar] [CrossRef]


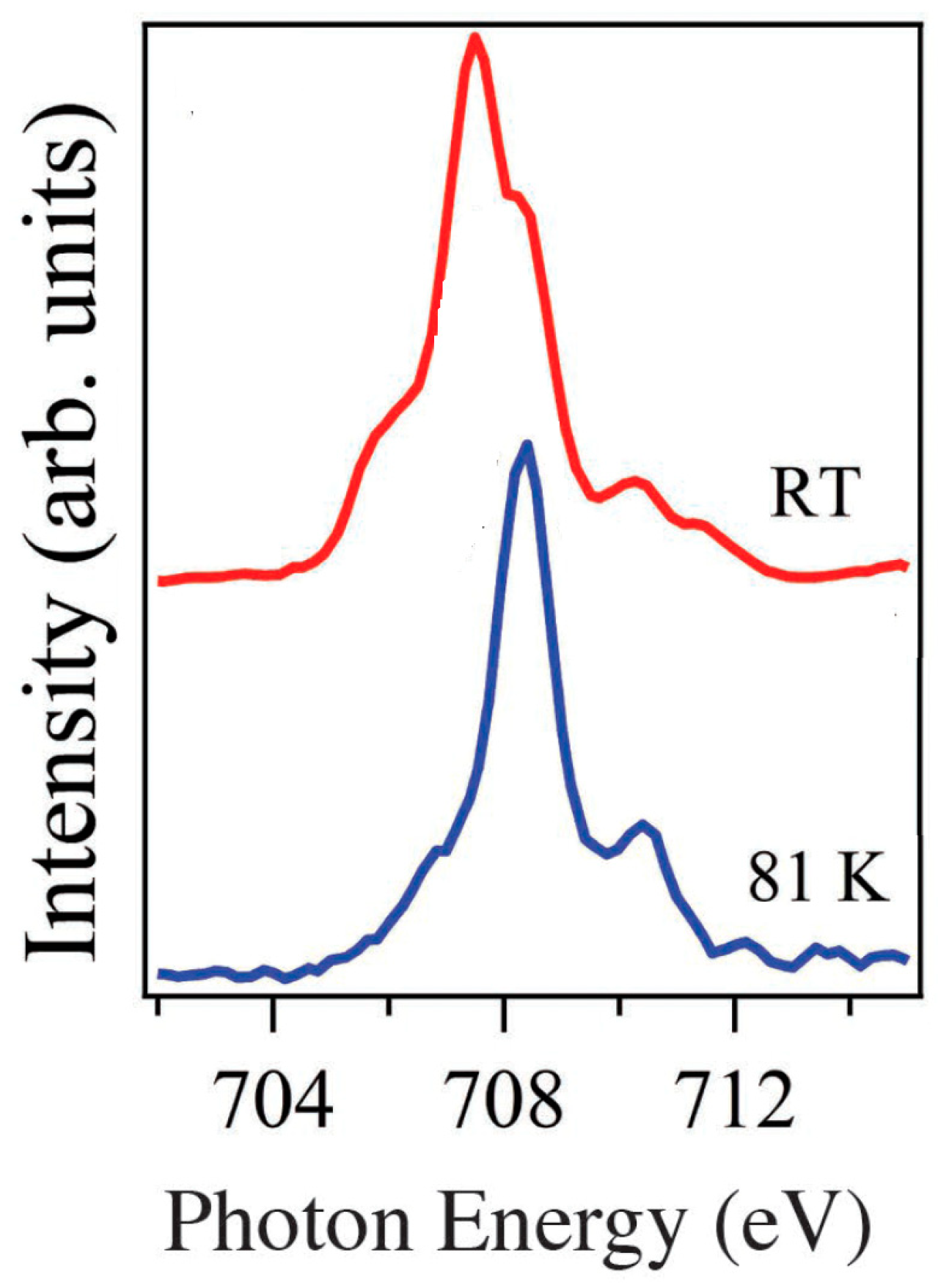
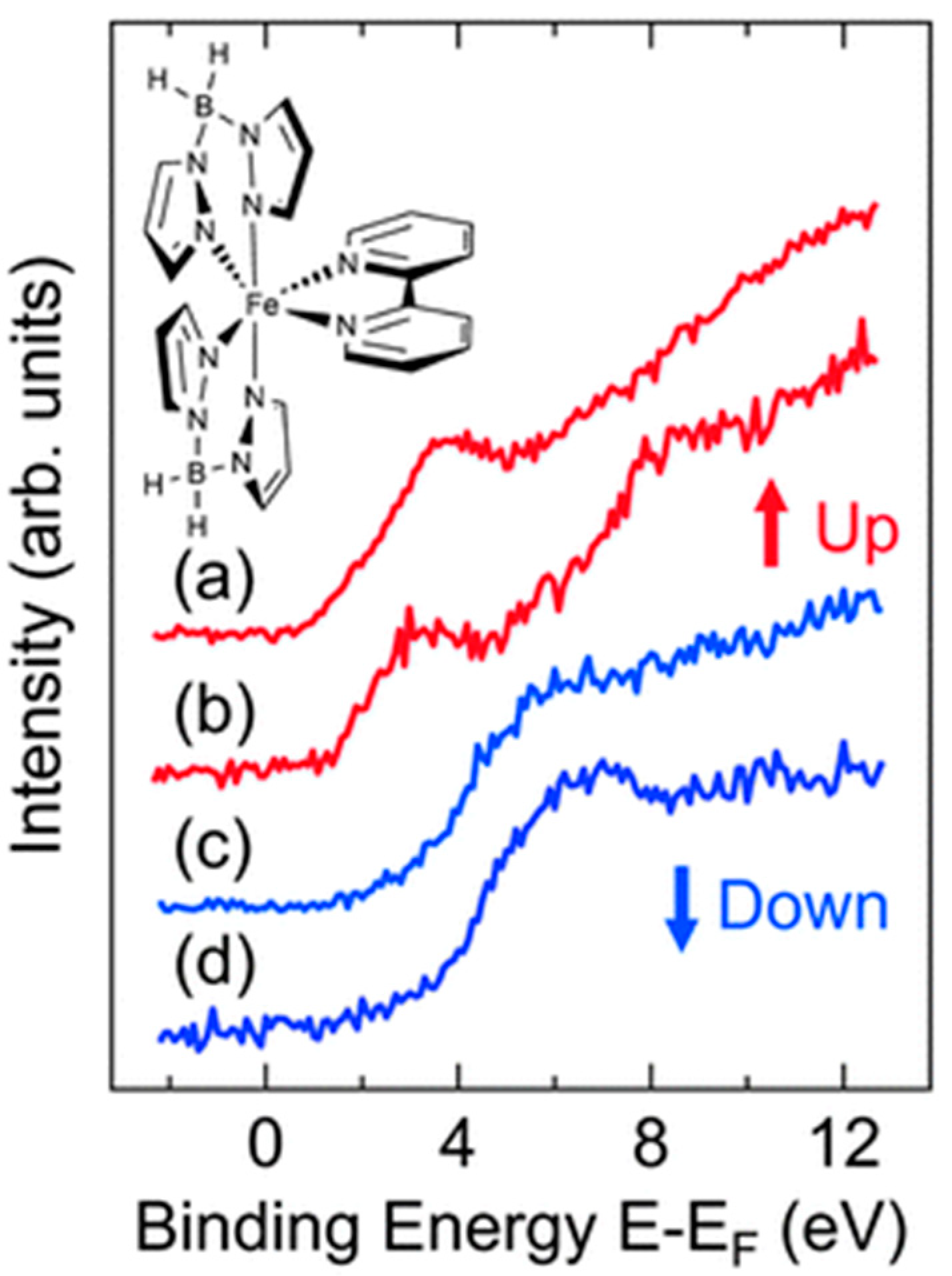
| On State | On/Off Ratio | On-State Current (A) | Off-State Current (A) | Switching Mechanism | Reference |
|---|---|---|---|---|---|
| Low spin state | 380 | 1.9 × 10−9 | 5 × 10−12 | Temperature controlled | [32] |
| Low spin state | 8 | - | - | Temperature controlled | [39] |
| Low spin state | 2 | 2 × 10−13 | 1 × 10−13 | Temperature controlled | [13] |
| Low spin state | 1.6 | 1 × 10−10 | 0.5 × 10−10 | Temperature controlled | [36] |
| High spin state | 11 | 5.5 × 10−9 | 0.5 × 10−9 | Temperature controlled | [31] |
| High spin state | 6 | 6 × 10−11 | 1 × 10−11 | Temperature controlled | [40] |
| High spin state | 1.7 | 9.2 × 10−10 | 5.2 × 10−10 | Temperature controlled | [34] |
| High spin state | 1.5 | 6 × 10−8 | 4 × 10−8 | Photo induced | [38] |
| High spin state | 1.5 | - | - | Temperature controlled | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaz, M.Z.; Ekanayaka, T.K.; Cheng, R.; Dowben, P.A. Variability of the Conductance Changes Associated with the Change in the Spin State in Molecular Spin Crossover Complexes. Magnetochemistry 2023, 9, 223. https://doi.org/10.3390/magnetochemistry9110223
Zaz MZ, Ekanayaka TK, Cheng R, Dowben PA. Variability of the Conductance Changes Associated with the Change in the Spin State in Molecular Spin Crossover Complexes. Magnetochemistry. 2023; 9(11):223. https://doi.org/10.3390/magnetochemistry9110223
Chicago/Turabian StyleZaz, M. Zaid, Thilini K. Ekanayaka, Ruihua Cheng, and Peter A. Dowben. 2023. "Variability of the Conductance Changes Associated with the Change in the Spin State in Molecular Spin Crossover Complexes" Magnetochemistry 9, no. 11: 223. https://doi.org/10.3390/magnetochemistry9110223
APA StyleZaz, M. Z., Ekanayaka, T. K., Cheng, R., & Dowben, P. A. (2023). Variability of the Conductance Changes Associated with the Change in the Spin State in Molecular Spin Crossover Complexes. Magnetochemistry, 9(11), 223. https://doi.org/10.3390/magnetochemistry9110223







