Solvothermal One-Pot Synthesis of a New Family of Chiral [Fe4O4]-Cubane Clusters with Redox Active Cores
Abstract
:1. Introduction
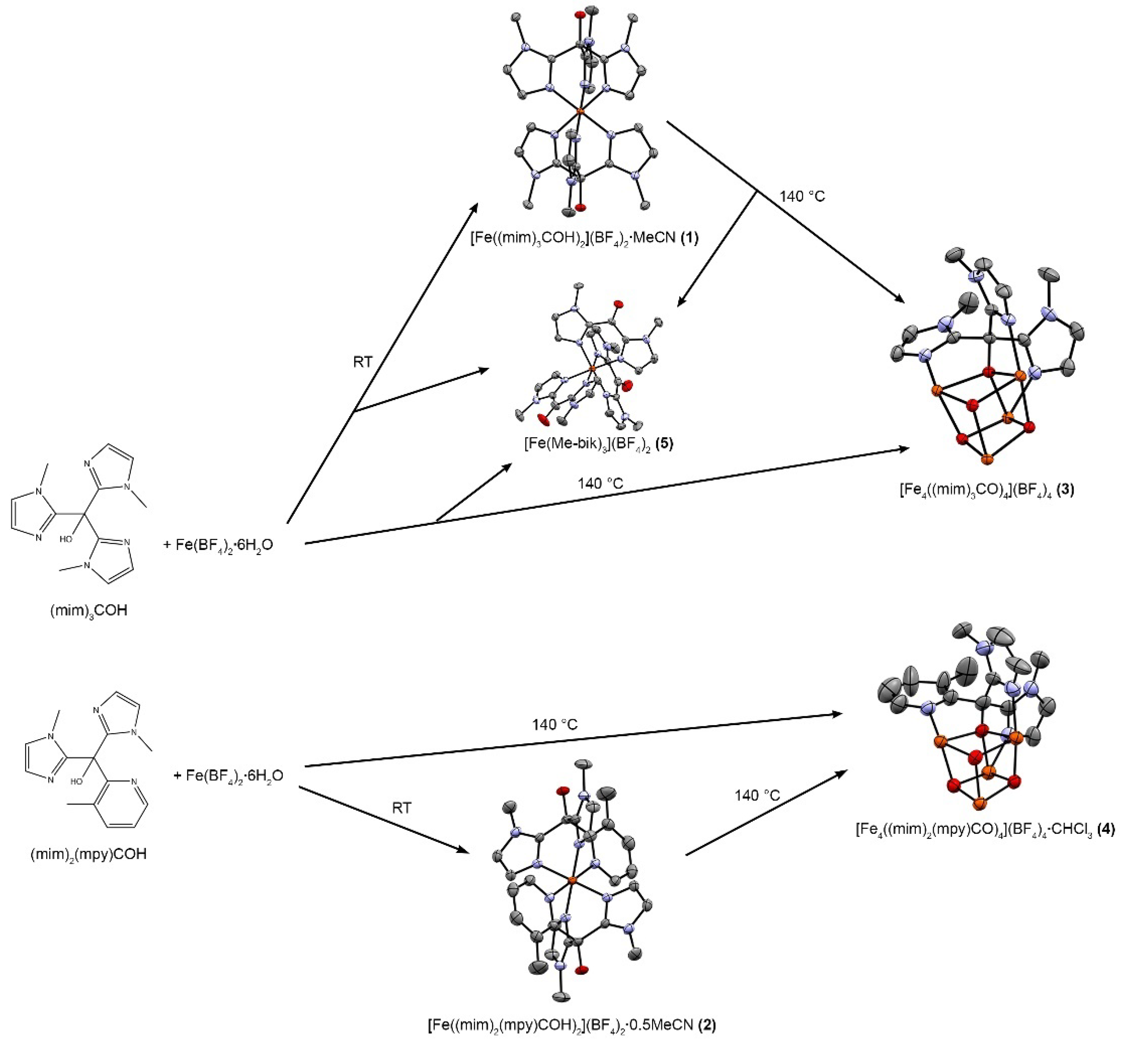
2. Results and Discussion
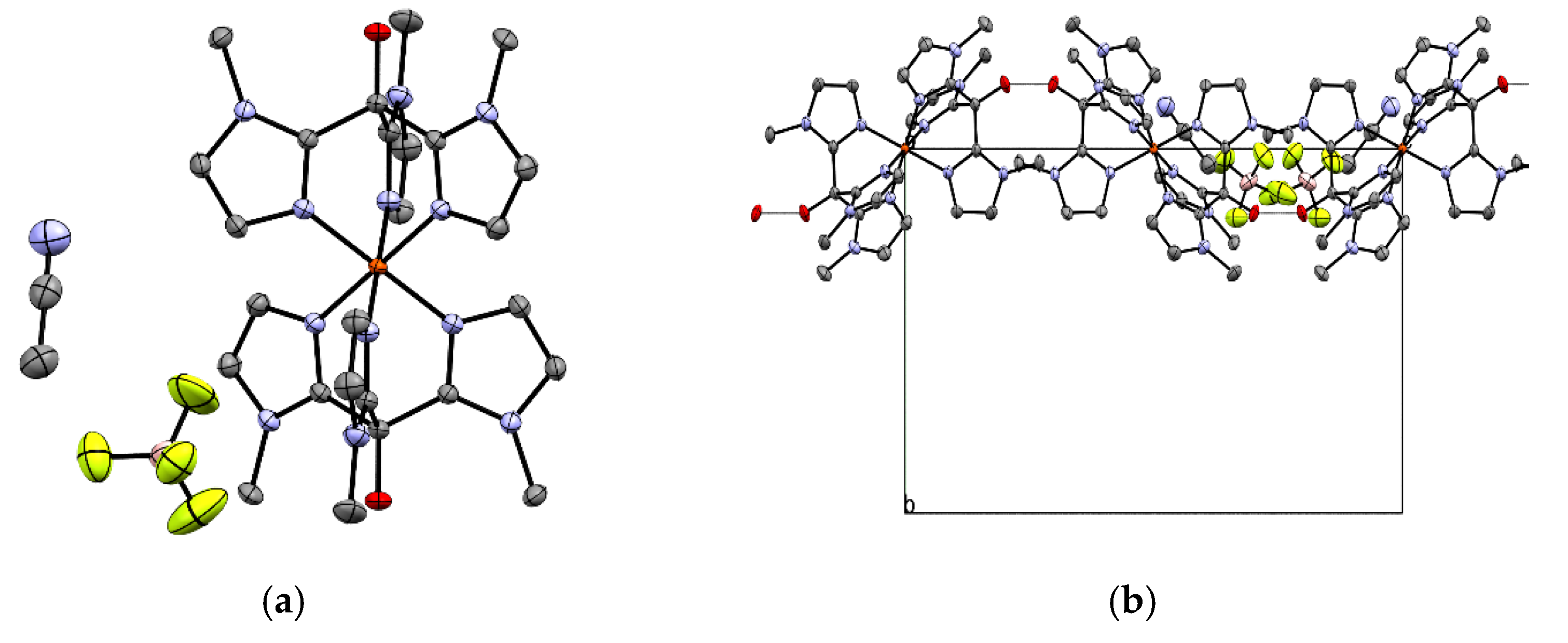
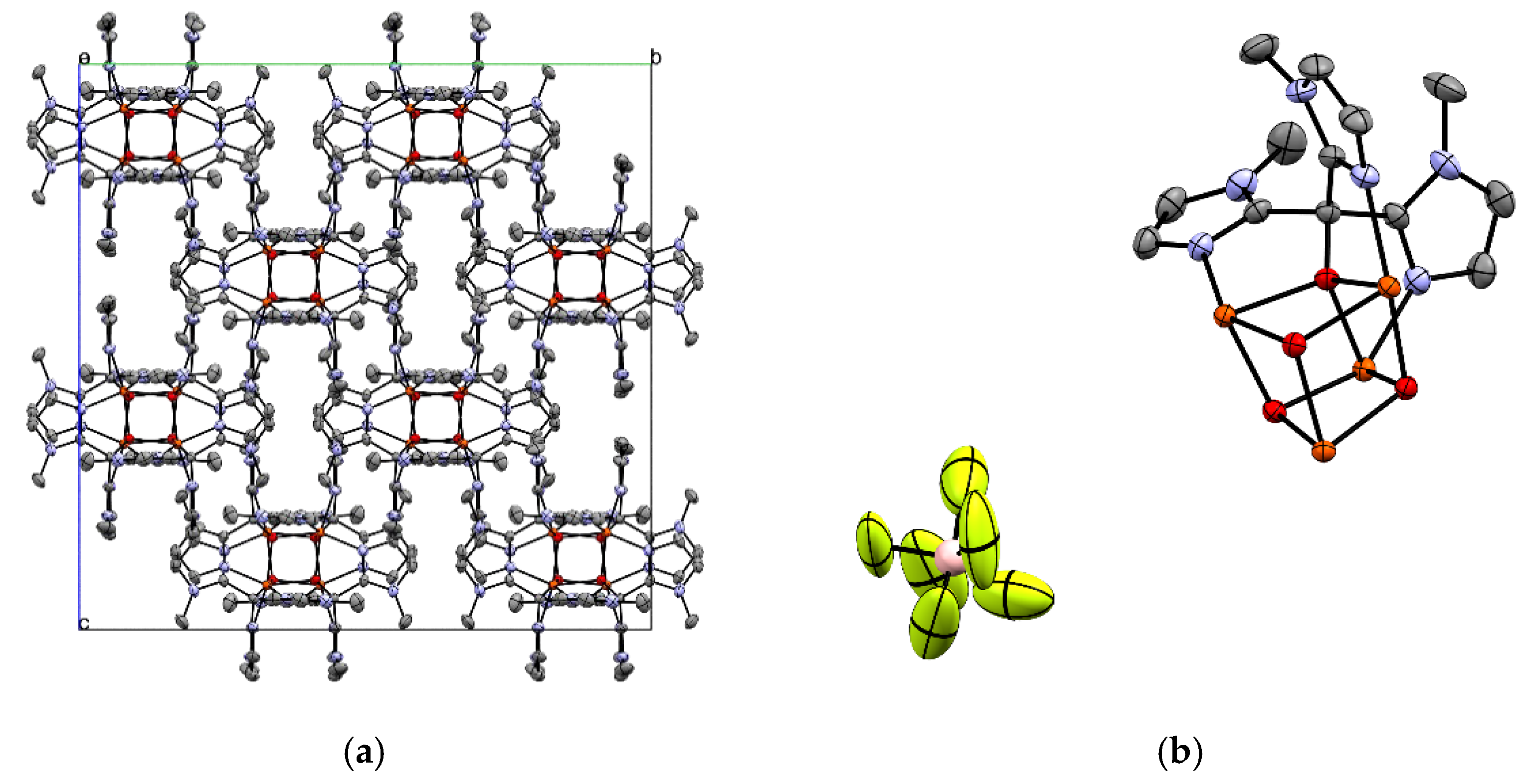
2.1. Structural Characterization
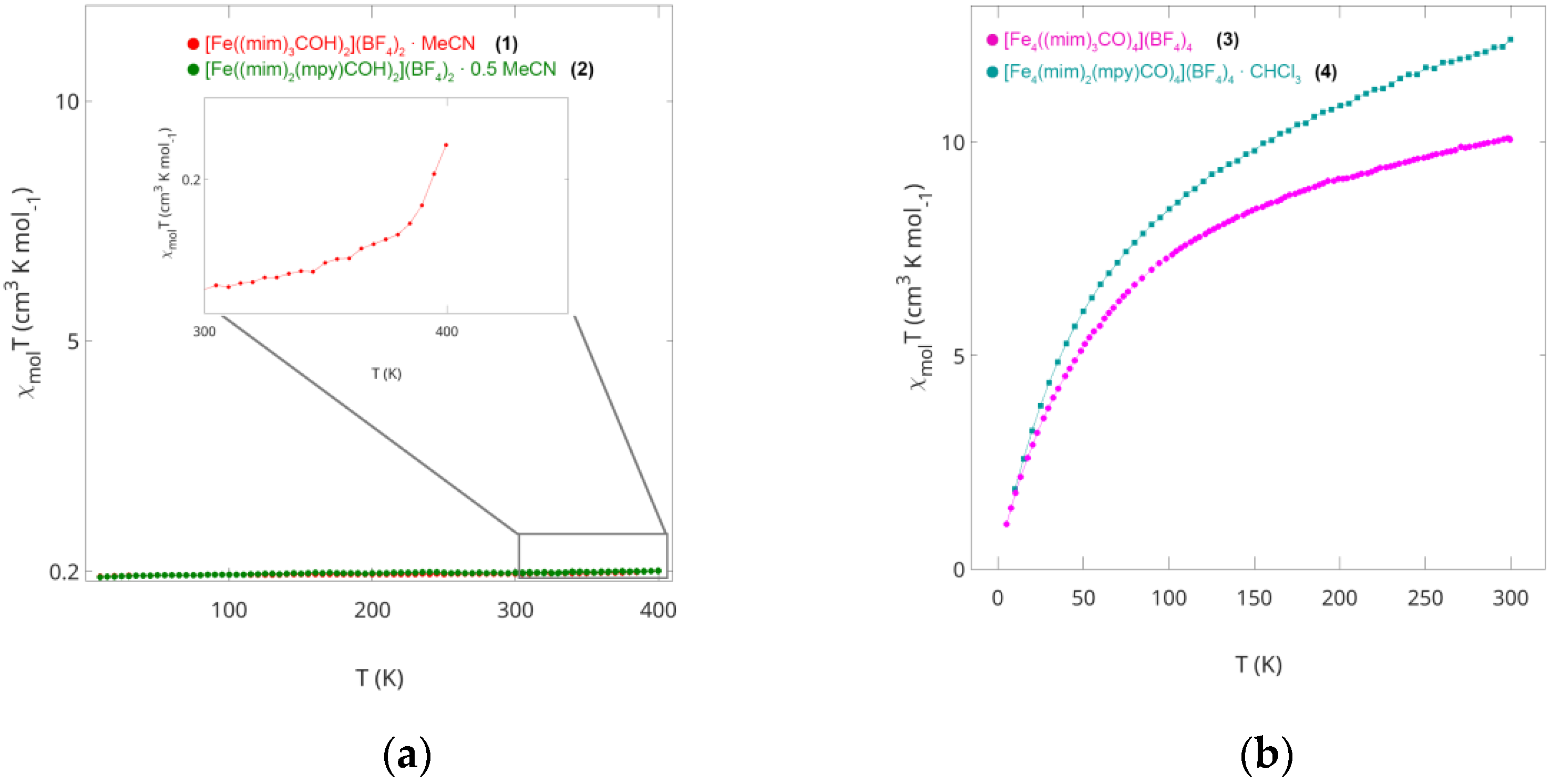
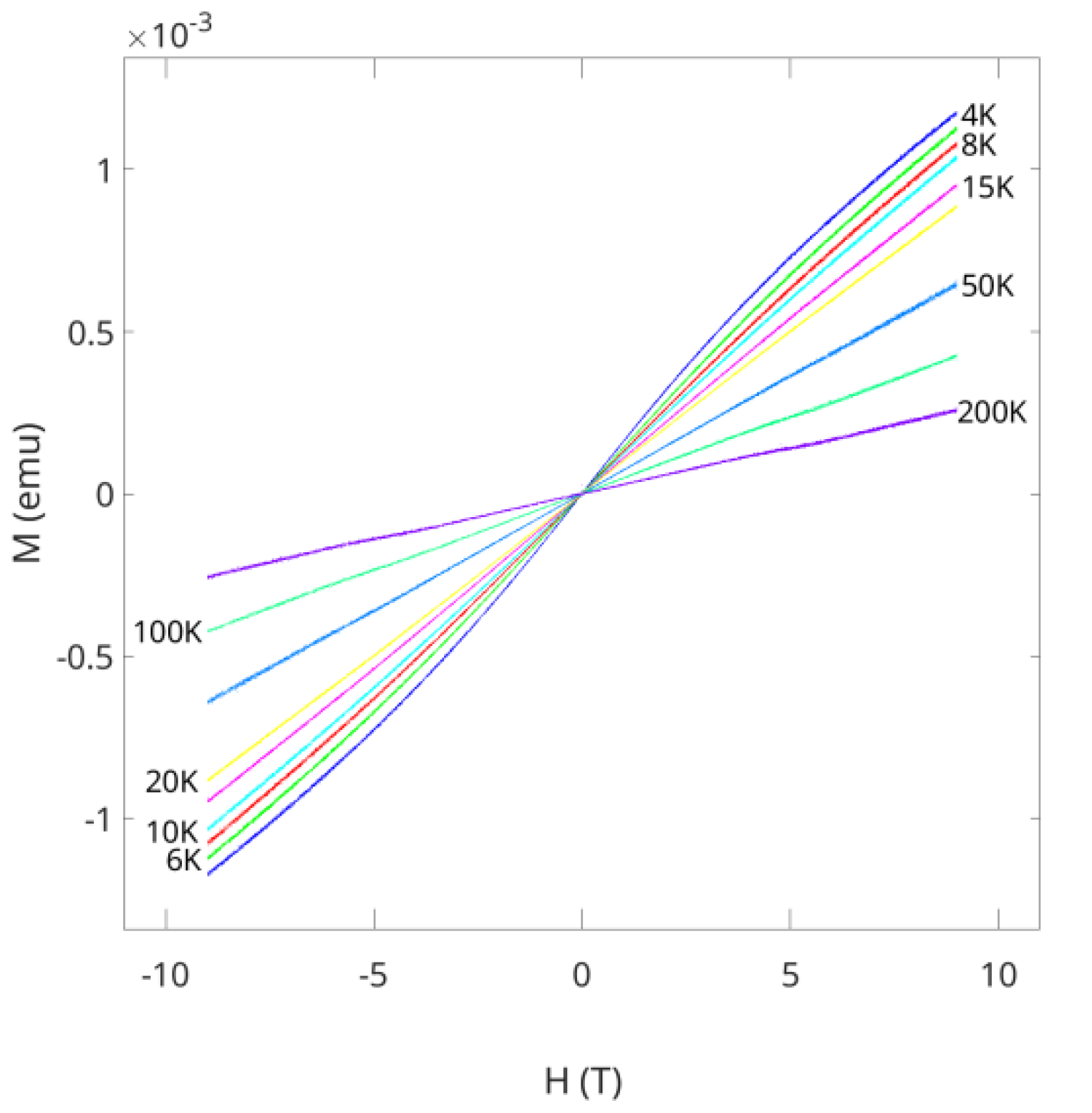
2.2. Magnetic Susceptibility
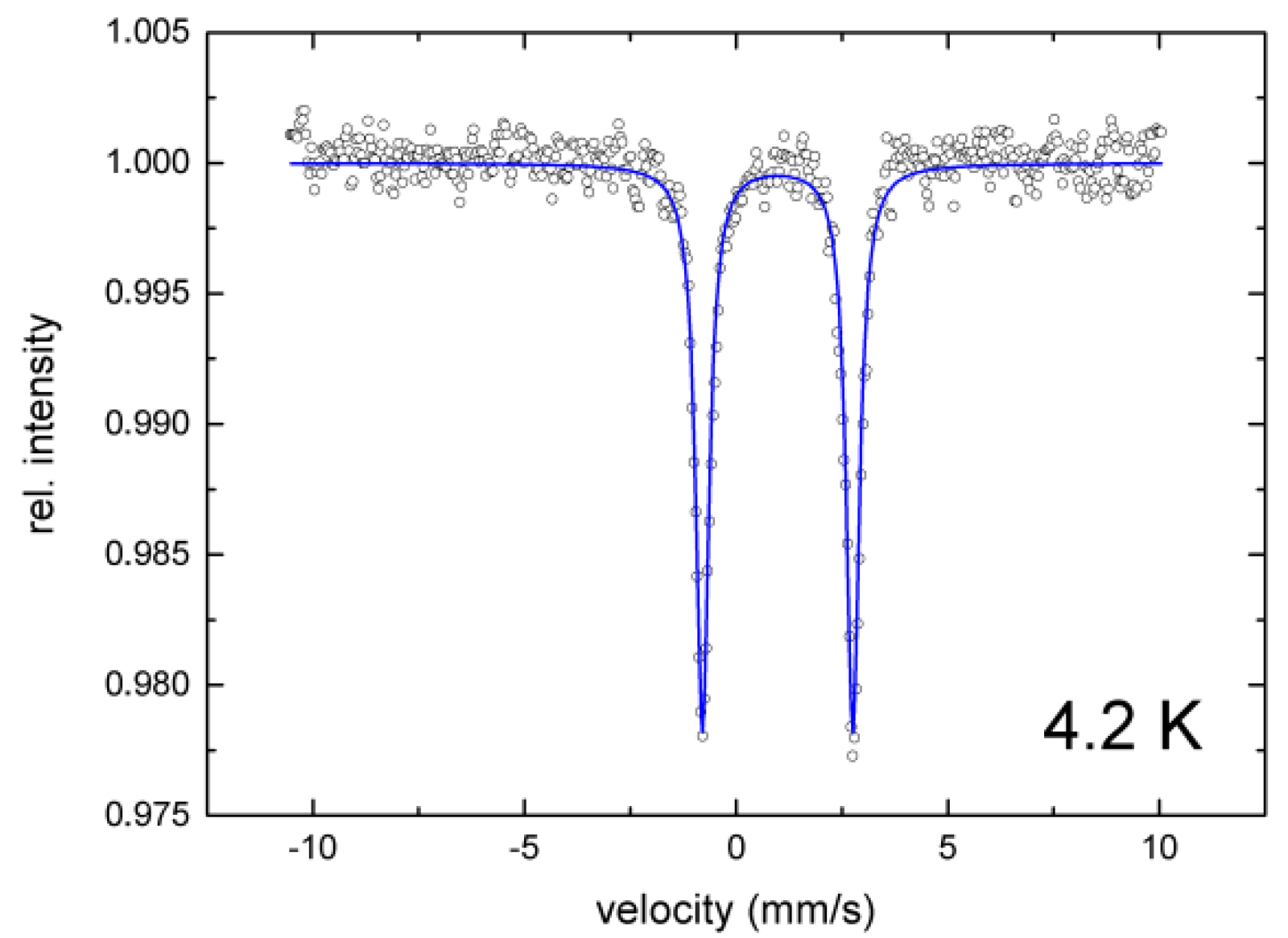
2.3. 57Fe-Mössbauer Spectroscopy
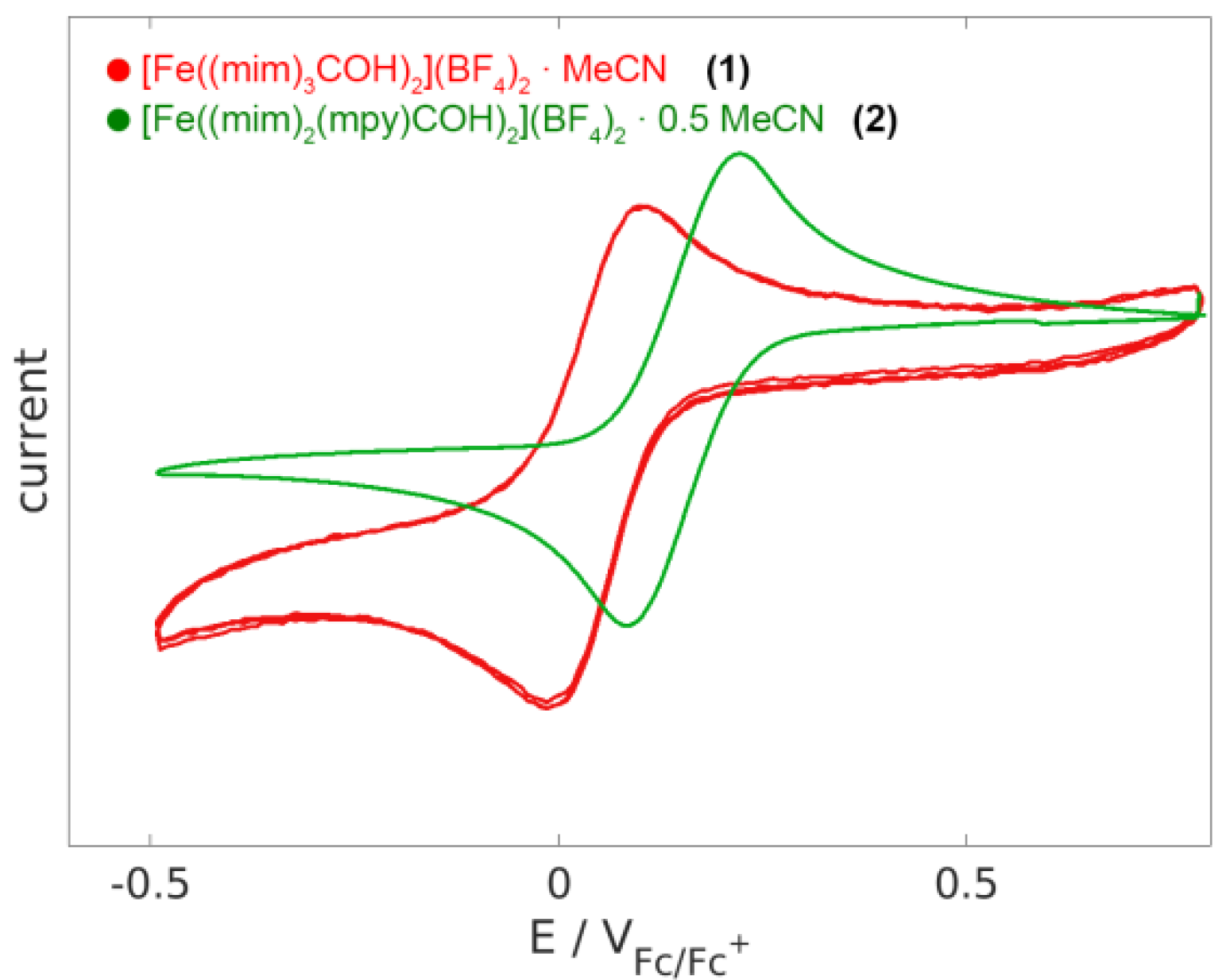
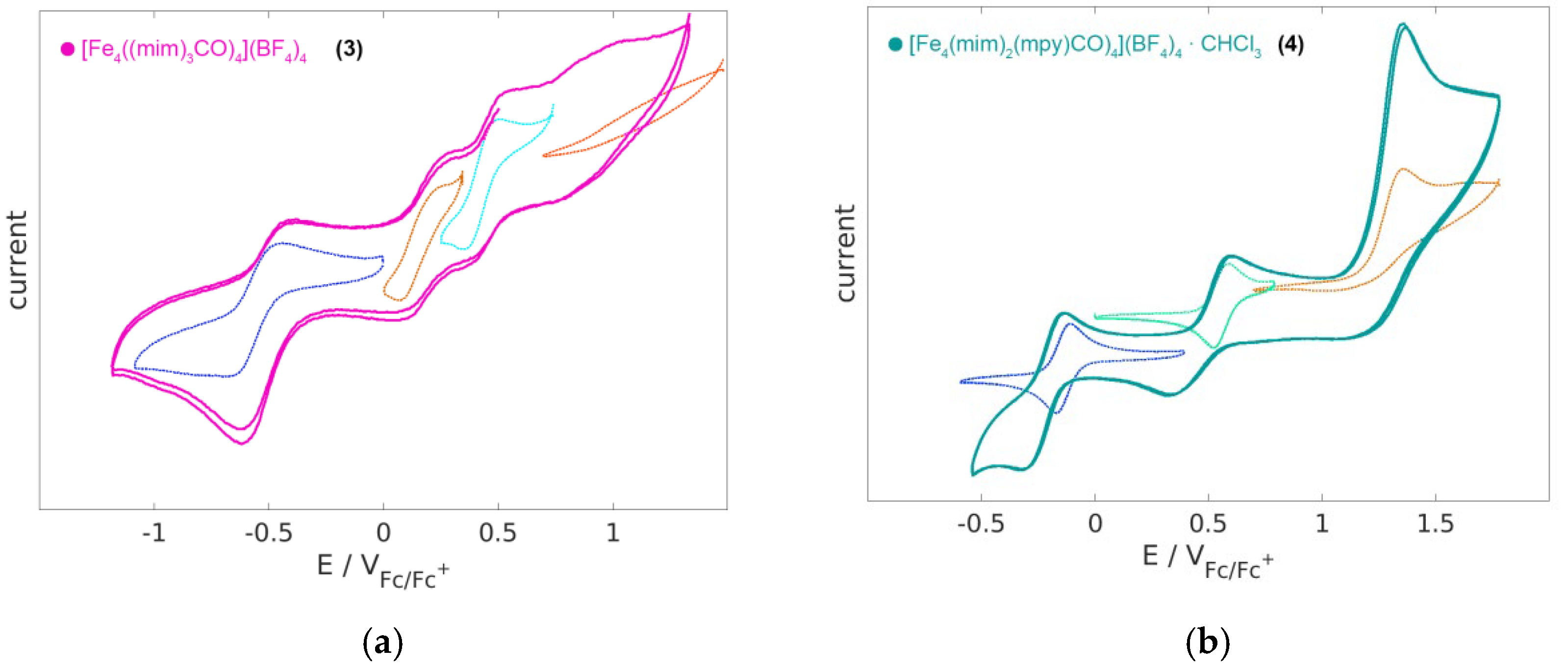
2.4. Electrochemistry
2.5. Vibrational Spectroscopy
3. Conclusions
4. Experimental Section
4.1. Methodology
4.2. Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Quesada, M.; Kooijman, H.; Gamez, P.; Costa, J.S.; van Koningsbruggen, P.J.; Weinberger, P.; Reissner, M.; Spek, A.L.; Haasnoot, J.G.; Reedijk, J. [Fe (μ-btzmp) 2 (btzmp) 2](ClO 4) 2: A doubly-bridged 1D spin-transition bistetrazole-based polymer showing thermal hysteresis behaviour. Dalton Trans. 2007, 46, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Koudriavtsev, A.B.; Stassen, A.F.; Haasnoot, J.G.; Grunert, M.; Weinberger, P.; Linert, W. Theoretical description of phenomena observed in a systematic study of the spin crossover in Fe (ii) complexes with halogenated ethyltetrazoles Part II. The quasi-chemical model (specific molecular interactions). Phys. Chem. Chem. Phys. 2003, 5, 3676–3683. [Google Scholar] [CrossRef]
- Halcrow, M.A. Iron (II) complexes of 2, 6-di (pyrazol-1-yl) pyridines—A versatile system for spin-crossover research. Coord. Chem. Rev. 2009, 253, 2493–2514. [Google Scholar] [CrossRef]
- Tang, C.C.; Davalian, D.; Huang, P.; Breslow, R. Models for Metal Binding Sites in Zinc Enzymes. Syntheses of tris [4 (5)-Imidazolyl] carbinol (4-TIC), tris (2-Imidazolyl) carbinol (2-TIC), and Related Ligands, and studies on metal complex binding constants and spectra. J. Am. Chem. Soc. 1978, 100, 3918–3922. [Google Scholar] [CrossRef]
- Brown, R.S.; Huguet, J. Synthesis and physical studies of pyridine and imidazole containing tridentate metal binding ligands. Can. J. Chem. 1980, 58, 889–901. [Google Scholar] [CrossRef]
- Wu, L.P.; Yamagiwa, Y.; Ino, I.; Sugimoto, K.; Kuroda-Sowa, T.; Kamikawa, T.; Munakata, M. Unique tetranuclear copper (II) cluster and monomeric iron (II),(III) complexes with a tris (imidazolyl) chelating ligand. Polyhedron 1999, 18, 2047–2053. [Google Scholar] [CrossRef]
- Baran, P.; Boca, R.; Chakraborty, I.; Giapintzakis, J.; Herchel, R.; Huang, Q.; McGrady, J.E.; Raptis, R.G.; Sanakis, Y.; Simopoulos, A. Synthesis, characterization, and study of octanuclear iron-oxo clusters containing a redox-active Fe4O4-cubane core. Inorg. Chem. 2008, 47, 645–655. [Google Scholar] [CrossRef]
- Shoner, S.C.; Power, P.P. Neutral catecholate derivatives of manganese and iron: Synthesis and characterization of the metal-oxygen cubane-like species M4 (DBCat) 4 (py) 6 (M= Mn, Fe), the trinuclear complex Mn3 (DBCat) 4 (py) 4, and the dimers M2 (DBCat) 2 (py) n (M= Mn, n= 6; M= Fe, n= 4, 6). Inorg. Chem. 1992, 31, 1001. [Google Scholar]
- Taft, K.L.; Caneschi, A.; Pence, L.E.; Delfs, C.D.; Papaefthymiou, G.C.; Lippard, S.J.J. Iron and manganese alkoxide cubes. Am. Chem. Soc. 1993, 115, 11753. [Google Scholar] [CrossRef]
- Taft, L.; Papaefthymiou, G.C.; Lippard, S.J. A mixed-valent polyiron oxo complex that models the biomineralization of the ferritin core. Science 1993, 259, 1302. [Google Scholar] [CrossRef]
- Taft, K.L.; Papaefthymiou, G.C.; Lippard, S.J. Synthesis, structure, and electronic properties of a mixed-valent dodecairon oxo complex, a model for the biomineralization of ferritin. Inorg. Chem. 1994, 33, 1510. [Google Scholar] [CrossRef]
- Dell’Amico, D.B.; Boschi, D.; Calderazo, F.; Ianelli, S.; Labella, L.; Marchetti, F.; Pelizzi, G.; Quadrelli, E.G.F. N, N-Dialkylcarbamato μ-oxo derivatives of iron (III). Inorg. Chim. Acta 2000, 300–302, 882. [Google Scholar]
- Oshio, H.; Hoshino, N.; Ito, T.J. Superparamagnetic behavior in an alkoxo-bridged iron (II) cube. Am. Chem. Soc. 2000, 122, 12602. [Google Scholar] [CrossRef]
- Oshio, H.; Hoshino, N.; Ito, T.; Nakano, M.J. Single-molecule magnets of ferrous cubes: Structurally controlled magnetic anisotropy. Am. Chem. Soc. 2004, 126, 8805. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Sorace, L.; Caneschi, A.; Lippard, S.J. Hydroxo-bridged cubane-type tetrairon (II) clusters supported by sterically-hindered carboxylate ligands. Inorg. Chem. 2001, 40, 6774. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Mackiewicz, C.; Verelst, M.; Dahan, F.; Bousseksou, A.; Sanakis, Y.; Tuchagues, J.-P. Synthesis, structure, and magnetic properties of tetranuclear cubane-like and chain-like iron (II) complexes based on the N4O pentadentate dinucleating ligand 1, 5-bis [(2-pyridylmethyl) amino] pentan-3-ol. Inorg. Chem. 2002, 41, 1478. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Hudson, T.A.; Robson, R.J. Highly symmetric networks derived from cubane-related octametallic complexes of a new oxyanion of carbon, C4O74-, each molecule attached to eight neighbors by 24 equivalent hydrogen bonds. Am. Chem. Soc. 2004, 126, 8624. [Google Scholar] [CrossRef]
- Hudson, T.A.; Berry, K.J.; Moubaraki, B.; Murray, K.; Robson, R. Citrate, in Collaboration with a Guanidinium Ion, as a Generator of Cubane-like Complexes with a Range of Metal Cations: Synthesis, Structures, and Magnetic Properties of [C (NH2) 3] 8 [(MII) 4 (cit) 4]⊙ 8H2O (M= Mg, Mn, Fe, Co, Ni, and Zn; cit = Citrate). Inorg. Chem. 2006, 45, 3549. [Google Scholar] [CrossRef]
- Gass, I.A.; Milios, C.J.; Whittaker, A.G.; Fabiani, F.P.A.; Parsons, S.; Murrie, M.; Perlepes, S.P.; Brechin, E.K. A cube in a tetrahedron: Microwave-assisted synthesis of an octametallic FeIII cluster. Inorg. Chem. 2006, 45, 5281. [Google Scholar] [CrossRef]
- De, S.; Tewary, S.; Garnier, D.; Li, Y.; Gontard, G.; Lisnard, L.; Flambard, A.; Breher, F.; Boillot, M.L.; Rajaraman, G.; et al. Solution and Solid-State Study of the Spin-Crossover [FeII (R-bik) 3](BF4) 2 Complexes (R = Me, Et, Vinyl). Eur. J. Inorg. Chem. 2018, 2018, 414–428. [Google Scholar] [CrossRef]
- Drew, M.G.B.; Harding, C.J.; McKee, V.; Morgan, G.G.; Nelson, J. Geometric Control of Manganese Redox State. J. Chem. Soc. Chem. Commun. 1995, 1035–1038. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths intransition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Bartlett, G.J.; Newberry, R.W.; VanVeller, B.; Raines, R.T.; Woolfson, D.N. Interplay of hydrogen bonds and n→π* interactions in proteins. J. Am. Chem. Soc. 2013, 135, 18682–18688. [Google Scholar] [CrossRef]
- Raptis, R.G.; Georgakaki, I.P.; Hockless, D.C. A FeIII/oxo cubane contained in an octanuclear complex of T symmetry that is stable over five oxidation states. Angew. Chem. Int. Ed. 1999, 38, 1632–1634. [Google Scholar] [CrossRef]
- Datta, S.; Rahaman, B. First principles study of electronic structure for cubane-like and ring-shaped structures of M4O4, M4S4 clusters (M= Mn, Fe, Co, Ni, Cu). AIP Adv. 2015, 5, 117231. [Google Scholar] [CrossRef]
- Armarego, W.L. Purification of Laboratory Chemicals; Butterworth-Heinemann: Amsterdam, The Netherlands; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Bruker Analytical X-ray Instruments, I. Available online: https://www.bruker.com/products (accessed on 22 July 2022).
- Müller, D.; Knoll, C.; Seifried, M.; Weinberger, P. ATR or transmission—A variable temperature study comparing both techniques using [Fe (3ditz) 3](BF4) 2 as model system. Vib. Spectrosc. 2016, 86, 198–205. [Google Scholar] [CrossRef]
- Bruker, I. Bruker AXS Inc.: Madison, WI, USA, 2012. Available online: https://www.bruker.com/products (accessed on 22 July 2022).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Sorrell, T.N.; Borovik, A.S. Synthesis, structure, and spectroscopic properties of an unusual copper (I) dimer having imidazole ligands. A model for the carbonyl derivative of hemocyanin and implications for the structure of deoxyhemocyanin. J. Am. Chem. Soc. 1987, 109, 4255–4260. [Google Scholar] [CrossRef]



| Bond | Length (Å) | Atoms | Angle (°) | Atoms | Angle (°) |
|---|---|---|---|---|---|
| Fe1-O1 | 2.095(7) | O1-Fe1-O3 | 79.3(3) | Fe1-O1-Fe2 | 100.7(3) |
| Fe1-O3 | 2.096(7) | O1-Fe1-O4 | 78.5(3) | Fe1-O1-Fe4 | 100.9(3) |
| Fe1-O4 | 2.124(7) | O3-Fe1-O4 | 77.9(3) | Fe2-O1-Fe4 | 101.5(3) |
| Fe2-O1 | 2.138(7) | O1-Fe2-O2 | 77.7(3) | Fe2-O2-Fe3 | 101.1(3) |
| Fe2-O2 | 2.107(7) | O1-Fe2-O3 | 77.2(3) | Fe2-O2-Fe4 | 101.9(3) |
| Fe2-O3 | 2.150(7) | O2-Fe2-O3 | 77.8(3) | Fe3-O2-Fe4 | 100.4(3) |
| Fe3-O2 | 2.188(8) | O2-Fe3-O3 | 76.6(3) | Fe1-O3-Fe2 | 100.3(3) |
| Fe3-O3 | 2.127(7) | O2-Fe3-O4 | 76.4(3) | Fe1-O3-Fe3 | 102.3(3) |
| Fe3-O4 | 2.145(7) | O3-Fe3-O4 | 76.8(3) | Fe2-O3-Fe3 | 101.7(3) |
| Fe4-O1 | 2.134(7) | O1-Fe4-O2 | 76.8(3) | Fe1-O4-Fe3 | 100.7(3) |
| Fe4-O2 | 2.151(7) | O1-Fe4-O4 | 77.7(3) | Fe1-O4-Fe4 | 100.4(3) |
| Fe4-O4 | 2.120(7) | O2-Fe4-O4 | 77.8(3) | Fe3-O4-Fe4 | 102.8(3) |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Formula | C15H18.5BF4Fe0.5N7O | C16H18.5BF4Fe0.5N5.5O | C52H60B4F16Fe4N24O4 | C61H62B4Cl3F16Fe4N20O4 |
| m [g mol−1] | 427.60 | 418.60 | 1655.88 | 1816.29 |
| T [K] | 200(2) | 200(2) | 200.15 | 200.15 |
| Color | Magenta | Red | Colorless | Yellow |
| Shape | Block | Block | Block | Block |
| CrystalSystem | monoclinic | triclinic | cubic | orthorhombic |
| Space Group | C2/c | P | Fd | P212121 |
| a [Å] | 16.0441(17) | 11.1878(13) | 24.8347(8) | 16.037(3) |
| b [Å] | 11.6518(12) | 12.6938(14) | 24.8347(8) | 22.753(4) |
| c [Å] | 20.049(2) | 13.6370(15) | 24.8347(8) | 23.418(5) |
| α [°] | 90 | 74.337(3) | 90 | 90 |
| β [°] | 102.904(2) | 82.058(3) | 90 | 90 |
| γ [°] | 90 | 86.255(3) | 90 | 90 |
| V [Å3] | 3653.3(7) | 1846.1(4) | 15317.1(15) | 8545(3) |
| Z | 8 | 4 | 8 | 4 |
| ρ calc. [g cm−3] | 1.555 | 1.506 | 1.436 | 1.412 |
| µ [mm−1] | 0.506 | 0.497 | 0.838 | 0.848 |
| Measured Refl’s. | 65735 | 51222 | 118123 | 133050 |
| Indep’t Refl’s | 7001 | 9124 | 1983 | 15661 |
| GooF | 1.016 | 1.017 | 1.106 | 1.026 |
| wR2 | 0.0980 | 0.1179 | 0.1969 | 0.1433 |
| R1 | 0.0431 | 0.0520 | 0.0636 | 0.0531 |
| CCDC | 2167517 | 2167516 | 2167518 | 2167519 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifried, M.; Kapsamer, F.M.; Reissner, M.; Welch, J.M.; Giester, G.; Müller, D.; Weinberger, P. Solvothermal One-Pot Synthesis of a New Family of Chiral [Fe4O4]-Cubane Clusters with Redox Active Cores. Magnetochemistry 2022, 8, 95. https://doi.org/10.3390/magnetochemistry8090095
Seifried M, Kapsamer FM, Reissner M, Welch JM, Giester G, Müller D, Weinberger P. Solvothermal One-Pot Synthesis of a New Family of Chiral [Fe4O4]-Cubane Clusters with Redox Active Cores. Magnetochemistry. 2022; 8(9):95. https://doi.org/10.3390/magnetochemistry8090095
Chicago/Turabian StyleSeifried, Marco, Frieda M. Kapsamer, Michael Reissner, Jan M. Welch, Gerald Giester, Danny Müller, and Peter Weinberger. 2022. "Solvothermal One-Pot Synthesis of a New Family of Chiral [Fe4O4]-Cubane Clusters with Redox Active Cores" Magnetochemistry 8, no. 9: 95. https://doi.org/10.3390/magnetochemistry8090095
APA StyleSeifried, M., Kapsamer, F. M., Reissner, M., Welch, J. M., Giester, G., Müller, D., & Weinberger, P. (2022). Solvothermal One-Pot Synthesis of a New Family of Chiral [Fe4O4]-Cubane Clusters with Redox Active Cores. Magnetochemistry, 8(9), 95. https://doi.org/10.3390/magnetochemistry8090095






