1. Introduction
Bacterial infections, especially those caused by multidrug-resistant bacteria, have become a global public health concern [
1]. The emergence of antimicrobial resistance is largely attributed to the indiscriminate and often abusive use of antimicrobials [
2]. It was clinically found that due to the abuse of antibiotics, there is a lack of effective antibacterial drugs to treat bacterial infections [
3]. It is estimated that deaths due to infectious diseases that are associated with resistant pathogens will lead to 10 million deaths per year by 2050 [
4]. Therefore, there is an urgent need to develop non-antibiotic drugs to treat multidrug-resistant bacterial infections [
5].
The early detection and identification of bacterial infections are also major clinical challenges [
6]. Moreover, the traditional detection and identification methods are time-consuming and tedious. In order to overcome the shortcomings of traditional antibacterial treatment and detection methods, various metals and metal oxide nanoparticles have been used for bacterial detection and treatment [
7]. MNPs have been widely used in the biological field in recent years due to their physical properties, good biocompatibility, and high binding capacity [
8,
9,
10], including in vivo and in vitro bacterial detection and separation imaging [
11], as well as the treatment of pathogenic bacteria. For example, magnetic materials were used to synthesize new structures [
12] and new magnetic material composite nanoparticles with improved structural stability [
13], biological activity [
14], and antibacterial properties [
15,
16,
17,
18] to realize the separability and recyclability of MNPs [
19,
20,
21].
Several previous reviews have summarized and compared the applications of MNPs in the field of biomedicine [
12,
22], but few reviews focused on the application of MNPs regarding bacterial infections. In this review, we summarize some recent advances in the diagnosis and treatment of bacterial infections with MNPs because MNPs can solve the two major clinical challenges of infectious diseases. First, we discuss the physical properties of MNPs, which account for their increasing application in detecting and treating bacterial infections. Second, we summarize the latest developments in MNPs for bacterial detection. Third, we discuss the recent progress in MNPs for the treatment of bacterial infections. Fourth, the dual application of multifunctional MNPs as antibacterial and imaging agents is discussed. Finally, the challenges and potential future development directions of MNPs in the diagnosis and treatment of bacterial infectious diseases are presented.
2. Physical Properties of MNPs
In the past few decades, MNPs have been considered as highly promising supporting materials and have been utilized in the bacterial detection and antibacterial therapies fields. Their wide application is mainly from their unique physical properties, including magnetic properties [
23,
24,
25], structural properties [
26], and the feature of easy modification [
27].
2.1. Magnetic Properties
MNPs show excellent magnetic properties that are responsible for their wide use in enhancing drug delivery efficiency [
28], bacterial separation, and detection. Moreover, the properties of nanosized magnetic particles are different from those of the corresponding conventional magnetic materials [
29,
30,
31]. In particular, MNPs with a core size ranging between 10 and 100 nm in diameter, exhibit special magnetic properties called “superparamagnetism” [
23,
24]. Superparamagnetic nanoparticles can induce magnetic resonance (MR) relaxation, which results in their application as contrast agents in magnetic resonance imaging (MRI) [
32,
33]. The mechanism behind MNP-based contrast agents involves affecting their energy by changing the dipole–dipole interactions between hydrogen nuclei and metal ions, thus improving the imaging contrast [
34]. The magnetic property of MNPs was also leveraged to synthesize a multi-branched Au nanoflower that was modified with a sulfhydryl amino ligand as a skeleton to encapsulate iron porphyrin, which showed high sensitivity as an immunosensor [
35]. Due to their response to an applied magnetic field, MNPs could also influence the movement of nanomaterials in the body under magnetic guidance and subsequent nanomaterial disassembly to release loaded drugs for tumor chemotherapy [
36]. Moreover, the magnetic property of MNPs enabled the improved rotation of nanomaterials under an external magnetic field to generate sufficient heat to ablate tumor cells via magnetothermal therapy [
37]. Therefore, these instances promote extensive leveraging of the magnetic properties of MNPs for the detection and treatment of bacterial infections.
2.2. Structural Properties
MNPs, including Fe, Co, Ni, and Mn, show great potential for antibacterial applications due to their unique structural properties. The size of the MNPs is one of the important parameters for determining the properties of MNPs [
23], and it can be controlled by varying different reaction parameters, including the precursor concentration, type of surfactant, reaction temperature, and time [
25]. For example, MNPs with a size between 10 to 100 nm show excellent relaxation performance and photothermal properties [
24,
38]. By controlling the size of MNPs, it is possible to manipulate the imaging contrast and the quantity of produced heat, making them promising for diagnosing diseases and magnetic hyperthermia-based treatment [
37]. Additionally, MNPs show the great characteristic of being easily functionalized. Because of this, MNPs can be modified with other metal elements, peptides, antibodies, or therapeutic drugs to synthesize new structures and new magnetic nanocomposites [
10]. The new magnetic nanocomposites have good drug-loading capability and controlled delivery ability. Moreover, with different magnetic fields, it is possible to manipulate the amount of produced heat.
3. Bacterial Detection
Due to the magnetic and MR properties of MNPs, they can be used for the detection of bacteria using MRI. MNPs can also bind other materials, thus enabling their use for the separation and enrichment of bacteria. The detection of bacterial infections can be achieved through colorimetric, fluorescence and surface-enhanced Raman scattering (SERS) means.
3.1. Colorimetric Detection
Colorimetric detection is a kind of color change that is induced by the target, which can be observed by the naked eye for qualitative or quantitative detection and quantitative analysis using ultraviolet-visible spectroscopy. The colorimetric method is easy to operate, fast, sensitive, and economical for detecting bacteria [
39].
Au nanoparticles are the most commonly used metal-based NPs for colorimetric detection due to their good solubility, excellent biocompatibility and easy surface modification [
40]. The combination of Au nanoparticles and MNPs can achieve bacterial aggregation and separation to afford a more sensitive colorimetric detection effect. Due to the combination of magnetic materials, bacterial aggregation can achieve a transition from low to high concentration, thereby improving detection sensitivity [
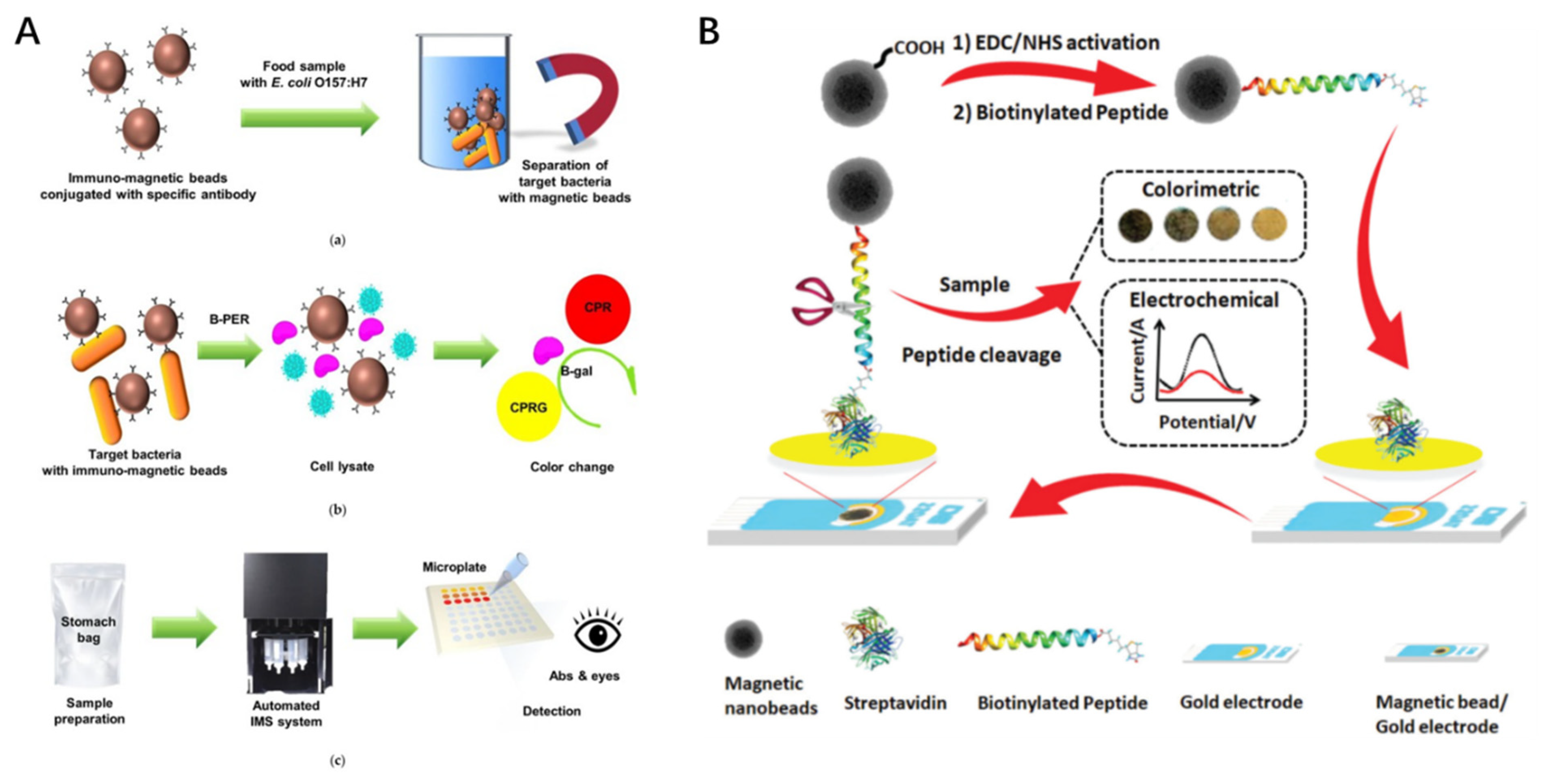
8]. For example, Mahheidari et al. used thiocyanate/Au nanoparticles combined with MNPs to detect
Vibrio cholerae bacteria and their toxins [
41]. In another study, Park et al. developed an automated immunomagnetic separation combined with a colorimetric assay for the rapid detection of
E. coli O157:H7 (
Figure 1A) [
42].
As the extinction coefficient of Au nanoparticles is several orders of magnitude larger than those of traditional organic dye molecules, upon formation of Au nanoparticle aggregates, the solution color will change from red to purple for Au nanoparticles, which can be easily visualized by naked eyes. Lei et al. reported a simple, reliable, and economical colorimetric assay using the peroxidase-like activity of chitosan-coated iron oxide MNPs [
43]. This simple colorimetric strategy allows for the rapid detection of bacterial cells down to 10
4 CFU/mL by the naked eye and 10
2 CFU/mL using spectrophotometry within 10 min.
Since the point-of-care facile and economical detection puts high demands on the early diagnosis and control of infections, Eissa et al. were inspired by the proteolytic activity of
Staphylococcus aureus (S. aureus) protease on specific peptide substrates on the same platform [
44]. A fast, simple, and cost-effective biosensor for
S. aureus was developed using dual colorimetric and electrochemical detection technology. Moreover, the biosensor was capable of specifically distinguishing
S. aureus (
Figure 1B).
Figure 1.
Schematic illustration of experimental procedures: (
A) detection of
Escherichia coli (
E. coli )O157:H7 using automated immunomagnetic separation and enzyme-based colorimetric assay (reproduced with permission from [
42]; published by MDPI, 2020); (
B) a dual electrochemical/colorimetric MNP/peptide-based platform for the detection of
S. aureus (reproduced with permission from [
44]; published by the Royal Society of Chemistry, 2020).
Figure 1.
Schematic illustration of experimental procedures: (
A) detection of
Escherichia coli (
E. coli )O157:H7 using automated immunomagnetic separation and enzyme-based colorimetric assay (reproduced with permission from [
42]; published by MDPI, 2020); (
B) a dual electrochemical/colorimetric MNP/peptide-based platform for the detection of
S. aureus (reproduced with permission from [
44]; published by the Royal Society of Chemistry, 2020).
3.2. Fluorescent Detection
Compared with colorimetric detection, fluorescent detection offers the advantages of high detection sensitivity and an enhanced limit of detection [
45]. Combining fluorescent dyes with MNPs can afford a detection method that integrates magnetic capture, fluorescent labeling, and detection. As a paradigm, Bhaisare et al. proposed a novel and simple method for detecting pathogenic bacteria, which uses fluorescence sensing and magnetic carbon dots (Mag-CD) to capture bacteria [
46]. Due to the magnetic driving force, the bacteria were firmly fixed on the hybrid material (Mag-CD) for highly sensitive fluorescence detection.
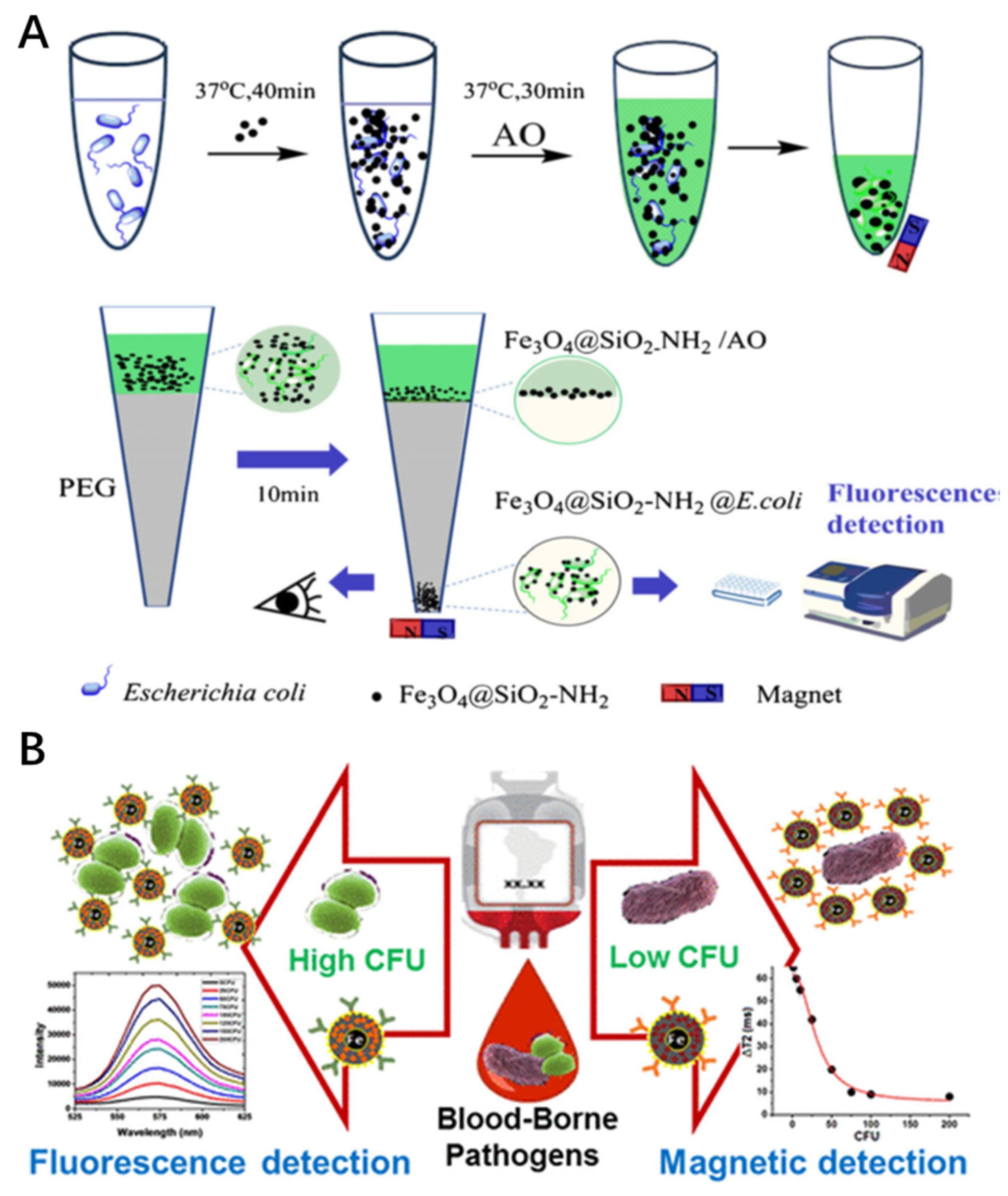
As pathogens usually exist in complex matrices at low concentrations, effective enrichment is essential for diagnosis. Che et al. established a rapid and sensitive analytical method to detect pathogenic bacteria, which combined magnetic enrichment and fluorescent labeling using polyethylene glycol (PEG) magnetophoretic chromatography [
47]. Pathogens were enriched with MNPs (Fe
3O
4 @ SiO
2-NH
2) through electrostatic interactions and the capture efficiency reached 95.4% for
E. coli (
Figure 2A). In addition, quantitative analysis of bacteria was performed using pyridine orange (AO) as a fluorescent probe for the captured
E. coli due to its abilities of staining series types of bacteria and rapid labeling. This is a method of semi-quantitative analysis via visual study and quantitative analysis via fluorescence detection.
In clinical settings, the detection of low bacterial contaminants is quite challenging. Banerjee et al. designed a method that allows for the specific interaction between the target pathogen and a functional magneto-fluorescent nanosensor (MFnS), resulting in the change of the magnetic relaxation time of water protons, which is indicative of the sensitive detection of the target bacteria from low to high colony-forming units (CFUs). The study established the design and synthesis of a dual-modal MFnS for the extensive detection of target pathogen by integrating MR and fluorescence modalities (
Figure 2B) [
48].
3.3. Surface-Enhanced Raman Scattering (SERS) Detection
SERS is a new method for bacterial identification. Raman spectroscopy can provide fingerprint information of pathogenic bacteria and is a very promising tool for rapid bacterial identification and antibiotic susceptibility testing [
49].
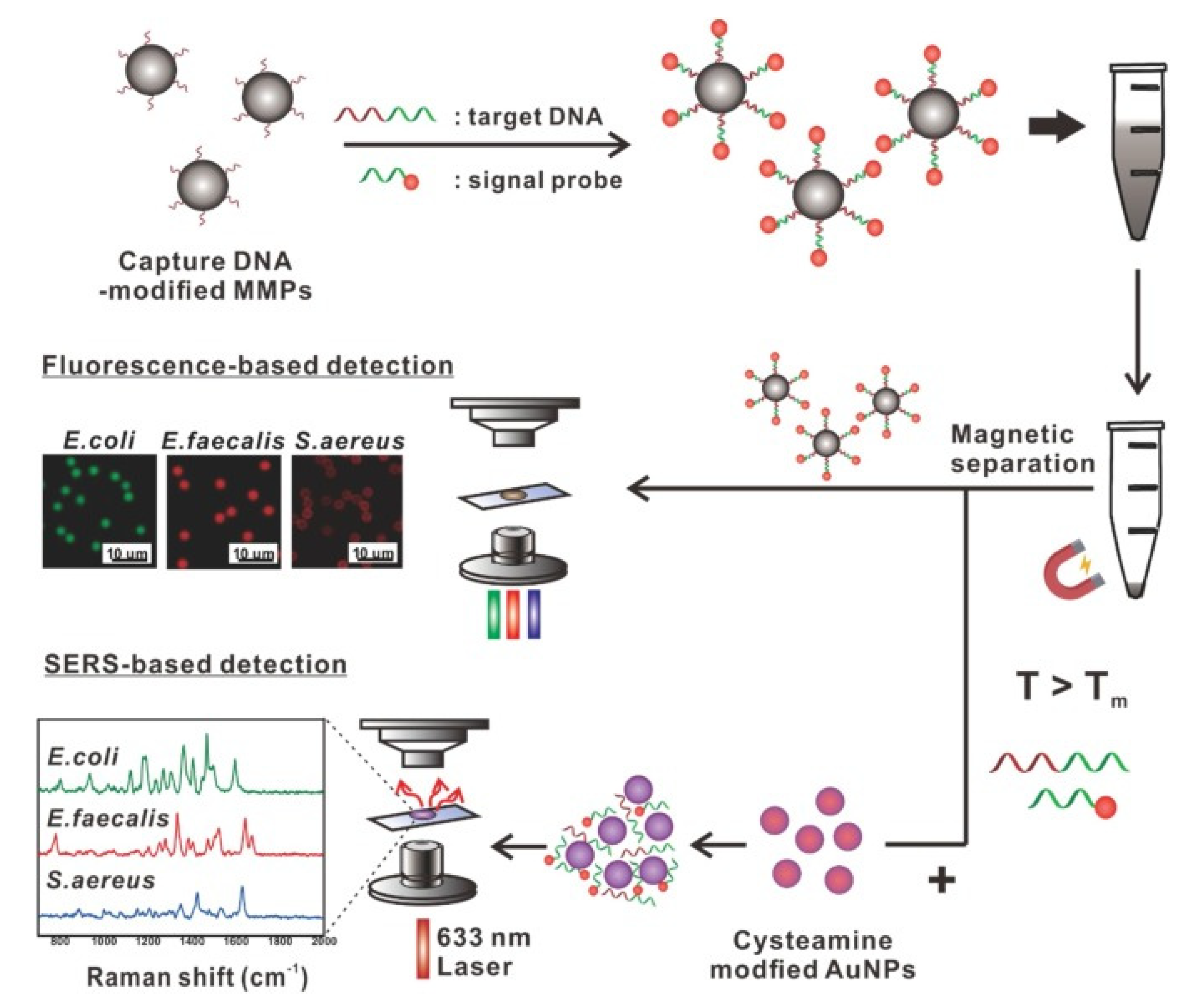
Due to the diversity of surface chemistry and easy separation of magnetic materials, they have been widely used in the detection of various protein-based biomarkers or nucleic acid targets. Hwang et al. designed a simple measurement method based on MMPs to target bacterial DNA with two different optical signals: fluorescence and Raman scattering [
50] (
Figure 3). Under optimized measurement conditions, the sensitivity and multiplexing capability of the two signals were strictly compared. The MMP-based detection method can detect the DNA of multiple target bacteria, such as
E. coli,
Enterococcus faecalis, and
S. aureus, with high reliability and sensitivity, as well as multiple detection capabilities. Detecting the genomic DNA of
E. coli using the SERS technique showed better sensitivity than fluorescence methods.
Based on the intuitive and fast imaging characteristics of fluorescence and SERS spectroscopy, it is possible to achieve quantitative and more sensitive analysis. Combining the advantages of these two spectral detection technologies, the fluorescence and SERS signal enhancement are integrated on the same substrate so that the substrate has both fluorescence imaging and SERS analysis capabilities. First, fluorescent signals are used for rapid positioning and then SERS technology is used for multi-target tracking and quantitative research. Jang et al. reported on the first preparation of dual nanoprobes based on SERS and fluorescence imaging modalities [
51]. These probes include layered nanostructures with metal nanoparticle clusters (MNPC) in combination with magnetic beads (MBs). The probes were deployed for the rapid multiplex detection of bacterial pathogens, which showed great potential for biodetection and bioimaging.
3.4. Nuclear Magnetic Resonance (NMR) Detection
NMR is an invaluable biophysical tool that is used in modern chemistry and the life sciences [
52]. As early as 1946, Block and Purcell reported this phenomenon and applied it to spectroscopy [
53]. The NMR sensor is a device that converts nuclear (or electronic) resonance information into electrical parameters and transmits it to the spectrometer based on the principle of nuclear magnetic resonance. Hash et al. used iron nanoparticles coated with target-specific biomarkers that are capable of binding to the DNA of the target microorganism to detect pathogenic levels of a microorganism (10
5 CFU/mL) with molecular mirroring [
54]. Zou et al. developed an Fe
3O
4 nanoparticle cluster biosensor for Salmonella detection using NMR [
55]. This method effectively detected Salmonella at 10
5 CFU/mL.
MRI was reported in 1974 by Paul Lauterbur and Peter Mansfield [
56] and has continuously been used in physics, chemistry, and clinical medicine. MRI provides biochemical and pathological statuses and information at the molecular level, which can be used for the early diagnosis or ultra-early diagnosis of edema, infection, inflammation, and degeneration in the human body. The contrast with soft tissues is large [
57]. With high resolution, it is very useful in determining the extent of inflammation, edema, and tumors. The rapid development of MR technology has been used to visualize infections caused by
S. aureus in osteomyelitis, endocarditis, and soft tissue infection models [
58]. Hoerr et al. provided a novel versatile tool to follow bacterial infections [
59], which were labeled with iron oxide particles to allow for the in vivo detection of small
S. aureus colonies in infection models using MRI.
4. MNPs as Antibacterial Therapies
The discovery of penicillin in 1928 by Alexander Fleming [
60] ushered in the “antibiotic era”; penicillin was truly a miracle drug: uniformly fatal infections could be cured. By the mid-1940s after the antibacterial efficacy of penicillin was clinically proven, antibiotics have begun to play a role in various diseases. The discovery of antibiotics has brought effective cures for many diseases to mankind [
61]. Yet, as human beings progress, bacteria are constantly evolving. When antibiotics are widely used, they are also constantly losing their antibacterial efficacy, leading to the problem of antibiotic resistance [
62,
63]. Therefore, the development of new antibiotic therapies is one of the important strategies that are used to combat super-resistant bacteria. In particular, finding new sterilization mechanisms has become a new hot spot in the development of new therapies for the treatment of infections.
Along with the extensive range of exotic NPs applications, the investigation of MNPs in vitro has ushered modern antibacterial studies into an increasingly attractive research area. The great potential of engineered MNPs in the treatment of various resistant bacteria has reduced the threat of deadly bacterial infections [
64]. The properties of MNPs allow them to be guided around the body by a magnetic field or into magnetic implants. This opens up the potential to combine various biological materials with nanoparticles, which can then be directed into the body for treatment [
22].
4.1. Magnetic Hyperthermia
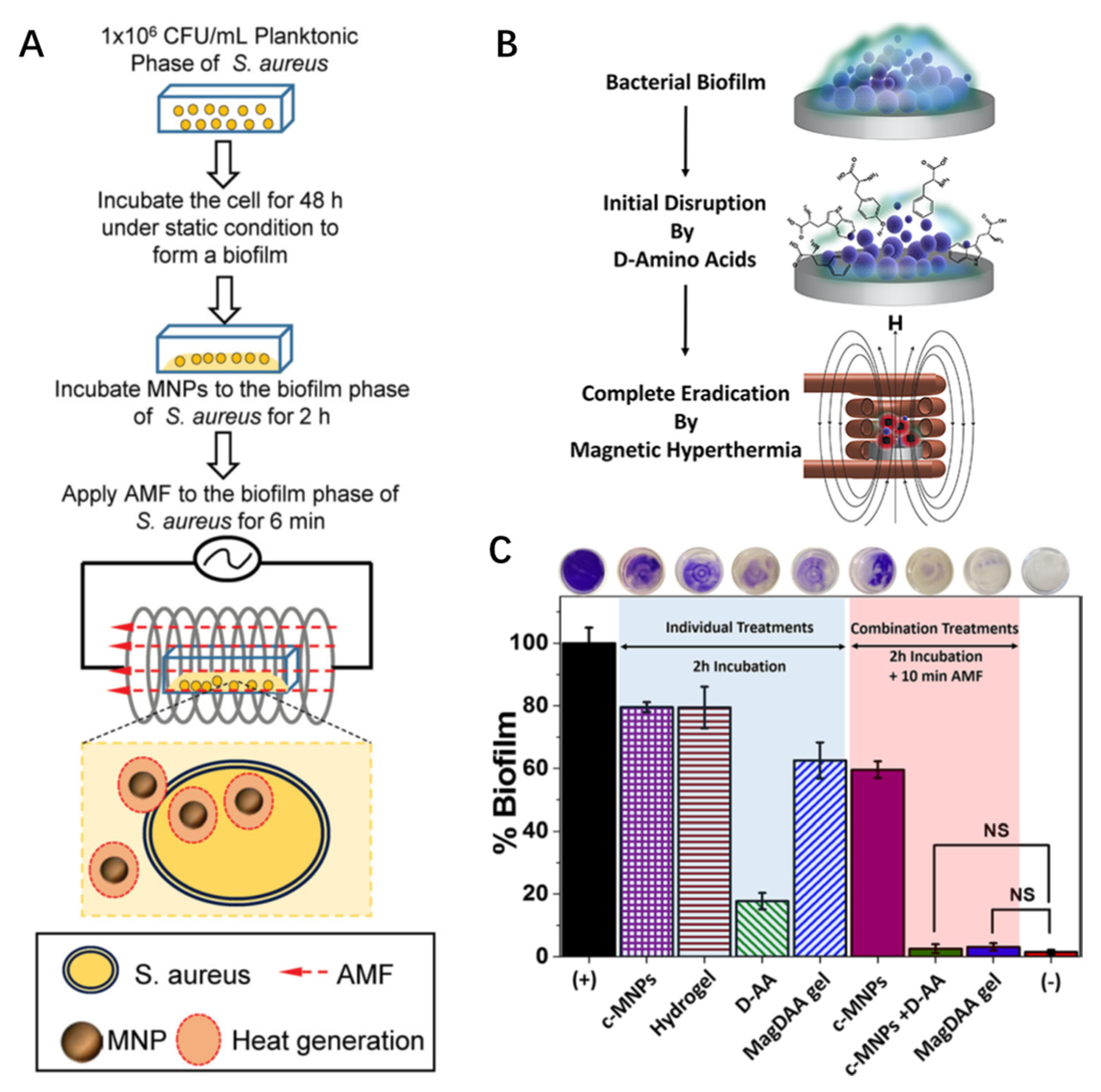
Magnetic hyperthermia usually uses the principle of electromagnetic waves to generate heat, which acts on soft tissues to achieve therapeutic effects. Generally, it can promote local blood circulation and accelerate the absorption of local inflammation, which can also promote the repair of injured soft tissues. Alumutairi et al. demonstrated that MNP/alternating magnetic field (MNP/AMF) hyperthermia has a synergistic effect with conventional antibiotics within a safe thermal dose threshold [
65]. This was performed by correlating MNP/AMF-induced thermal ablation in an in vitro cell culture setting. For this purpose, varying combinations of MNPs concentration and AMF strength were applied to the culture of
S. aureus biofilm and the change in ambient temperature in the media of
S. aureus biofilm was measured (
Figure 4A). This contributed to the innate immune response of host macrophages to eliminate intracellular bacteria. Abenojar et al. developed thermally responsive magnetic nanocomposites to eliminate pre-formed biofilms [
66]. Loading the hydrogel nanocomposite with MNPs allows for a combined heat treatment after magnetic field (magnetic hyperthermia) stimulation. First, the D-amino acids in the hydrogel nanocomposite inhibited
S. aureus biofilm formation. Then, complete eradication via magnetic hyperthermia occurred, thus realizing an antibacterial treatment (
Figure 4B). The use of the magnetic glycolchitin-based, D-AA-loaded hydrogel—which involves initial treatment for 2 h, followed by additional AMF treatment for 10 min at AC field amplitude of 5 kA/m and a frequency of 380 kHz—showed complete biofilm disruption. NS indicates that the difference in the means of the treatments was not statistically significant at
p > 0.05 (
Figure 4C). Chen et al. evaluated magnetothermal therapy mediated by magnetotactic bacteria with magnetic nanocrystals and exhibiting magneto-controlled swimming [
67]. The nanocrystals effectively eradicated
S. aureus both in vitro and in vivo, indicating that magnetotactic bacteria-mediated magnetothermal therapy can be used as a treatment method for skin or wound infections that are induced by
S. aureus. In addition, the adhesion between magnetotactic bacteria and
S. aureus increases the killing efficiency of hyperthermia to more than 50%.
In addition to iron-based composite magnetic nanomaterials, Mn- and/or Ni-doped magnetic nanomaterials were also used in bacterial therapy [
68].
4.2. Multifunctional MNPs for Theranostics
Clinically, the diagnosis and treatment of diseases are two relatively independent processes. The two administrations of diagnostic medication and therapeutic medication will put more pressure on the patient’s metabolism, increase the patient’s pain, and increase the risk of more side effects. In order to overcome these serious problems, based on the concept of visual therapy, a new concept of the integration of diagnosis and therapy that organically combines imaging and therapy was proposed.
The concept of “theranostics” was first coined by John Funkhouser in 1998 [
69]. He defined the term “theranostics” as “the ability to affect therapy or treatment of a disease state.” With the rapid and vigorous development of the integration of diagnosis and treatment, its definition has also expanded. It is now generally believed that the integration of diagnosis and treatment is a new type of biomedical technology that organically combines the diagnosis or monitoring of diseases with treatment [
70]. In recent years, researchers have developed a series of nanomedicines that can realize the integration of diagnosis and treatment, bringing new hope for humans to overcome diseases [
71,
72]. The following is a brief introduction to the latest research progress of the integrated diagnosis and treatment of bacterial infections.
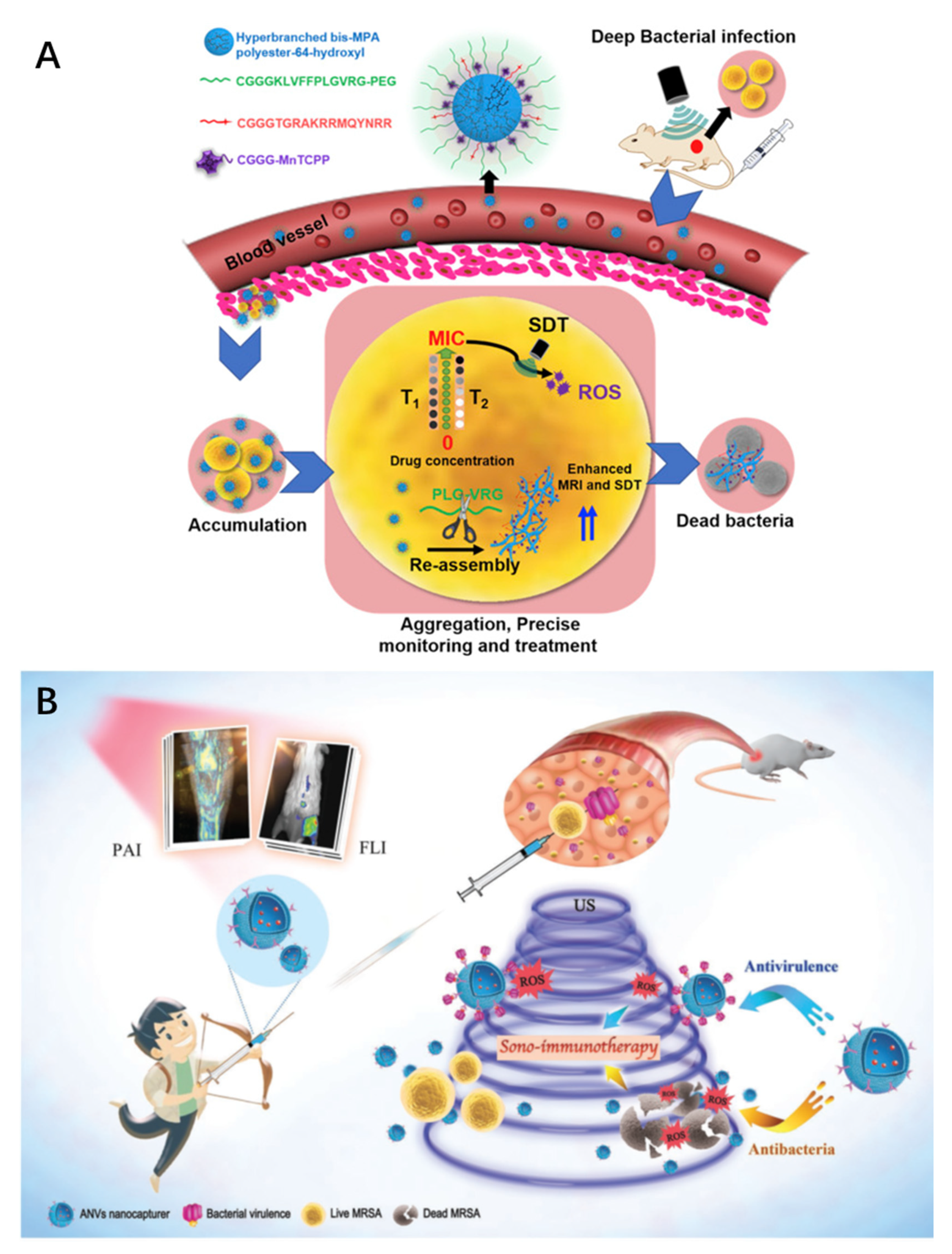
In order to accurately treat drug-resistant deep-seated bacterial infections, Wang et al. built a polymer–peptide–porphyrin conjugate (PPPC) that can monitor the site of infection in real time based on an in vivo self-assembly strategy and used it to perform precise sonodynamic therapy (SDT) for deep infections [
73]. MRI was used to monitor the porphyrin sonosensitizer (MnTCPP) contained in PPPCs in the infection site in situ. When the concentration of PPPC-1 reaches the minimum inhibitory concentration (MIC), the infected area of drug-resistant bacteria can be exposed to ultrasound irradiation and the bacteria can be killed accurately and efficiently (
Figure 5A).
In another study, Pang et al. proposed a simple and bioinspired strategy to bridge antibacterial SDT and antiviral immunotherapy [
74]. It was used as a novel method of biomimetic nanovesicle (ANV) nanocapture for non-invasive fluorescence/photoacoustic (PA) diagnosis and powerful sono-immunotherapy for methicillin-resistant
Staphylococcus aureus (MRSA) infections. After ANVs were activated using ultrasound, the ultrasound sensitizer effectively produced reactive oxygen species to kill bacteria and accelerate the elimination of virulence. In vivo optical imaging showed that the antibody-directed nanotrap successfully located MRSA infections and accurately distinguished between lesions and sterile inflammation. In situ MRI and oxygenated hemoglobin saturation detection visualized the progress of treatment, showing that mouse MRSA myositis was completely eradicated during the ultrasound immunotherapy (
Figure 5B).
5. Summary and Perspectives
MNPs have been extensively studied in bacterial diagnosis and treatment, mainly due to their unique superparamagnetic properties, large specific surface area, and easy surface modification. MNPs also possess excellent antibacterial properties that are mainly driven by magnetic fields and active oxygen generation, which are used to treat bacteria in vitro and in vivo. This article reviewed their application in the detection and treatment of bacterial infections. By also leveraging their unique superparamagnetism, MNPs were used to develop various detection schemes for detecting signal amplification or direct signal transduction. Different detection strategies, including colorimetric, fluorescence, SERS, PAI, and NMR, were developed to detect bacteria. The application of MNPs in the treatment of bacterial infections was also discussed in detail in this review with emphasis on magnetic hyperthermia and theranostics.
MNPs were shown to be superior bacterial detection and therapeutic agents. However, before their clinical application as a diagnostic and therapeutic drug, there are still some challenges to overcome. Hitherto, the utilization of MNPs for the detection of bacteria is still in the proof-of-concept stage, where they were evaluated in facile laboratory samples rather than body fluids. Large-scale tests should be carried out in different complex biological samples to simulate a real environment. Various surface modifications are needed to further improve detection selectivity and material stability, and biocompatibility is an important criterion for clinical applications. Without affecting their antibacterial activity, minimizing the toxicity of MNPs to normal eukaryotic cells is still a very challenging problem. Although there has been a lot of progress in the past few decades on the development of MNPs for bacterial detection, there are still great challenges that need to be resolved before their clinical application, such as the stability of the MNPs, which is essential in the practical application, and non-specifically aggregated particles may be formed in a complex biological system, leading to false positive or false negative signals. In order to overcome these problems, effective surface modification could be used to improve their stability and reduce background signals. Furthermore, the selectivity could be further improved for the detection of bacteria. Suitable surface modification using various strategies to identify agents for the MNPs could be conducted to solve this problem.
Nanomaterials with both photothermal and sonodynamic effects have higher application potential, and their preclinical evaluation needs to be further studied. Despite the therapeutic effects of MNPs summarized in this review, their applications are just in the laboratory stage, which mostly involves in vitro testing. It is common knowledge that various parameters change on transiting from in vitro to in vivo applications. Despite significant progress in MNPs for the treatment of bacterial infections, there are still some issues to be resolved. Toxicity and side effects of the MNPs need to be carefully and extensively investigated to confirm that these nanomaterials are suitable for clinical application. It is necessary to study the toxicity of MNPs, especially improving the bio-compatibility, to reduce non-specific toxicity in vitro and in vivo. Surface modification of MNPs with biocompatible components, such as peptides or polysaccharides, could be the future direction. Finally, the addition of selective recognition agents, such as specific antibiotics or antibodies of identified pathogens, could be explored to further improve the selectivity of the MNPs. Finally, the use of multifunctional MNPs can perform detection and treatment at the same time, realize image-guided treatment, and provide a new direction for the development of nanomedicine. It is ultimately expected that this report will significantly help in developing effective and broad-spectrum magnetic nanomedicines for the treatment of bacterial infections.
Author Contributions
Conceptualization, L.X., O.U.A., C.X. and A.W.; methodology, L.X.; software, L.X.; validation, L.X., O.U.A. and C.X.; formal analysis, L.X.; investigation, L.X.; resources, L.X.; data curation, L.X.; writing—original draft preparation, L.X.; writing—review and editing, O.U.A. and C.X.; visualization, A.W.; supervision, A.W.; project administration, A.W.; funding acquisition, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
National Key R&D Program of China, Grant/Award Numbers: 2019YFA0405603, 2018YFC0910601; National Natural Science Foundation of China, Grant/Award Numbers: 32025021, 31971292, 51873225; Zhejiang Province Financial Supporting, Grant/Award Number: 2020C03110, LQ19H180002, LQ20H180002, 2020C03110; Natural Science Foundation of Ningbo, Grant/Award Number: 2020Z094; Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province, Grant/Award Number: 2019E10020; Key Program of Zhejiang Province Medical Scientific Research Foundation, Grant/Award Number: WKJ-ZJ-1807.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.
Acknowledgments
The authors acknowledge the support of the National Key R&D Program of China (2019YFA0405603, 2018YFC0910601), National Natural Science Foundation of China (32025021, 31971292, 51873225), Funding in Zhejiang Province (2020C03110, LQ19H180002, LQ20H180002, 2020C03110), the Science & Technology Bureau of Ningbo City (2020Z094), Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province (2019E10020), and Key Program of Zhejiang Province Medical Scientific Research Foundation (WKJ-ZJ-1807).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Jin, Q.; Ji, J. Emerging antibacterial nanomedicine for enhanced antibiotic therapy. Biomater. Sci. 2020, 8, 6825–6839. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.W.; Barnett, M.L.; MacFadden, D.R.; Brownstein, J.S.; Hernandez-Diaz, S.; Lipsitch, M.; Grad, Y.H. The distribution of antibiotic use and its association with antibiotic resistance. Elife 2018, 7, e39435. [Google Scholar] [CrossRef] [PubMed]
- Campanini-Salinas, J.; Andrades-Lagos, J.; Melia-Raipan, J.; Vasquez-Velasquez, D. Novel Classes of Antibacterial Drugs in Clinical Development, a Hope in a Post-antibiotic Era. Curr. Top. Med. Chem. 2018, 18, 1188–1202. [Google Scholar] [CrossRef]
- Kollef, M.H.; Bassetti, M.; Francois, B.; Burnham, J.; Dimopoulos, G.; Garnacho-Montero, J.; Lipman, J.; Luyt, C.E.; Nicolau, D.P.; Postma, M.J.; et al. The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Intensive Care Med. 2017, 43, 1187–1197. [Google Scholar] [CrossRef]
- Bhatia, B.D.; Basu, S. Newer diagnostic tests for bacterial diseases. Indian J. Pediatr. 2007, 74, 673–677. [Google Scholar] [CrossRef]
- Yuan, P.; Ding, X.; Yang, Y.Y.; Xu, Q.H. Metal Nanoparticles for Diagnosis and Therapy of Bacterial Infection. Adv. Healthc. Mater. 2018, 7, e1701392. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, Y.; Qiu, Y.; Yang, X.Z.; Cao, H.; Wu, Y. Design of Functional Magnetic Nanocomposites for Bioseparation. Colloids Surf. B Biointerfaces 2020, 191, 111014. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Zheng, J.J.; Wu, A.G. Applications of Iron Oxide-Based Magnetic Nanoparticles in the Diagnosis and Treatment of Bacterial Infections. Front. Bioeng. Biotechnol. 2019, 7, 00141. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, T.; Iqbal, M.Z.; Yang, F.; Hampp, N.; Wu, A.; Luo, L. Applications of magnetic materials separation in biological nanomedicine. Electrophoresis 2019, 40, 2011–2028. [Google Scholar] [CrossRef]
- Bell, C.S.; Mejias, R.; Miller, S.E.; Greer, J.M.; McClain, M.S.; Cover, T.L.; Giorgio, T.D. Magnetic Extraction of Acinetobacter baumannii Using ColistinFunctionalized gamma-Fe2O3/Au Core/Shell Composite Nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 26719–26730. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L.; Sica de Toledo, L.d.A. Pharmaceutical Applications of Iron-Oxide Magnetic Nanoparticles. Magnetochemistry 2019, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Matai, I.; Garg, M.; Rana, K.; Singh, S. Polydopamine functionalized hydrogel beads as magnetically separable antibacterial materials. RSC Adv. 2019, 9, 13444–13457. [Google Scholar] [CrossRef] [Green Version]
- Chandrappa, M.; Swathi, K.; Vijayalakshmi, U.; Pullela, P.K.; Kumar, S.G. Magnetic Nanoparticle Assisted Bulk Scale Synthesis of Quinazoline Synthon. Adv. Sci. Lett. 2018, 24, 5936–5941. [Google Scholar] [CrossRef]
- Bucki, R.; Niemirowicz-Laskowska, K.; Deptula, P.; Wilczewska, A.Z.; Misiak, P.; Durnas, B.; Fiedoruk, K.; Piktel, E.; Mystkowska, J.; Janmey, P.A. Susceptibility of microbial cells to the modified PIP2-binding sequence of gelsolin anchored on the surface of magnetic nanoparticles. J. Nanobiotechnol. 2019, 17, 81. [Google Scholar] [CrossRef] [Green Version]
- Abdulhady, Y.A.M.; El-Shazly, M.M.; El-Kased, R.F. Evaluation of antibacterial activity and toxic metal removal of chemically synthesized magnetic iron oxide titanium coated nanoparticles and application in bacterial treatment. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 205–212. [Google Scholar] [CrossRef]
- Chen, C.; Xia, D.-L.; Guo, L.-Y.; Chen, Y.-P.; Li, X.-D.; Wang, Y.-F.; Zhang, D.; Wang, Y.-Y.; Zhang, Y.-X.; He, H.; et al. Extracorporeal magnetic approach to reduce the unwanted side-effects and improve antibacterial activity of Ag/Fe3O4 nanocomposites in rat. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2029–2036. [Google Scholar] [CrossRef]
- Ai, C.; Wu, S.; Li, L.; Lei, Y.; Shao, X. Novel magnetically separable gamma-Fe2O3/Ag/AgCl/g-C3N4 composite for enhanced disinfection under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123981. [Google Scholar] [CrossRef]
- Lei, J.; Chen, S.; Yanyi, W.; Zhongjie, Z.; Jia, L.; Sirui, H.; Shu, H.; Xiufeng, L.; Wei, S. Antibacterial activity and long-term stable antibacterial performance of nisin grafted magnetic GO nanohybrids. Mater. Sci. Eng. C 2020, 111, 110809. [Google Scholar] [CrossRef]
- Akter, N.; Chowdhury, L.; Uddin, J.; Ullah, A.K.M.A.; Shariare, M.H.; Azam, M.S. N-halamine functionalization of polydopamine coated Fe3O4 nanoparticles for recyclable and magnetically separable antimicrobial materials. Mater. Res. Express 2018, 5, 115007. [Google Scholar] [CrossRef]
- Al-Jabari, M.H.; Sulaiman, S.; Ali, S.; Barakat, R.; Mubarak, A.; Khan, S.A. Adsorption study of levofloxacin on reusable magnetic nanoparticles: Kinetics and antibacterial activity. J. Mol. Liq. 2019, 291, 111249. [Google Scholar] [CrossRef]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, S.M.; Nivetha, A.; Prabha, I. Superparamagnetic Properties and Significant Applications of Iron Oxide Nanoparticles for Astonishing Efficacy—A Review. J. Supercond. Nov. Magn. 2018, 32, 127–144. [Google Scholar] [CrossRef]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, M.; Ghoorchian, A.; Dashtian, K.; Kamalabadi, M.; Madrakian, T.; Afkhami, A. Application of magnetic nanomaterials in electroanalytical methods: A review. Talanta 2021, 225, 121974. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasir, S.; Anwar, A.; Kawish, M.; Anwar, A.; Shah, M.R.; Siddiqui, R.; Khan, N.A. Metronidazole conjugated magnetic nanoparticles loaded with amphotericin B exhibited potent effects against pathogenicAcanthamoeba castellaniibelonging to the T4 genotype. AMB Express 2020, 10, 127. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahreinizad, H.; Amiri, Z.; Aliabadi, H.A.M.; Salimi-Bani, M.; Nakisa, A.; Davoodi, F.; Tahmasebi, B.; Ahmadpour, F.; Radinekiyan, F.; et al. Functionalized magnetic nanoparticles for the separation and purification of proteins and peptides. TrAC Trends Anal. Chem. 2021, 141, 116291. [Google Scholar] [CrossRef]
- Huang, R.Y.; Liu, Z.H.; Weng, W.H.; Chang, C.W. Magnetic nanocomplexes for gene delivery applications. J. Mater. Chem. B 2021, 9, 4267–4286. [Google Scholar] [CrossRef]
- Guo, G.Y.; Wang, Y.K.; Chen, Y.Y. Ab initio studies of the electronic structure and magnetic properties of bulk and nano-particle CeCo2. J. Magn. Magn. Mater. 2004, 272, E1193–E1194. [Google Scholar] [CrossRef]
- Saha, S.; Das, K.; Bandyopadhyay, S.; Das, I. A comparative study of magnetic field induced meta-magnetic transition in nanocrystalline and bulk Pr0.65(Ca0.7Sr0.3)0.35MnO3 compound. J. Magn. Magn. Mater. 2017, 432, 271–275. [Google Scholar] [CrossRef]
- Dutta, P.; Seehra, M.S.; Thota, S.; Kumar, J. A comparative study of the magnetic properties of bulk and nanocrystalline Co3O4. J. Phys. Condens. Matter 2008, 20, 015218. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.L.; Gillis, P.; Roch, A.; Gossuin, Y. Magnetic resonance relaxation induced by superparamagnetic particles used as contrast agents in magnetic resonance imaging: A theoretical review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1468. [Google Scholar] [CrossRef]
- Jarockyte, G.; Daugelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.C.; Hsu, S.H.; Karabanovas, V.; Rotomskis, R. Accumulation and Toxicity of Superparamagnetic Iron Oxide Nanoparticles in Cells and Experimental Animals. Int. J. Mol. Sci. 2016, 17, 1193. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2020, 33, 1906539. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Huang, X.L.; Zhang, W.J.; Ji, Y.W.; Chen, R.; Xiong, Y.H. Multi-branched gold nanoflower-embedded iron porphyrin for colorimetric immunosensor. Biosens. Bioelectron. 2018, 102, 9–16. [Google Scholar] [CrossRef]
- Chenyang, Y.; Fang, Y.; Li, S.; Yuanyuan, M.; Stanciu, S.G.; Zihou, L.; Chuang, L.; Akakuru, O.U.; Lipeng, X.; Norbert, H.; et al. Magnetically switchable mechano-chemotherapy for enhancing the death of tumour cells by overcoming drug-resistance. Nano Today 2020, 35, 100967. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A.; Norbakhsh, H.; Pournajaf, R.; Tayebi, M. Synthesis of mesoporous cobalt ferrite/hydroxyapatite core-shell nanocomposite for magnetic hyperthermia and drug release applications. Ceram. Int. 2021, 47, 18167–18176. [Google Scholar] [CrossRef]
- Crăciunescu, I.; Palade, P.; Iacob, N.; Ispas, G.M.; Stanciu, A.E.; Kuncser, V.; Turcu, R.P. High-Performance Functionalized Magnetic Nanoparticles with Tailored Sizes and Shapes for Localized Hyperthermia Applications. J. Phys. Chem. C 2021, 125, 11132–11146. [Google Scholar] [CrossRef]
- Wang, H.; Wu, T.; Li, M.; Tao, Y. Recent advances in nanomaterials for colorimetric cancer detection. J. Mater. Chem. B 2021, 9, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [Green Version]
- Mahheidari, N.; Rashidiani, J.; Kooshki, H.; Eskandari, K. An Effort to Making a Colorimitric Nano-Biosensor for Vibrio cholera Detection. Curr. Nanosci. 2020, 16, 793–804. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, K.; Ok, G.; Chang, H.J.; Park, T.J.; Choi, S.W.; Lim, M.C. Detection of Escherichia coli O157:H7 Using Automated Immunomagnetic Separation and Enzyme-Based Colorimetric Assay. Sensors 2020, 20, 1395. [Google Scholar] [CrossRef] [Green Version]
- Le, T.N.; Tran, T.D.; Kim, M.I. A Convenient Colorimetric Bacteria Detection Method Utilizing Chitosan-Coated Magnetic Nanoparticles. Nanomaterials 2020, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Eissa, S.; Zourob, M. A dual electrochemical/colorimetric magnetic nanoparticle/peptide-based platform for the detection ofStaphylococcus aureus. Analyst 2020, 145, 4606–4614. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Han, L.; Li, Q.J.; Li, L.Y.; Liu, Y.; Song, Y.; Tan, W.H.; Song, E.R. Gradient Magnetic Separation and Fluorescent Imaging-Based Heterogeneous Circulating Tumor Cell Subpopulations Assay with Biomimetic Multifunctional Nanoprobes. Adv. Funct. Mater. 2021, 31, 2009937. [Google Scholar] [CrossRef]
- Bhaisare, M.L.; Gedda, G.; Khan, M.S.; Wu, H.F. Fluorimetric detection of pathogenic bacteria using magnetic carbon dots. Anal. Chim. Acta 2016, 920, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.L.; Xu, Y.; Wang, R.J.; Chen, L. Rapid fluorescence detection of pathogenic bacteria using magnetic enrichment technique combined with magnetophoretic chromatography. Anal. Bioanal. Chem. 2017, 409, 4709–4718. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Tummala, T.; Elliott, R.; Jain, V.; Brantley, W.; Hadorn, L.; Santra, S. Multimodal Magneto-Fluorescent Nanosensor for Rapid and Specific Detection of Blood-Borne Pathogens. ACS Appl. Nano Mater. 2019, 2, 5587–5593. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.B.; Hu, Z.W.; Yu, G.X.; Yang, D.T.; Zhao, J.S. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. [Google Scholar] [CrossRef]
- Hwang, M.J.; Jang, A.S.; Lim, D.-K. Comparative study of fluorescence and surface-enhanced Raman scattering with magnetic microparticle-based assay for target bacterial DNA detection. Sens. Actuators B Chem. 2021, 329, 129134. [Google Scholar] [CrossRef]
- Jang, H.; Hwang, E.Y.; Kim, Y.; Choo, J.; Jeong, J.; Lim, D.W. Surface-Enhanced Raman Scattering and Fluorescence-Based Dual Nanoprobes for Multiplexed Detection of Bacterial Pathogens. J. Biomed. Nanotechnol. 2016, 12, 1938–1951. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.B.; Huang, Y.H.; Lu, H.F.; Qiu, T.Y.; Guo, D.; Agback, T.; Orekhov, V.; Chen, Z. Accelerated Nuclear Magnetic Resonance Spectroscopy with Deep Learning. Angew. Chem. Int. Ed. 2020, 59, 10297–10300. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, W.C. The Time Average Magnetic Field at the Nucleus in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1951, 81, 717–731. [Google Scholar] [CrossRef]
- Hash, S.; Martinez-Viedma, M.P.; Fung, F.; Han, J.E.; Yang, P.; Wong, C.; Doraisamy, L.; Menon, S.; Lightner, D. Nuclear magnetic resonance biosensor for rapid detection of Vibrio parahaemolyticus. Biomed. J. 2019, 42, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.C.; Jin, L.; Wu, B.; Hu, L.W.; Chen, X.G.; Huang, G.H.; Zhang, J.S. Rapid detection of Salmonella in milk by biofunctionalised magnetic nanoparticle cluster sensor based on nuclear magnetic resonance. Int. Dairy J. 2019, 91, 82–88. [Google Scholar] [CrossRef]
- Geva, T. Magnetic resonance imaging: Historical perspective. J. Cardiovasc. Magn. Reson. 2006, 8, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, M.H.M.; Lauterbur, P.C. Contrast Agents for Nuclear-Magnetic-Resonance Imaging. Biol. Trace Elem. Res. 1987, 13, 229–239. [Google Scholar] [CrossRef]
- Ohlsen, K.; Hertlein, T. Towards clinical application of non-invasive imaging to detect bacterial infections. Virulence 2018, 9, 943–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoerr, V.; Tuchscherr, L.; Hueve, J.; Nippe, N.; Loser, K.; Glyvuk, N.; Tsytsyura, Y.; Holtkamp, M.; Sunderkoetter, C.; Karst, U.; et al. Bacteria tracking by in vivo magnetic resonance imaging. BMC Biol. 2013, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Diggins, F.W.E. The true history of the discovery of penicillin, with refutation of the misinformation in the literature. Br. J. Biomed. Sci. 1999, 56, 83–93. [Google Scholar]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sexton, P.M. Targeting Antibiotic Resistance: From Diagnostics to Novel Antibiotics. ACS Pharmacol. Transl. Sci. 2020, 3, 371–372. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T. Antibiotics Special Issue: Challenges and Opportunities in Antibiotic Discovery and Development. ACS Infect. Dis. 2020, 6, 1286–1288. [Google Scholar] [CrossRef]
- Allafchian, A.; Hosseini, S.S. Antibacterial magnetic nanoparticles for therapeutics: A review. IET Nanobiotechnol. 2019, 13, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Alumutairi, L.; Yu, B.; Filka, M.; Nayfach, J.; Kim, M.H. Mild magnetic nanoparticle hyperthermia enhances the susceptibility of Staphylococcus aureus biofilm to antibiotics. Int. J. Hyperth. 2020, 37, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abenojar, E.C.; Wickramasinghe, S.; Ju, M.; Uppaluri, S.; Klika, A.; George, J.; Barsoum, W.; Frangiamore, S.J.; Higuera-Rueda, C.A.; Samia, A.C.S. Magnetic Glycol Chitin-Based Hydrogel Nanocomposite for Combined Thermal and D-Amino-Acid-Assisted Biofilrn Disruption. ACS Infect. Dis. 2018, 4, 1246–1256. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, L.J.; Yi, Y.; Chen, C.F.; Wu, L.F.; Song, T. Killing of Staphylococcus aureus via Magnetic Hyperthermia Mediated by Magnetotactic Bacteria. Appl. Environ. Microbiol. 2016, 82, 2219–2226. [Google Scholar] [CrossRef] [Green Version]
- Ates, T.; Dorozhkin, S.V.; Kaygili, O.; Kom, M.; Ercan, I.; Bulut, N.; Firdolas, F.; Keser, S.; Gursoy, N.C.; Ozercan, I.H.; et al. The effects of Mn and/or Ni dopants on the in vitro/in vivo performance, structural and magnetic properties of beta-tricalcium phosphate bioceramics. Ceram. Int. 2019, 45, 22752–22758. [Google Scholar] [CrossRef]
- Idee, J.-M.; Louguet, S.; Ballet, S.; Corot, C. Theranostics and contrast-agents for medical imaging: A pharmaceutical company viewpoint. Quant. Imaging Med. Surg. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lovell, J.F. Advanced Functional Nanomaterials for Theranostics. Adv. Funct. Mater. 2017, 27, 1603524. [Google Scholar] [CrossRef] [Green Version]
- Chiari-Andreo, B.G.; Abucafy, M.P.; Manaia, E.B.; da Silva, B.L.; Rissi, N.C.; Oshiro, J.A.; Chiavacci, L.A. Drug Delivery Using Theranostics: An Overview of its Use, Advantages and Safety Assessment. Curr. Nanosci. 2020, 16, 3–14. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, D.B.; Ji, L.; Niu, L.J.; Zhang, X.H.; Cong, Y.; Cao, R.H.; Zhou, L.; Bai, F.; Qiao, Z.Y.; et al. Precise magnetic resonance imaging-guided sonodynamic therapy for drug-resistant bacterial deep infection. Biomaterials 2021, 264, 120386. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Liu, X.; Cheng, Y.; Zhang, C.; Ren, E.; Liu, C.; Zhang, Y.; Zhu, J.; Chen, X.; Liu, G. Sono-Immunotherapeutic Nanocapturer to Combat Multidrug-Resistant Bacterial Infections. Adv. Mater. 2019, 31, e1902530. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).