Exploring the Slow Magnetic Relaxation of a Family of Photoluminescent 3D Lanthanide–Organic Frameworks Based on Dicarboxylate Ligands
Abstract
1. Introduction
2. Results and Discussion
2.1. General Comments on the Synthesis
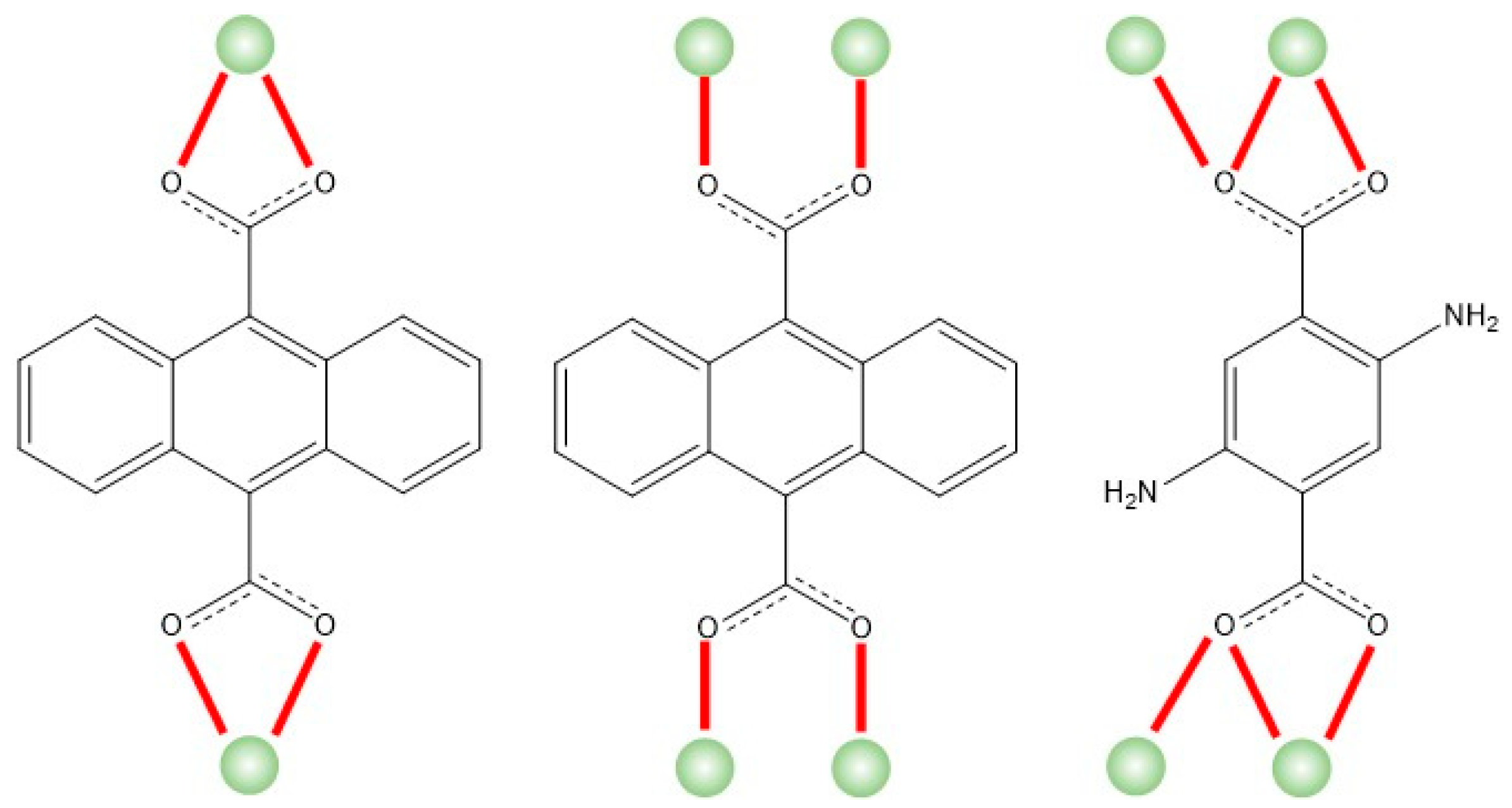
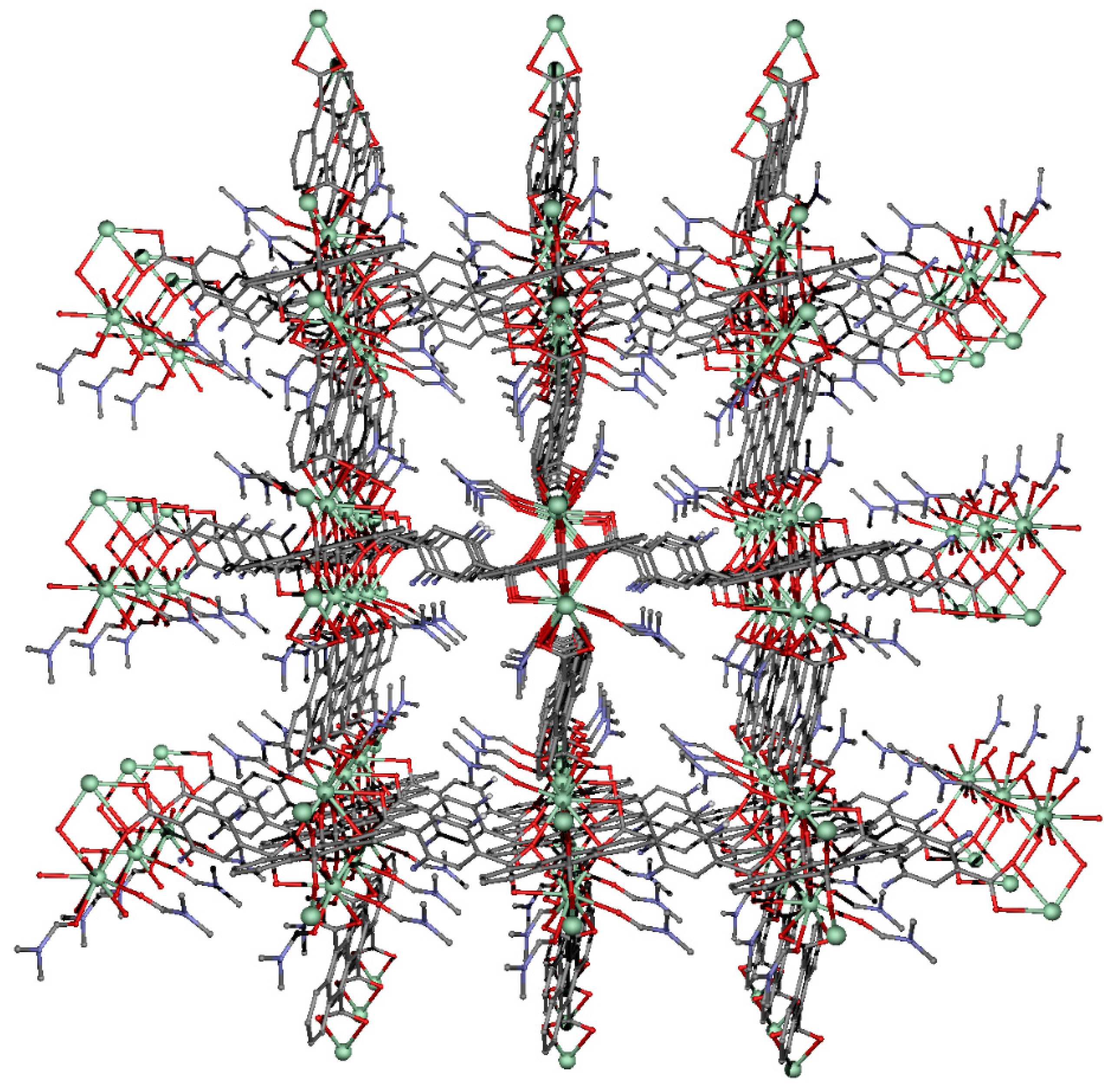
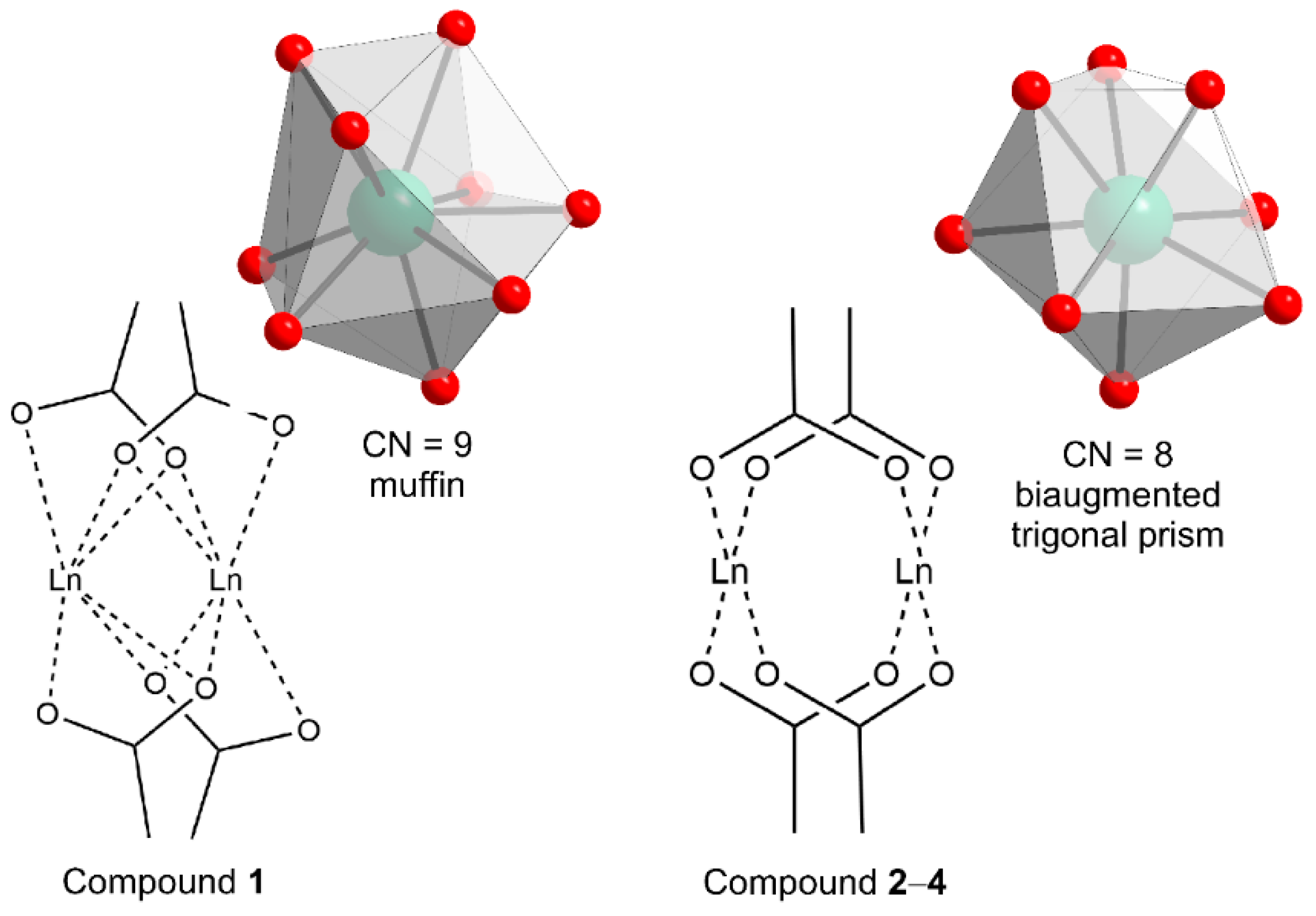
2.2. Structural Description of Compound {[Nd2(ant)2((NH2)2-bdc)(DMF)4]·2DMF}n (1)
2.3. Structural Description of Compounds {[Ln2(ant)2((NH2)2-bdc)(DMF)4]·2DMF·2H2O}n (Ln = Tb (2), Ho (3) and Er (4))
3. Magnetic Properties
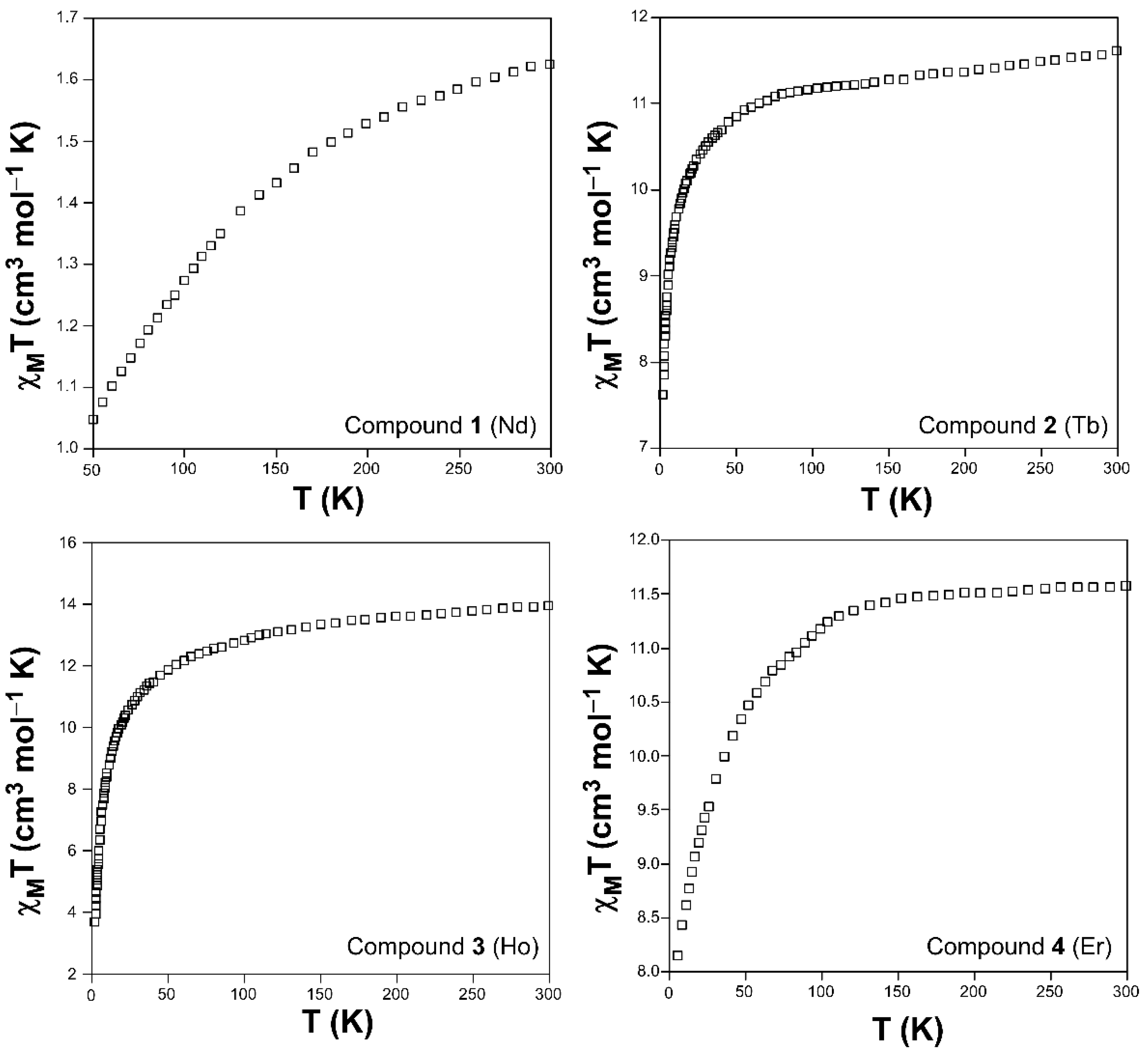
3.1. DC Magnetic Properties
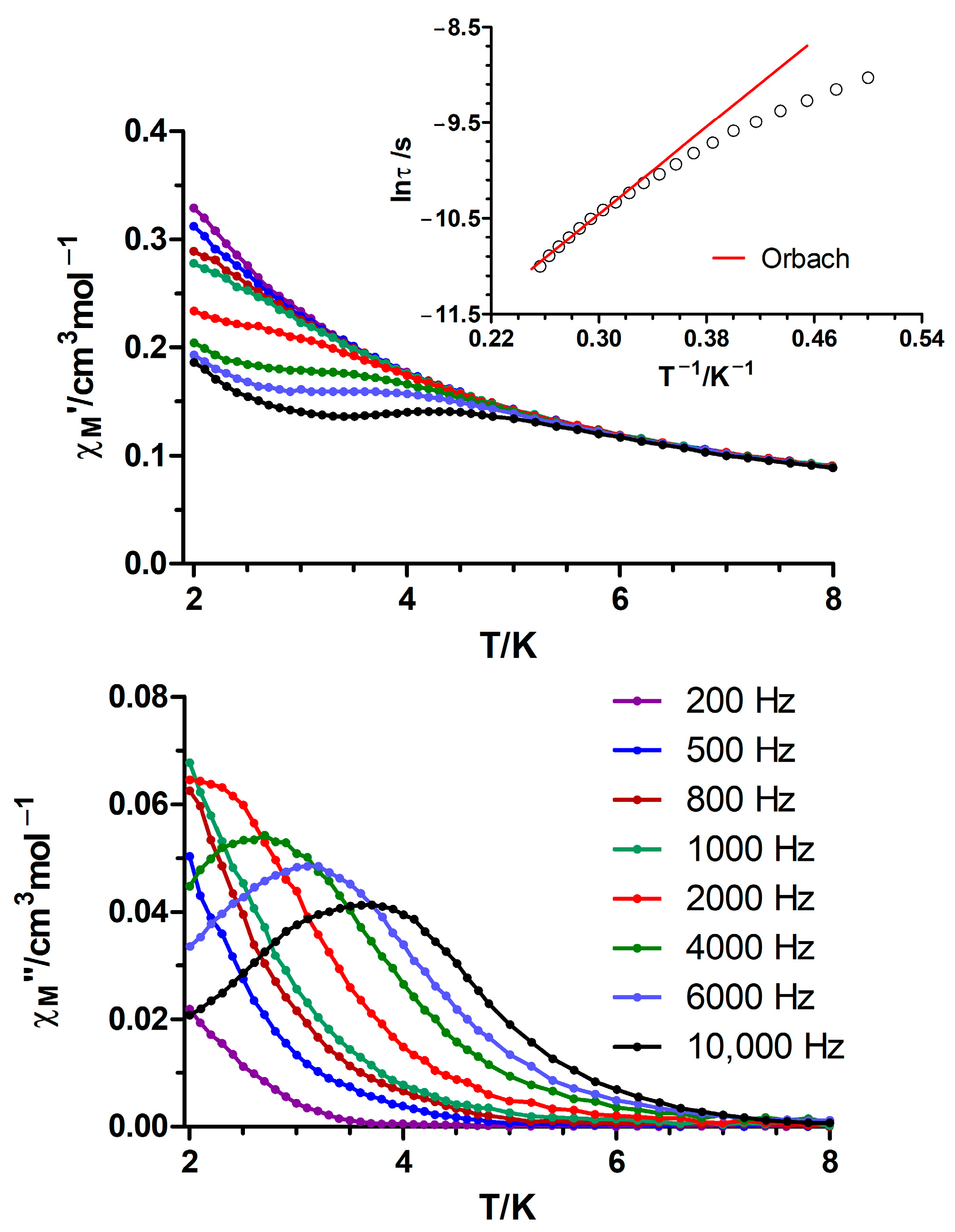
3.2. Ac Magnetic Properties
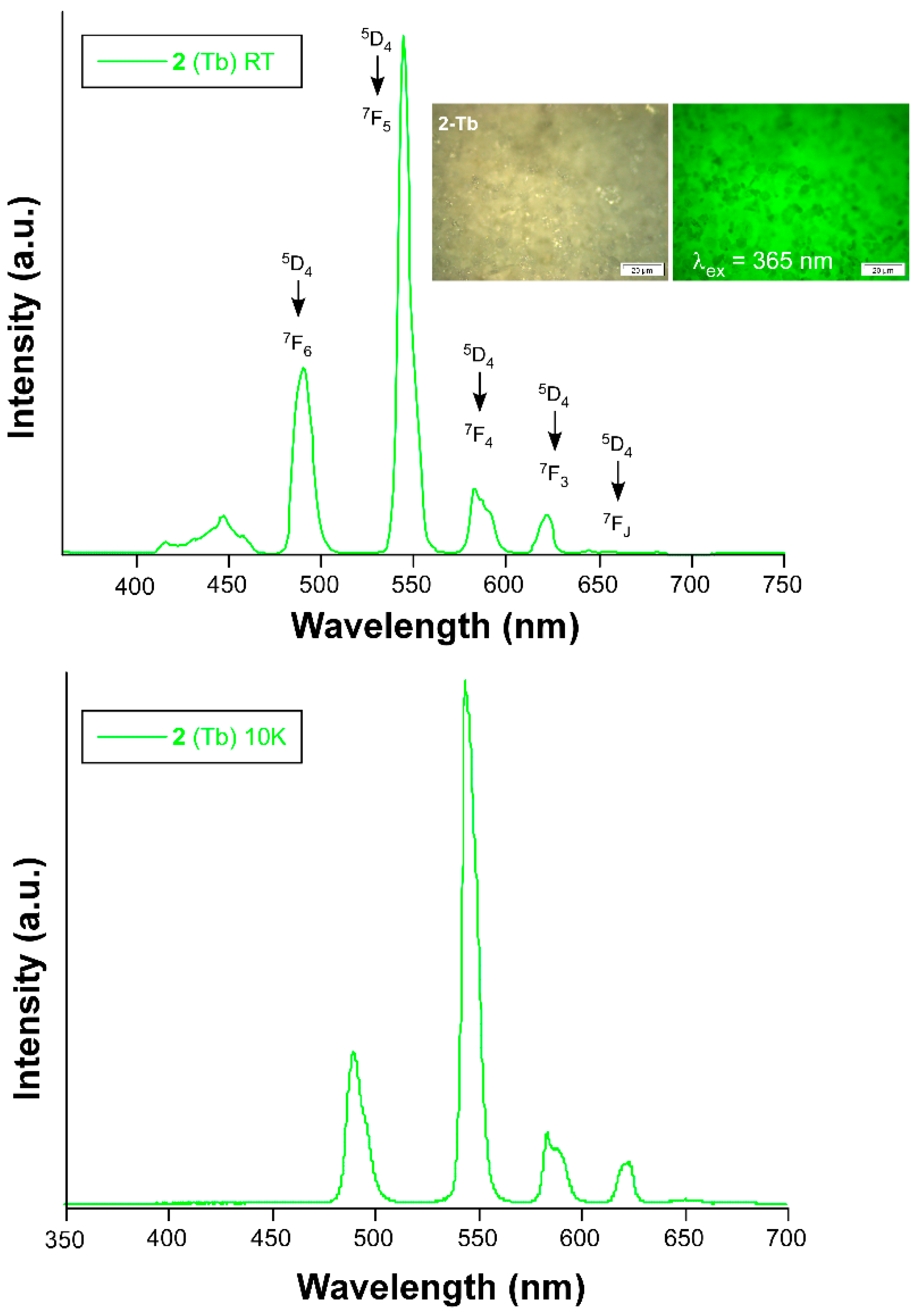
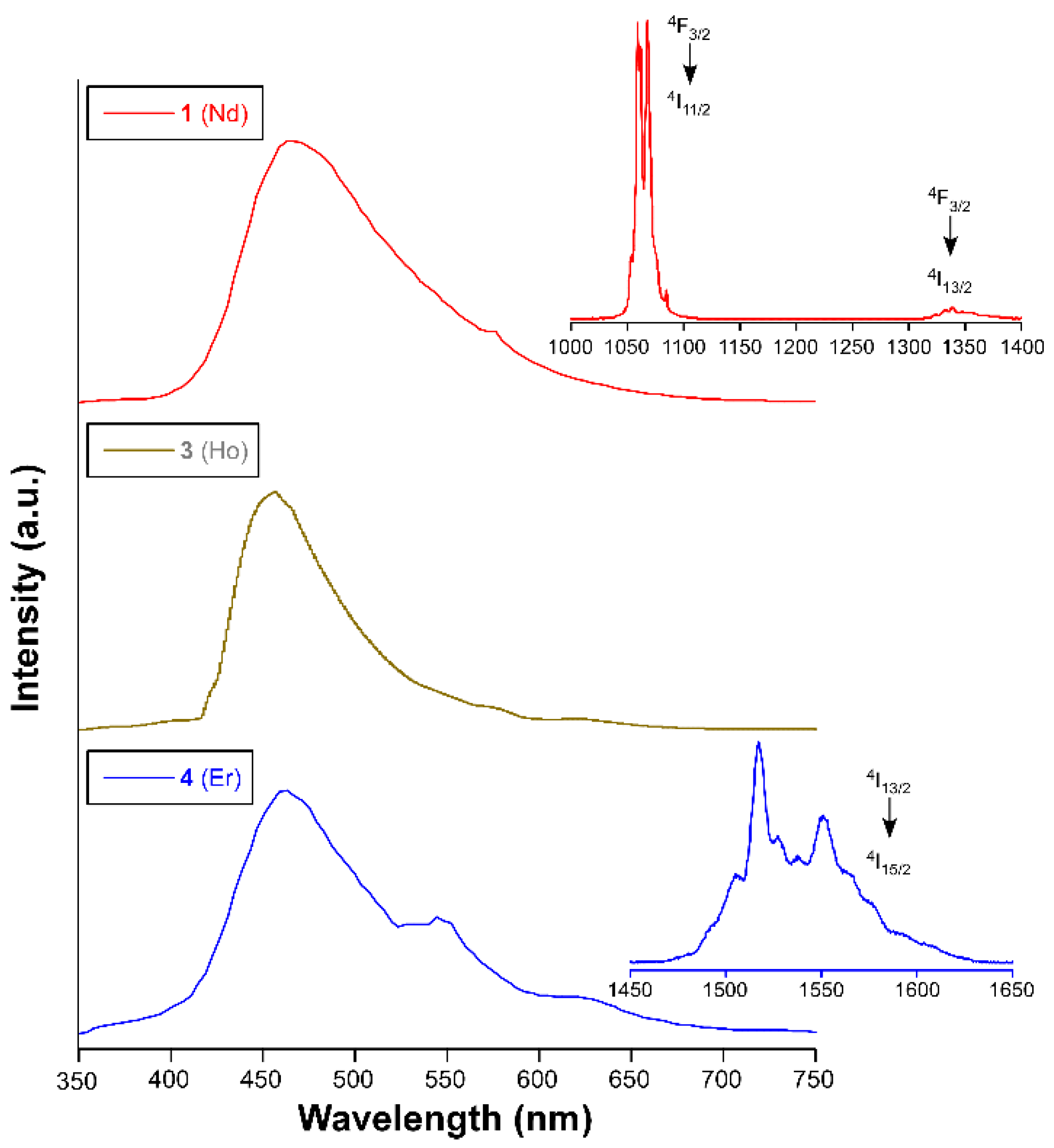
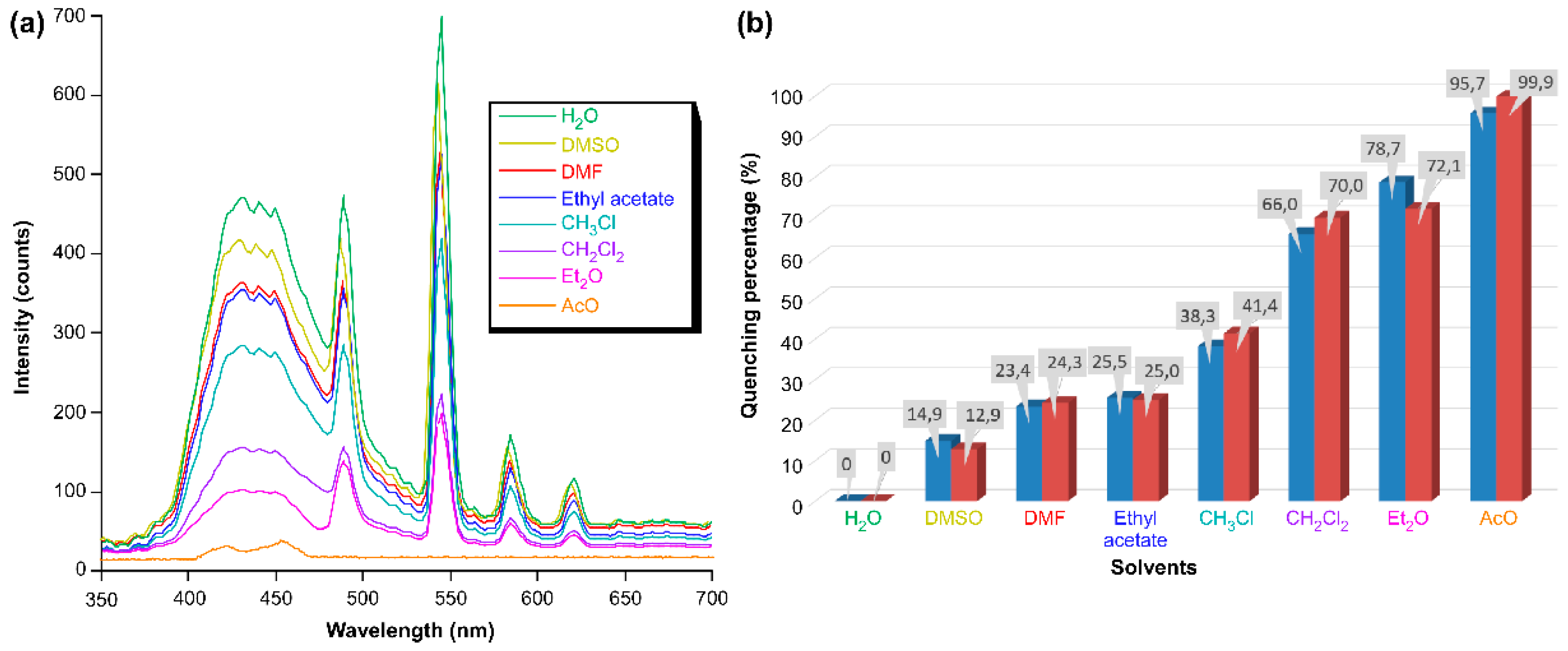
4. Photoluminescence Properties
5. Materials and Methods
5.1. Chemicals
5.2. Synthesis of Compounds 1–4
5.3. Physical Measurements
5.4. Single-Crystal Structure Determination and PXRD Measurements
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-Organic Frameworks: A Rapidly Growing Class of Versatile Nanoporous Materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef]
- Cheetham, A.K.; Rao, C.N.R. There´s Room in the Middle. Science 2007, 318, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nat. Cell Biol. 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bosch, M.; Iii, T.G.; Zhou, H.-C. Rational design of metal–organic frameworks with anticipated porosities and functionalities. CrystEngComm 2014, 16, 4069–4083. [Google Scholar] [CrossRef]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: from synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Voorde, B.V.D.; Bueken, B.; Denayer, J.; Vos, D. De Adsorptive separation on metal-organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic gas removal—Metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef]
- Saha, S.; Chandra, S.; Garai, B.; Banerjee, R. Carbon dioxide capture by metal organic frameworks. Indian J. Chem. 2012, 51, 1223–1230. [Google Scholar]
- Mendiratta, S.; Lee, C.-H.; Usman, M.; Lu, K.-L. Metal–organic frameworks for electronics: Emerging second order nonlinear optical and dielectric materials. Sci. Technol. Adv. Mater. 2015, 16, 054204. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
- Minguez Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Saraci, F.; Quezada-Novoa, V.; Donnarumma, P.R.; Howarth, A.J. Rare-earth metal–organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zhang, D.Q.; Hao, X.; Zhu, D. Ben Luminescence and slow magnetic relaxation of isostructural 2D lanthanide metal-organic frameworks derived from both nicotinate N-oxide and glutarate. RSC Adv. 2015, 5, 92980–92987. [Google Scholar] [CrossRef]
- Luo, L.-L.; Qu, X.-L.; Li, Z.; Li, X.; Sun, H.-L. Isostructural lanthanide-based metal–organic frameworks: Structure, photoluminescence and magnetic properties. Dalton Trans. 2017, 47, 925–934. [Google Scholar] [CrossRef]
- García-García, A.; Zabala-Lekuona, A.; Goñi-Cárdenas, A.; Cepeda, J.; Seco, J.M.; Salinas-Castillo, A.; Choquesillo-Lazarte, D.; Rodríguez-Diéguez, A. Magnetic and luminescent properties of isostructural 2d coordination polymers based on 2-pyrimidinecarboxylate and lanthanide ions. Crystals 2020, 10, 571. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.M.; Harriman, K.L.M.; Murugesu, M. The rise of 3-d single-ion magnets in molecular magnetism: Towards materials from molecules? Chem. Sci. 2016, 7, 2470–2491. [Google Scholar] [CrossRef]
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef]
- Jia, J.-H.; Li, Q.-W.; Chen, Y.-C.; Liu, J.-L.; Tong, M.-L. Luminescent single-molecule magnets based on lanthanides: Design strategies, recent advances and magneto-luminescent studies. Coord. Chem. Rev. 2019, 378, 365–381. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, C.; Zhao, Y.-F.; Zhang, Y.-Q.; Jiang, S.-D.; Wang, B.-W.; Han, J.-B.; Sun, J.-L.; Bian, Z.-Q.; Wang, Z.-M.; et al. Thermostability and photoluminescence of Dy(iii) single-molecule magnets under a magnetic field. Chem. Sci. 2016, 7, 5020–5031. [Google Scholar] [CrossRef]
- Almeida Paz, F.A.A.; Klinowski, J.; Vilela, S.M.F.; Tomé, J.P.C.; Cavaleiro, J.A.S.; Rocha, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2011, 41, 1088–1110. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Coronado, E.; Gaita-Ariño, A.; Gamer, C.; Giménez-Marqués, M.; Minguez Espallargas, G.M. A SIM-MOF: Three-Dimensional Organisation of Single-Ion Magnets with Anion-Exchange Capabilities. Chem. Eur. J. 2014, 20, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Mao, J.-G. Structures and luminescent properties of lanthanide phosphonates. Co-ord. Chem. Rev. 2007, 251, 1493–1520. [Google Scholar] [CrossRef]
- Barry, D.E.; Caffrey, D.F.; Gunnlaugsson, T. Lanthanide-directed synthesis of luminescent self-assembly supramolecular structures and mechanically bonded systems from acyclic coordinating organic ligands. Chem. Soc. Rev. 2016, 45, 3244–3274. [Google Scholar] [CrossRef] [PubMed]
- Huizi-Rayo, U.; Zabala-Lekuona, A.; Terenzi, A.; Cruz, C.M.; Cuerva, J.M.; Rodríguez-Diéguez, A.; García, J.A.; Seco, J.M.; Sebastian, E.S.; Cepeda, J. Influence of thermally induced structural transformations on the magnetic and luminescence properties of tartrate-based chiral lanthanide organic-frameworks. J. Mater. Chem. C 2020, 8, 8243–8256. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Voort, P.V.D. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef]
- Calahorro, A.J.; Oyarzabal, I.; Fernández, B.; Seco, J.M.; Tian, T.; Fairen-Jimenez, D.; Colacio, E.; Rodríguez-Diéguez, A. Rare earth anthracenedicarboxylate metal–organic frameworks: Slow relaxation of magnetization of Nd3+, Gd3+, Dy3+, Er3+ and Yb3+ based materials. Dalton Trans. 2015, 45, 591–598. [Google Scholar] [CrossRef]
- Oyarzabal, I.; Fernández, B.; Cepeda, J.; Gómez-Ruiz, S.; Calahorro, A.J.; Seco, J.M.; Rodríguez-Diéguez, A. Slow relaxation of magnetization in 3D-MOFs based on dysprosium dinuclear entities bridged by dicarboxylic linkers. CrystEngComm 2016, 18, 3055–3063. [Google Scholar] [CrossRef]
- Cepeda, J.; Balda, R.; Beobide, G.; Castillo, O.; Fernández, J.; Luque, A.; Pérez-Yáñez, S.; Román, P. Synthetic Control to Achieve Lanthanide(III)/Pyrimidine-4,6-dicarboxylate Compounds by Preventing Oxalate Formation: Structural, Magnetic, and Luminescent Properties. Inorg. Chem. 2012, 51, 7875–7888. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Cepeda, J.; Oyarzabal, I.; Rodríguez-Diéguez, A.; García, J.A.; Seco, J.M.; Colacio, E. Rational design of triple-bridged dinuclear ZnIILnIII-based complexes: A structural, magnetic and luminescence study. CrystEngComm 2017, 19, 256–264. [Google Scholar] [CrossRef]
- Cepeda, J.; Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; García, J.Á.; Luque, A. Photoluminescence Modulation in Lanthanide(III)/Pyrazine-2,5-dicarboxylato/Nitrato Frameworks. Eur. J. Inorg. Chem. 2015, 2015, 4318–4328. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, face-, point-, Schläfli-, and Delaney-symbols in nets, polyhedra and tilings: Recommended terminology. CrystEngComm 2009, 12, 44–48. [Google Scholar] [CrossRef]
- Alvarez, S. Polyhedra in (inorganic) chemistry. Dalton Trans. 2005, 13, 2209–2233. [Google Scholar] [CrossRef] [PubMed]
- Sorace, L.; Benelli, C.; Gatteschi, D. Lanthanides in molecular magnetism: Old tools in a new field. Chem. Soc. Rev. 2011, 40, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Mota, A.J.; Rodriguez-Dieguez, A.; Oyarzabal, I.; Seco, J.M.; Colacio, E. Rational design of ferromagnetic coupled diphenoxocarboxylate triply bridged dinuclear nickel(ii) complexes: Orbital countercomplementarity of the bridging ligands. Dalton Trans. 2012, 41, 14265–14273. [Google Scholar] [CrossRef] [PubMed]
- McKee, V.; Zvagulis, M.; Reed, C.A. ChemInform Abstract: Further insight into magnetostructural corelations in binuclear copper(II) species related to methemocyanin: X-ray crystal structure of a 1,2-μ-nitro complex. Inorg. Chem. Inf. 1985, 16, 2914–2919. [Google Scholar] [CrossRef]
- Nishida, Y.; Kida, S. Crystal structures and magnetism of binuclear copper(II) complexes with alkoxide bridges. Importance of orbital complementarity in spin coupling through two different bridging groups. J. Chem. Soc. Dalton Trans. 1986, 2633–2640. [Google Scholar] [CrossRef]
- Pérez-Yáñez, S.; Castillo, O.; Cepeda, J.; García-Terán, J.P.; Luque, A.; Román, P. Analysis of the interaction between adenine nucleobase and metal-malonato complexes. Eur. J. Inorg. Chem. 2009. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Slow magnetic relaxation in homoleptic trispyrazolylborate complexes of neodymium(iii) and uranium(iii). Dalton Trans. 2012, 41, 13572–13574. [Google Scholar] [CrossRef]
- Gómez-Coca, S.; Urtizberea, A.; Cremades, E.; Alonso, P.J.; Camón, A.; Ruiz, E.; Luis, F. Origin of slow magnetic relaxation in Kramers ions with non-uniaxial anisotropy. Nat. Commun. 2014, 5, 4300. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral structures with an odd number of vertices: Nine-atom clusters and supramolecular architectures. J. Chem. Soc. Dalt. Trans. 2008, 19, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.P.; Titos-Padilla, S.; Oyarzabal, I.; Gupta, T.; Duhayon, C.; Rajaraman, G.; Colacio, E. Effect of Ligand Substitution around the DyIII on the SMM Properties of Dual-Luminescent Zn–Dy and Zn–Dy–Zn Complexes with Large Anisotropy Energy Barriers: A Combined Theoretical and Experimental Magnetostructural Study. Inorg. Chem. 2016, 55, 4428–4440. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Evans, R.C.; Douglas, P.; Winscom, C.J. Coordination complexes exhibiting room-temperature phosphorescence: Evaluation of their suitability as triplet emitters in organic light emitting diodes. Co-ord. Chem. Rev. 2006, 250, 2093–2126. [Google Scholar] [CrossRef]
- San Sebastian, E.S.; Rodríguez-Diéguez, A.; Seco, J.M.; Cepeda, J. Coordination Polymers with Intriguing Photoluminescence Behavior: The Promising Avenue for Greatest Long-Lasting Phosphors. Eur. J. Inorg. Chem. 2018, 2018, 2155–2174. [Google Scholar] [CrossRef]
- Liu, Q.-D.; Wang, R.; Wang, S. Blue phosphorescent Zn(ii) and orange phosphorescent Pt(ii) complexes of 4,4′-diphenyl-6,6′-dimethyl-2,2′-bipyrimidine. Dalton Trans. 2004, 35, 2073–2079. [Google Scholar] [CrossRef]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem. Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef]
- Hagan, A.K.; Zuchner, T. Lanthanide-based time-resolved luminescence immunoassays. Anal. Bioanal. Chem. 2011, 400, 2847–2864. [Google Scholar] [CrossRef]
- Kim, N.; Son, S.-H. Development of Dissociation-Enhanced Lanthanide Fluoroimmunoassay for Measuring Leptin. J. Fluoresc. 2016, 26, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Dotsenko, A.V.; Glebov, L.B.; Tsekhomsky, A.V.; Kokorina, V.F. Phosphor Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; Volume 23. [Google Scholar]
- Soares-Santos, P.C.R.; Cunha-Silva, L.; Paz, F.A.A.; Ferreira, R.A.S.; Rocha, J.; Carlos, L.D.; Nogueira, H.I.S. Photoluminescent Lanthanide-Organic Bilayer Networks with 2,3-Pyrazinedicarboxylate and Oxalate. Inorg. Chem. 2010, 49, 3428–3440. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, J.; Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; García, J. Ángel; Lanchas, M.; Luque, A. Enhancing luminescence properties of lanthanide(iii)/pyrimidine-4,6-dicarboxylato system by solvent-free approach. Dalton Trans. 2015, 44, 6972–6986. [Google Scholar] [CrossRef]
- De Bettencourt-Dias, A.; Barber, P.S.; Viswanathan, S.; De Lill, D.T.; Rollett, A.; Ling, G.; Altun, S. Para-Derivatized Pybox Ligands as Sensitizers in Highly Luminescent Ln(III) Complexes. Inorg. Chem. 2010, 49, 8848–8861. [Google Scholar] [CrossRef] [PubMed]
- Jüstel, T.; Nikol, H.; Ronda, C. New Developments in the Field of Luminescent Materials for Lighting and Displays. Angew. Chem. 1998, 37, 3084–3103. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Ruiz-Muelle, A.B.; García-García, A.; García-Valdivia, A.A.; Oyarzabal, I.; Cepeda, J.; Seco, J.M.; Colacio, E.; Rodríguez-Diéguez, A.; Fernández, I. Design and synthesis of a family of 1D-lanthanide-coordination polymers showing luminescence and slow relaxation of the magnetization. Dalton Trans. 2018, 47, 12783–12794. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165–1176. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; Rodríguez-Diéguez, A.; Beobide, G.; Pérez-Yáñez, S.; García, J.A.; San Sebastian, E.S.; Seco, J.M.; Cepeda, J. Alkaline-earth and aminonicotinate based coordination polymers with combined fluorescence/long-lasting phosphorescence and metal ion sensing response. J. Mater. Chem. C 2019, 7, 6997–7012. [Google Scholar] [CrossRef]
- Chests, C.A.; Avila, V.; Soltermann, A.T.; Previtali, C.M.; Cosa, J.J.; Crystallb, P.B.; Phillipsb, D. Solvent effects on the fluorescence quenching rate constant of an intramolecular exciplex by poly (chlorobenzenes ). J. Chem. Soc. Faraday Trans. 1996, 92, 3327–3332. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Pleshkov, D.N.; Lyssenko, K.A.; Lepnev, L.S.; Bünzli, J.-C.G.; Kuzmina, N.P. Highly Luminescent and Triboluminescent Coordination Polymers Assembled from Lanthanide β-Diketonates and Aromatic BidentateO-Donor Ligands. Inorg. Chem. 2010, 49, 9300–9311. [Google Scholar] [CrossRef]
- Lakowicz, J.R. General Features of Protein Fluorescence; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Earnshaw, A. Introduction to Magnetochemistry; Academic Press: London, UK, 1968. [Google Scholar]
- Bruker AXS Inc. APEX2 User Manual. 2004. Available online: http://xraysweb.lbl.gov/bl1131/1131website/doc/brukerapex2%20user%20manual.pdf (accessed on 21 February 2021).
- Sheldrick, G.M. SADABS 1996, Program for Empirical Adsorption Correction; University of Göttingen: Göttingen, Germany, 1996; p. 467. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. FULLPROF 2000; Version 2.5d; Laboratoire Léon Brillouin (CEA-CNRS), Centre d’Études deSaclay: Gif sur Yvette Cedex, France, 2003. [Google Scholar]


| Nd1-O1A | 2.417(5) | Nd1-O1C(i) | 2.704(6) |
| Nd1-O2A(i) | 2.442(5) | Nd1-O2C(i) | 2.486(6) |
| Nd1-O1B | 2.518(6) | Nd1-O1M | 2.458(5) |
| Nd1-O2B | 2.492(6) | Nd1-O1N | 2.447(6) |
| Nd1-O1C | 2.446(5) | - | - |
| Compound | 1 |
|---|---|
| Chemical formula | C29H32N4O9Nd |
| CCDC | 1995112 |
| M (g mol−1) | 724.82 |
| T (K) | 100 |
| Cryst. syst. | Triclinic |
| Space group | P-1 |
| a (Å) | 10.4488(6) |
| b (Å) | 11.4058(6) |
| c (Å) | 13.2404(8) |
| α (°) | 73.550(2) |
| β (°) | 86.550(2) |
| γ (°) | 87.175(2) |
| V/Å3 | 1509.76(15) |
| Z | 2 |
| ρ/g cm−3 | 1.594 |
| µ/mm−1 | 1.778 |
| R1 a/wR2 b [I>2σ(I)] | 0.0620/0.1411 |
| R1 a/wR2 b [all data] | 0.0816/0.1520 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyarzabal, I.; Rojas, S.; Parejo, A.D.; Salinas-Castillo, A.; García, J.Á.; Seco, J.M.; Cepeda, J.; Rodríguez-Diéguez, A. Exploring the Slow Magnetic Relaxation of a Family of Photoluminescent 3D Lanthanide–Organic Frameworks Based on Dicarboxylate Ligands. Magnetochemistry 2021, 7, 41. https://doi.org/10.3390/magnetochemistry7030041
Oyarzabal I, Rojas S, Parejo AD, Salinas-Castillo A, García JÁ, Seco JM, Cepeda J, Rodríguez-Diéguez A. Exploring the Slow Magnetic Relaxation of a Family of Photoluminescent 3D Lanthanide–Organic Frameworks Based on Dicarboxylate Ligands. Magnetochemistry. 2021; 7(3):41. https://doi.org/10.3390/magnetochemistry7030041
Chicago/Turabian StyleOyarzabal, Itziar, Sara Rojas, Ana D. Parejo, Alfonso Salinas-Castillo, José Ángel García, José M. Seco, Javier Cepeda, and Antonio Rodríguez-Diéguez. 2021. "Exploring the Slow Magnetic Relaxation of a Family of Photoluminescent 3D Lanthanide–Organic Frameworks Based on Dicarboxylate Ligands" Magnetochemistry 7, no. 3: 41. https://doi.org/10.3390/magnetochemistry7030041
APA StyleOyarzabal, I., Rojas, S., Parejo, A. D., Salinas-Castillo, A., García, J. Á., Seco, J. M., Cepeda, J., & Rodríguez-Diéguez, A. (2021). Exploring the Slow Magnetic Relaxation of a Family of Photoluminescent 3D Lanthanide–Organic Frameworks Based on Dicarboxylate Ligands. Magnetochemistry, 7(3), 41. https://doi.org/10.3390/magnetochemistry7030041








