Magnetic Field Perturbations to a Soft X-ray-Activated Fe (II) Molecular Spin State Transition
Abstract
:1. Introduction
2. Results
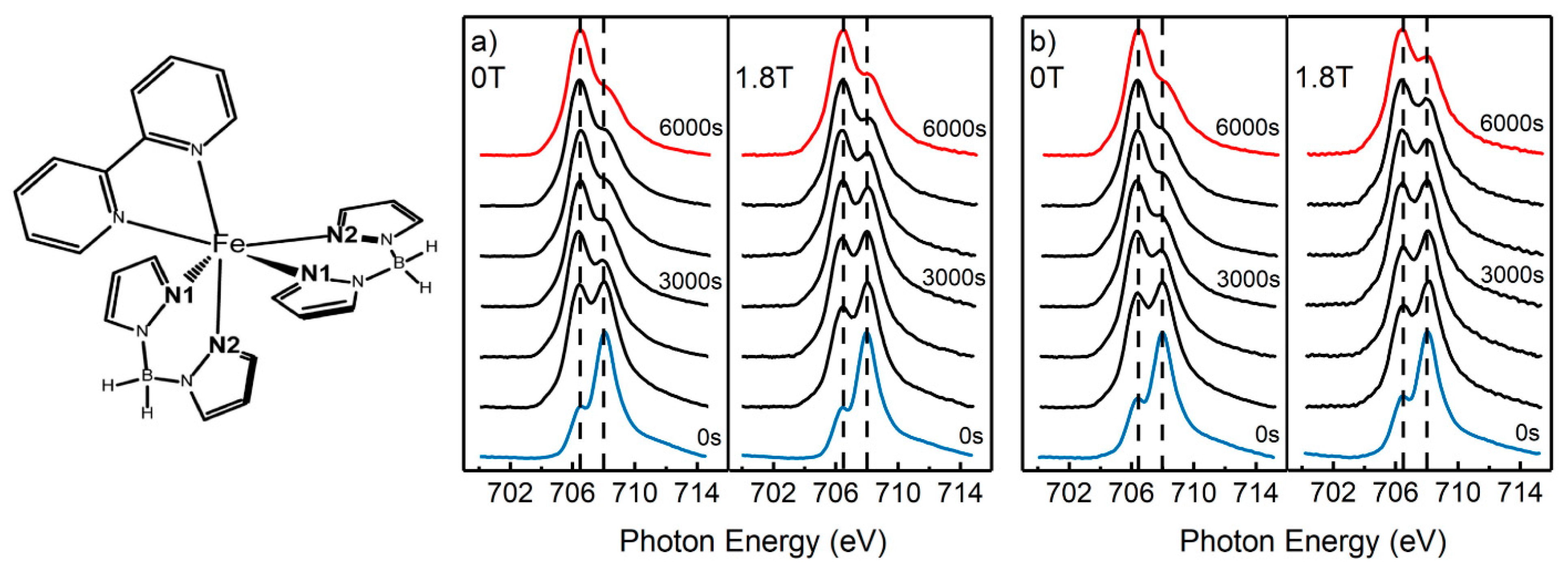
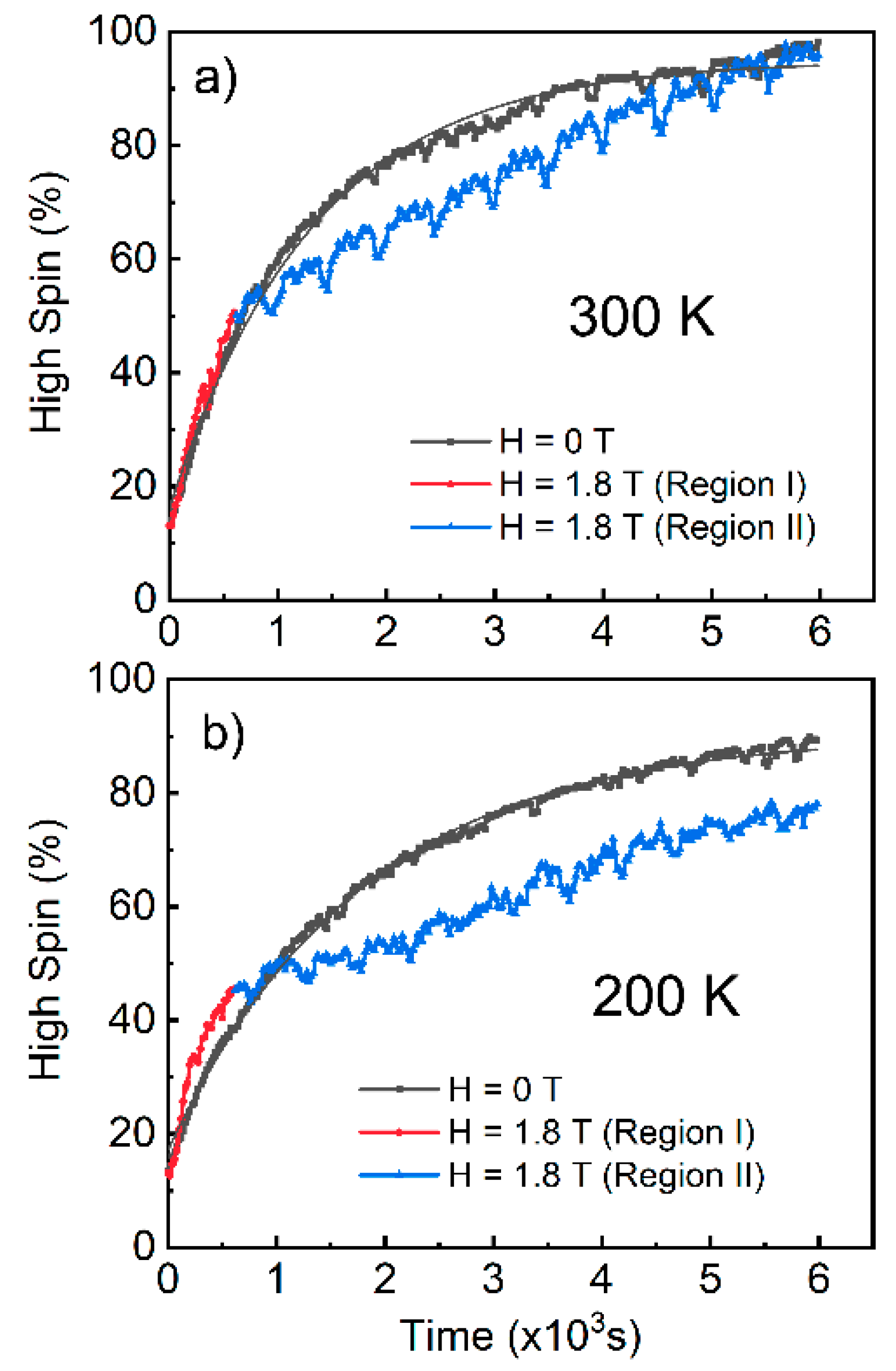
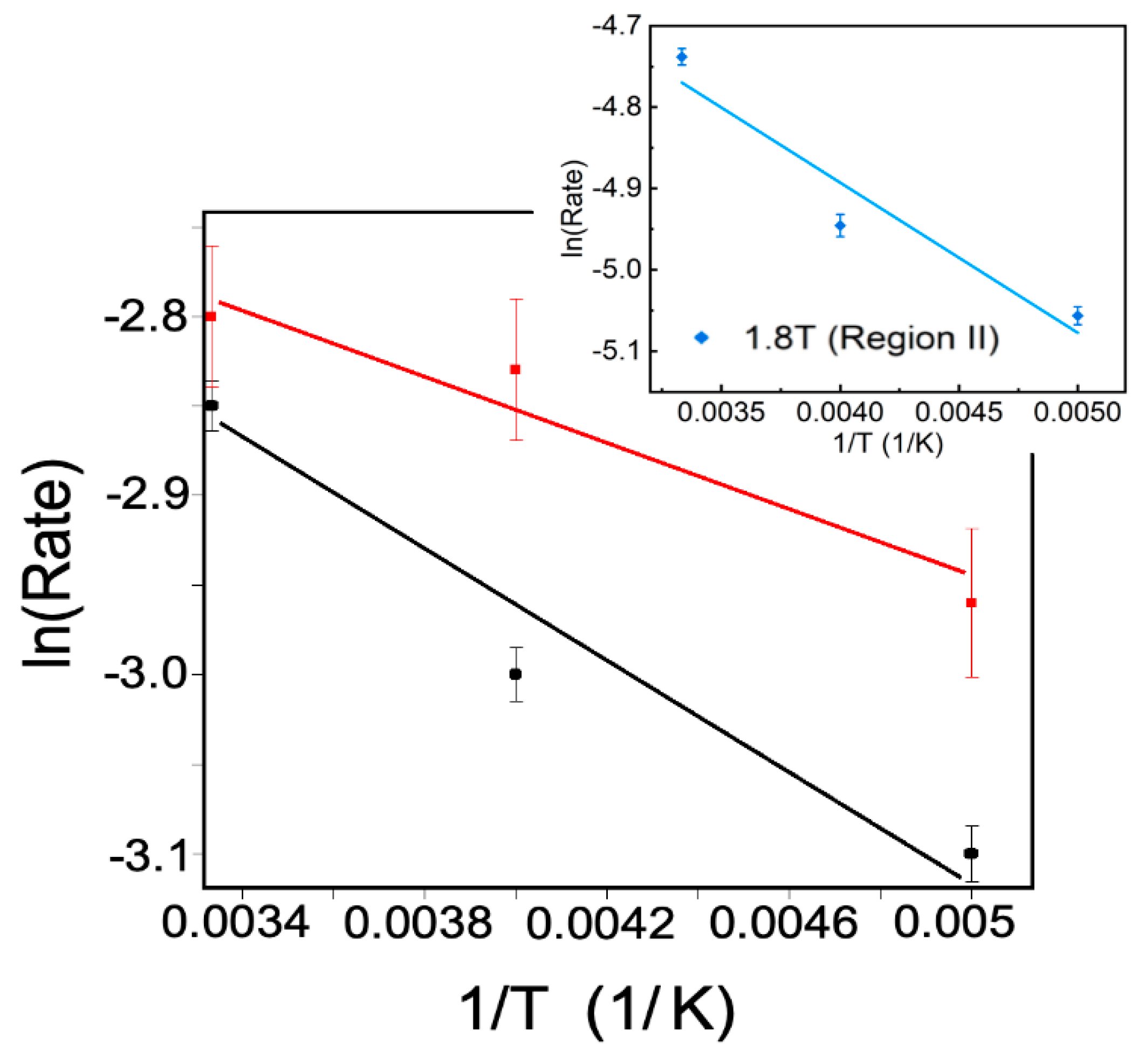
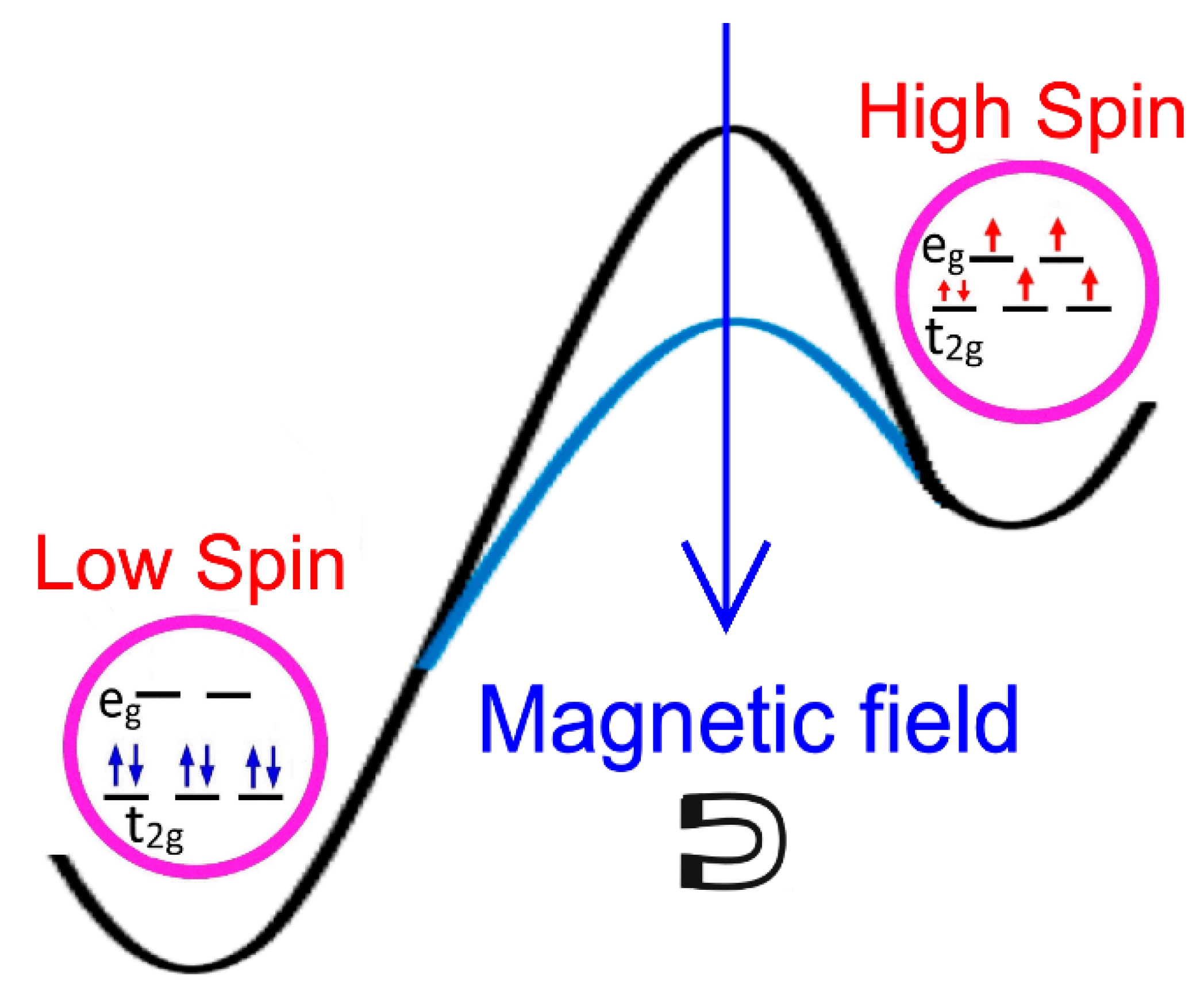
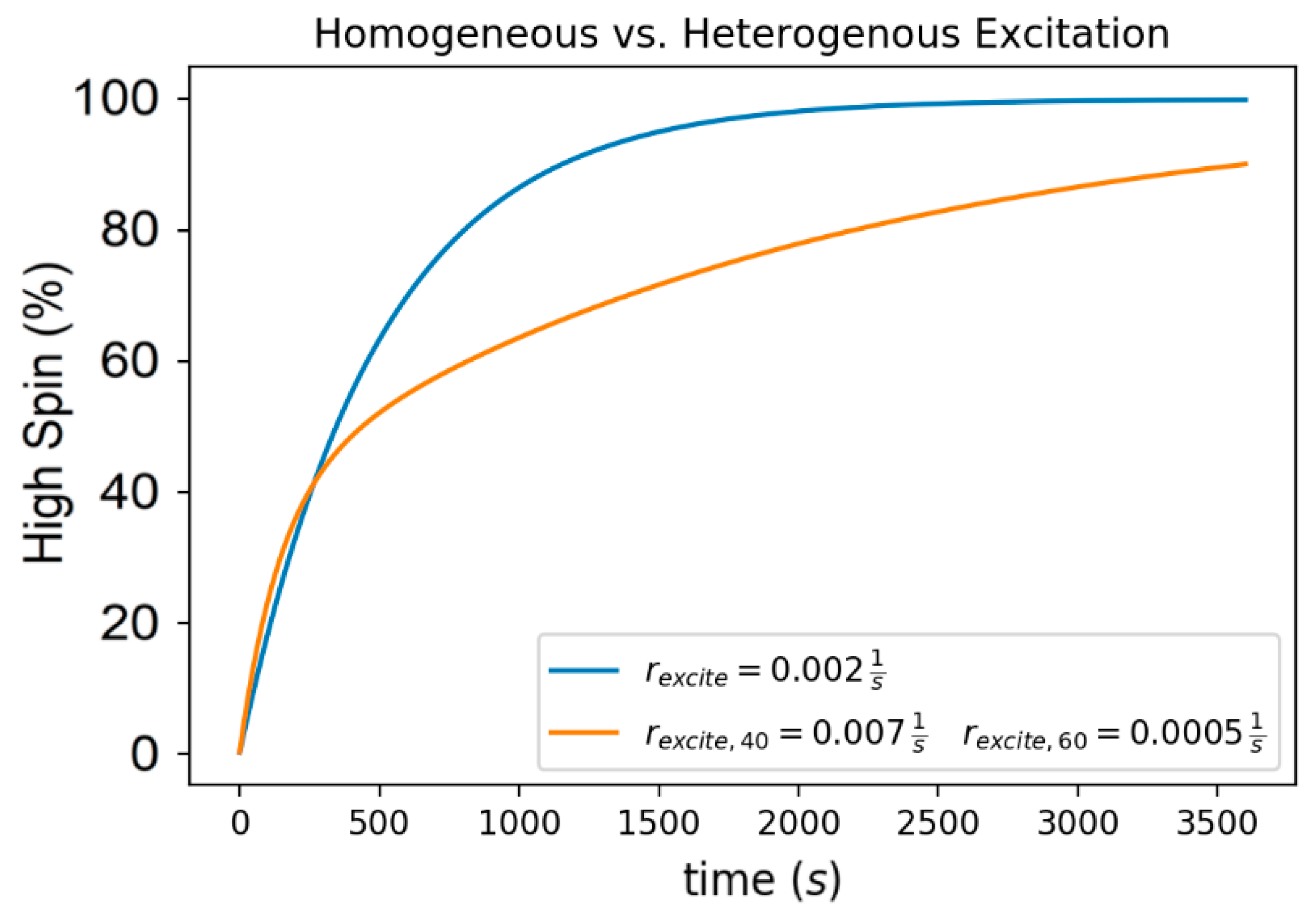
2.1. Magnetic Field Effects on the Excitation of a Spin Crossover Molecule
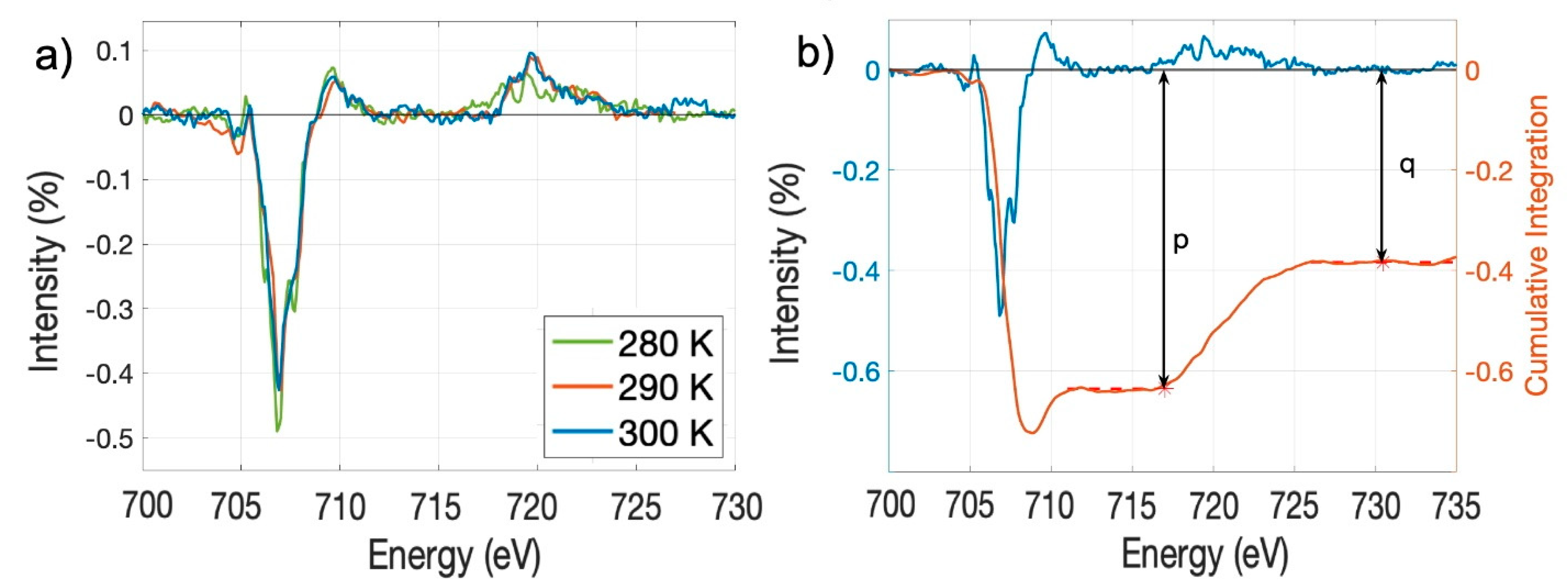
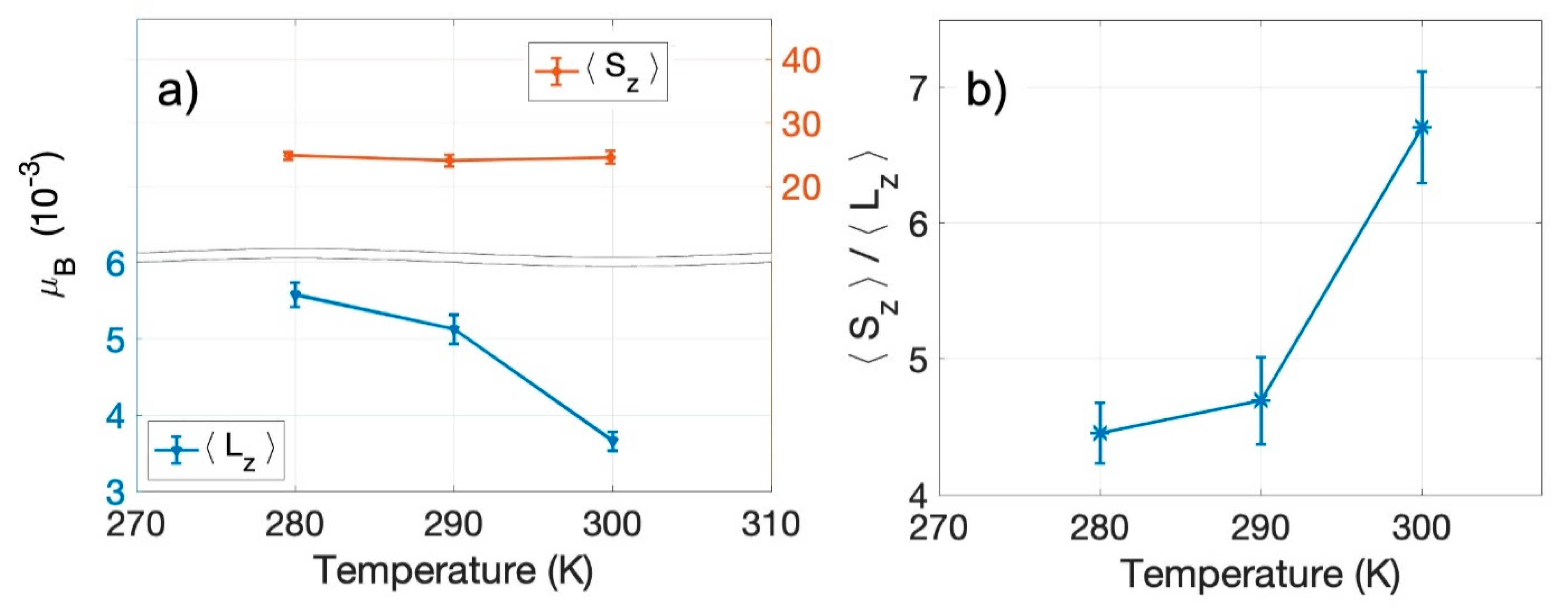
2.2. The Temperature Dependence of the Spin-to-Orbital Moment Ratio
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiebig, M. Revival of the magnetoelectric effect. J. Phys. D Appl. Phys. 2005, 38, R123–R152. [Google Scholar] [CrossRef]
- Curie, P. Sur la symétrie dans les phénomènes physiques, symétrie d’un champ électrique et d’un champ magnétique. J. Phys. Théorique Appliquée 1894, 3, 93–415. [Google Scholar] [CrossRef]
- Sharma, N.; Bird, J.P.; Binek, C.; Dowben, A.P.; Nikonov, D.E.; Marshall, A. Evolving magneto-electric device technologies. Semicond. Sci. Technol. 2020, 35, 073001. [Google Scholar] [CrossRef]
- International Roadmap for Devices and Systems 2020 Edition, Beyond CMOS; 3.4.4 Magnetoelectric Logic; IEEE, 2020; pp. 38–40. Available online: https://irds.ieee.org/editions/2020 (accessed on 5 October 2020).
- Chikara, S.; Gu, J.; Zhang, X.G.; Cheng, H.P.; Smythe, N.; Singleton, J.; Scott, B.; Krenkel, E.; Eckert, J.; Zapf, V.S. Magnetoelectric behavior via a spin state transition. Nat. Commun. 2019, 10, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuki, Y.; Kimura, S.; Awaji, S.; Nakano, M. Magnetocapacitance effect and magnetostriction by the field-induced spin-crossover in [MnIII(taa)]. AIP Adv. 2019, 9, 085219. [Google Scholar] [CrossRef]
- Jakobsen, V.B.; Chikara, S.; Yu, J.-X.; Dobbelaar, E.; Kelly, C.T.; Ding, X.; Weickert, F.; Trzop, E.; Collet, E.; Cheng, H.-P.; et al. Giant Magnetoelectric Coupling and Magnetic-Field-Induced Permanent Switching in a Spin Crossover Mn(III) Complex. Inorg. Chem. 2021, 60, 6167–6175. [Google Scholar] [CrossRef]
- Yum, J.-X.; Chen, D.-T.; Gu, J.; Chen, J.; Jiang, J.; Zhang, L.; Yu, Y.; Zhang, X.-G.; Zapf, V.S.; Cheng, H.-P. Three Jahn-Teller States of Matter in Spin-Crossover System Mn(taa). Phys. Rev. Lett. 2020, 124, 227201. [Google Scholar] [CrossRef]
- Zapf, V.S.; Sengupta, P.; Batista, C.D.; Nasreen, F.; Wolff-Fabris, F.; Paduan-Filho, A. Magnetoelectric effects in an organometallic quantum magnet. Phys. Rev. B 2011, 83, 140405. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Narumi, Y.; Kindo, K.; Nakano, M.; Matsubayashi, G.-E. Field-induced spin-crossover transition of [MnIII(taa)] studied under pulsed magnetic fields. Phys. Rev. B 2005, 72, 064448. [Google Scholar] [CrossRef]
- Bousseksou, A.; Negre, N.; Goiran, M.; Salmon, L.; Tuchagues, J.-P.; Boillot, M.-L.; Boukheddaden, K.; Varret, F. Dynamic triggering of a spin-transition by a pulsed magnetic field. Eur. Phys. J. B 2000, 13, 451–456. [Google Scholar] [CrossRef]
- Zhang, X.; N’Diaye, A.T.; Jiang, X.; Zhang, X.; Yin, Y.; Chen, X.; Hong, X.; Xu, X.; Dowben, P.A. Indications of magnetic coupling effects in spin cross-over molecular thin films. Chem. Commun. 2018, 54, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Ketsle, G.A.; Levshin, L.V.; Mel’nikov, G.V.; Minaev, B.F. Effect of a magnetic field on delayed fluorescence of anthracene exciplexes. J. Appl. Spectrosc. 1981, 34, 287–291. [Google Scholar] [CrossRef]
- Hodges, M.P.P.; Grell, M.; Morley, N.A.; Allwood, D.A. Wide Field Magnetic Luminescence Imaging. Adv. Funct. Mater. 2017, 27, 1606613. [Google Scholar] [CrossRef] [Green Version]
- Klein, G.; Voltz, R.; Schott, M. Magnetic field effect on prompt fluorescence in anthracene: Evidence for singlet exciton fission. Chem. Phys. Lett. 1972, 16, 340–344. [Google Scholar] [CrossRef]
- Kalinowski, J.; Godlewski, J. Magnetic field effects on recombination radiation in tetracene crystal. Chem. Phys. Lett. 1975, 36, 345–348. [Google Scholar] [CrossRef]
- Ern, V.; Merrifield, R.E. Magnetic Field Effect on Triplet Exciton Quenching in Organic Crystals. Phys. Rev. Lett. 1968, 21, 609–611. [Google Scholar] [CrossRef]
- Piland, G.B.; Burdett, J.J.; Kurunthu, D.; Bardeen, C.J. Magnetic Field Effects on Singlet Fission and Fluorescence Decay Dynamics in Amorphous Rubrene. J. Phys. Chem. C 2013, 117, 1224–1236. [Google Scholar] [CrossRef]
- Zhang, X.; Costa, P.S.; Hooper, J.; Miller, D.P.; N’Diaye, A.T.; Beniwal, S.; Jiang, X.; Yin, Y.; Rosa, P.; Routaboul, L.; et al. Locking and Unlocking the Molecular Spin Crossover Transition. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, S.; Chastanet, G.; Daro, N.; Palamarciuc, T.; Rosa, P.; Létard, J.-F.; Liu, J.; Sterbinsky, G.E.; Arena, D.A.; et al. Complexities in the Molecular Spin Crossover Transition. J. Phys. Chem. C 2015, 119, 16293–23302. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, G.; Wang, X.; Mosey, A.; Zhang, X.; Yu, L.; Yost, A.J.; Zhang, X.; DiChiara, A.D.; N’Diaye, A.T.; et al. Tunable spin-state bistability in a spin crossover molecular complex. J. Phys. Condens. Matter 2019, 31, 315401. [Google Scholar] [CrossRef]
- Warner, B.; Oberg, J.C.; Gill, T.G.; El Hallak, F.; Hirjibehedin, C.F.; Serri, M.; Heutz, S.; Arrio, M.-A.; Sainctavit, P.; Mannini, M.; et al. Temperature- and Light-Induced Spin Crossover Observed by X-ray Spectroscopy on Isolated Fe(II) Complexes on Gold. J. Phys. Chem. Lett. 2013, 4, 1546–1552. [Google Scholar] [CrossRef] [Green Version]
- Mosey, A.; Dale, A.S.; Hao, G.; N’Diaye, A.; Dowben, P.A.; Cheng, R. Quantitative Study of the Energy Changes in Voltage-Controlled Spin Crossover Molecular Thin Films. J. Phys. Chem. Lett. 2020, 11, 8231–8237. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Mosey, A.; Jiang, X.; Yost, A.J.; Sapkota, K.R.; Wang, G.T.; Zhang, X.; Zhang, J.; N’Diaye, A.T.; Cheng, R.; et al. Nonvolatile voltage controlled molecular spin state switching. Appl. Phys. Lett. 2019, 114, 032901. [Google Scholar] [CrossRef] [Green Version]
- Kipgen, L.; Bernien, M.; Nickel, F.; Naggert, H.; Britton, A.J.; Arruda, L.; Schierle, E.; Weschke, E.; Tuczek, F.; Kuch, W. Soft-x-ray-induced spin-state switching of an adsorbed Fe(II) spin-crossover complex. J. Physics. Condens. Matter 2017, 29, 394003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipgen, L.; Bernien, M.; Ossinger, S.; Nickel, F.; Britton, A.J.; Arruda, L.; Naggert, H.; Luo, C.; Lotze, C.; Ryll, H.; et al. Evolution of cooperativity in the spin transition of an iron(II) complex on a graphite surface. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Hao, G.; N’Diaye, A.T.; Routaboul, L.; Braunstein, P.; Zhang, X.; Zhang, J.; Doudin, B.; Enders, A.; Dowben, P.A. Perturbing the spin crossover transition activation energies in Fe(H2B(pz)2)2(bipy) with zwitterionic additions. J. Physics. Condens. Matter 2018, 30, 305503. [Google Scholar] [CrossRef] [PubMed]
- Palamarciuc, T.; Oberg, J.C.; El Hallak, F.; Hirjibehedin, C.F.; Serri, M.; Heutz, S.; Létard, J.-F.; Rosa, P. Spin crossover materials evaporated under clean high vacuum and ultra-high vacuum conditions: From thin films to single molecules. J. Mater. Chem. 2012, 22, 9690. [Google Scholar] [CrossRef]
- Moliner, N.; Salmon, L.; Capes, L.; Muñoz, M.C.; Létard, J.-F.; Bousseksou, A.; Tuchagues, J.-P.; McGarvey, J.J.; Dennis, A.C.; Castro, M.; et al. Thermal and Optical Switching of Molecular Spin States in the {[FeL[H2B(pz)2]2} Spin-Crossover System (L = bpy, phen). J. Phys. Chem. B 2002, 106, 4276–4283. [Google Scholar] [CrossRef]
- Real, J.A.; Muñoz, M.C.; Faus, J.; Solans, X. Spin Crossover in Novel Dihydrobis(1-pyrazolyl)borate [H2B(pz)2]-Containing Iron(II) Complexes. Synthesis, X-ray Structure, and Magnetic Properties of [FeL{H2B(pz)2}2] (L = 1,10-Phenanthroline and 2,2′-Bipyridine). Inorg. Chem. 1997, 36, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Idzerda, Y.U.; Lin, H.-J.; Smith, N.V.; Meigs, G.; Chaban, E.; Ho, G.H.; Pellegrin, E.; Sette, F. Experimental Confirmation of the X-Ray Magnetic Circular Dichroism Sum Rules for Iron and Cobalt. Phys. Rev. Lett. 1995, 75, 152–155. [Google Scholar] [CrossRef]
- Zhang, X.; Palamarciuc, T.; Létard, J.-F.; Rosa, P.; Lozada, E.V.; Torres, F.; Rosa, L.G.; Doudin, B.; Dowben, P.A. The spin state of a molecular adsorbate driven by the ferroelectric substrate polarization. Chem. Commun. 2014, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, B. Koordinationschemie Grundlagen und aktuelle Trends; Springer Spectrum, Springer Verlag: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Hao, G.; Cheng, R.; Dowben, P.A. The emergence of the local moment molecular spin transistor. J. Phys. Condens. Matter 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.; Zhang, X.; Wei, W.; Guo, W.; Pant, A.; Xu, X.; Shen, J.; Ma, L.; Hou, D. Nanostructural origin of semiconductivity and large magnetoresistance in epitaxial NiCo2O4/Al2O3 thin films. J. Phys. D. Appl. Phys. 2018, 51, 145308. [Google Scholar] [CrossRef] [Green Version]
- Mellinger, C.; Waybright, J.; Zhang, X.; Schmidt, C.; Xu, X. Perpendicular magnetic anisotropy in conducting NiCo2O4 films from spin-lattice coupling. Phys. Rev. B 2020, 101, 014413. [Google Scholar] [CrossRef] [Green Version]
- Beamline 4-ID-C. Available online: https://www.aps.anl.gov/Sector-4/4-ID-C/Instrumentation (accessed on 5 October 2020).
- Shepit, M.; Paidi, V.K.; Roberts, C.A.; Reddy, G.K.; van Lierop, J. Unusual magnetism in CuxCo3-xO4 nanoparticles. Phys. Rev. B 2021, 103, 024448. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, G.; N’Diaye, A.T.; Ekanayaka, T.K.; Dale, A.S.; Jiang, X.; Mishra, E.; Mellinger, C.; Yazdani, S.; Freeland, J.W.; Zhang, J.; et al. Magnetic Field Perturbations to a Soft X-ray-Activated Fe (II) Molecular Spin State Transition. Magnetochemistry 2021, 7, 135. https://doi.org/10.3390/magnetochemistry7100135
Hao G, N’Diaye AT, Ekanayaka TK, Dale AS, Jiang X, Mishra E, Mellinger C, Yazdani S, Freeland JW, Zhang J, et al. Magnetic Field Perturbations to a Soft X-ray-Activated Fe (II) Molecular Spin State Transition. Magnetochemistry. 2021; 7(10):135. https://doi.org/10.3390/magnetochemistry7100135
Chicago/Turabian StyleHao, Guanhua, Alpha T. N’Diaye, Thilini K. Ekanayaka, Ashley S. Dale, Xuanyuan Jiang, Esha Mishra, Corbyn Mellinger, Saeed Yazdani, John W. Freeland, Jian Zhang, and et al. 2021. "Magnetic Field Perturbations to a Soft X-ray-Activated Fe (II) Molecular Spin State Transition" Magnetochemistry 7, no. 10: 135. https://doi.org/10.3390/magnetochemistry7100135
APA StyleHao, G., N’Diaye, A. T., Ekanayaka, T. K., Dale, A. S., Jiang, X., Mishra, E., Mellinger, C., Yazdani, S., Freeland, J. W., Zhang, J., Cheng, R., Xu, X., & Dowben, P. A. (2021). Magnetic Field Perturbations to a Soft X-ray-Activated Fe (II) Molecular Spin State Transition. Magnetochemistry, 7(10), 135. https://doi.org/10.3390/magnetochemistry7100135






