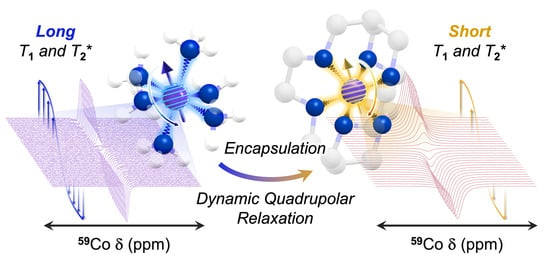
Ligand Control of 59Co Nuclear Spin Relaxation Thermometry
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations
2.2. Variable-Temperature 59Co-NMR Spectroscopy
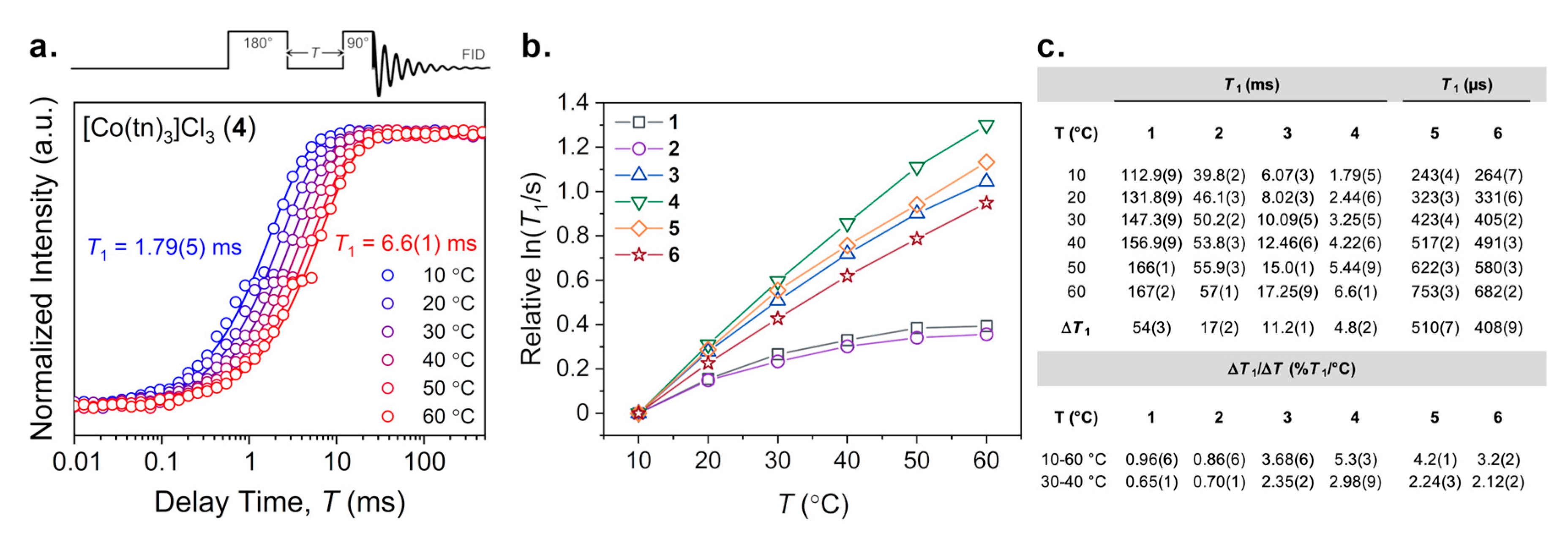
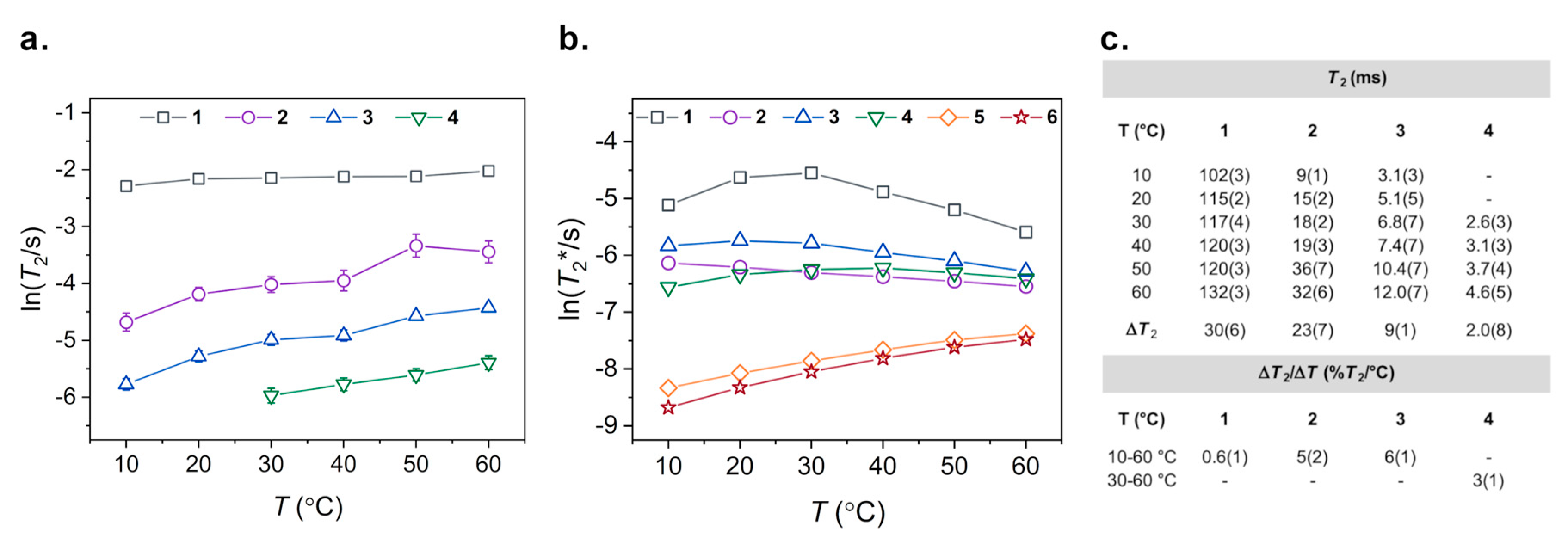
2.3. Variable-Temperature 59Co Inversion Recovery and CPMG Experiments
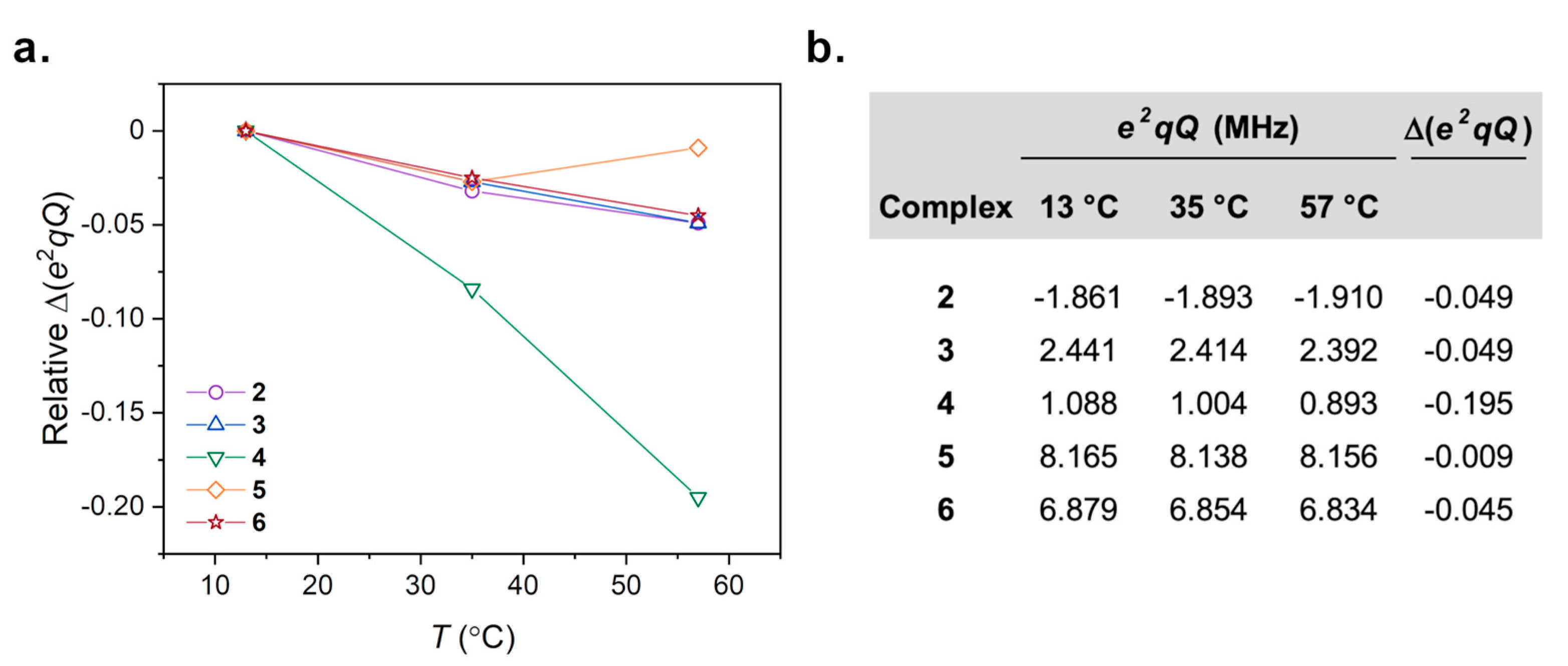
2.4. Computation of 59Co Quadrupolar Coupling Constants
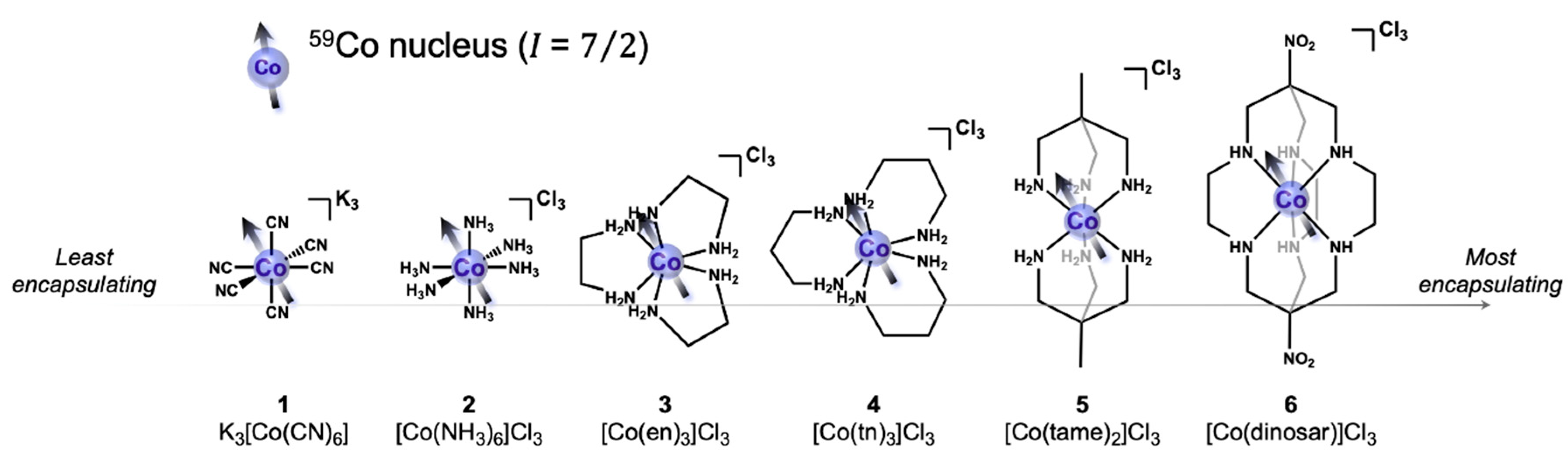
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sotoma, S.; Epperla, C.P.; Chang, H.-C. Diamond Nanothermometry. ChemNanoMat 2018, 4, 15–27. [Google Scholar] [CrossRef]
- Toyli, D.M.; de las Casas, C.F.; Christle, D.J.; Dobrovitski, V.V.; Awschalom, D.D. Fluorescence thermometry enhanced by the quantum coherence of single spins in diamond. Proc. Natl. Acad. Sci. USA 2013, 110, 8417–8421. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.Y.; Chen, O.Y.; Azuma, T.; Chang, B.-M.; Hsieh, F.-J.; Chang, H.-C. All-Optical Thermometry with Nitrogen-Vacancy Centers in Nanodiamond-Embedded Polymer Films. J. Phys. Chem. C 2019, 123, 15366–15374. [Google Scholar] [CrossRef]
- Thiele, S.; Balestro, F.; Ballou, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Electrically driven nuclear spin resonance in single-molecule magnets. Science 2014, 344, 1135–1138. [Google Scholar] [CrossRef]
- Gershenfeld, N.A.; Chuang, I.L. Bulk Spin-Resonance Quantum Computation. Science 1997, 275, 350–356. [Google Scholar] [CrossRef]
- Havel, T.F.; Cory, D.G.; Lloyd, S.; Boulant, N.; Fortunato, E.M.; Pravia, M.A.; Teklemariam, G.; Weinstein, Y.S.; Bhattacharyya, A.; Hou, J. Quantum information processing by nuclear magnetic resonance spectroscopy. Am. J. Phys. 2002, 70, 345–362. [Google Scholar] [CrossRef]
- Vandersypen, L.M.; Steffen, M.; Breyta, G.; Yannoni, C.S.; Sherwood, M.H.; Chuang, I.L. Experimental realization of Shor’s quantum factoring algorithm using nuclear magnetic resonance. Nature 2001, 414, 883–887. [Google Scholar] [CrossRef]
- Brace, C. Thermal Tumor Ablation in Clinical Use. IEEE Pulse 2011, 2, 28–38. [Google Scholar] [CrossRef]
- Tseng, H.; Lin, S.-E.; Chang, Y.-L.; Chen, M.-H.; Hung, S.-H. Determining the critical effective temperature and heat dispersal pattern in monopolar radiofrequency ablation using temperature-time integration. Exp. Ther. Med. 2016, 11, 763–768. [Google Scholar] [CrossRef]
- Yuan, J.; Mei, C.-S.; Panych, L.P.; McDannold, N.J.; Madore, B. Towards fast and accurate temperature mapping with proton resonance frequency-based MR thermometry. Quant. Imaging Med. Surg. 2012, 2, 21–32. [Google Scholar]
- Van Rhoon, G.C.; Wust, P. Introduction: Non-invasive thermometry for thermotherapy. Int. J. Hyperth. 2005, 21, 489–495. [Google Scholar] [CrossRef]
- Levy, G.C.; Terry Bailey, J.; Wright, D.A. A sensitive NMR thermometer for multinuclei FT NMR. J. Magn. Reson. 1980, 37, 353–356. [Google Scholar] [CrossRef]
- Bornais, J.; Brownstein, S. A low-temperature thermometer for 1H, 19F, and 13C. J. Magn. Reson. 1978, 29, 207–211. [Google Scholar] [CrossRef]
- Quast, H.; Heubes, M.; Dunger, A.; Limbach, H.-H. A high-precision carbon-13 shift thermometer for the temperature range 100–300 K. J. Magn. Reson. 1998, 134, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.S.; Bowers, J.L.; Metz, K.R.; Nosaka, T.; Sherry, A.D.; Clouse, M.E. TmDOTP5−: A substance for NMR temperature measurements in vivo. Magn. Reson. Med. 1996, 36, 955–959. [Google Scholar] [CrossRef]
- Sargeson, A.M. Developments in the synthesis and reactivity of encapsulated metal ions. Pure Appl. Chem. 1986, 58, 1511–1522. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Huang, C.-W.; Yap, L.-P.; Park, R.; Shan, H.; Li, Z.; Conti, P.S. The Efficient Synthesis and Biological Evaluation of Novel Bi-Functionalized Sarcophagine for 64Cu Radiopharmaceuticals. Theranostics 2012, 2, 589–596. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Conti, P.S. Development of Multi-Functional Chelators Based on Sarcophagine Cages. Molecules 2014, 19, 4246–4255. [Google Scholar] [CrossRef]
- Ozvat, T.M.; Peña, M.E.; Zadrozny, J.M. Influence of ligand encapsulation on cobalt-59 chemical-shift thermometry. Chem. Sci. 2019, 10, 6727–6734. [Google Scholar] [CrossRef]
- Krause, R.; Megargel, E. Student synthesis of tris(ethylenediamine)cobalt(III) chloride. J. Chem. Educ. 1976, 53, 667. [Google Scholar] [CrossRef]
- Bailar, J.C.; Work, J.B. Some Coördination Compounds of Cobalt Containing Trimethylenediamine and Neopentanediamine. J. Am. Chem. Soc. 1946, 68, 232–235. [Google Scholar] [CrossRef]
- Geue, R.J.; Snow, M.R. Structure, conformational analysis and optical activity of a bis(tridentate)cobalt(III) complex. (+)589-Δλλ-Bis[1,1,1-tris(aminomethyl)ethane]cobalt(III) chloride (+)589-(R,R)-tartrate hydrate. Inorg. Chem. 1977, 16, 231–241. [Google Scholar] [CrossRef]
- Qin, C.-J.; James, L.; Chartres, J.D.; Alcock, L.J.; Davis, K.J.; Willis, A.C.; Sargeson, A.M.; Bernhardt, P.V.; Ralph, S.F. An Unusually Flexible Expanded Hexaamine Cage and Its CuII Complexes: Variable Coordination Modes and Incomplete Encapsulation. Inorg. Chem. 2011, 50, 9131–9140. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, G.; Clark, I.; Creaser, I.; Engelhardt, L.; Geue, R.; Hagen, K.; Harrowfield, J.; Lawrance, G.; Lay, P.; Sargeson, A.; et al. The Synthesis and Structure of Encapsulating Ligands: Properties of Bicyclic Hexamines. Aust. J. Chem. 1994, 47, 143–179. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Ozvat, T.M.; Sterbinsky, G.E.; Campanella, A.J.; Rappé, A.K.; Zadrozny, J.M. EXAFS investigations of temperature-dependent structure in cobalt-59 molecular NMR thermometers. Dalton Trans. 2020. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Kirby, C.W.; Puranda, C.M.; Power, W.P. Cobalt-59 nuclear magnetic relaxation studies of aqueous octahedral cobalt(III) complexes. J. Phys. Chem. 1996, 100, 14618–14624. [Google Scholar] [CrossRef]
- Ader, R.; Loewenstein, A. Nuclear magnetic relaxation studies in solutions of symmetric cobalt (III) complexes. J. Magn. Reson. 1969 1971, 5, 248–261. [Google Scholar] [CrossRef]
- Chacko, V.P.; Bryant, R.G. Electric field gradient modulation and nuclear magnetic relaxation in hexacyanocobaltate ion. J. Magn. Reson. 1984, 57, 79–84. [Google Scholar] [CrossRef]
- Doddrell, D.M.; Bendall, M.R.; Healy, P.C.; Smith, G.; Kennard, C.H.L.; Raston, C.L.; White, A.H. 59Co and 13C Nuclear Spin Relaxation Studies in Solutions of Symmetric, Bidentate Cobalt(III) Complexes. On the Mechanism of 59Co Spin Relaxation. Crystal Structure Determination of Tris(tropolonato)cobalt(III). Aust. J. Chem. 1979, 32, 1219–1230. [Google Scholar] [CrossRef]
- Boeré, R.T.; Kidd, R.G. Rotational Correlation Times in Nuclear Magnetic Relaxation. In Annual Reports on NMR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1983; Volume 13, pp. 319–385. ISBN 978-0-12-505313-6. [Google Scholar]
- Foster, R.J.; Damion, R.A.; Ries, M.E.; Smye, S.W.; McGonagle, D.G.; Binks, D.A.; Radjenovic, A. Imaging of nuclear magnetic resonance spin–lattice relaxation activation energy in cartilage. R. Soc. Open Sci. 2018, 5, 180221. [Google Scholar] [CrossRef] [PubMed]
- Rieke, V.; Pauly, K.B. MR Thermometry. J. Magn. Reson. Imaging 2008, 27, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Anet, F.A.L.; O’leary, D.J. The shielding tensor part II: Understanding its strange effects on relaxation. Concepts Magn. Reson. 1992, 4, 35–52. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozvat, T.M.; Johnson, S.H.; Rappé, A.K.; Zadrozny, J.M. Ligand Control of 59Co Nuclear Spin Relaxation Thermometry. Magnetochemistry 2020, 6, 58. https://doi.org/10.3390/magnetochemistry6040058
Ozvat TM, Johnson SH, Rappé AK, Zadrozny JM. Ligand Control of 59Co Nuclear Spin Relaxation Thermometry. Magnetochemistry. 2020; 6(4):58. https://doi.org/10.3390/magnetochemistry6040058
Chicago/Turabian StyleOzvat, Tyler M., Spencer H. Johnson, Anthony K. Rappé, and Joseph M. Zadrozny. 2020. "Ligand Control of 59Co Nuclear Spin Relaxation Thermometry" Magnetochemistry 6, no. 4: 58. https://doi.org/10.3390/magnetochemistry6040058
APA StyleOzvat, T. M., Johnson, S. H., Rappé, A. K., & Zadrozny, J. M. (2020). Ligand Control of 59Co Nuclear Spin Relaxation Thermometry. Magnetochemistry, 6(4), 58. https://doi.org/10.3390/magnetochemistry6040058