Magnetic Behavior of Luminescent Dinuclear Dysprosium and Terbium Complexes Derived from Phenoxyacetic Acid and 2,2′-Bipyridine
Abstract
1. Introduction
2. Results
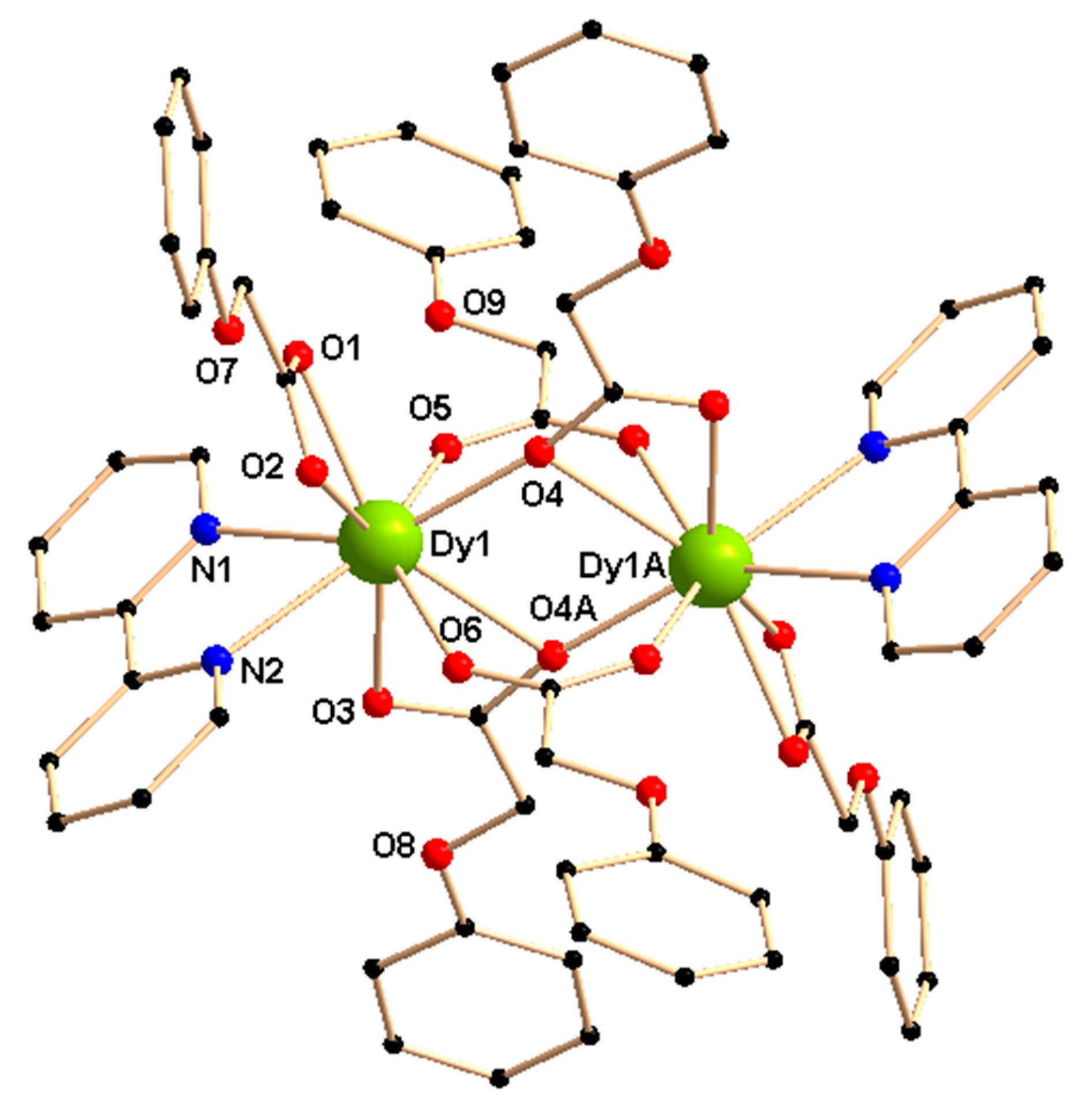
2.1. Description of Crystal Structures
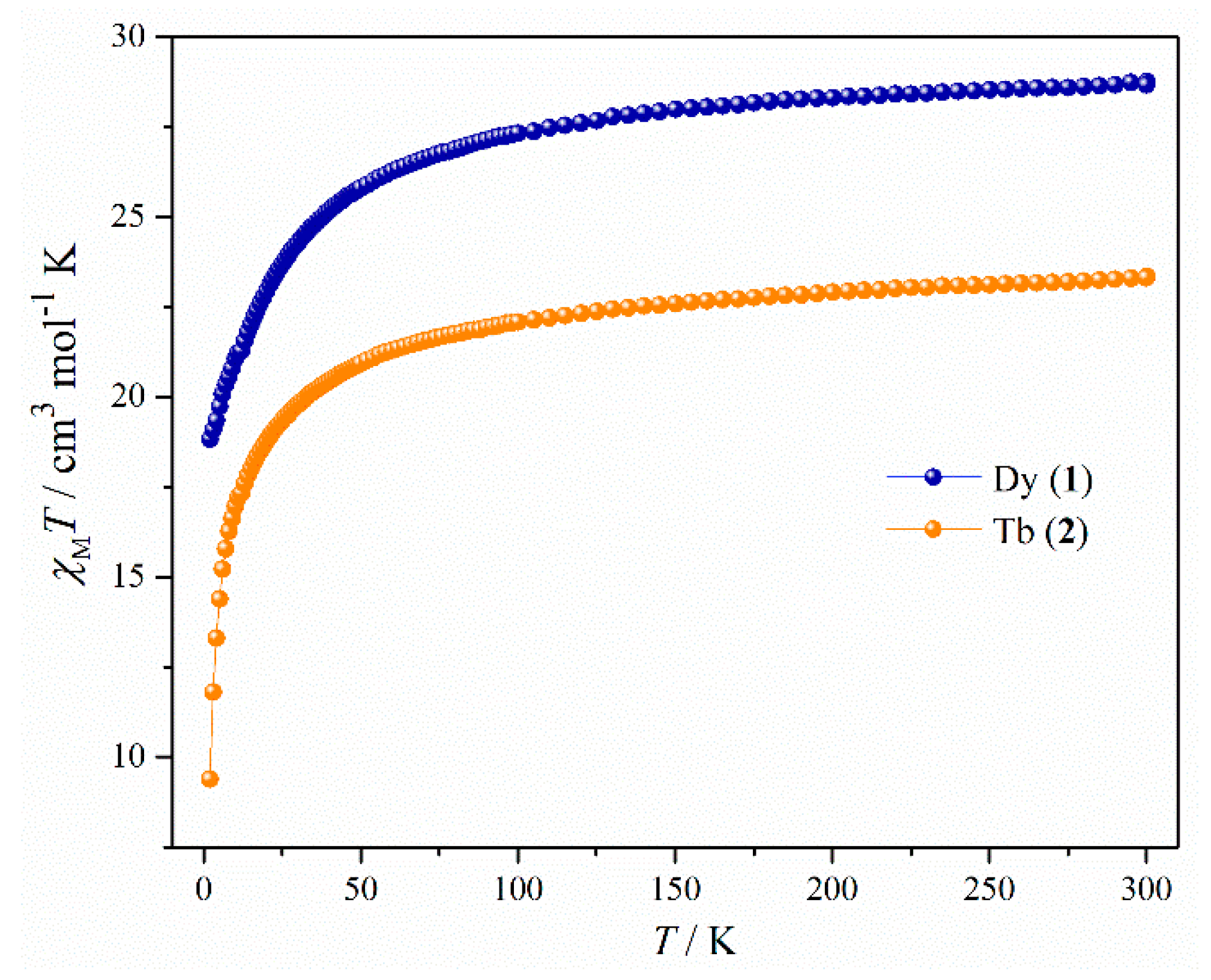
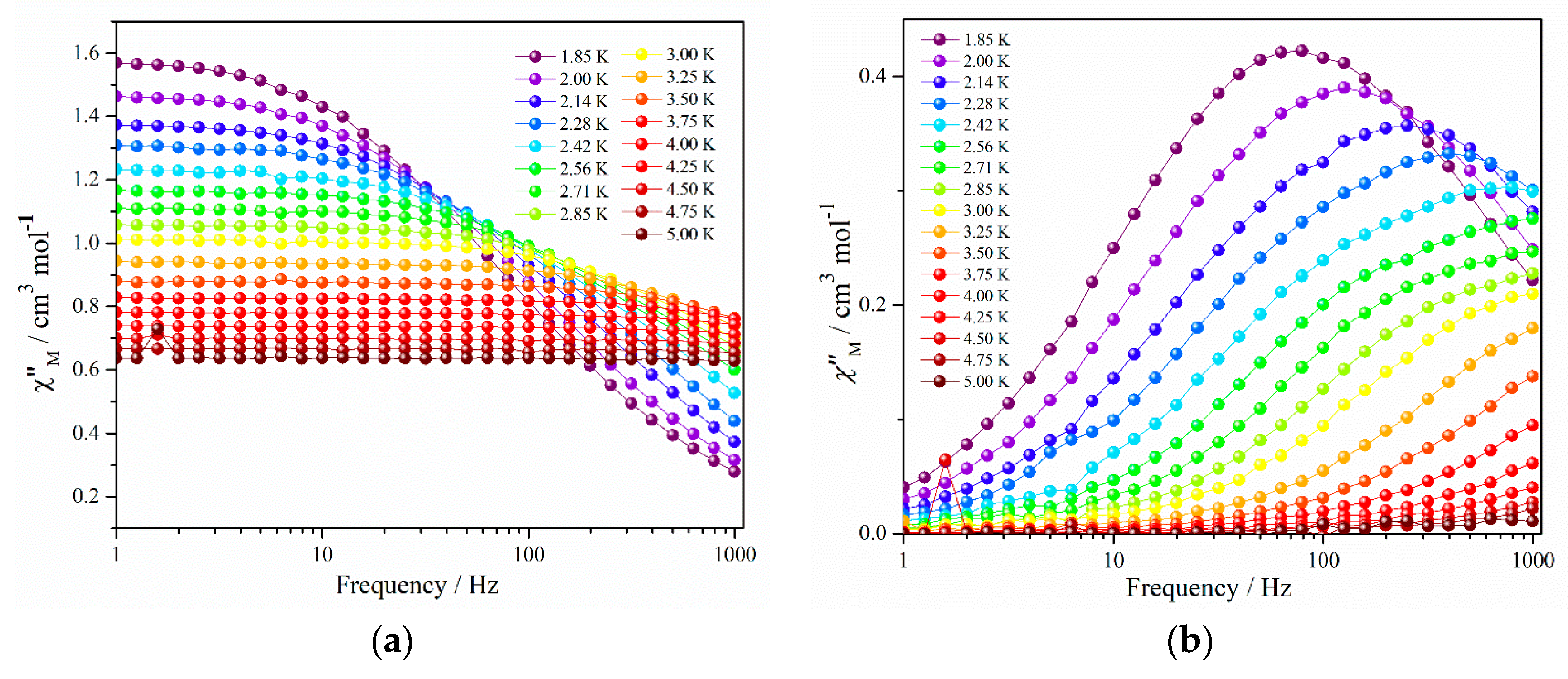
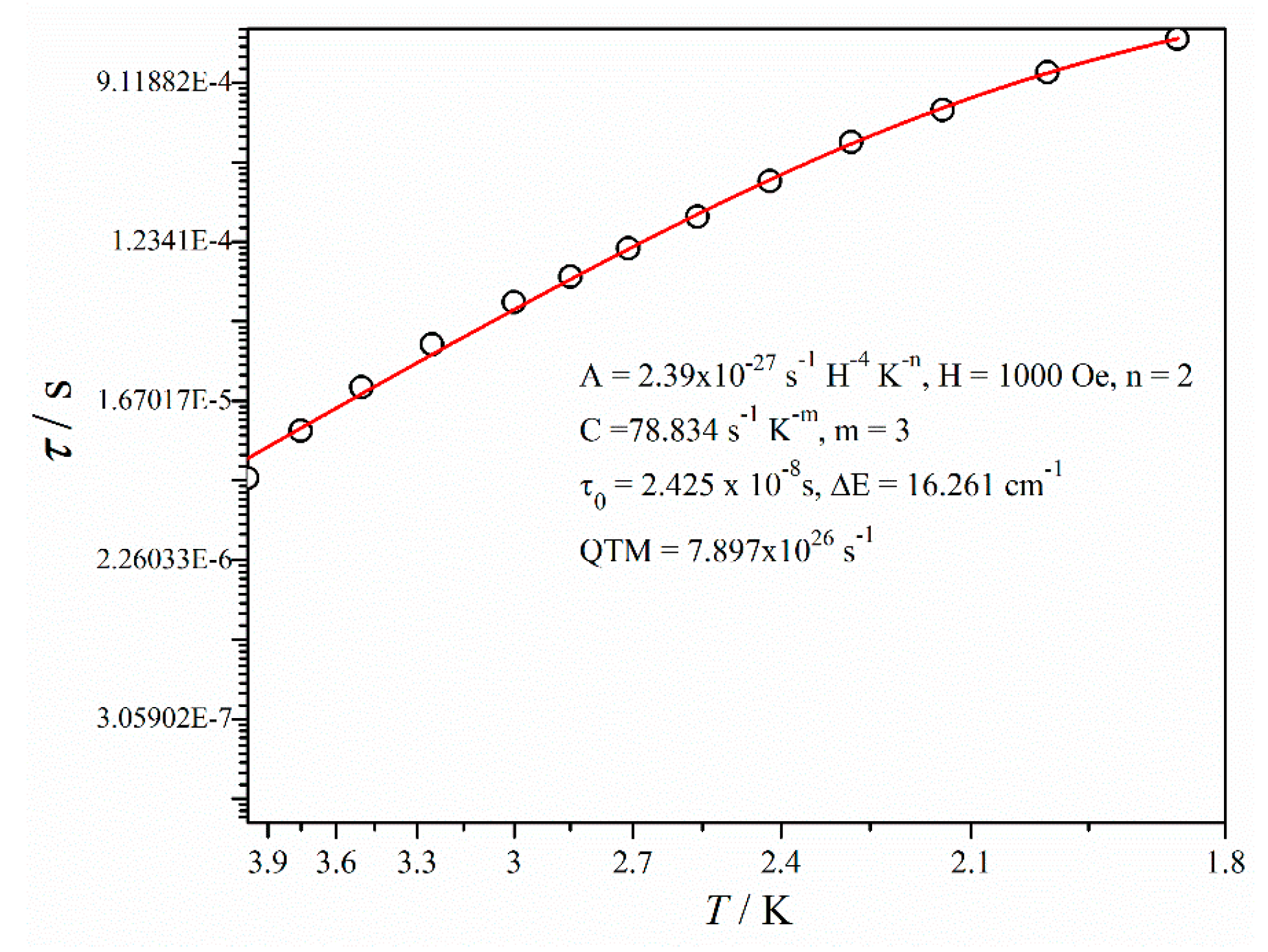
2.2. Magnetic Properties
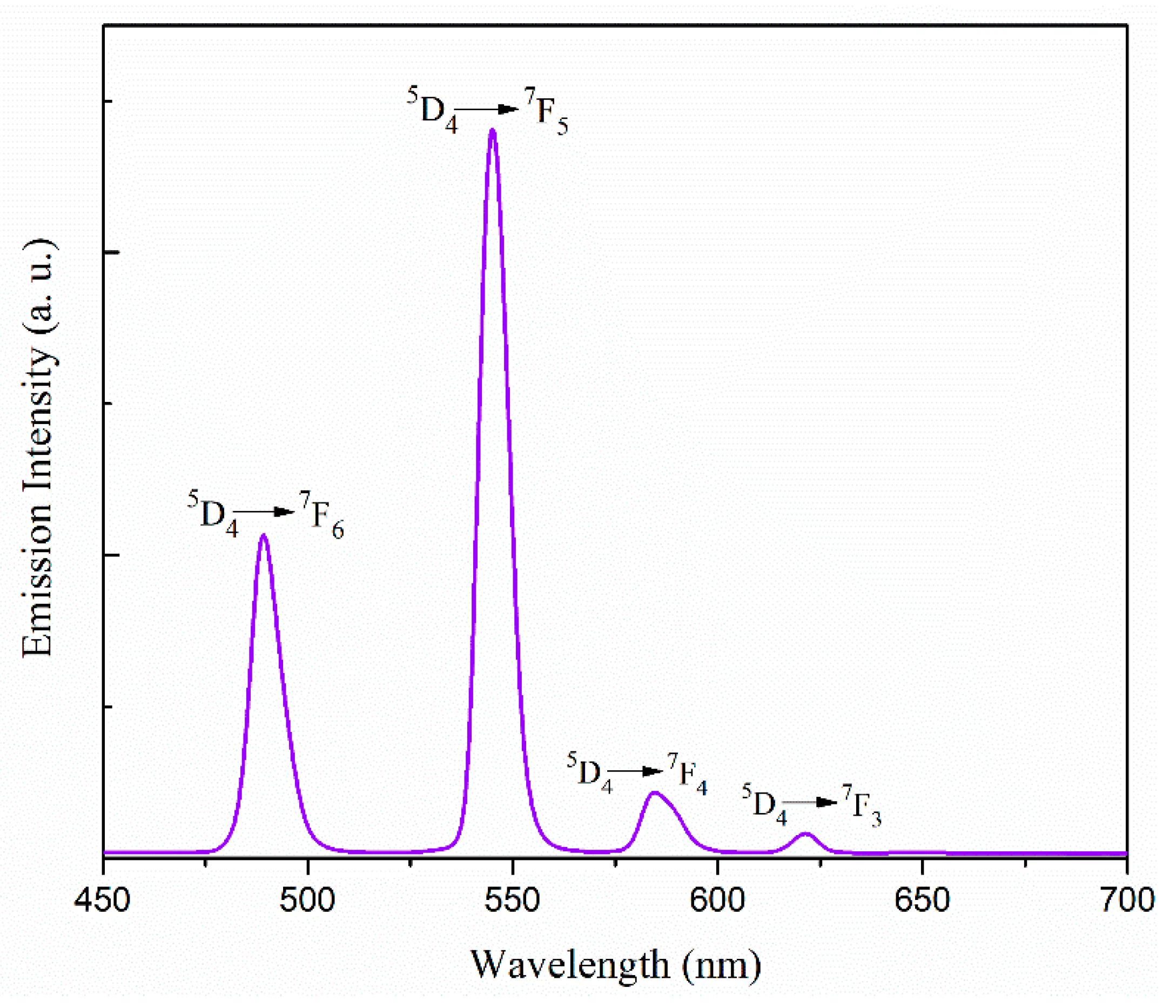
2.3. Photophysical Properties
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nature Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Domingo, N.; Bellido, E.; Ruiz-Molina, D. Advances on structuring, integration and magnetic characterization of molecular nanomagnets on surfaces and devices. Chem. Soc. Rev. 2012, 41, 258–302. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, M.; Roubeau, O.; Palacios, E.; Camón, A.; Hooper, T.N.; Brechin, E.K.; Alonso, J.J. Cryogenic Magnetocaloric Effect in a Ferromagnetic Molecular Dimer. Angew. Chem. Int. Ed. 2011, 50, 6606–6609. [Google Scholar] [CrossRef] [PubMed]
- Aromí, G.; Aguilá, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar] [CrossRef]
- Candini, A.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Affronte, M. Graphene Spintronic Devices with Molecular Nanomagnets. Nano Lett. 2011, 11, 2634–2639. [Google Scholar] [CrossRef]
- Biswas, S.; Mondal, A.K.; Konar, S. Densely Packed Lanthanide Cubane Based 3D Metal−Organic Frameworks for Efficient Magnetic Refrigeration and Slow Magnetic Relaxation. Inorg. Chem. 2016, 55, 2085–2090. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-Y.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Giusti, A.; Charron, G.; Mazerat, S.; Compain, J.-D.; Mialane, P.; Dolbecq, A.; Rivière, E.; Wernsdorfer, W.; Biboum, R.N.; Keita, B.; et al. Magnetic Bistability of Individual Single-Molecule Magnets Grafted on Single-Wall Carbon Nanotubes. Angew. Chem. Int. Ed. 2009, 48, 4949–4952. [Google Scholar] [CrossRef]
- Sorace, L.; Benelli, C.; Gatteschi, D. Lanthanides in molecular magnetism: old tools in a new field. Chem. Soc. Rev. 2011, 40, 3092–3104. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Meng, X.; Shi, W.; Cheng, P.; Powell, A.K. Constraining the coordination geometries of lanthanide centers and magnetic building blocks in frameworks: A new strategy for molecular nanomagnets. Chem. Soc. Rev. 2016, 45, 2423–2439. [Google Scholar] [CrossRef] [PubMed]
- Liddle, S.T.; Slageren, J.V. Improving f-element single molecule magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, Y.-N.; Tang, J. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Tian, H.; Wang, M.; Zhao, L.; Guo, Y.-N.; Guo, Y.; Tang, J.; Liu, Z. A Discrete Dysprosium Trigonal Prism Showing Single-Molecule Magnet Behaviour. Chem. Eur. J. 2012, 18, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Chilton, N.F.; Winpenny, R.E.P.; Zheng, Y.-Z. On Approaching the Limit of Molecular Magnetic Anisotropy: A Near-Perfect Pentagonal Bipyramidal Dysprosium (III) Single-Molecule Magnet. Angew. Chem. Int. Ed. 2016, 55, 16071–16074. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Wang, C.; Xue, S.; Lin, S.-Y.; Tang, J. Equatorially Coordinated Lanthanide Single Ion Magnets. J. Am. Chem. Soc. 2014, 136, 4484–4487. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Guo, M.; Xu, Y.; Wu, J.; Zhao, L.; Tang, J. Geometry and magnetic interaction modulations in dinuclear Dy2 single-molecule magnets. Dalton Trans. 2017, 46, 8252–8258. [Google Scholar] [CrossRef]
- Habib, F.; Murugesu, M. Lessons learned from dinuclear lanthanide nano-magnets. Chem. Soc. Rev. 2013, 42, 3278–3288. [Google Scholar] [CrossRef]
- Ghosh, S.; Mandal, S.; Singh, M.K.; Liu, C.-M.; Rajaraman, G.; Mohanta, S. Experimental and theoretical exploration of magnetic exchange interactions and single-molecule magnetic behaviour of bis(η1:η2:μ2-carboxylate)GdIII2 /DyIII2 systems. Dalton Trans. 2018, 47, 11455–11469. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Bernot, K.; Pointillart, F.; Poneti, G.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Sessoli, R. A Luminescent and Sublimable DyIII-Based Single-Molecule Magnet. Chem. Eur. J. 2012, 18, 11379–11387. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Gal, Y.L.; Golhen, S.; Cador, O.; Ouahab, L. Single-Molecule Magnet Behaviour in a Tetrathiafulvalene-Based Electroactive Antiferromagnetically Coupled Dinuclear Dysprosium (III) Complex. Chem. Eur. J. 2011, 17, 10397–10404. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Wernsdorfer, W.; Ungur, L.; Guo, Y.; Tang, J.; Zhang, H.-J.; Chibotaru, L.F.; Powell, A.K. Strong Axiality and Ising Exchange Interaction Suppress Zero-Field Tunneling of Magnetization of an Asymmetric Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 11948–11951. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.Y.; Bolvin, H.; Campbell, V.E.; Guillot, R.; Kampf, J.W.; Wernsdorfer, W.; Gendron, F.; Autschbach, J.; Pecoraro, V.L.; Mallah, T. Assessing the exchange coupling in binuclear lanthanide(III) complexes and the slow relaxation of the magnetization in the antiferromagnetically coupled Dy2 derivative. Chem. Sci. 2015, 6, 4148–4159. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Brunet, G.; Vieru, V.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Significant enhancement of energy barriers in dinuclear dysprosium single-molecule magnets through electron-withdrawing effects. J. Am. Chem. Soc. 2013, 135, 13242–13245. [Google Scholar] [CrossRef]
- Pineda, E.M.; Chilton, N.F.; Marx, R.; Dörfel, M.; Sells, D.O.; Neugebauer, P.; Jiang, S.-D.; Collison, D.; Slageren, J.V.; McInnes, E.J.L.; et al. Direct measurement of dysprosium(III)·dysprosium(III) interactions in a single-molecule magnet. Nat. Commun. 2014, 5, 5243. [Google Scholar] [CrossRef]
- Giansiracusa, M.J.; Moreno-Pineda, E.; Hussain, R.; Marx, R.; Prada, M.M.; Neugebauer, P.; Al-Badran, S.; Collison, D.; Tuna, F.; Slageren, J.V.; et al. Measurement of Magnetic Exchange in Asymmetric Lanthanide Dimetallics: Toward a Transferable Theoretical Framework. J. Am. Chem. Soc. 2018, 140, 2504–2513. [Google Scholar] [CrossRef]
- Ji, B.; Deng, D.; He, X.; Liu, B.; Miao, S.; Ma, N.; Wang, W.; Ji, L.; Liu, P.; Li, X. Syntheses, Structures, Luminescence, and Magnetic Properties of One-dimensional Lanthanide Coordination Polymers with a Rigid 2,2′-Bipyridine-3,3′,6,6′-tetracarboxylic Acid Ligand. Inorg. Chem. 2012, 51, 2170–2177. [Google Scholar] [CrossRef]
- Ye, J.-W.; Wang, J.; Zhang, J.-Y.; Zhang, P.; Wang, Y. Construction of 2-D lanthanide coordination frameworks: syntheses, structures and luminescent property. CrystEngComm 2007, 9, 515–523. [Google Scholar] [CrossRef]
- Lu, Y.-B.; Jiang, X.-M.; Zhu, S.-D.; Du, Z.-Y.; Liu, C.-M.; Xie, Y.-R.; Liu, L.-X. Anion Effects on Lanthanide (III) Tetrazole-1-acetate Dinuclear Complexes Showing Slow Magnetic Relaxation and Photofluorescent Emission. Inorg. Chem. 2016, 55, 3738–3749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-M.; Ren, N.; Zhang, J.-J. Lanthanide complexes with 3-methoxybenzoic acid and 5,5′-dimethyl-2,2′-bipyridine: Crystal structures, luminescence and magnetic property. Inorg. Chim. Acta 2018, 480, 140–148. [Google Scholar] [CrossRef]
- Armelao, L.; Dell’Amico, D.B.; Bellucci, L.; Bottaro, G.; Ciattini, S.; Labella, L.; Manfroni, G.; Marchetti, F.; Mattei, C.A.; Samaritani, S. Homodinuclear Lanthanide Complexes with the Divergent Heterotopic 4,4′-Bipyridine N-Oxide (bipyMO) Ligand. Eur. J. Inorg. Chem. 2018, 4421–4428. [Google Scholar] [CrossRef]
- Chen, G.-J.; Guo, Y.-N.; Tian, J.-L.; Tang, J.; Gu, W.; Liu, X.; Yan, S.-P.; Cheng, P.; Liao, D.-Z. Enhancing Anisotropy Barriers of Dysprosium (III) Single-Ion Magnets. Chem. Eur. J. 2012, 18, 2484–2487. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-H.; Li, Q.-W.; Chen, Y.-C.; Liu, J.-L.; Tong, M.-L. Luminescent single-molecule magnets based on lanthanides: Design strategies, recent advances and magneto-luminescent studies. Coord. Chem. Rev. 2019, 378, 365–381. [Google Scholar] [CrossRef]
- Long, J.; Guari, Y.; Ferreira, R.A.S.; Carlos, L.D.; Larionova, J. Recent advances in luminescent lanthanide based Single-Molecule Magnets. Coord. Chem. Rev. 2018, 363, 57–70. [Google Scholar] [CrossRef]
- Wu, D.-F.; Liu, Z.; Ren, P.; Liu, X.-H.; Wang, N.; Cui, J.-Z.; Gao, H.-L. A new family of dinuclear lanthanide complexes constructed from an 8-hydroxyquinoline Schiff base and β-diketone: Magnetic properties and near-infrared luminescence. Dalton Trans. 2019, 48, 1392–1403. [Google Scholar] [CrossRef]
- Biswas, S.; Jena, H.S.; Goswami, S.; Sanda, S.; Konar, S. Synthesis and Characterization of Two Lanthanide (Gd3+ and Dy3+)-Based Three-Dimensional Metal Organic Frameworks with Squashed Metallomacrocycle Type Building Blocks and Their Magnetic, Sorption, and Fluorescence Properties Study. Cryst. Growth Des. 2014, 14, 1287–1295. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Biswas, S.; Mandal, L.; Shen, Y.; Yamashita, M. Exploration of SMM behavior of Ln2-complexes derived from thianaphthene-2-carboxylic acid. Dalton Trans. 2019, 48, 14096–14102. [Google Scholar] [CrossRef]
- Biswas, S.; Jena, H.S.; Sanda, S.; Konar, S. Proton-Conducting Magnetic Coordination Polymers. Chem. Eur. J. 2015, 21, 13793–13801. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Xu, Z.-L.; Bao, S.-S.; Wang, T.-T.; Zheng, Z.-H.; Ferreira, R.A.S.; Zheng, L.-M.; Carlos, L.D. Lanthanide salen-type complexes exhibiting single ion magnet and photoluminescent properties. Dalton Trans. 2016, 45, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Jeletic, M.; Lin, P.-H.; Roy, J.J.L.; Korobkov, I.; Gorelsky, S.I.; Murugesu, M. An Organometallic Sandwich Lanthanide Single-Ion Magnet with an Unusual Multiple Relaxation Mechanism. J. Am. Chem. Soc. 2011, 133, 19286–19289. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Lin, P.-H.; Long, J.; Korobkov, I.; Wernsdorfer, W.; Murugesu, M. The Use of Magnetic Dilution to Elucidate the Slow Magnetic Relaxation Effects of a Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 8830–8833. [Google Scholar] [CrossRef]
- Gavey, E.L.; Hareri, M.A.; Regier, J.; Carlos, L.D.; Ferreira, R.A.S.; Razavi, F.S.; Rawson, J.M.; Pilkington, M. Placing a crown on DyIII—A dual property LnIII crown ether complex displaying optical properties and SMM behaviour. J. Mater. Chem. C 2015, 3, 7738–7747. [Google Scholar] [CrossRef]
- Jiang, Z.-X.; Liu, J.-L.; Chen, Y.-C.; Liu, J.; Jia, J.-H.; Tong, M.-L. Lanthanoid single-ion magnets with the LnN10 coordination geometry. Chem. Commun. 2016, 52, 6261–6264. [Google Scholar]
- Morita, T.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Controlling the Dipole−Dipole Interactions between Terbium (III) Phthalocyaninato Triple-Decker Moieties through Spatial Control Using a Fused Phthalocyaninato Ligand. Inorg. Chem. 2013, 52, 13555–13561. [Google Scholar] [CrossRef]
- Blagg, R.J.; Ungur, L.; Tuna, F.; Speak, J.; Comar, P.; Collison, D.; Wernsdorfer, W.; McInnes, E.J.L.; Chibotaru, L.F.; Winpenny, R.E.P. Magnetic relaxation pathways in lanthanide single-molecule magnets. Nat. Chem. 2013, 5, 673–678. [Google Scholar] [CrossRef]
- Mori, F.; Nyui, T.; Ishida, T.; Nogami, T.; Choi, K.-Y.; Nojiri, H. Oximate-Bridged Trinuclear Dy-Cu-Dy Complex Behaving as a Single-Molecule Magnet and Its Mechanistic Investigation. J. Am. Chem. Soc. 2006, 128, 1440–1441. [Google Scholar] [CrossRef]
- Liang, Z.; Damjanović, M.; Kamila, M.; Cosquer, G.; Breedlove, B.K.; Enders, M.; Yamashita, M. Proton Control of the Lanthanoid Single-Ion Magnet Behavior of a Double-Decker Complex with an Indolenine-Substituted Annulene Ligand. Inorg. Chem. 2017, 56, 6512–6521. [Google Scholar] [CrossRef]
- Mandal, L.; Biswas, S.; Cosquer, G.; Shen, Y.; Yamashita, M. Anion-driven structures and SMM behavior of dinuclear terbium and ytterbium complexes. Dalton Trans. 2018, 47, 17493–17499. [Google Scholar] [CrossRef]
- Meihaus, K.R.; Minasian, S.G.; Lukens, W.W.; Kozimor, J.S.A.; Shuh, D.K.; Tyliszczak, T.; Long, J.R. Influence of Pyrazolate vs N-Heterocyclic Carbene Ligands on the Slow Magnetic Relaxation of Homoleptic Trischelate Lanthanide (III) and Uranium (III) Complexes. J. Am. Chem. Soc. 2014, 136, 6056–6068. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, J.J.; Yuan, A.; Song, Y. Slow magnetic relaxation in luminescent mononuclear dysprosium (III) and erbium (III) pentanitrate complexes with the same LnO10 coordination geometry. Dalton Trans. 2017, 46, 15812–15818. [Google Scholar] [CrossRef]
- Kalita, P.; Goura, J.; Martinez, J.M.H.; Colacio, E.; Chandrasekhar, V. Homodinuclear {LnIII2} (LnIII = GdIII, TbIII, HoIII, and DyIII) Complexes: Field-Induced SMM Behavior of the DyIII and TbIII Analogues. Eur. J. Inorg. Chem. 2019, 2019, 212–220. [Google Scholar] [CrossRef]
- Boulon, M.-E.; Cucinotta, G.; Luzon, J.; Degl’Innocenti, C.; Perfetti, M.; Bernot, K.; Calvez, G.; Caneschi, A.; Sessoli, R. Magnetic Anisotropy and Spin-Parity Effect Along the Series of Lanthanide Complexes with DOTA. Angew. Chem. Int. Ed. 2013, 52, 350–354. [Google Scholar] [CrossRef]
- Xiao, Q.; Yanbin, Z.; Xia, L. Synthesis, Crystal structure and fluorescence of a new europium complex with 2-bromobenzoate and 2,2′-bipyridine. J. Rare Earths 2009, 27, 797–800. [Google Scholar]
- Zhao, Y.-F.; Chu, H.-B.; Bai, F.; Gao, D.-Q.; Zhang, H.-X.; Zhou, Y.-S.; Wei, X.-Y.; Shan, M.-N.; Li, H.-Y.; Zhao, Y.-L. Synthesis, crystal structure, luminescent property and antibacterial activity of lanthanide ternary complexes with 2,4,6-tri(2-pyridyl)-s-triazine. J. Organomet. Chem. 2012, 716, 167–174. [Google Scholar] [CrossRef]
- Liu, C.-S.; Du, M.; Sañudo, E.C.; Echeverria, J.; Hu, M.; Zhang, Q.; Zhou, L.-M.; Fang, S.-M. A luminescent linear trinuclear DyIII complex exhibiting slow magnetic relaxation of single ion origin. Dalton Trans. 2011, 40, 9366–9369. [Google Scholar] [CrossRef]
- Wen, H.-R.; Xie, X.-R.; Liu, S.-J.; Bao, J.; Wang, F.-F.; Liu, C.-M.; Liao, J.-S. Homochiral luminescent lanthanide dinuclear complexes derived from a chiral carboxylate. RSC Adv. 2015, 5, 98097–98104. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, G.-H.; Xie, L.-Q.; Wu, Y.-S.; Wu, H.-L.; Zhou, A.-J.; Wu, Z.-Y.; Wang, J.; Chen, Y.-C.; Tong, M.-L. Magnetic and luminescent properties of lanthanide coordination polymers with asymmetric biphenyl-3,2′,5′-tricarboxylate. Dalton Trans. 2015, 44, 14424–14435. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.-Y.; Zou, Y.-Q. Synthesis, Structure and Luminescence Properties of Four Novel Terbium 2-Fluorobenzoate Complexes. Eur. J. Inorg. Chem. 2005, 2005, 2909–2918. [Google Scholar] [CrossRef]
- Samuel, A.P.S.; Xu, J.; Raymond, K.N. Predicting Efficient Antenna Ligands for Tb (III) Emission. Inorg. Chem. 2009, 48, 687–698. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Wu, J. Luminescence of Europium (III) and Terbium (III) Complexes Incorporated in Poly (Vinyl Pyrrolidone) Matrix. J. Phys. Chem. B 2001, 105, 12293–12296. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.38.46; Rigaku Oxford Diffraction: Tokyo, Japan, 2015.
- Crystal Clear-SM, version 1.4.0 SP1; Rigaku Corporation: Tokyo, Japan, 2008.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-2014/7, Crystal Structure Refinement Program. University of Göttingen. Acta Crystallogr. Sect. C 2014, 71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
| Ligands Involved in the Bond | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| ɳ2-chelating carboxylate | Dy1–O1 | 2.452(7) | Tb1–O1 | 2.479(3) | Y1–O1 | 2.449(2) |
| Dy1–O2 | 2.427(7) | Tb1–O2 | 2.436(3) | Y1–O2 | 2.411(2) | |
| μ-ɳ2: ɳ1-tridentate | Dy1–O3 | 2.430(6) | Tb1–O3A | 2.443(3) | Y1–O3 | 2.415(2) |
| bridging carboxylate | Dy1–O4 | 2.306(6) | Tb1–O4 | 2.337(3) | Y1–O4A | 2.303(2) |
| Dy1–O4A | 2.677(6) | Tb1–O4A | 2.679(3) | Y1–O4 | 2.687(2) | |
| μ-ɳ1:ɳ1-bidentate | Dy1–O5 | 2.330(6) | Tb1–O5A | 2.337(3) | Y1–O5A | 2.314(2) |
| bridging carboxylate | Dy1–O6 | 2.330(6) | Tb1–O6 | 2.335(3) | Y1–O6 | 2.307(2) |
| 2,2′-bipyridine | Dy1–N1 | 2.556(7) | Tb1–N1 | 2.569(4) | Y1–N1 | 2.556(3) |
| Dy1–N2 | 2.507(7) | Tb1–N2 | 2.546(3) | Y1–N2 | 2.525(3) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, L.; Biswas, S.; Yamashita, M. Magnetic Behavior of Luminescent Dinuclear Dysprosium and Terbium Complexes Derived from Phenoxyacetic Acid and 2,2′-Bipyridine. Magnetochemistry 2019, 5, 56. https://doi.org/10.3390/magnetochemistry5040056
Mandal L, Biswas S, Yamashita M. Magnetic Behavior of Luminescent Dinuclear Dysprosium and Terbium Complexes Derived from Phenoxyacetic Acid and 2,2′-Bipyridine. Magnetochemistry. 2019; 5(4):56. https://doi.org/10.3390/magnetochemistry5040056
Chicago/Turabian StyleMandal, Leena, Soumava Biswas, and Masahiro Yamashita. 2019. "Magnetic Behavior of Luminescent Dinuclear Dysprosium and Terbium Complexes Derived from Phenoxyacetic Acid and 2,2′-Bipyridine" Magnetochemistry 5, no. 4: 56. https://doi.org/10.3390/magnetochemistry5040056
APA StyleMandal, L., Biswas, S., & Yamashita, M. (2019). Magnetic Behavior of Luminescent Dinuclear Dysprosium and Terbium Complexes Derived from Phenoxyacetic Acid and 2,2′-Bipyridine. Magnetochemistry, 5(4), 56. https://doi.org/10.3390/magnetochemistry5040056