Abstract
High-pressure (HP) structural and magnetic properties of a magnetic coordination polymer {[NiII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (Ni2Nb) are presented, discussed and compared with its two previously reported analogs {[MnII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (Mn2Nb) and {[FeII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (Fe2Nb). Ni2Nb shows a significant decrease of the long-range ferromagnetic ordering under high pressure when compared to Mn2Nb, where the pressure enhances the Tc (magnetic ordering temperature), or to Fe2Nb exhibiting a pressure-induced spin crossover. The different HP magnetic responses of the three compounds were rationalized and correlated with the structural models as determined by single-crystal X-ray diffraction.
1. Introduction
Structural and magnetic measurements under high pressure are the most reliable source of straightforward magneto-structural correlations in crystalline magnetic solids. These types of studies make it possible to fine-tune the structure and physical properties in a continuous manner—a feature that cannot be achieved via chemical modifications, which often introduce unexpected complications (different packing modes, additional intra- and intermolecular contacts). The application of high pressure is known to be extremely useful for enhancing the magnetic ordering temperature of extended coordination systems [1,2,3,4,5], ligand field and magnetic anisotropy tuning of mononuclear complexes [6,7] and control of the spin crossover behavior [8,9]. The combined high-pressure single-crystal X-ray diffraction (scXRD) and SQUID magnetometry make a perfect set of tools to study and understand the changes induced by this type of mechanical stimulus. scXRD structural analysis experiments are commonly performed using diamond anvil cells (DACs) [10] and, in the case of high-pressure SQUID magnetometry, the most common environment chamber is a piston-cylinder cell (PCC) made of diamagnetic copper-beryllium alloy [11].
Magnetic coordination polymers are known for their high responsiveness to mechanical stress and high pressure. In particular, cyanide-bridged Prussian Blue analogs [12] and the related octacyanometallate-based bimetallic assemblies [2] show significant magnetic changes under high pressure.
Herein, we present the quenching of the long-range ferromagnetic ordering in a cyanide-bridged coordination polymer {[NiII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (Ni2Nb) based on nickel(II) (S = 1) and niobium(IV) (S = ½). We also discuss its properties in comparison to MnII and FeII analogs: {[MnII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (Mn2Nb) and {[FeII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (Fe2Nb) [13].
2. Results and Discussion
2.1. X-ray Crystal Structure Description under High Pressure
Ni2Nb shows some interesting structural distortions when pressurized using a Merrill–Bassett DAC [14]. Its structure under pressure was determined by scXRD (Figure 1 and Table 1) [13]. Ni2Nb crystallizes in a tetragonal space group I41/a and forms a three-dimensional (3-D) CN-bridged skeleton with a flattened diamond-like topology where the niobium and nickel ions are all linked by cyanide ligands. The niobium(IV) centers play the role of the four-fold tetrahedral nodes in the 3-D framework of Ni2Nb (Figure 1a,b).
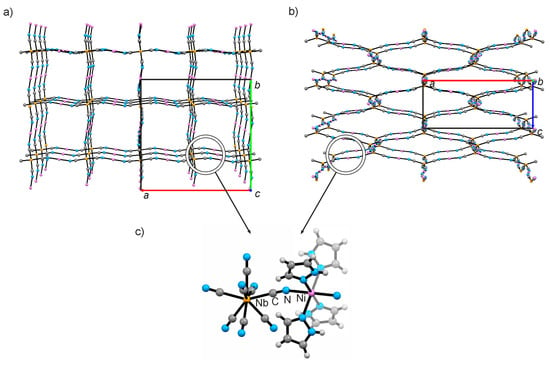
Figure 1.
Structural diagrams presenting the cyanido-bridged framework of {[NiII(pyrazole)4]2[NbIV(CN)8]·4H2O}n down crystallographic axis z (a) and axis x (b). H2O, pyrazole and non-bridging CN− are omitted for clarity. (c) The local geometry of the NbIV-CN-NiII structural motif.

Table 1.
Single-crystal X-ray diffraction (scXRD) unit-cell parameters for Ni2Nb at room temperature and high pressure.
The high-pressure compression of Ni2Nb is presented in Table 1 and Figure 2 as the pressure dependence of the normalized unit-cell volume V/V0, where V0 is the unit-cell volume at 1000 hPa, along with the relevant data for the two analogs Mn2Nb and Fe2Nb published previously [8]. The unit-cell volumes of Mn2Nb, Fe2Nb and Ni2Nb are significantly compressed up to 87.6%, 84.6% and 87.7% of the initial value at ca. 2.4 GPa, respectively. The V/V0 vs. p dependences were fitted using the third-order Birch-Murnaghan equation of state (BMEOS) [15,16] (Equation (1)):
where p—pressure, V0—volume at zero pressure, in this case the ambient pressure, V—volume, K0—isothermal bulk modulus at zero pressure, K’0—dimensionless first derivative of K0 with respect to pressure. The solid lines in Figure 2, which represent the best fit to Equation (1), match the experimental data for Ni2Nb and Mn2Nb in the investigated pressure range. In the case of Fe2Nb there is a strong deviation from the BMEOS above 1 GPa. The best fit parameters K0 and K0’ are: 10.7 ± 0.9 and 8.7 ± 1.8 GPa for Mn2Nb, 10.4 ± 1.6 and 8.2 ± 5.6 GPa for Fe2Nb (from the 0–1 GPa range fit) and 11.9 ± 1.2 and 6.9 ± 2.0 GPa in the case of Ni2Nb. The bulk modulus K0 value is identical for all isomorphs within the experimental error and quite similar to other molecule-based coordination compounds [6,17,18]. Noteworthy, the K0 for the studied coordination polymers are nearly two orders of magnitude smaller than for diamond (440 GPa) and only one order larger than for rubber (1 GPa) [19]. This places the mechanical properties of coordination polymers somewhere between typical inorganic solids and soft matter.
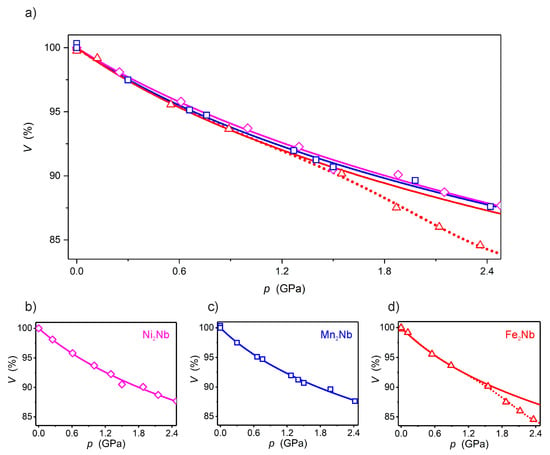
Figure 2.
Combined graph (a) of V/V0(p) dependencies for Ni2Nb (magenta) (b), Mn2Nb (blue) (c) and Fe2Nb (red) (d). The solid lines are the best fit to the Birch–Murnaghan equation of state (BMEOS). The dotted line for Fe2Nb above 1.2 GPa is only a guide for the eye.
The analysis of the coordination spheres of NiII and NbIV in Ni2Nb under high pressure leads to similar observations as for Mn2Nb [8]: the Nb–C and C–N distances as well as Nb–C–N angles remain roughly unchanged, while the Ni–N bonds (Figure 3a) shrink significantly in a linear fashion (Figure 3b). This fact and the good match between the V/V0(p) dependence and the BMEOS both indicate that no pressure-induced phase transition occurs at room temperature in Ni2Nb and Mn2Nb. In fact, the behavior of these two solids is very similar while that of Fe2Nb is quite different and strongly deviates from BMEOS due to the SCO (SCO - spin crossover) behavior. Also, the Fe–N distances shrink in a non-linear fashion in Fe2Nb above 1.5 GPa.
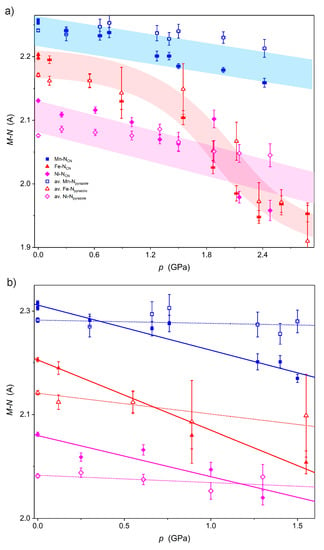
Figure 3.
Pressure dependence of the M–NCN bond lengths (full symbols) and M–Npyrazole (open symbols) in the full pressure range (a) and in the pressure range where only linear changes are observed (b) for Ni2Nb (magenta), Mn2Nb (blue) and Fe2Nb (red). Highlights in (a) are only for guiding the eye, while the lines in (b) are the best linear fit: solid for M–NCN and dotted for M–Npyrazole.
A more detailed analysis of M–N bonds up to 1.5 GPa (Ni2Nb, Mn2Nb and Fe2Nb follow the BMEOS in this pressure range) demonstrates that M–NCN shrinkage is much larger than that of M–Npyrazole (Figure 3b and Table 2). As a result, the elongated octahedral coordination spheres of FeII in Fe2Nb and NiII in Ni2Nb become closer to a perfect octahedron at around 0.7 and 1.2 GPa, respectively.

Table 2.
Shrinkage of the M–NCN and M–Npyrazole bonds under pressure. The Δ(M–N)/Δp values are the slopes of the best linear fit from Figure 3b.
2.2. Magnetic Properties under High Pressure
The magnetic properties of Mn2Nb and Fe2Nb (Figure 4 and Figure 5, respectively) have been discussed in detail in a previous report [8]. It was established that at ambient pressure both Mn2Nb and Fe2Nb are ferrimagnets with long-range magnetic ordering temperatures (Tc) of 23.4 and 9.4 K, respectively. However, the behavior of these compounds under high pressure is very different. Mn2Nb (Figure 4) displays a very strong and linear increase of the Tc from 23.4 K at ambient pressure to 36.5 K at 1.03 GPa. The linear fit of the Tc(p) dependence (Figure 4c) leads to dTc/dp = 12.4 ± 0.2 K GPa−1, typical for octacyanidoniobate(IV)-based systems and Prussian Blue analogs [2]. Fe2Nb, on the other hand, exhibits almost complete quenching of the long-range magnetic ordering under pressure, resulting in paramagnetic properties above 0.7 GPa (Figure 5).
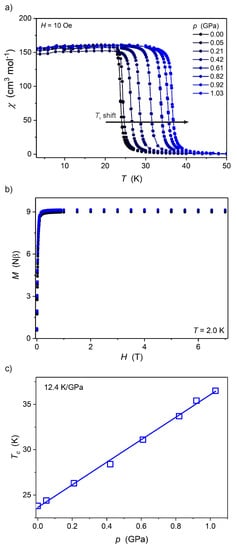
Figure 4.
Temperature dependence of molar magnetic susceptibility χ(T) at 10 Oe (a), M(H) at 2.0 K (b) and Tc(p) (c) for Mn2Nb under high pressure. The solid line in (a) is only a guide for the eye, while in (c) it represents the best linear fit.
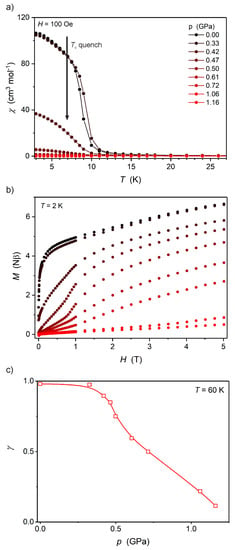
Figure 5.
Temperature dependence of molar magnetic susceptibility χ(T) at 100 Oe (a), M(H) at 2.0 K (b) and fraction of the high-spin FeII γ(p) at 60 K (c) for Fe2Nb under high pressure. The solid lines in (a,c) are only guides for the eye.
Ni2Nb is a ferromagnet due to the local ferromagnetic interactions between NbIV and NiII ions [13] with the Curie temperature (TC) of 13.2 K. It exhibits a completely different type of magnetic response to high pressure (Figure 6) when compared to the other two analogs Mn2Nb and Fe2Nb. The TC of Ni2Nb decreases with increasing pressure, which is characteristic for ferromagnets [20,21], and confirms the presence of local ferromagnetic interactions between NbIV and NiII. The TC shift is 1.9 K GPa−1, as obtained from the linear fit of the TC(p) dependence (Figure 6c, TC is the position of the dχ/dT peak in the χ(T) measurement at 10 Oe). The pressure response of Ni2Nb is much weaker and opposite to Mn2Nb, where the antiferromagnetic interactions between NbIV and MnII are present. Hence, the underlying local magnetic interactions in Ni2Nb (ferromagnetic) vs. Mn2Nb (antiferromagnetic) are the source of the observed difference. The shortening of the Ni–NCN bonds favors the resonance integral and makes the antiferromagnetic contribution to the total exchange interaction stronger while destabilizing the ferromagnetic one. Overall, the JNiNb coupling constant decreases under pressure.
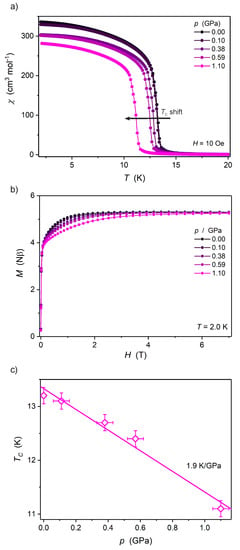
Figure 6.
Temperature dependence of molar magnetic susceptibility χ(T) at 10 Oe (a), M(H) at 2.0 K (b) and Tc(p) (c) for Ni2Nb under high pressure. The solid lines in (a,b) are only guides for the eye, while in (c) it represents the best linear fit.
Ni2Nb does not present a loss of the magnetic moment under high pressure, which is confirmed by the pressure-independent magnetization at saturation values of 5.3 Nβ (at 2.0 K and 7 T; Figure 6b) up to 1.10 GPa and the structural pressure response that matches the BMEOS. The magnetization at saturation values are close to 5.2 Nβ, as expected for two NiII (S = 1) and one NbIV (S = ½) coupled ferromagnetically (assuming gNb = 2.0 and gNi = 2.1 [22]).
Ni2Nb also shows interesting pressure-induced changes in the M(H) dependencies in the 0.1–5 T magnetic field range (Figure 6b). Following the initial sharp increase of the magnetization around 0.05 T in each case, there is a clear dependence with M(H) attaining lower values at higher pressure. All M(H) curves “meet” again above 5 T, converging to the same saturation value of 5.3 Nβ. This behavior is most probably related to the changes of the magnetic anisotropy of the NiII centers arising from the shortening of the Ni–NCN bonds along the CN–Ni–CN axis of the NiII coordination sphere, as evidenced by high-pressure structural studies (Figure 3b). The contribution of [NbIV(CN)8]4− to the pressure-induced magnetic anisotropy change in the M2Nb family can be excluded based on the fact that M(H) for Mn2Nb does not change at all with increasing pressure (Figure 4b).
3. Materials and Methods
3.1. Materials
Chemicals used in this study were of analytical grade and were obtained from commercial sources (Sigma-Aldrich Co., Avantor, Alfa-Aesar). K4[Nb(CN)8]·2H2O was prepared according to the newest available procedure [23]. {[NiII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (Ni2Nb) was obtained according to the literature procedure and its purity/identity was confirmed by elemental analysis and powder X-ray diffraction, which were identical to those published previously [13].
3.2. Single-Crystal X-ray Diffraction under Pressure
The single crystal diffraction data for Ni2Nb were collected at room temperature for a single crystal placed in a Merrill–Bassett DAC filled with Fluorinert 77 as the pressure transmitting medium and a chip of ruby. The ruby fluorescence method was used for the pressure determination inside the DAC chamber. The diffraction frames were collected on KUMA KM4-CCD and Xcalibur EOS machines (running Crysalis software; instruments presently manufactured by Rigaku Oxford Diffraction) using a Mo Kα radiation source and a graphite monochromator (λ = 0.71073 Å). The data were corrected for the sample and DAC absorption as well as the for the gasket shadow [24]. Overlaps with the diamond reflections were excluded from the refinement. Non-H atoms were refined anisotropically (weighted full-matrix least-squares on F2) [25]. The summary of crystallographic data can be found in Table 1. The Cambridge Crystallographic Data Center (CCDC) 1909715–1909722 contains the detailed supplementary crystallographic information for this paper. The crystallographic information files (CIFs) can be obtained free of charge from the CCDC via ww.ccdc.cam.ac.uk/data_request/cif.
The details of the related single-crystal XRD measurements under pressure for Mn2Nb and Fe2Nb were reported previously [8].
3.3. Magnetic Measurements under Pressure
Magnetic measurements under pressure for Mn2Nb and Fe2Nb were reported previously [8]. Ni2Nb was characterized in a similar fashion using a Quantum Design MPMS3 SQUID-VSM (Sand Diego, USA) magnetometer. A powdered sample of Ni2Nb was loaded into the CuBe piston-cylinder cell (manufactured by HMD, Japan; purchased from Quantum Design) with a piece of high-purity lead as the manometer and Daphne 7373 oil as the pressure-transmitting medium. The pressure determination at low temperature was performed with 0.02 GPa accuracy by using the linear pressure dependence of the superconducting transition of Pb (−0.379 K GPa−1). Magnetic data were corrected for the diamagnetic contribution of the sample and the pressure cell.
4. Conclusions
A combined high-pressure magneto-structural study of a magnetic coordination polymer {[NiII(pyrazole)4]2[NbIV(CN)8]·4H2O}n Ni2Nb was carried out and revealed a strong magnetic response of this material to mechanical stress. The magnetic ordering temperature of Ni2Nb shifted linearly towards lower temperatures at higher pressure due to the ferromagnetic character of the exchange coupling between NbIV and NiII ions. Such behavior is completely different from that observed for the two analogs {[MnII(pyrazole)4]2[NbIV(CN)8]·4H2O}n Mn2Nb and {[FeII(pyrazole)4]2[NbIV(CN)8]·4H2O}n Fe2Nb. Mn2Nb exhibited an opposite effect—a strong enhancement of Tc under pressure due to the antiferromagnetic exchange coupling between the constituent magnetic ions. Our study confirms the usefulness of combined high-pressure studies to understand the magnetic properties of molecular magnets and the possibility to fine-tune their properties by applying a mechanical stimulus—namely, high pressure.
Author Contributions
D.P. conceived and designed the experiments, performed the magnetic measurements and wrote the first draft of the paper; G.H. participated in the high-pressure magnetic measurements; H.T. and A.K. performed the high pressure scXRD measurements and analyzed the data; all authors participated in the preparation of the final version of the manuscript.
Funding
This research was funded by the Polish National Science Centre within the Sonata Bis project (2016/22/E/ST5/00055).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Woodall, C.H.; Craig, G.A.; Prescimone, A.; Misek, M.; Cano, J.; Faus, J.; Probert, M.R.; Parsons, S.; Moggach, S.; Martínez-Lillo, J.; et al. Pressure induced enhancement of the magnetic ordering temperature in rhenium(IV) monomers. Nat. Commun. 2016, 7, 13870. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Kurpiewska, K.; Lewiński, K.; Bałanda, M.; Mihalik, M.; Sieklucka, M.Z.B. High-pressure single-crystal XRD and magnetic study of a octacyanoniobate-based magnetic sponge. CrystEngComm 2012, 14, 5224–5229. [Google Scholar] [CrossRef]
- Coronado, E.; Giménez-López, M.C.; Korzeniak, T.; Levchenko, G.; Romero, F.M.; Segura, A.; Garcia-Baonza, V.; Cezar, J.C.; de Groot, F.M.; Milner, A.; et al. Pressure-Induced Magnetic Switching and Linkage Isomerism in K0.4Fe4[Cr(CN)6]2.8·16H2O: X-ray Absorption and Magnetic Circular Dichroism Studies. J. Am. Chem. Soc. 2008, 130, 15519–15532. [Google Scholar] [CrossRef] [PubMed]
- Ohba, M.; Kaneko, W.; Kitagawa, S.; Maeda, T.; Mito, M. Pressure Response of Three-Dimensional Cyanide-Bridged Bimetallic Magnets. J. Am. Chem. Soc. 2008, 130, 4475–4484. [Google Scholar] [CrossRef] [PubMed]
- Shum, W.W.; Her, J.H.; Stephens, P.W.; Lee, Y.; Miller, J.S. Observation of the Pressure Dependent Reversible Enhancement of Tc and Loss of the Anomalous Constricted Hysteresis for [Ru2(O2CMe)4]3[Cr(CN)6]. Adv. Mater. 2007, 19, 2910–2913. [Google Scholar] [CrossRef]
- Craig, G.A.; Sarkar, A.; Woodall, C.H.; Hay, M.A.; Marriott, K.E.; Kamenev, K.V.; Moggach, S.A.; Brechin, E.K.; Parsons, S.; Rajaraman, G.; et al. Rajaraman and M. Murrie. Probing the origin of the giant magnetic anisotropy in trigonal bipyramidal Ni(ii) under high pressure. Chem. Sci. 2018, 9, 1551–1559. [Google Scholar] [CrossRef]
- Prescimone, A.; Sanchez-Benitez, J.; Kamenev, K.K.; Moggach, S.A.; Warren, J.E.; Lennie, A.R.; Murrie, M.; Parsons, S.; Brechin, E.K. High pressure studies of hydroxo-bridged Cu(ii) dimers. Dalton Trans. 2010, 39, 113–123. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Rams, M.; Mišek, M.; Kamenev, K.V.; Tomkowiak, H.; Katrusiak, A.; Sieklucka, B. Enforcing Multifunctionality: A Pressure-Induced Spin-Crossover Photomagnet. J. Am. Chem. Soc. 2015, 137, 8795–8802. [Google Scholar] [CrossRef]
- Gütlich, P.; Ksenofontov, V.; Gaspar, A.B. Pressure effect studies on spin crossover systems. Coord. Chem. Rev. 2005, 249, 1811–1829. [Google Scholar] [CrossRef]
- Katrusiak, A. High-pressure devices. In International Tables for Crystallography, Volume H, Powder Diffraction; Gilmore, C.J., Kaduk, J.A., Schenk, H., Eds.; John Wiley & Sons: New York, NY, USA, 2018; pp. 156–173. [Google Scholar] [CrossRef]
- Kamarád, J.; Machátová, Z.; Arnold, Z. High pressure cells for magnetic measurements—Destruction and functional tests. Rev. Sci. Instrum. 2004, 75, 5022–5025. [Google Scholar] [CrossRef]
- Awaga, K.; Sekine, T.; Okawa, M.; Fujita, W.; Holmes, S.M.; Girolami, G.S. High-pressure effects on a manganese hexacyanomanganate ferrimagnet with TN = 29K. Chem. Phys. Lett. 1998, 293, 352–356. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Pełka, R.; Drath, O.; Nitek, W.; Bałanda, M.; Majcher, A.M.; Poneti, G.; Sieklucka, B. Nature of Magnetic Interactions in 3D {[MII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (M = Mn, Fe, Co, Ni) Molecular Magnets. Inorg. Chem. 2010, 49, 7565–7576. [Google Scholar] [CrossRef]
- Merrill, L.; Bassett, W.A. Miniature diamond anvil pressure cell for single crystal x-ray diffraction studies. Rev. Sci. Instrum. 1974, 45, 290–294. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Birch, F. Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high pressures and 300 K. J. Geophys. Res. Solid Earth 1978, 83, 1257–1268. [Google Scholar] [CrossRef]
- Byrne, P.J.; Richardson, P.J.; Chang, J.; Kusmartseva, A.F.; Allan, D.R.; Jones, A.C.; Kamenev, K.V.; Tasker, P.A.; Parsons, S. Piezochromism in Nickel Salicylaldoximato Complexes: Tuning Crystal-Field Splitting with High Pressure. Chem. Eur. J. 2012, 18, 7738–7748. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.R.; Overgaard, J.; Stalke, D.; Iversen, B.B. High-pressure single crystal X-ray diffraction study of the linear metal chain compound Co3(dpa)4Br2·CH2Cl2. Dalton Trans. 2015, 44, 9038–9043. [Google Scholar] [CrossRef] [PubMed]
- Tabor, D. The bulk modulus of rubber. Polymer 1994, 35, 2759–2763. [Google Scholar] [CrossRef]
- Motokawa, N.; Miyasaka, H.; Yamashita, M. Pressure effect on the three-dimensional charge-transfer ferromagnet [{Ru2(m-FPhCO2)4}2(BTDA-TCNQ)]. Dalton Trans. 2010, 39, 4724–4726. [Google Scholar] [CrossRef] [PubMed]
- Mito, M.; Matsumoto, K.; Komorida, Y.; Deguchi, H.; Takagi, S.; Tajiri, T.; Iwamoto, T.; Kawae, T.; Tokita, M.; Takeda, K. Volume shrinkage dependence of ferromagnetic moment in lanthanide ferromagnets gadolinium, terbium, dysprosium, and holmium. J. Phys. Chem. Solids 2009, 70, 1290–1296. [Google Scholar] [CrossRef]
- Kataev, V.; Golze, C.; Alfonsov, A.; Klingeler, R.; Büchner, B.; Goiran, M.; Broto, J.M.; Rakoto, H.; Mennerich, C.; Klauss, H.H.; et al. Magnetism of a novel tetranuclear nickel(II) cluster in strong magnetic fields. J. Phys. Conf. Ser. 2006, 51, 351–354. [Google Scholar] [CrossRef]
- Handzlik, G.; Magott, M.; Sieklucka, B.; Pinkowicz, D. Alternative Synthetic Route to Potassium Octacyanidoniobate (IV) and Its Molybdenum Congener. Eur. J. Inorg. Chem. 2016, 2016, 4872–4877. [Google Scholar] [CrossRef]
- Katrusiak, A. Shadowing and absorption corrections of single-crystal high-pressure data. Zeitschrift Für Kristallographie Crystalline Materials 2004, 219, 461–467. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).