1. Introduction
Copper acetate is one of the old-fashioned metal complexes with a paddle-wheel-like or lantern-like cluster and has attracted much attention since the discovery of the unique dinuclear structure with antiferromagnetic spin-coupling [
1,
2,
3,
4,
5,
6,
7]. We have engaged in the synthesis of copper acetate analogues and their coordination polymer complexes [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. Previously, we reported that a chain compound of copper(II) benzoate with pyrazine shows an adsorption property for N
2 because of the hydrophobic micropore that is formed by the aromatic benzoate groups [
12,
24]. This is an interesting feature of this type of coordination polymers. In order to extend copper acetate analogues, we introduced some organic radicals to copper(II) propionate to make adducts with organic radicals, by using pyridyl nitronyl nitroxides [
14]. Similar studies on copper acetate type complexes with pyridyl nitronyl nitroxides were reported by Wei et al. [
25,
26], Ouahab et al. [
27], and Miller et al. [
28]. We also prepared copper acetate analogues with free radical carboxylic acids, 4-carboxy-2,2,6,6-tetramethylpiperidinyloxy (Hcatempo), and 3-carboxy-2,2,5,5-tetramethyl-1-pyrrolidinyloxy (Hcaproxy) (
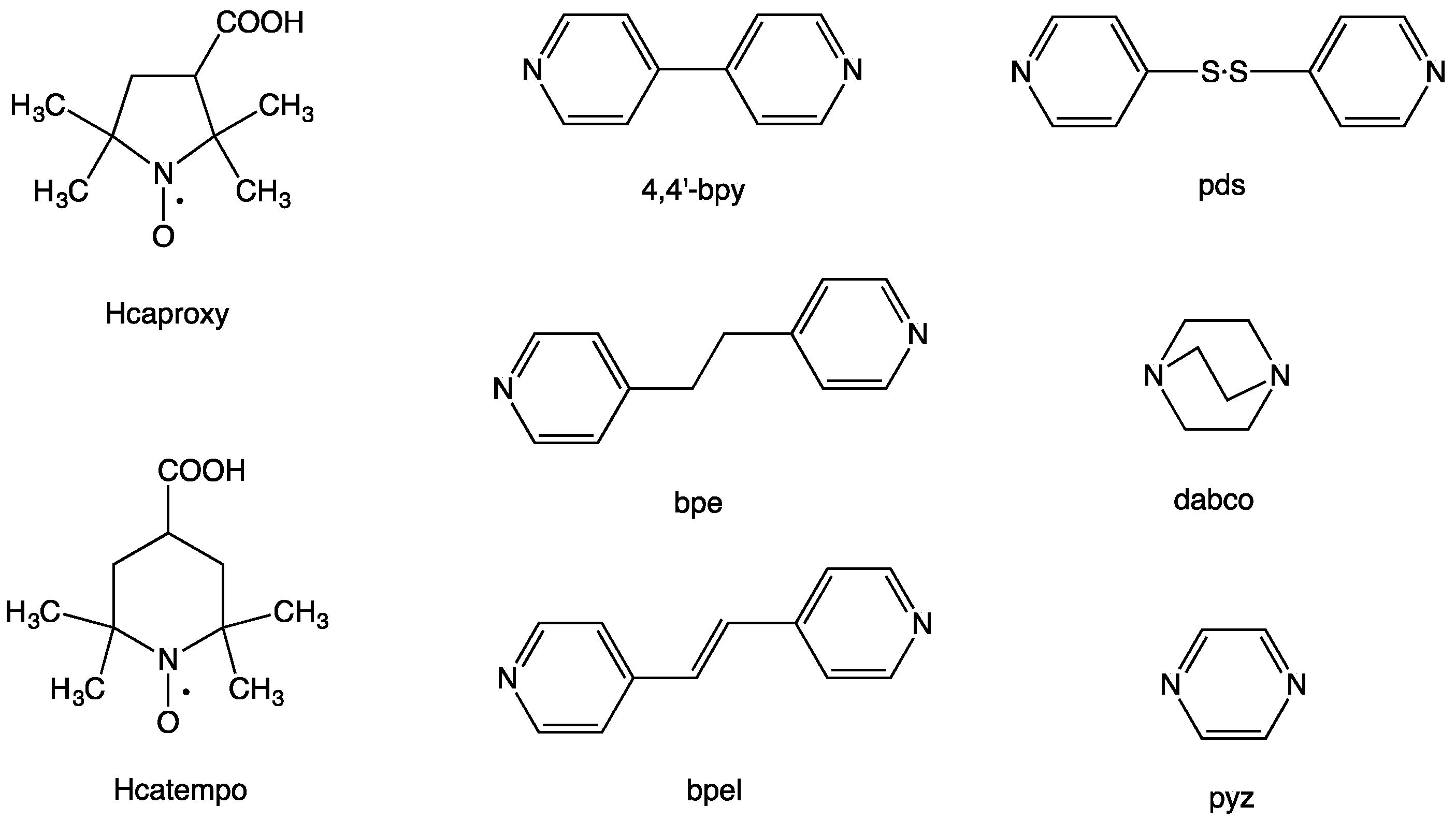
Figure 1) [
18]. In order to construct new coordination polymers with an interesting structures and properties, we combined these copper acetate type units and
N,
N’-bidentate linking ligands, 4,4′-bipyridine (4,4′-bpy), 1,2-bis(4-pyridyl)ethane (bpe),
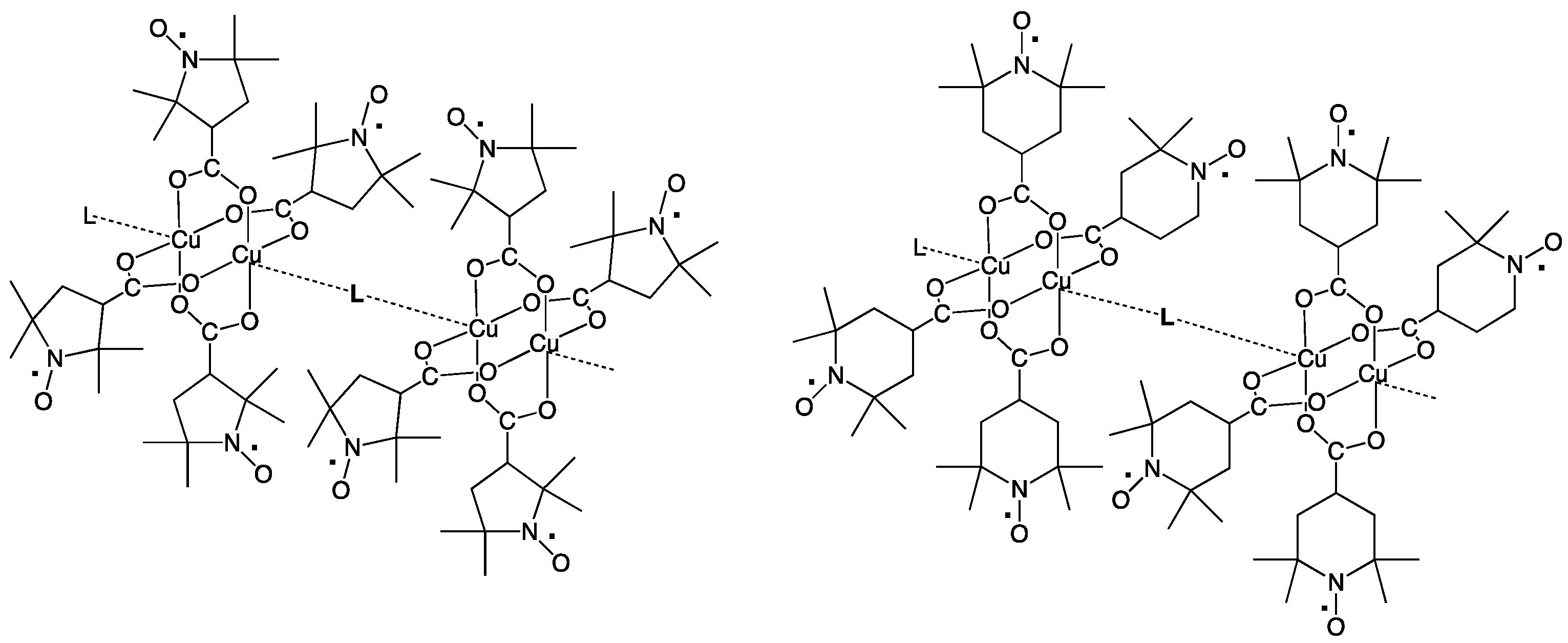
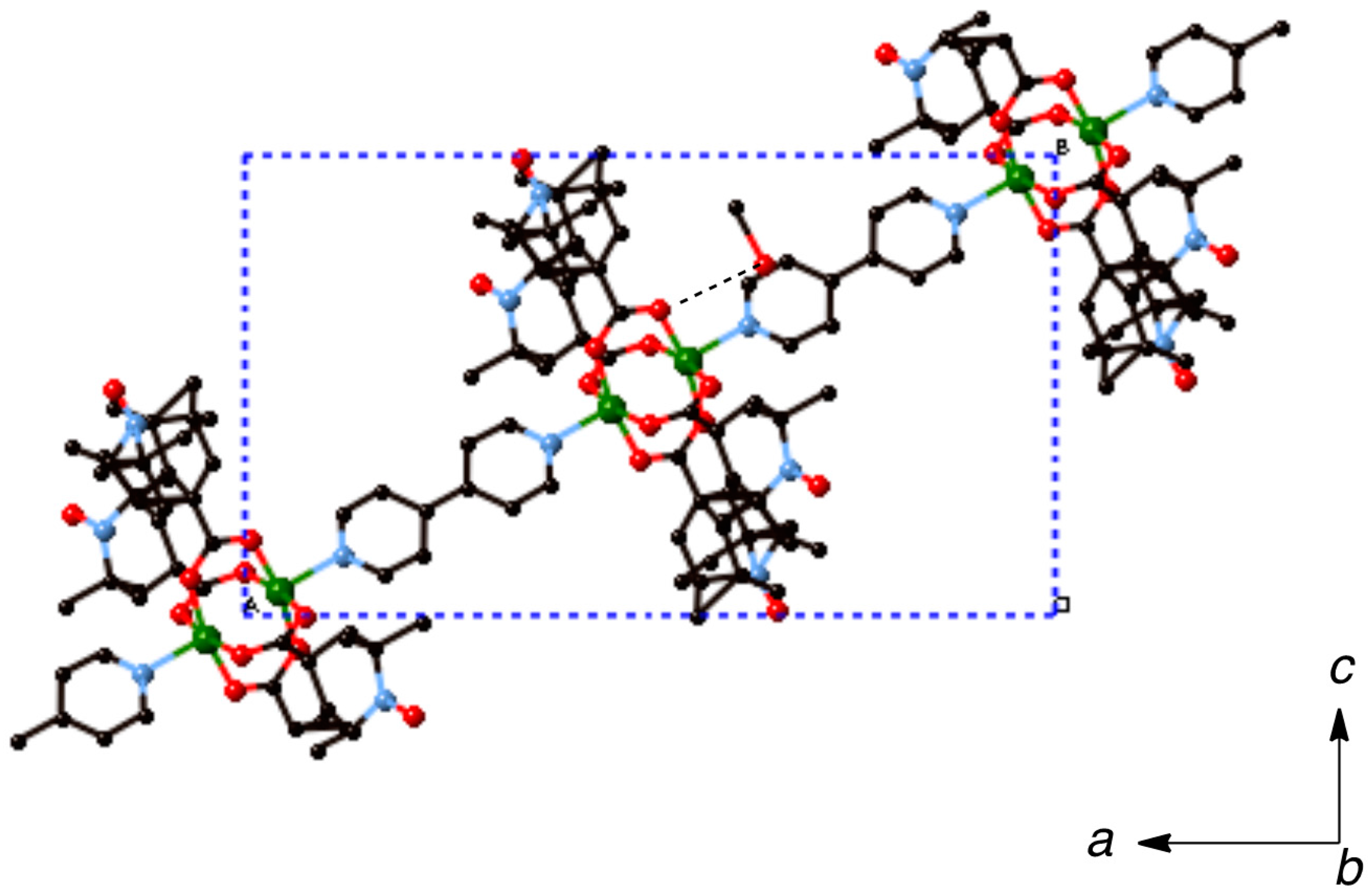
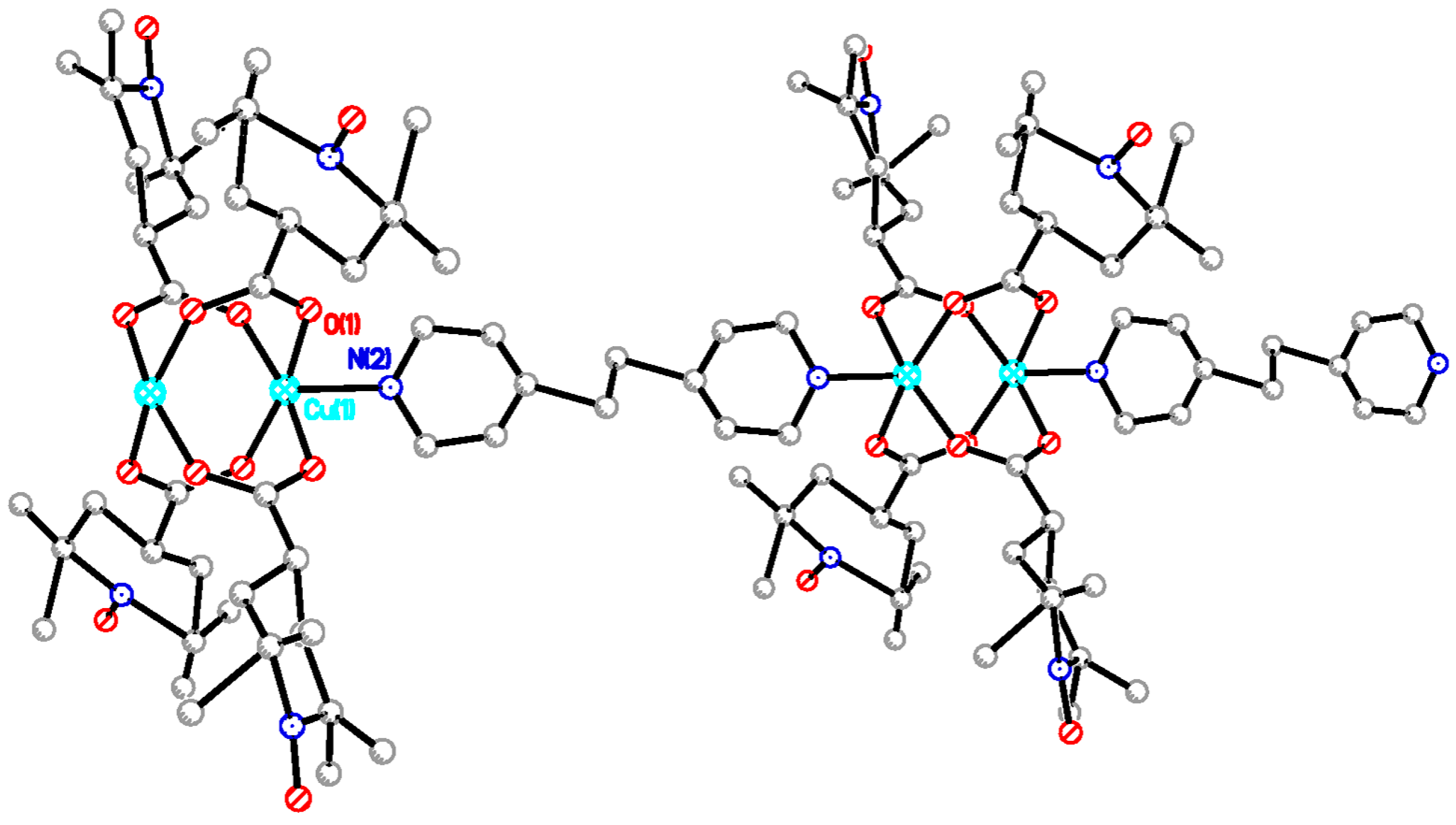
trans-1,2-bis(4-pyridyl)ethylene (bpel), 4,4′-dipyridyl disulfide (pds), and 1,4-diazabicyclo[2.2.2]octane (dabco), and pyrazine (pyz), aiming at chain compounds that are shown in
Figure 2. Among the present linking ligands, the pds ligand is interesting, having a twisted structure and accompanying the axial chirality with the
P- and the
M-forms of optical antipodes [
19]. We report here on the synthesis, magnetic properties, and crystal structures of these coordination polymers.
3. Materials and Methods
All of the chemicals were commercial products and were used as supplied. The parent dinuclear carboxylates, [Cu
2(caproxy)
4(H
2O)
2] (1) and [Cu
2(catempo)
4(H
2O)
2] (2) were prepared according to a method reported in the literature [
18]. The crude products were recrystallized once from distilled acetonitrile, filtered and dried in vacuo above P
2O
5 prior to subsequent synthesis. Anal. Found for 1: C, 47.58; H, 7.23; N, 5.67%. Calcd. for C
36H
60Cu
2N
4O
12·2.5H
2O: C, 47.36; H, 7.18; N, 6.14%. Found for 2: C, 50.25; H, 7.51; N, 6.22%. Calcd. for C
40H
68Cu
2N
4O
12·2H
2O: C, 50.04; H, 7.56; and, N, 5.84%.
Synthesis of [Cu2(caproxy)4(4,4′-bpy)]n·0.5nH2O (3). To an acetonitrile solution of 4,4′-bpy (5.8 mg, 0.037 mmol), an acetonitrile solution of 1 (30.2 mg, 0.033 mmol) was added, forming a light-green precipitate. After the reaction mixture was stirred for 1h, the mixture was left for one day. The solid was filtered off and desiccated in vacuo. Yield: 30.8 mg, 90% (based on the parent complex). Found C 53.57, H 7.03, N 7.73%. Calcd for C46H68Cu2N6O12·0.5H2O: C 53.48, H 6.73, N 8.13%.
Synthesis of [Cu2(caproxy)4(bpe)]n·0.5nCH3CN·2nH2O (4). Compound 4 was prepared as for 3 using bpe (5.8 mg, 0.031 mmol) and 1 (25.8 mg, 0.029 mmol). Yield: 23.4 mg, 77.9% (based on the parent complex). Found C 52.84, H 6.88, N 8.09%. Calcd for C48H72Cu2N6O12·0.5CH3CN·2H2O: C 53.08, H 7.05, N 8.21%.
Synthesis of [Cu2(caproxy)4(bpel)]n·0.5nH2O (5). Compound 5 was prepared as for 3 using bpel (5.8 mg, 0.032 mmol) and 1 (25.2 mg, 0.028 mmol). Yield: 27.0 mg, 92.2% (based on the parent complex). Found C 54.08, H 6.25, N 7.97%. Calcd for C48H70Cu2N6O12·0.5H2O: C 54.43, H 6.76, N 7.93%.
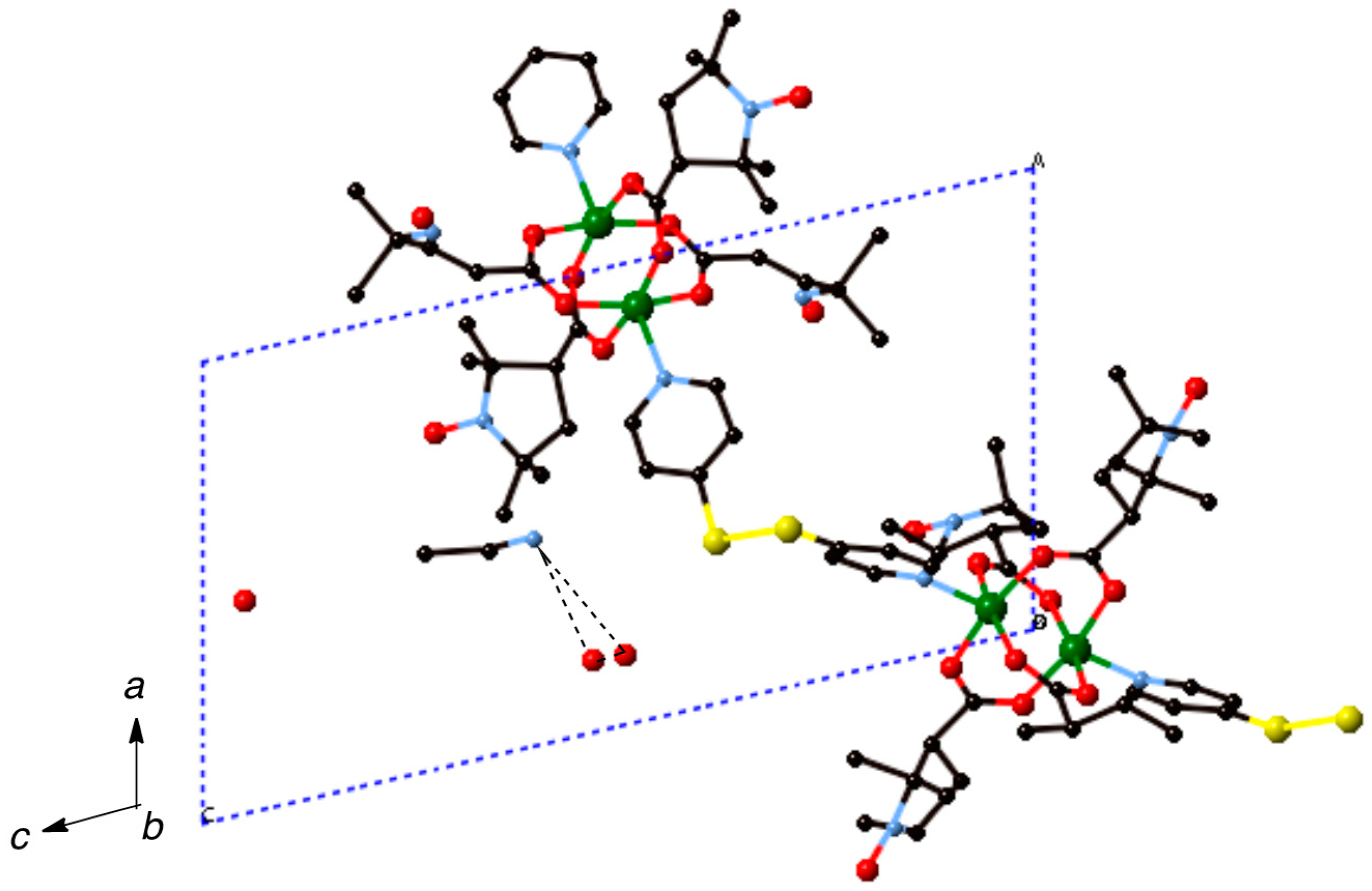
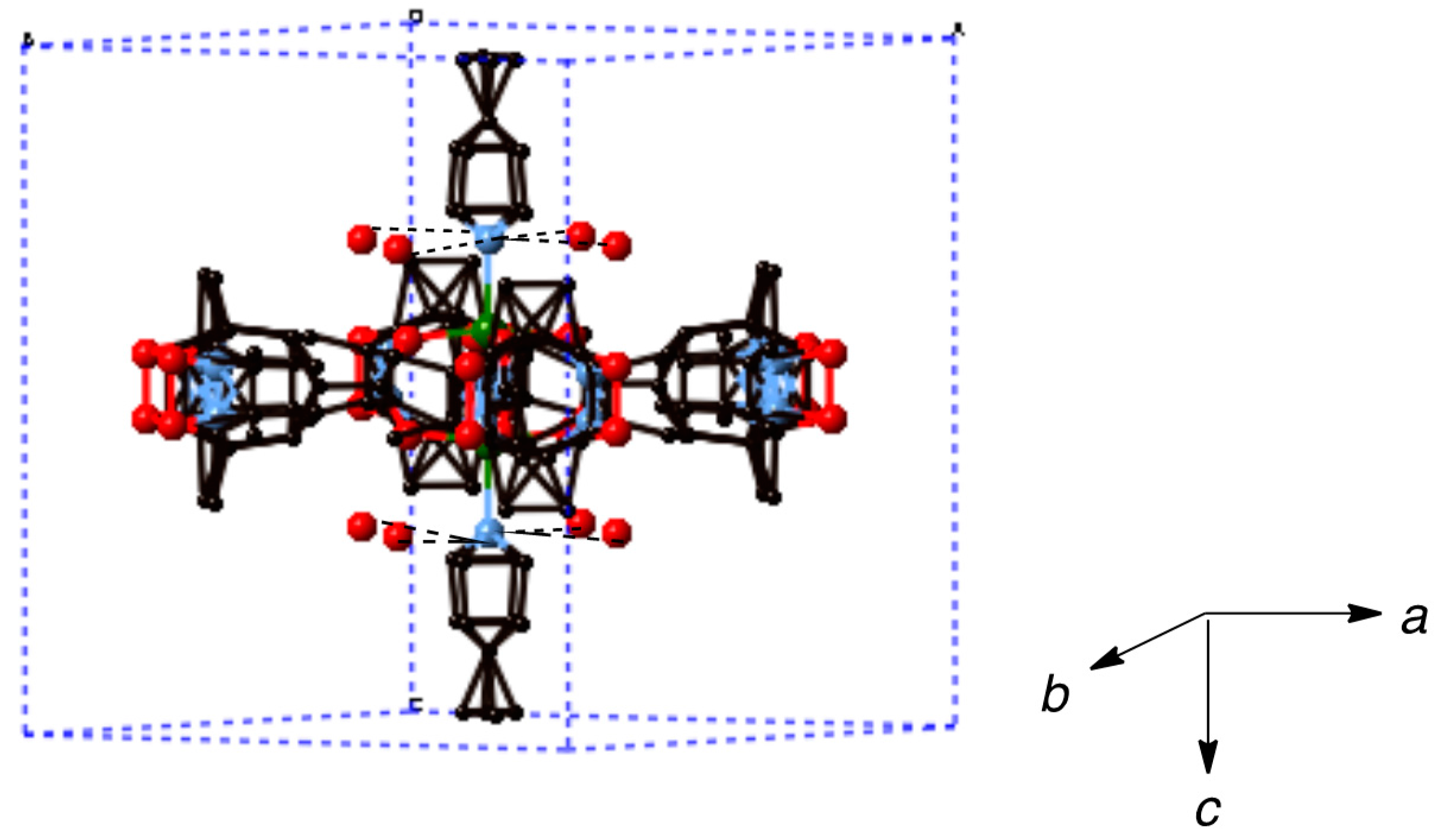
Synthesis of [Cu2(caproxy)4(pds)]n·0.5nCH3CN·2nH2O (6). Compound 6 was prepared as for 3 using pds (8.1 mg, 0.037 mmol) and 1 (30.8 mg, 0.034 mmol). Yield: 29.8 mg, 80.4% (based on the parent complex). Found C 49.10, H 6.00, N 8.38%. Calcd for C46H68Cu2N6O12S2·0.5CH3CN·2H2O: C 49.31, H 6.47, N 7.95%. X-ray quality crystals were grown by slow diffusion process using H-formed tube at ambient temperature.
Synthesis of [Cu2(caproxy)4(dabco)]n (7). Compound 7 was prepared as for 3 using dabco (4.8 mg, 0.043 mmol) and 1 (30.2 mg, 0.033 mmol). Yield: 29.2 mg, 89.2% (based on the parent complex). Found C 50.99, H 6.95, N 8.59%. Calcd for C42H72Cu2N6O12: C 51.47, H 7.40, N 8.57%.
Synthesis of [Cu2(caproxy)4(pyz)]n·2nH2O (8). Compound 8 was prepared as for 3 using pyz (3.4 mg, 0.042 mmol) and 1 (25.6 mg, 0.028 mmol). Yield: 16.1 mg, 60% (based on the parent complex). Found C 48.72, H 6.67, N 8.37%. Calcd for C40H64Cu2N6O12·2H2O: C 48.82, H 6.96, N 8.54%.
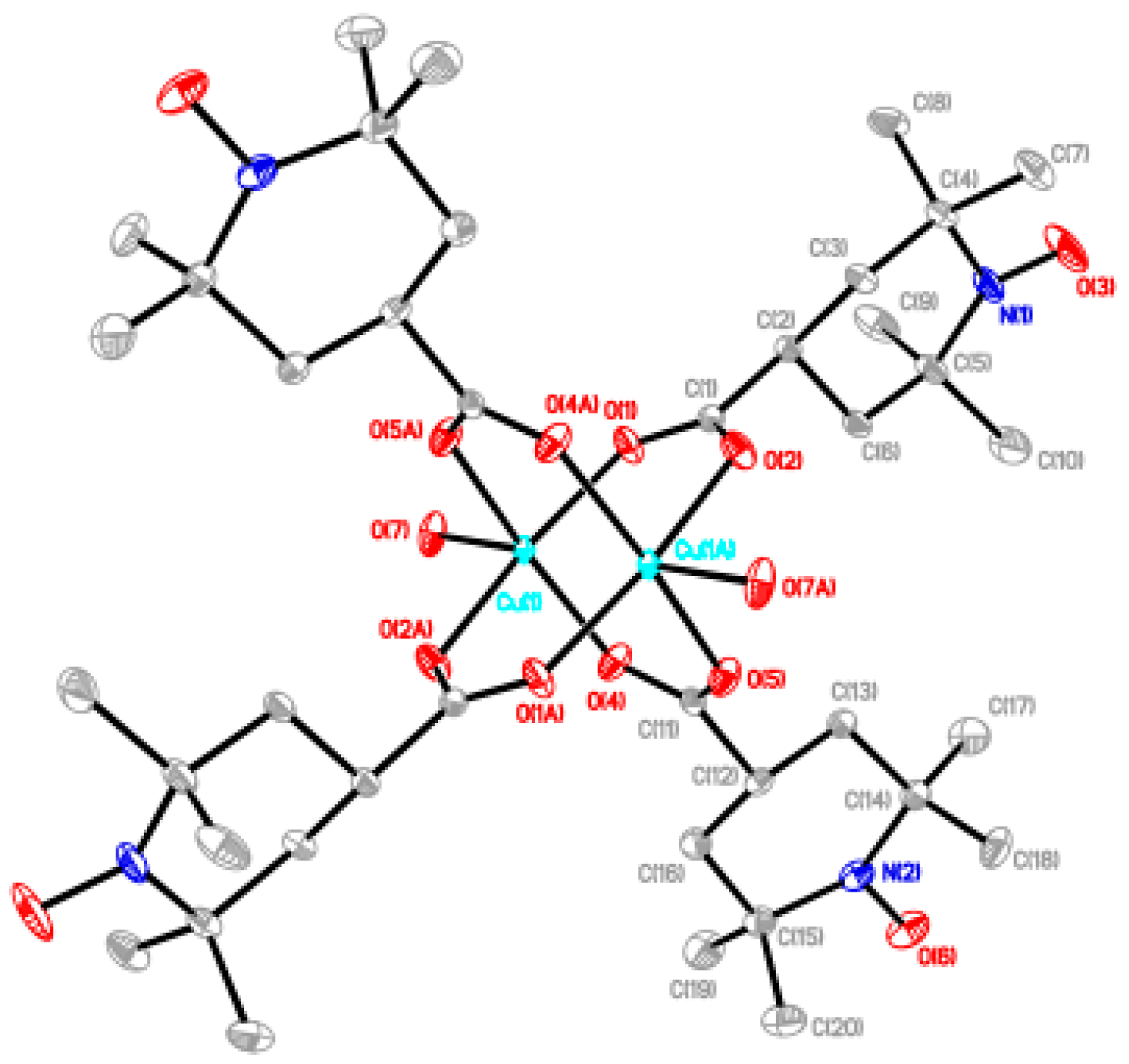
Synthesis of [Cu2(catempo)4(4,4′-bpy)]n (9). To an acetonitrile solution of 4,4′-bpy (6.8 mg, 0.044 mmol), an acetonitrile solution of 2 (40 mg, 0.042 mmol) was added, forming a light-green precipitate. After the reaction mixture was stirred for 1h, the mixture was left for one day. The solid was filtered off and was desiccated in vacuo. Yield: 40.0 mg, 88.9% (based on the parent complex). Found C 55.83, H 6.59, N 7.73%. Calcd for C50H76Cu2N6O12: C 55.59, H 7.09, N 7.78%. X-ray quality crystals were grown by slow diffusion process at ambient temperature.
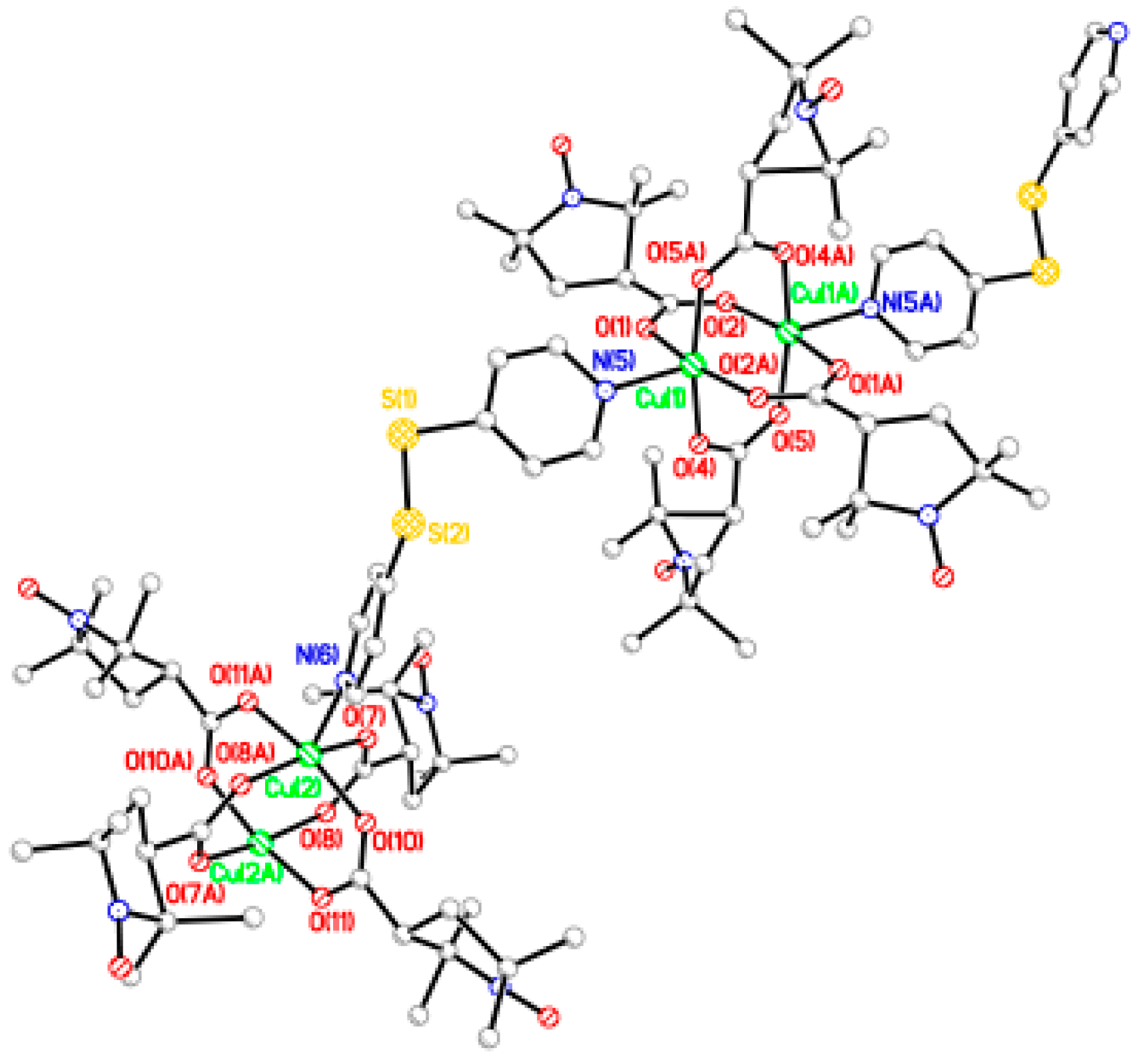
Synthesis of [Cu2(catempo)4(bpe)]n·1.5nH2O (10). Compound 10 was prepared as for 9 using bpe (8.0 mg, 0.043 mmol) and 2 (40.1 mg, 0.042 mmol). Yield: 40.4 mg, 87.3% (based on the parent complex). Found C 55.02, H 7.20, N 7.75%. Calcd for C52H80Cu2N6O12·1.5H2O: C 55.01, H 7.37, N 7.40%. X-ray quality crystals were grown by slow diffusion process at ambient temperature.
Synthesis of [Cu2(catempo)4(bpel)]n·nCH3CN·0.5nH2O (11). Compound 11 was prepared as for 9 using bpel (7.9 mg, 0.043 mmol) and 2 (40.2 mg, 0.042 mmol). Yield: 26.0 mg, 56.1% (based on the parent complex). Found C 55.77, H 6.84, N 8.69%. Calcd for C52H78Cu2N6O12·CH3CN·0.5H2O: C 56.09, H 7.15, N 8.48%.
Synthesis of [Cu2(catempo)4(pds)]n·0.5nCH3CN (12). Compound 12 was prepared as for 9 using pds (7.0 mg, 0.032 mmol) and 2 (30.0 mg, 0.031 mmol). Yield: 26.8 mg, 74.9% (based on the parent complex). Found C 53.03, H 6.23, N 7.57%. Calcd for C50H76Cu2N6O12S2·0.5CH3CN: C 52.58, H 6.71, N 7.82%.
Synthesis of [Cu2(catempo)4(dabco)]n·nH2O (13). Compound 13 was prepared as for 9 using dabco (3.7 mg, 0.033 mmol) and 2 (30.0 mg, 0.031 mmol). Yield: 26.5 mg, 81.8% (based on the parent complex). Found C 52.18, H 7.60, N 8.12%. Calcd for C46H80Cu2N6O12·H2O: C 52.41, H 7.84, N 7.97%.
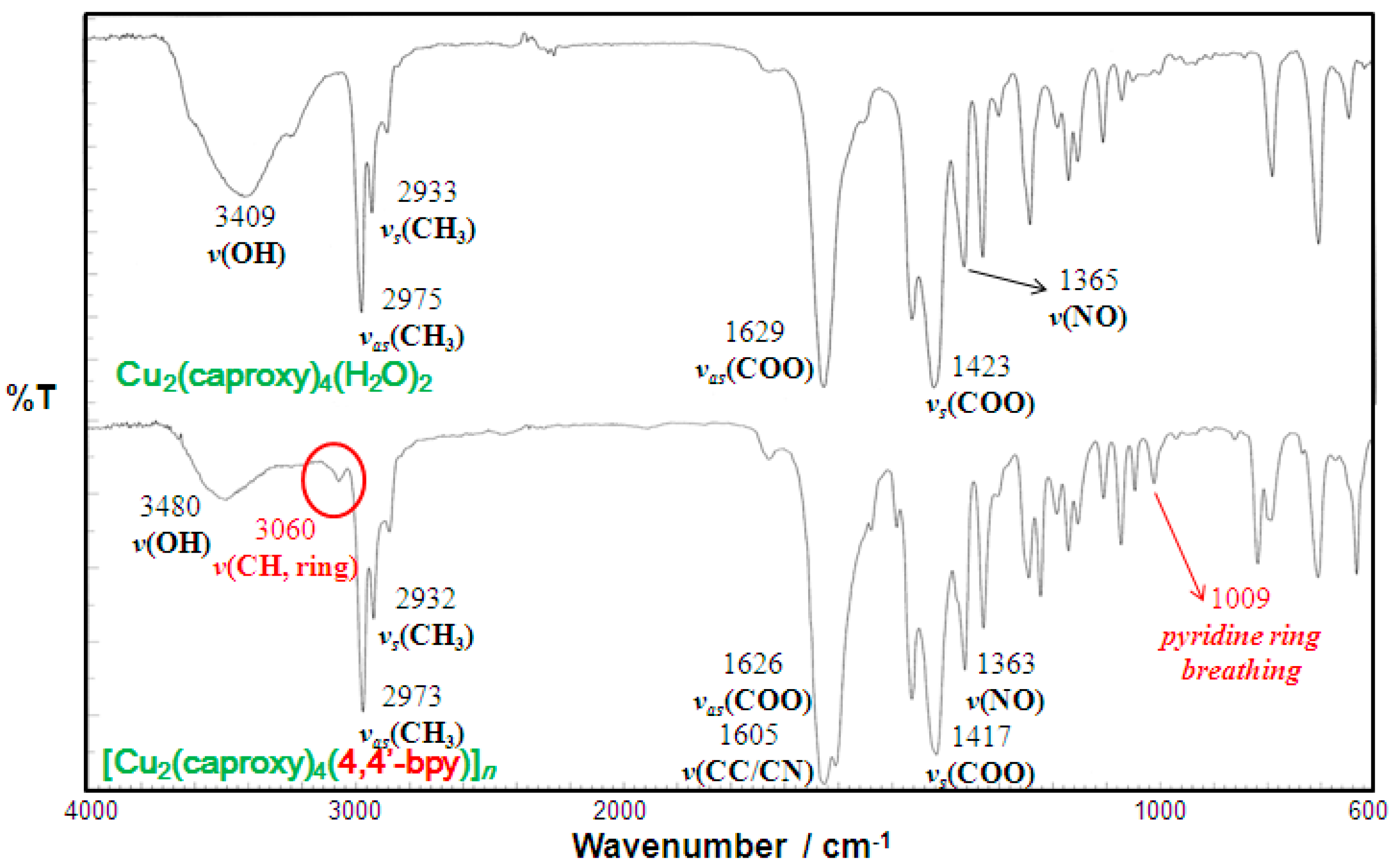
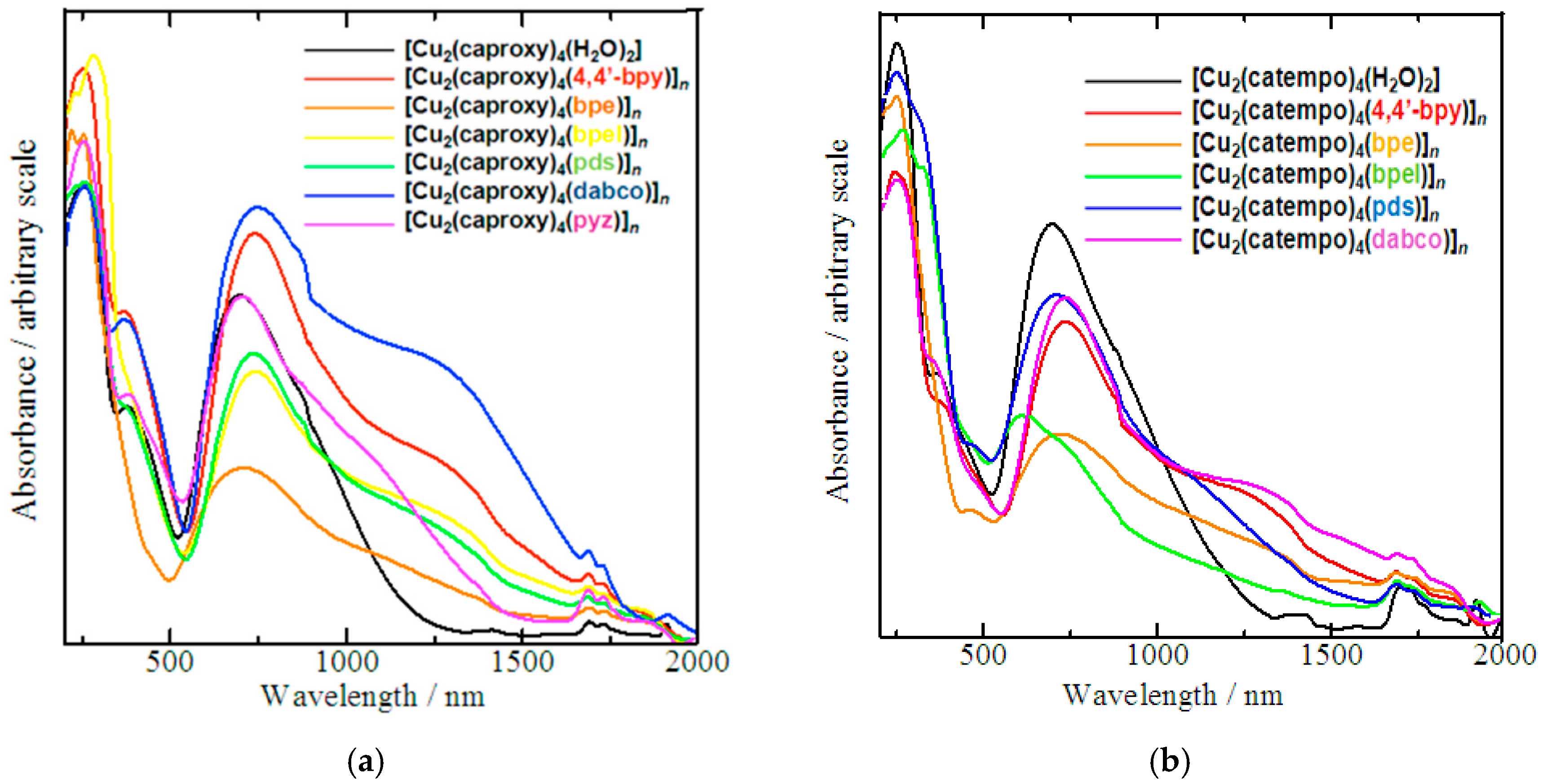
Elemental analyses for C, H, and N were performed using a Thermo-Finnigan FLASH EA1112 series CHNO-S analyzer. Infrared spectra were measured with a JASCO MFT-2000 FT-IR Spectrophotometer in the 4000–600 cm−1 region. Solution spectra (in CH3OH) were recorded on a Shimadzu UV-vis-NIR Recording Spectrophotometer Model UV-3100 in the 200–1000 nm region. Diffused reflectance spectra were measured with a Shimadzu UV-vis-NIR Recording Spectrophotometer Model UV-3100 that was equipped with an integrating sphere in the 200–2000 nm region. Magnetic susceptibilities were measured with a Quantum Design MPMS-XL7 SQUID susceptometer over a temperature range of 4.5–300 K.
Single-crystal diffraction data were measured on a Bruker Smart APEX CCD diffractometer equipped with a graphite crystal and incident beam monochromator using Mo Kα radiation (
λ = 0.71073 Å). The structure was solved by direct methods, and was refined by full-matrix least-squares methods. The hydrogen atoms were inserted at their calculated positions and fixed there. All of the calculations were carried out utilizing the SHELXTL software package [
40]. Crystallographic data have been deposited with Cambridge Crystallographic Data Centre: Deposit numbers CCDC-1836763-1836765 and 1836862. Copies of the data can be obtained free of charge via
http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB2 1EZ, UK; Fax: +44 1223 336033; e-mail:
deposit@ccdc.cam.ac.uk).
4. Conclusions
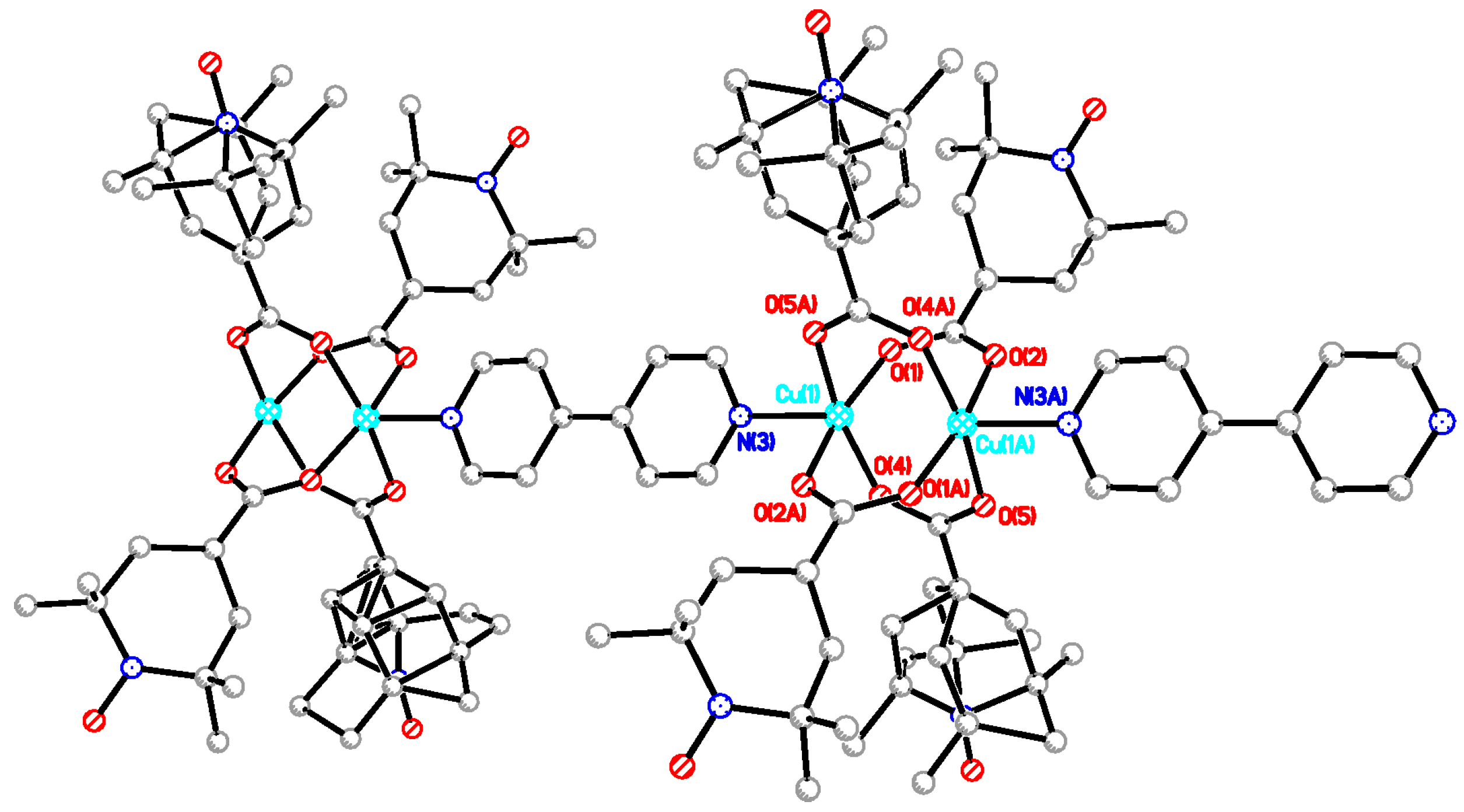
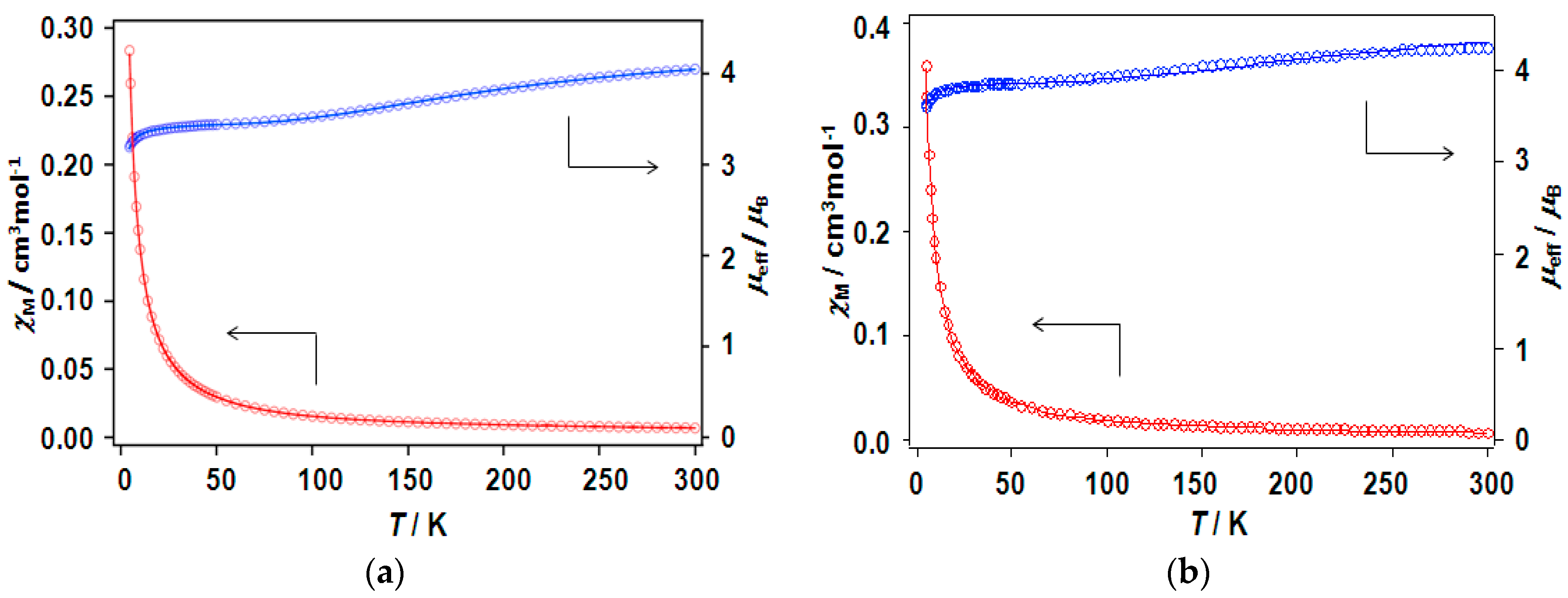
In this study, two dinuclear copper(II) complexes of free radical carboxylic acid, [Cu2(caproxy)4(H2O)2] and [Cu2(catempo)4(H2O)2], have been evolved into coordination polymer by using six types of N,N’-bidentate ligands. Eleven chain adducts, formulated as [Cu2(caproxy)4(L)]n (L = 4,4′-bpy (3), bpe (4), bpel (5), pds (6), dabco (7), pyz (8)) and [Cu2(catempo)4(L)]n (L = 4,4′-bpy (9), bpe (10), bpel (11), pds (12), dabco (13)), were prepared in satisfactory yield as well as characterized. The crystal structures of [Cu2(caproxy)4(pds)]n, [Cu2(catempo)4(4,4′-bpy)]n and [Cu2(catempo)4(bpe)]n revealed that the bridging carboxylate radicals (caproxy– and catempo–) are integrated in the dinuclear copper core, thus configuring a ‘paddle-wheel’ type structure, and each dinuclear cluster is connected by the linking ligand (pds, 4,4′-bpy, and bpe) to form a chain molecule. For the other complexes, a chain structure with an alternated arrangement of the Cu2(caproxy)4 or Cu2(catempo)4 dinuclear unit and the N,N’-bidentate ligands can be proposed based on the analytical data, infrared and electronic spectra, as well as the bridging nature of the N,N’-bidentate ligands. The magnetic interaction via the N,N’-bidentate spacer ligand was found to be generally weak and antiferromagnetic. Although, the present linking ligands did not occur a stronger interaction between the copper(II) clusters, it is important to find out that several linking ligands can work connect the clusters with multiple radicals to assemble the spins to construct magnetic materials as the first step.