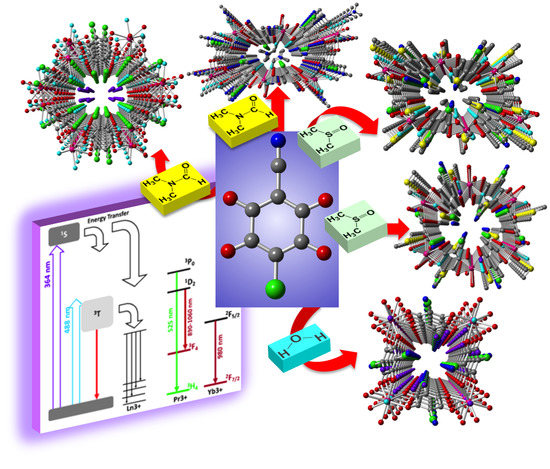
Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand
Abstract
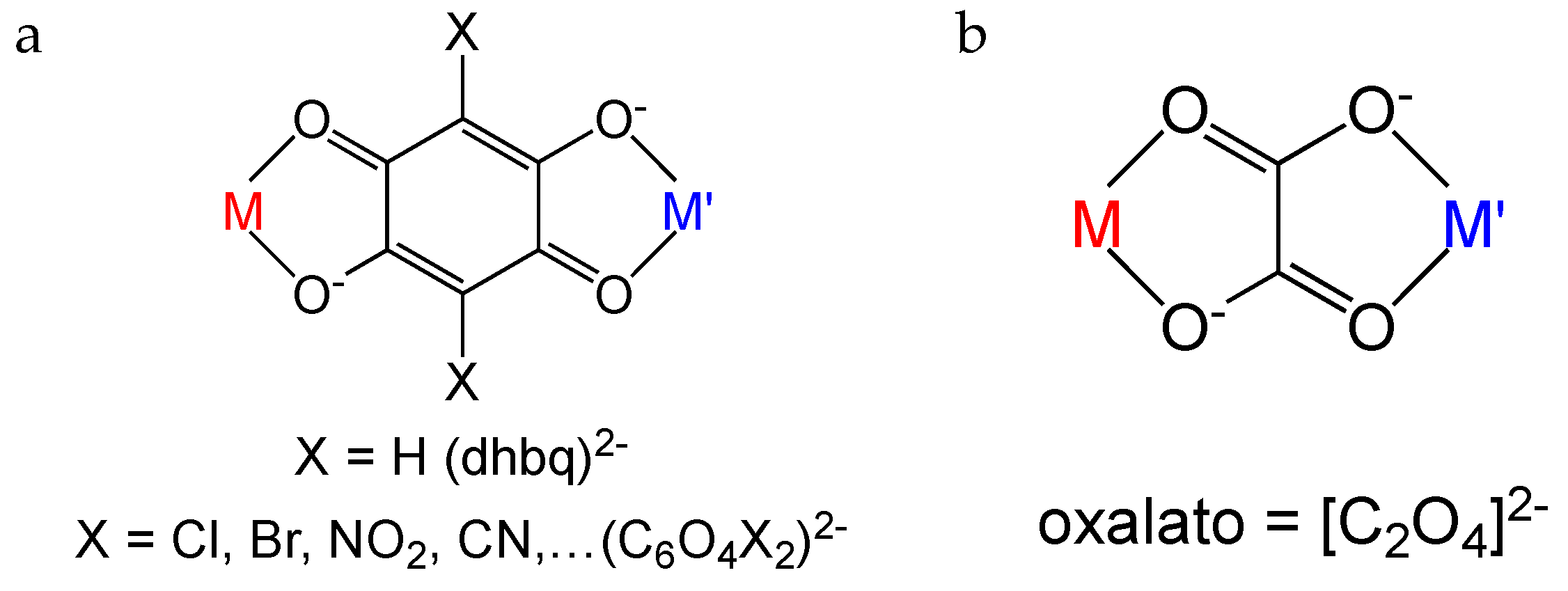
1. Introduction
2. Results and Discussion
2.1. Syntheses of the Complexes
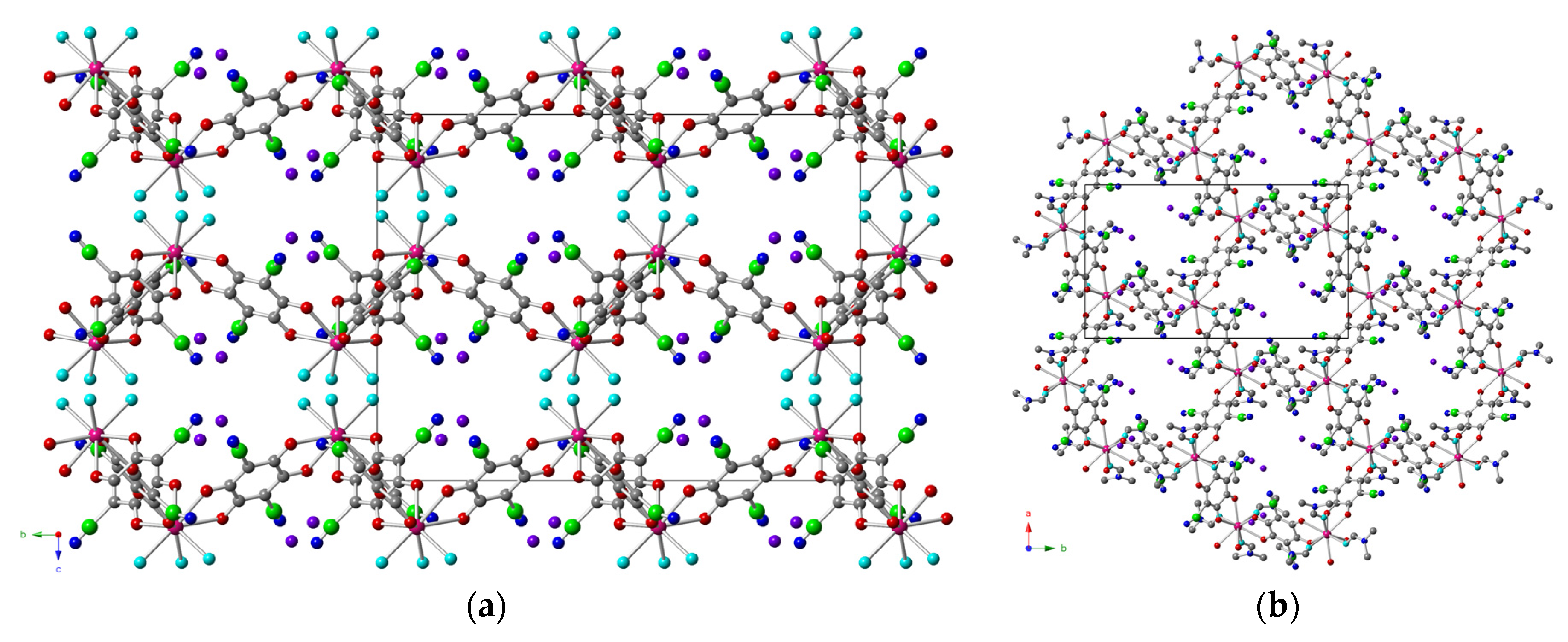
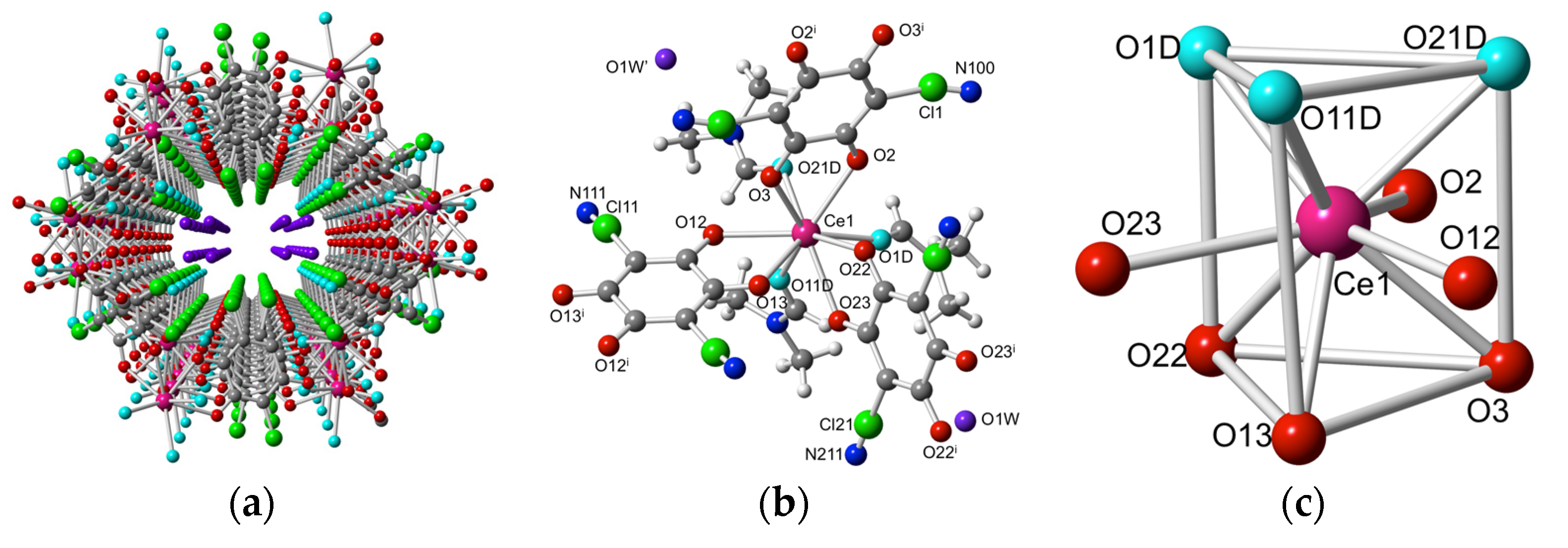
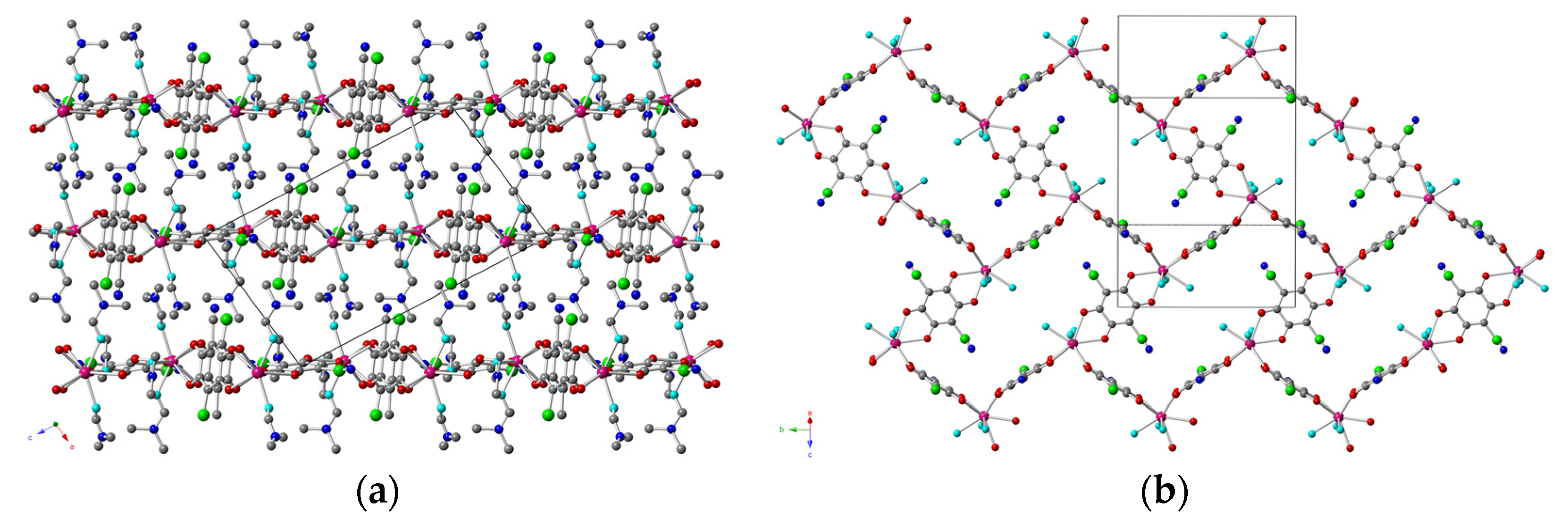
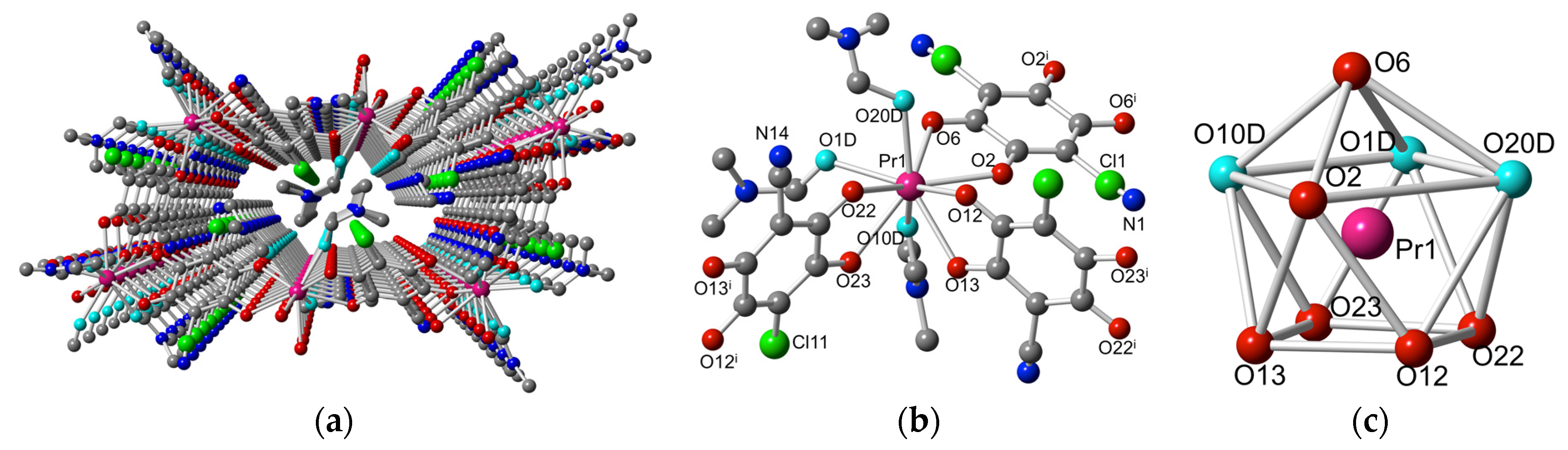
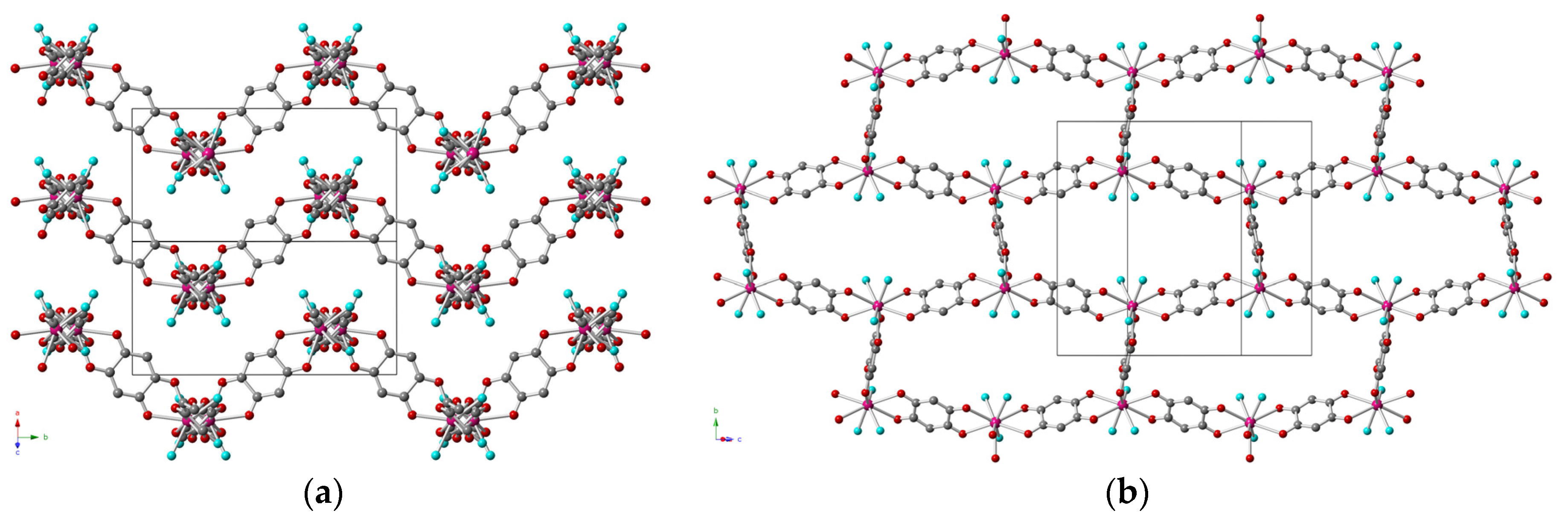
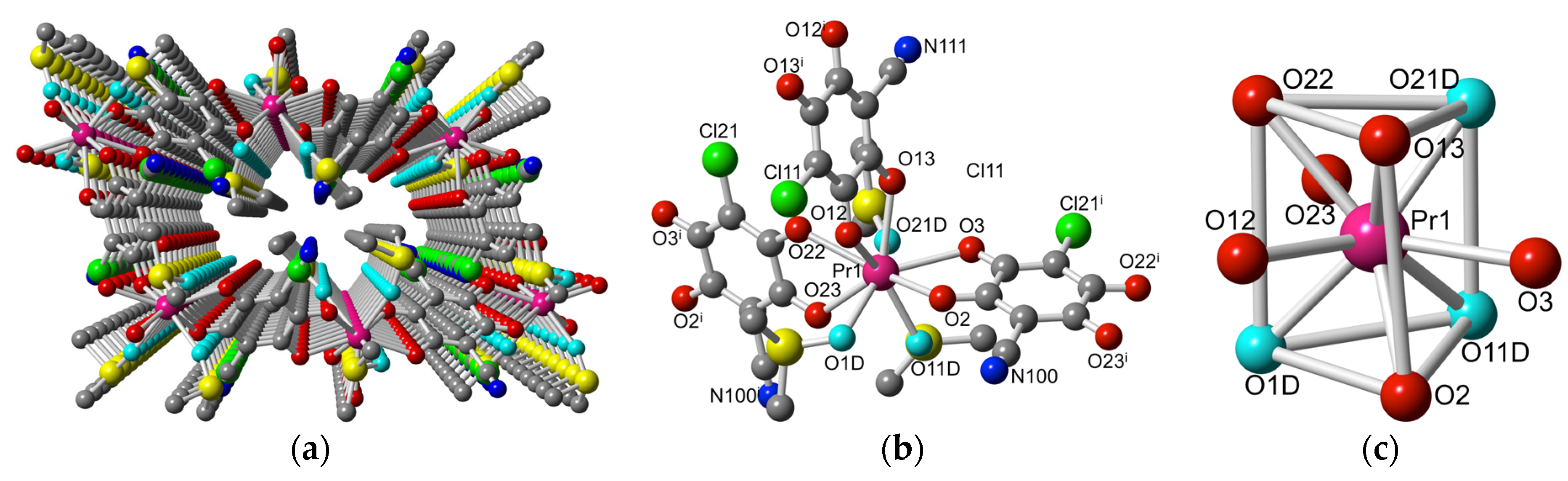
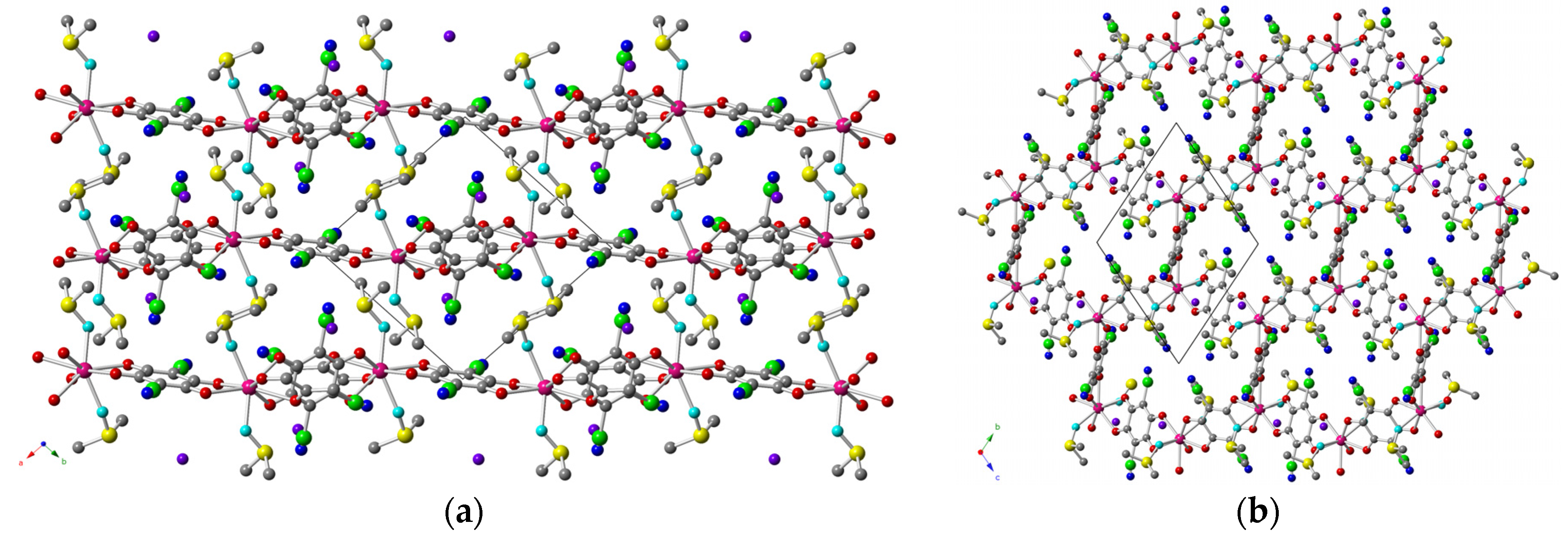
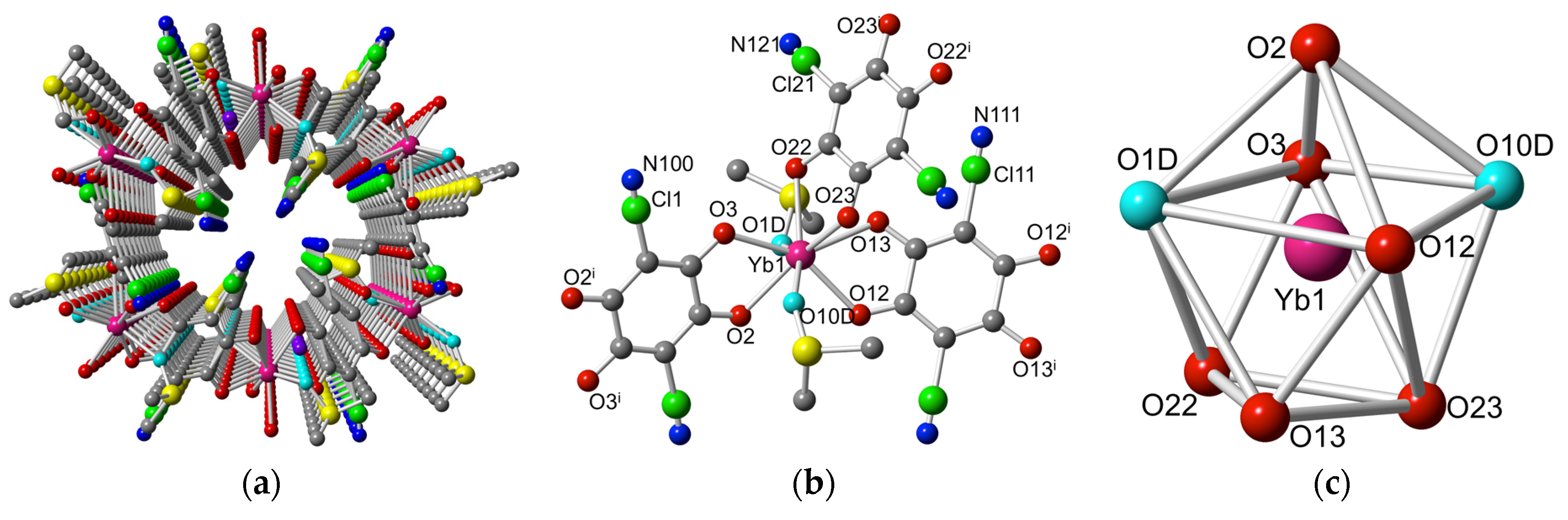
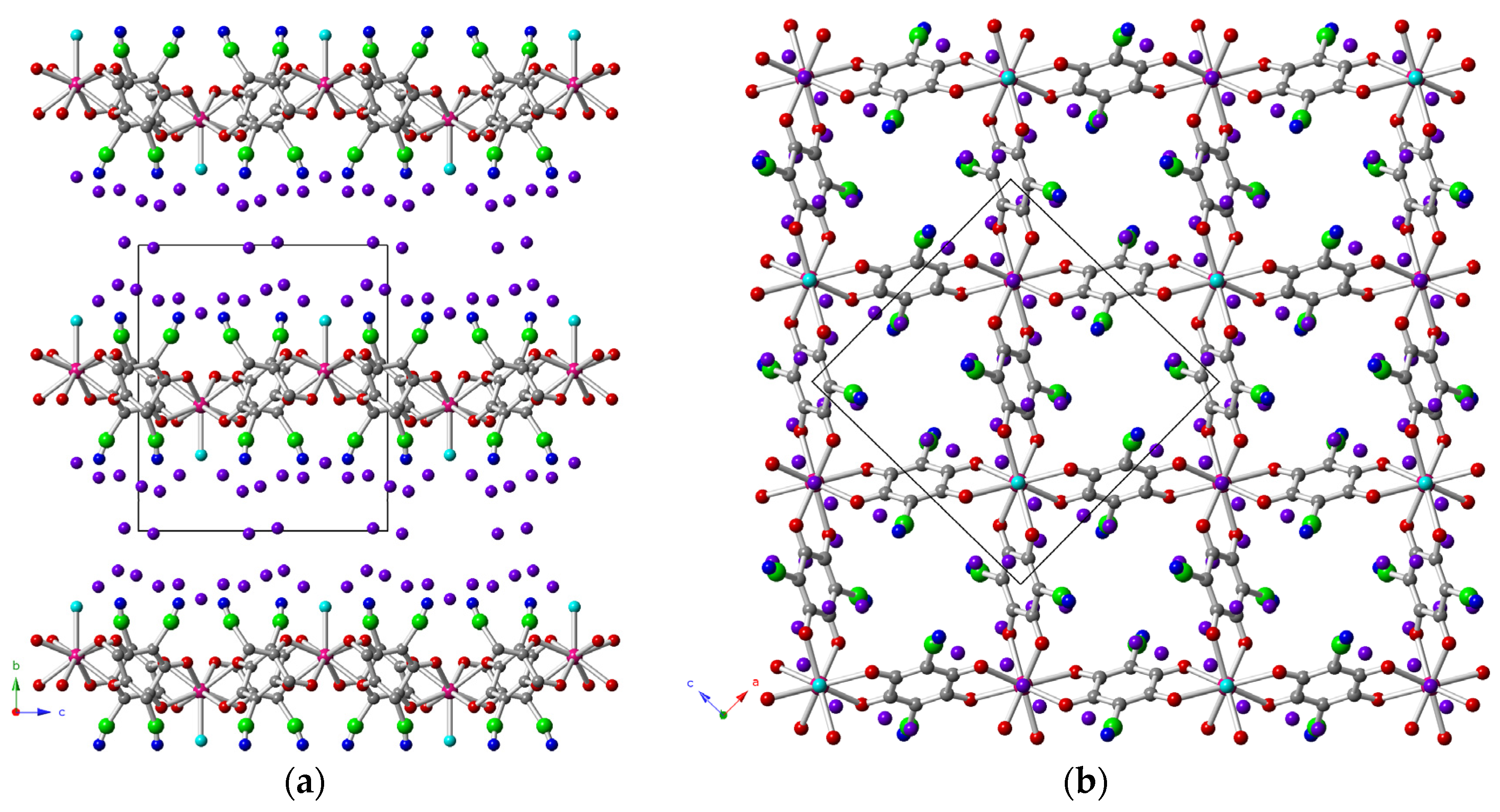
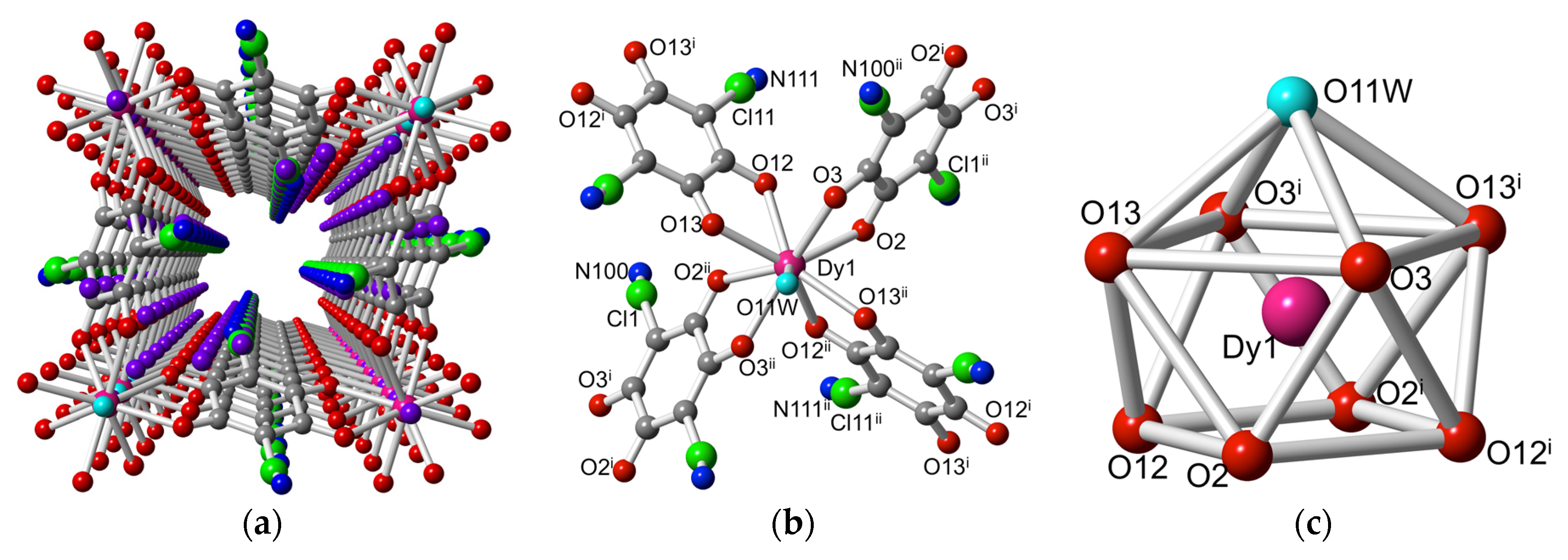
2.2. Description of the Structures
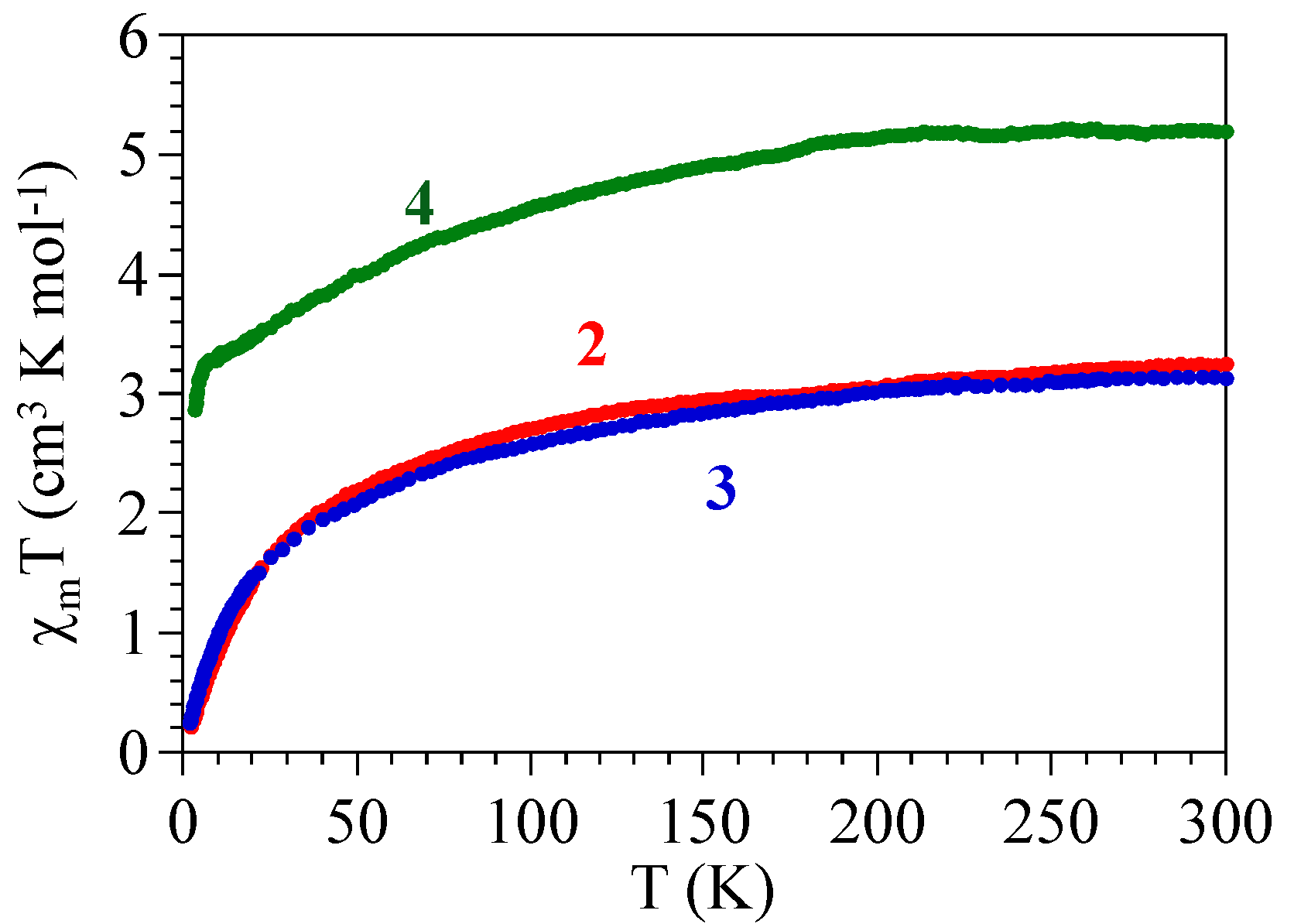
2.3. Magnetic Properties
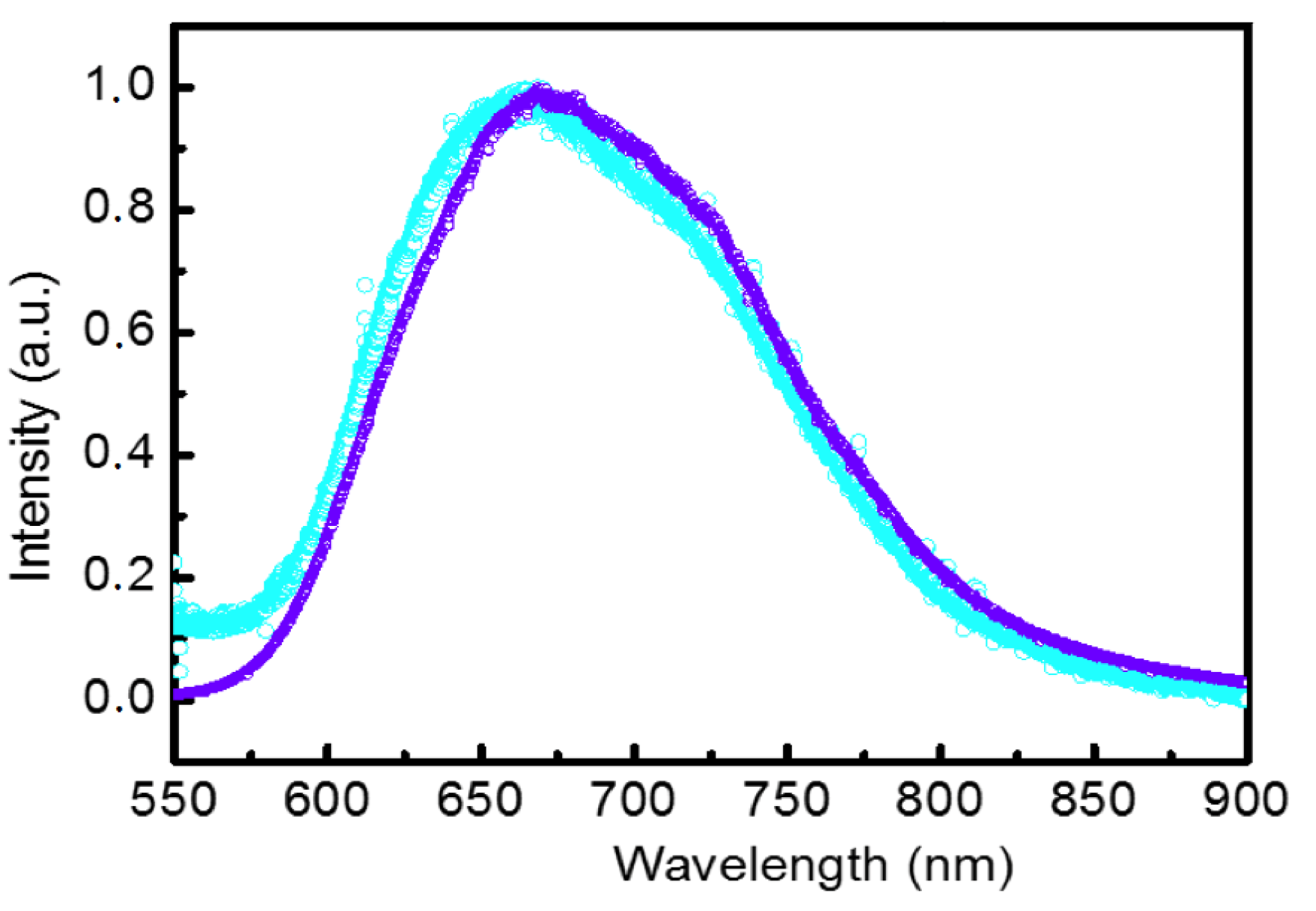
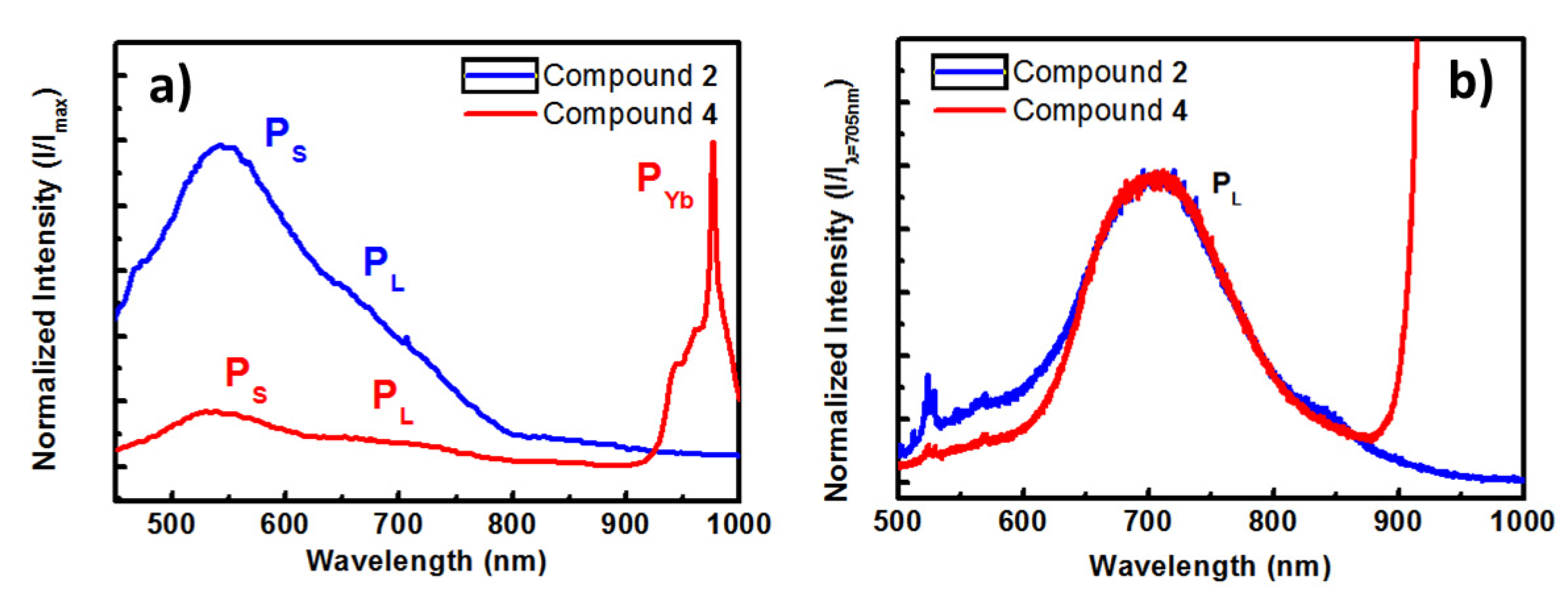
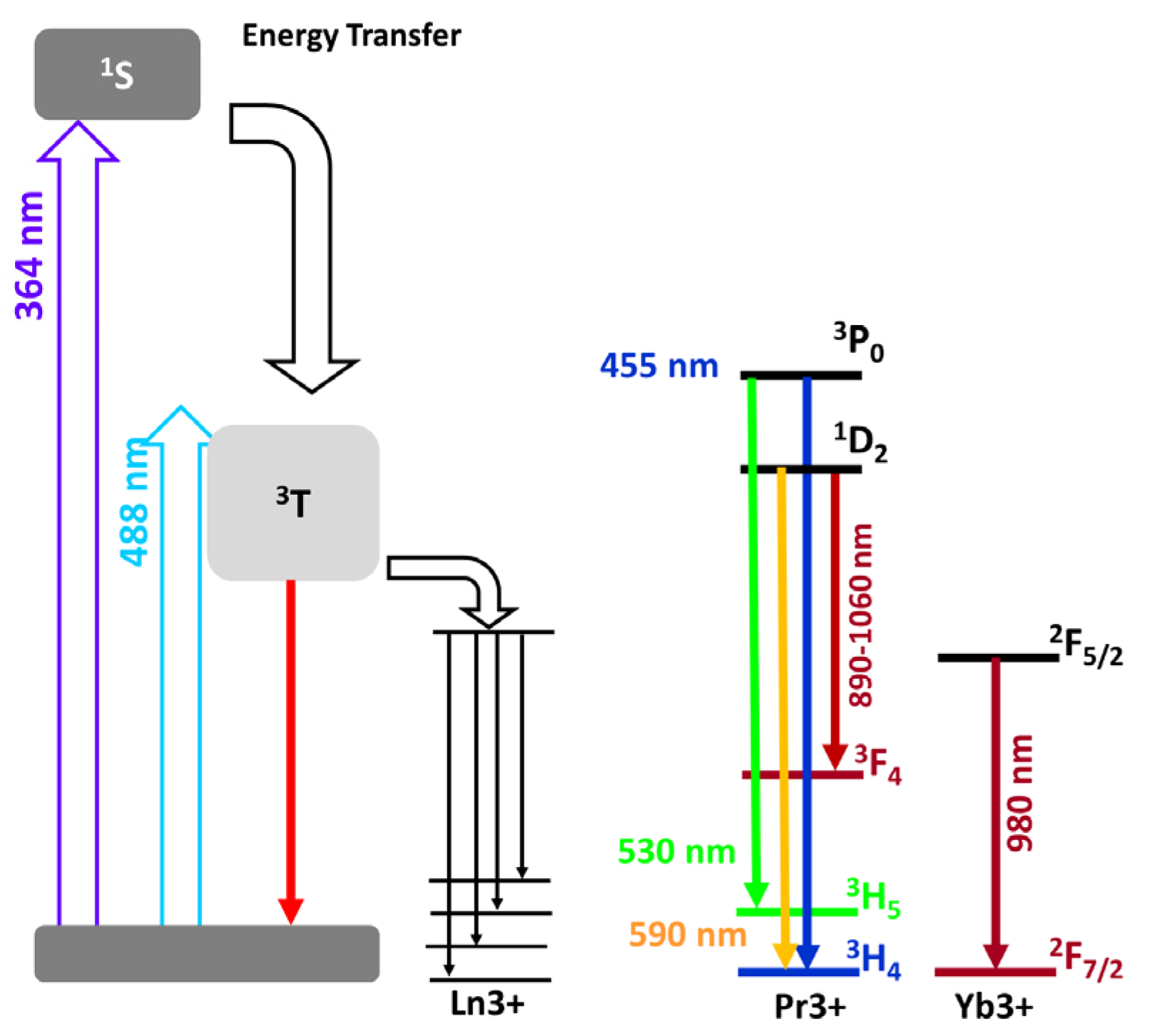
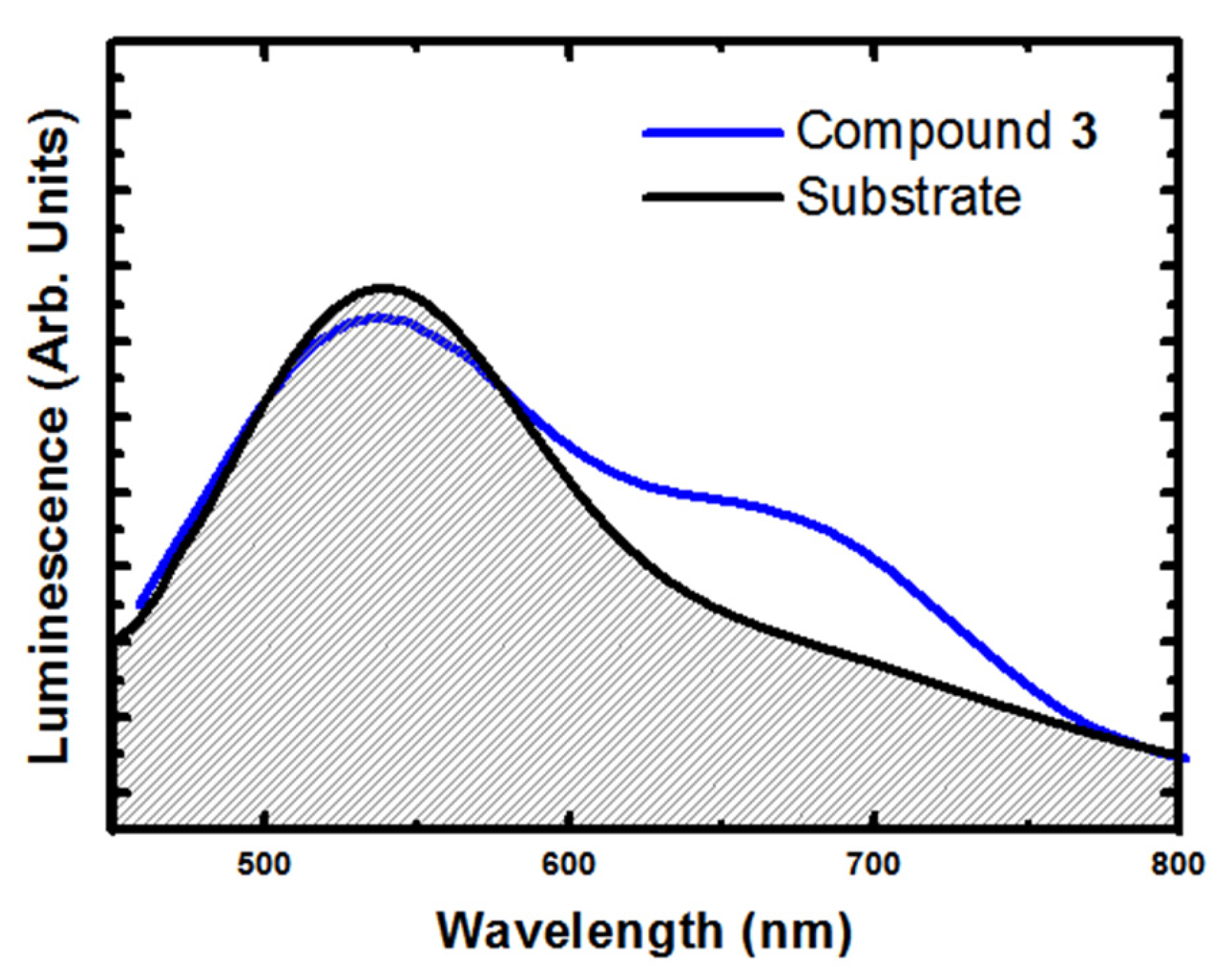
2.4. Luminescent Properties
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of [Ce2(C6O4Cl(CN))3(DMF)6]·2H2O (1)
3.3. Synthesis of [Pr2(C6O4(CN)Cl)3(DMF)6] (2)
3.4. Synthesis of [Pr2(C6O4(CN)Cl)3(DMSO)6] (3)
3.5. Synthesis of [Yb2(C6O4(CN)Cl)3(DMSO)4]·2H2O (4)
3.6. Synthesis of (H3O)[Dy(C6O4Cl(CN))2(H2O) ]·4H2O (5)
3.7. Magnetic Measurements
3.8. Luminesce Measurements
3.9. Crystallographic Data Collection and Refinement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Barea, E.; Montoro, C.; Navarro, J.A. Toxic gas removal-metal-organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal-organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1, 16034. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P. Metal–organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Dinca, M. Metal-Organic Frameworks as Active Materials in Electronic Sensor Devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Fordham, S.; Wang, X.; Bosch, M.; Zhou, H. Lanthanide Metal-Organic Frameworks: Syntheses, Properties, and Potential Applications. In Structure and Bonding; Springer: Berlin, Germany, 2015; Volume 163, pp. 1–27. [Google Scholar]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, W.; Bouwman, E. One-step growth of lanthanoid metal-organic framework (MOF) films under solvothermal conditions for temperature sensing. Chem. Commun. (Camb.) 2016, 52, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, W.; Wang, R.; Ren, C.; Li, Q.; Fan, Y.; Liu, B.; Liu, P.; Wang, Y. Effect of Coordinated Solvent Molecules on Metal Coordination Sphere and Solvent-Induced Transformations. Cryst. Growth Des. 2017, 17, 517–526. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Li, X.; Fu, Z.; Su, Y.; Xu, G. Porous Cadmium(II) Anionic Metal-Organic Frameworks Based on Aromatic Tricarboxylate Ligands: Encapsulation of Protonated Flexible Bis(2-methylimidazolyl) Ligands and Proton Conductivity. Cryst. Growth Des. 2015, 15, 4543–4548. [Google Scholar] [CrossRef]
- Sun, L.; Qi, Y.; Che, Y.; Batten, S.R.; Zheng, J. Three Unprecedented Entangled Metal-Organic Frameworks: Self-Penetration and Hydrothermal in Situ Ligand Formation. Cryst. Growth Des. 2009, 9, 2995–2998. [Google Scholar] [CrossRef]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.C. Tuning the topology and functionality of metal-organic frameworks by ligand design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kawata, S. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chem. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry 2017, 3, 17. [Google Scholar] [CrossRef]
- Atzori, M.; Artizzu, F.; Sessini, E.; Marchio, L.; Loche, D.; Serpe, A.; Deplano, P.; Concas, G.; Pop, F.; Avarvari, N.; et al. Halogen-bonding in a new family of tris(haloanilato)metallate(III) magnetic molecular building blocks. Dalton Trans. 2014, 43, 7006–7019. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Gómez-Claramunt, P.; Vallés-García, C.; Mínguez Espallargas, G.; Gómez García, C.J. Key Role of the Cation in the Crystallization of Chiral Tris(Anilato)Metalate Magnetic Anions. Cryst. Growth Des. 2016, 16, 518–526. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Grannas, M.J.; Hudson, T.A.; Hughes, S.A.; Pranoto, N.H.; Robson, R. Synthesis, structure and host-guest properties of (Et4N)2[SnIVCaII(chloranilate)4], a new type of robust microporous coordination polymer with a 2D square grid structure. Dalton Trans. 2011, 40, 12242–12247. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Benmansour, S.; Mínguez Espallargas, G.; Clemente-León, M.; Abhervé, A.; Gómez-Claramunt, P.; Coronado, E.; Artizzu, F.; Sessini, E.; Deplano, P.; et al. A Family of Layered Chiral Porous Magnets Exhibiting Tunable Ordering Temperatures. Inorg. Chem. 2013, 52, 10031–10040. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Vallés-García, C.; Gómez-Claramunt, P.; Mínguez Espallargas, G.; Gómez-García, C.J. 2D and 3D Anilato-Based Heterometallic M(I)M(III) Lattices: The Missing Link. Inorg. Chem. 2015, 54, 5410–5418. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Gómez-García, C.J. A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand. Polymers 2016, 8, 89. [Google Scholar] [CrossRef]
- Benmansour, S.; Abhervé, A.; Gómez-Claramunt, P.; Vallés-García, C.; Gómez-García, C.J. Nanosheets of Two-Dimensional Magnetic and Conducting Fe(II)/Fe(III) Mixed-Valence Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 26210–26218. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Coleiro, J.; Ha, K.; Hoskins, B.F.; Orchard, S.D.; Robson, R. Dihydroxybenzoquinone and chloranilic acid derivatives of rare earth metals. J. Chem. Soc. Dalton Trans. 2002, 1586–1594. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Coleiro, J.; Hoskins, B.F.; Robson, R. Gas hydrate-like pentagonal dodecahedral M2(H2O)18 cages (M = lanthanide or Y) in 2,5-dihydroxybenzoquinone-derived coordination polymers. Chem. Commun. 1996, 603–604. [Google Scholar] [CrossRef]
- López-Martínez, G. Multifunctionality in Molecular Materials Based on Anilato-Type Ligands. Ph.D. Thesis, University of Valencia, Valencia, Spain, June 2017. [Google Scholar]
- Christian, R. Complexes with substituted 2,5-dihydroxy-p-benzoquinones: The inclusion compounds [Y(H2O)3]2 (C6Cl2O4)3·6.6H2O and [Y(H2O)3]2 (C6Br2O4)3·6H2O. Mater. Res. Bull. 1987, 22, 1483–1491. [Google Scholar]
- Riley, P.E.; Haddad, S.F.; Raymond, K.N. Preparation of praseodymium(III) chloranilate and the crystal structures of Pr2(C6Cl2O4)3·8C2H5OH and Na3[C6H2O(OH)(SO3)2] H2O. Inorg. Chem. 1983, 22, 3090–3096. [Google Scholar] [CrossRef]
- Benmansour, S.; Pérez-Herráez, I.; López-Martínez, G.; Gómez García, C.J. Solvent-modulated structures in anilato-based 2D coordination polymers. Polyhedron 2017, 135, 17–25. [Google Scholar] [CrossRef]
- Benmansour, S.; López-Martínez, G.; Canet-Ferrer, J.; Gómez-García, C.J. A Family of Lanthanoid Dimers with Nitroanilato Bridges. Magnetochemistry 2016, 2, 32. [Google Scholar] [CrossRef]
- Benmansour, S.; Hernández-Paredes, A.; Gómez-García, C.J. Size-dependence of the structure in a series of lanthanoid-anilato 2D lattices. J. Coord. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Atzori, M.; Artizzu, F.; Marchio, L.; Loche, D.; Caneschi, A.; Serpe, A.; Delano, P.; Avarvari, N.; Mercuri, M.L. Switching-on luminescence in anilate-based molecular materials. Dalton Trans. 2015, 44, 15786–15802. [Google Scholar] [CrossRef] [PubMed]
- Rehwoldt, R.E.; Chasen, B.; Li, J. 2-Chloro-5-Cyano-3,6-Dihydroxybenzoquinone, a New Analytical Reagent for the Spectrophotometric Determination of Calcium(II). Anal. Chem. 1966, 38, 1018–1019. [Google Scholar] [CrossRef]
- Diaz-Torres, R.; Alvarez, S. Coordinating ability of anions and solvents towards transition metals and lanthanides. Dalton Trans. 2011, 40, 10742–10750. [Google Scholar] [CrossRef] [PubMed]
- Imaz, I.; Mouchaham, G.; Roques, N.; Brandès, S.; Sutter, J. Tetradihydrobenzoquinonate and Tetrachloranilate Zr(IV) Complexes: Single-Crystal-to-Single-Crystal Phase Transition and Open-Framework Behavior for K4Zr(DBQ)4. Inorg. Chem. 2013, 52, 11237–11243. [Google Scholar] [CrossRef] [PubMed]
- Mouchaham, G.; Roques, N.; Duhayon, C.; Imaz, I.; Sutter, J. Extended H-bond networks based on guanidinium H-donors and [Zr(A)4]4− H-acceptor units: Modulation of the assemblage and guest accessible volume by chemical design (A = oxalate, dihydrobenzoquinonate, chloranilate). New J. Chem. 2013, 37, 3476–3487. [Google Scholar] [CrossRef]
- Sorace, L.; Gatteschi, D. Electronic Structure and Magnetic Properties of Lanthanide Molecular Complexes. In Lanthanides and Actinides in Molecular Magnetism; Layfield, R.A., Murugesu, M., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 1, pp. 1–25. [Google Scholar]
- Sontakke, A.D.; Ueda, J.; Katayama, Y.; Zhuang, Y.; Dorenbos, P. Role of electron transfer in Ce3+ sensitized Yb3+ luminescence in borate glass. J. Appl. Phys. 2015, 117, 013105. [Google Scholar] [CrossRef]
- Kristianpoller, N.; Shmilevich, A.; Weiss, D.; Chen, R.; Khaidukov, N. Luminescence of LiKYF5:Pr3 crystals. Radiat. Meas. 2001, 33, 637–640. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
| Ln(III) | Compound | Phase | Cavity | Ref. |
|---|---|---|---|---|
| La | [La2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [31] |
| Ce | [Ce2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [31] |
| Pr | [Pr2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Nd | [Nd2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Sm | [Sm2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Eu | [Eu2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Gd | [Gd2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [31] |
| Tb | [Tb2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Dy | [Dy2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Ho | [Ho2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Er | [Er2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Tm | [Tm2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Yb | [Yb2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [31] |
| Ln(III) | Compound | Phase | Cavity | Ref. |
|---|---|---|---|---|
| La | [La2(CA)3(H2O)6]·14H2O a | I | hex-4E-2F | [31] |
| Ce | [Ce2(CA)3(H2O)6]·14H2O b | I | hex-4E-2F | [31] |
| Pr | [Pr2(CA)3(H2O)6]·14H2O | I | hex-4E-2F | [31] |
| Nd | [Nd2(CA)3(H2O)6]·14H2O c | I | hex-4E-2F | [31] |
| Sm | [Sm2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [33] |
| Eu | [Eu2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [31] |
| Gd | [Gd2(CA)3(H2O)6]·12H2O d | II | hex-4E-2F | [31] |
| Tb | [Tb2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [31] |
| Dy | [Dy2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [33] |
| Ho | [Ho2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [33] |
| Er | [Er2(CA)3(H2O)6]·10H2O | III | hex-2E-4F | [33] |
| Tm | [Tm2(CA)3(H2O)6]·8H2O | IV | rect-4E-2F | [33] |
| Yb | [Yb2(CA)3(H2O)6]·8H2Oe | IV | rect-4E-2F | [31] |
| Ln(III) | D = H2O/G = H2O | Phase | Cavity | Ref. |
|---|---|---|---|---|
| La | [La2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Ce | [Ce2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Pr | [Pr2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Nd | [Nd2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Sm | [Sm2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Eu | [Eu2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Gd | [Gd2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Tb | [Tb2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Dy | [Dy2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Ho | [Ho2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Er | [Er2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [36] |
| Tm | [Tm2(BA)3(H2O)6]·8H2O | II | rect-4E-2F | [33] |
| Yb | [Yb2(BA)3(H2O)6]·8H2O | II | rect-4E-2F | [33] |
| Ln(III) | D = H2O/G = H2O | Phase | Ref. |
|---|---|---|---|
| Sm | [Sm2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Eu | [Eu2(NA)3(H2O)10]·6H2O | dimer | [33] |
| Gd | [Gd2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Tb | [Tb2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Dy | [Dy2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Ho | [Ho2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Er | [Er2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Formula | C39H46Cl3 N9O20Ce2 | C39H42Cl3 N9O18Pr2 | C33H36Cl3 N3O18S6Pr2 | C29H28Cl3 N3O18S4Yb2 | C14H13Cl2N2 O14Dy |
| F. Wt. | 1347.42 | 1312.98 | 1343.21 | 1287.24 | 666.66 |
| Space group (#) | C2/c (15) | P21/n (14) | P21/n (14) | P-1 (2) | P2/n (13) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic | Monoclinic |
| a (Å) | 13.8141(3) | 10.6585(10) | 9.6262(3) | 9.9354 | 11.9237(4) |
| b (Å) | 23.3672(4) | 13.7163(10) | 16.3853(4) | 10.6195 | 14.0496(8) |
| c (Å) | 17.9855(4) | 18.3196(14) | 15.3240(4) | 10.7752 | 12.2263(5) |
| α (°) | 90 | 90 | 90 | 112.010 | 90 |
| β (°) | 98.983(2) | 98.863 | 91.765 | 93.135 | 90.254(4) |
| γ (°) | 90 | 90 | 90 | 95.19 | 90 |
| V/Å3 | 5734.5(2) | 2646.3(4) | 2415.88(11) | 1044.39(7) | 2048.17(16) |
| Z | 4 | 2 | 2 | 1 | 1 |
| T (K) | 120 | 120 | 120 | 120 | 120 |
| ρcalc/g cm−3 | 1.638 | 1.633 | 1.797 | 2.021 | 1.189 |
| μ/mm−1 | 1.921 | 2.045 | 2.488 | 4.916 | 2.009 |
| F(000) | 2796 | 1280 | 1256 | 606 | 700 |
| R(int) | 0.0314 | 0.0898 | 0.1180 | 0.0594 | 0.0623 |
| θ range (deg) | 3.31–25.08 | 2.81–25.14 | 2.76–25.07 | 3.05–25.03 | 3.33–25.05 |
| Total reflections | 19,380 | 35,858 | 38,300 | 10,448 | 14,772 |
| Unique reflections | 5071 | 4705 | 4278 | 3180 | 3604 |
| Data with I > 2σ (I) | 4450 | 3541 | 3154 | 3207 | 3264 |
| Nvar | 342 | 327 | 311 | 289 | 186 |
| R1 a on I > 2σ (I) | 0.0599 | 0.0442 | 0.0606 | 0.0394 | 0.0839 |
| wR2 b (all) | 0.1773 | 0.1139 | 0.1497 | 0.1010 | 0.2384 |
| GOF c on F2 | 1.089 | 1.052 | 1.067 | 1.071 | 1.113 |
| Δρmax (eÅ−3) | 1.810 | 1,616 | 1.419 | 1.775 | 4.534 |
| Δρmin (eÅ−3) | −1.980 | −0,865 | −1.004 | −1.100 | −1.419 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Claramunt, P.; Benmansour, S.; Hernández-Paredes, A.; Cerezo-Navarrete, C.; Rodríguez-Fernández, C.; Canet-Ferrer, J.; Cantarero, A.; Gómez-García, C.J. Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry 2018, 4, 6. https://doi.org/10.3390/magnetochemistry4010006
Gómez-Claramunt P, Benmansour S, Hernández-Paredes A, Cerezo-Navarrete C, Rodríguez-Fernández C, Canet-Ferrer J, Cantarero A, Gómez-García CJ. Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry. 2018; 4(1):6. https://doi.org/10.3390/magnetochemistry4010006
Chicago/Turabian StyleGómez-Claramunt, Patricia, Samia Benmansour, Antonio Hernández-Paredes, Christian Cerezo-Navarrete, Carlos Rodríguez-Fernández, Josep Canet-Ferrer, Andrés Cantarero, and Carlos J. Gómez-García. 2018. "Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand" Magnetochemistry 4, no. 1: 6. https://doi.org/10.3390/magnetochemistry4010006
APA StyleGómez-Claramunt, P., Benmansour, S., Hernández-Paredes, A., Cerezo-Navarrete, C., Rodríguez-Fernández, C., Canet-Ferrer, J., Cantarero, A., & Gómez-García, C. J. (2018). Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry, 4(1), 6. https://doi.org/10.3390/magnetochemistry4010006