Investigating Size- and Temperature-Dependent Coercivity and Saturation Magnetization in PEG Coated Fe3O4 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
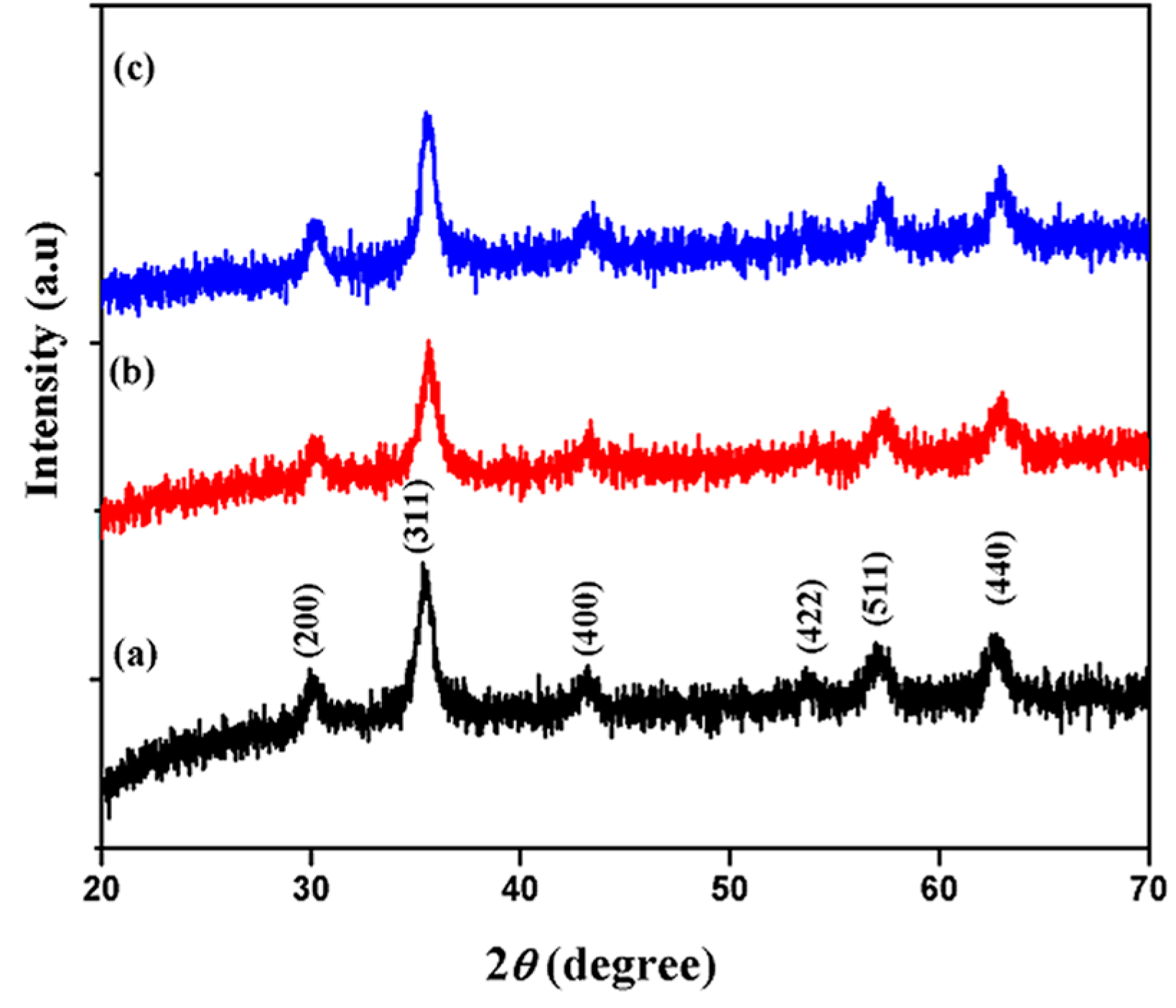
3.1. Structural and Phase Analysis
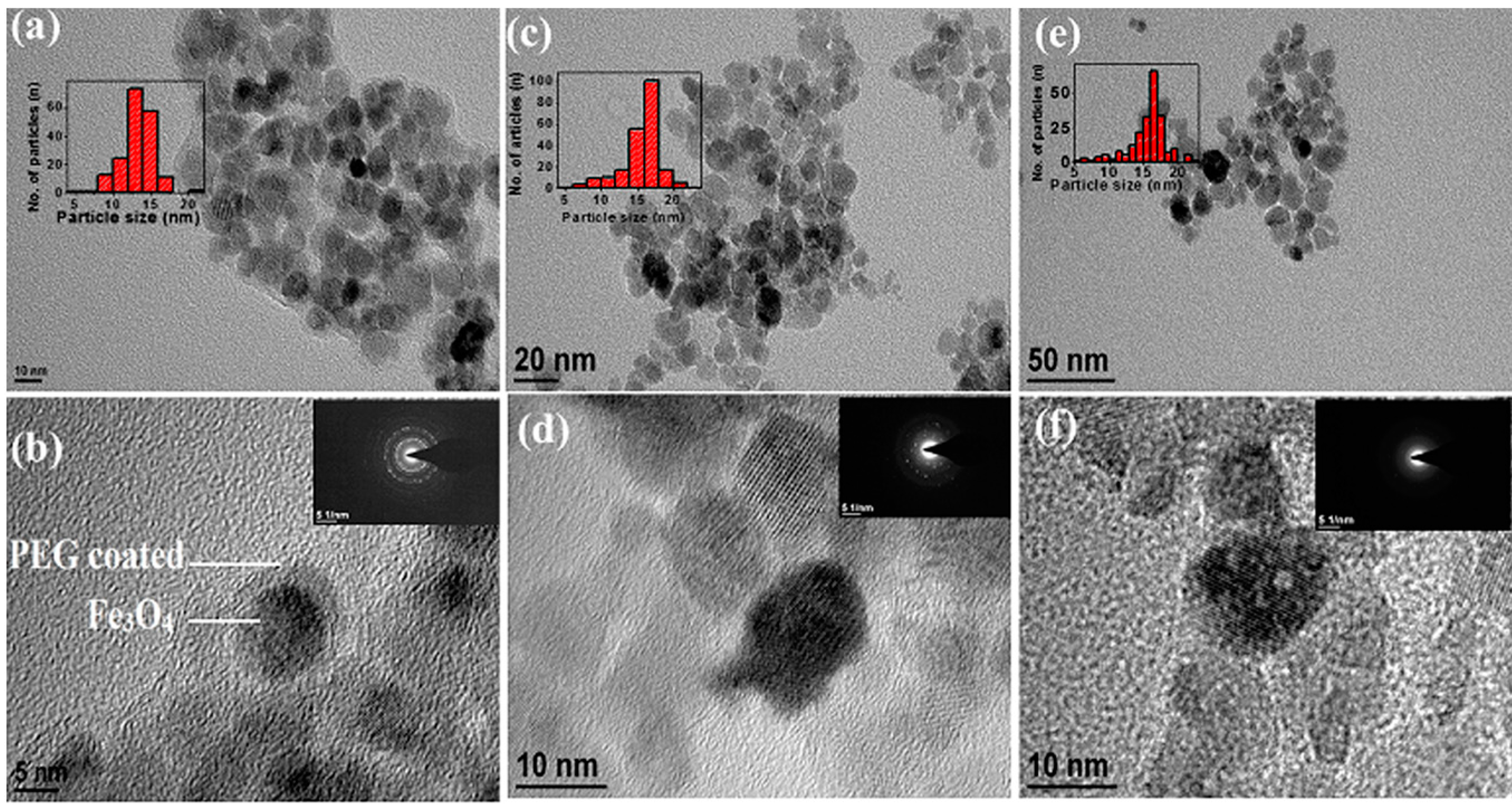
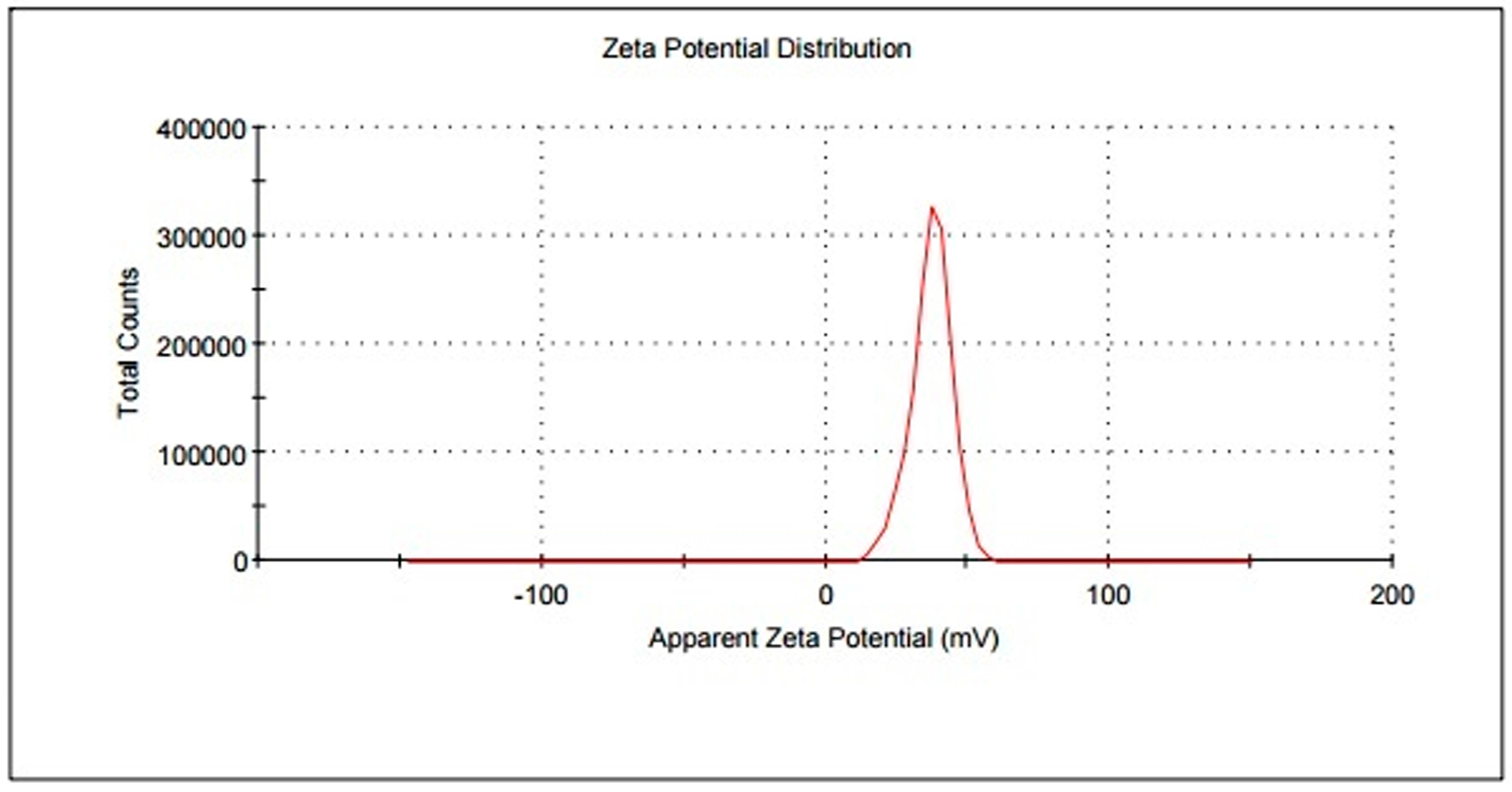
3.2. Morphological Characterization
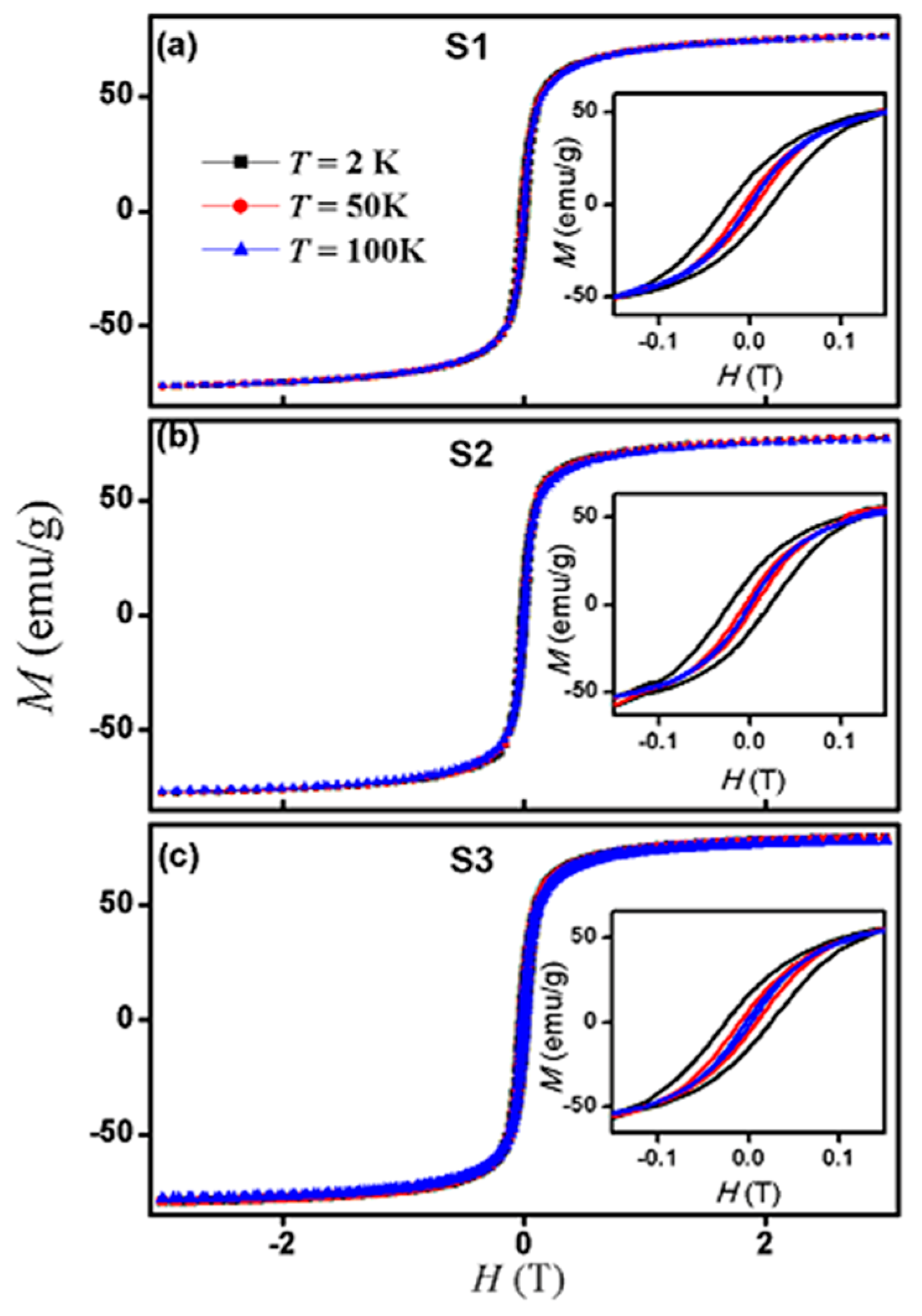
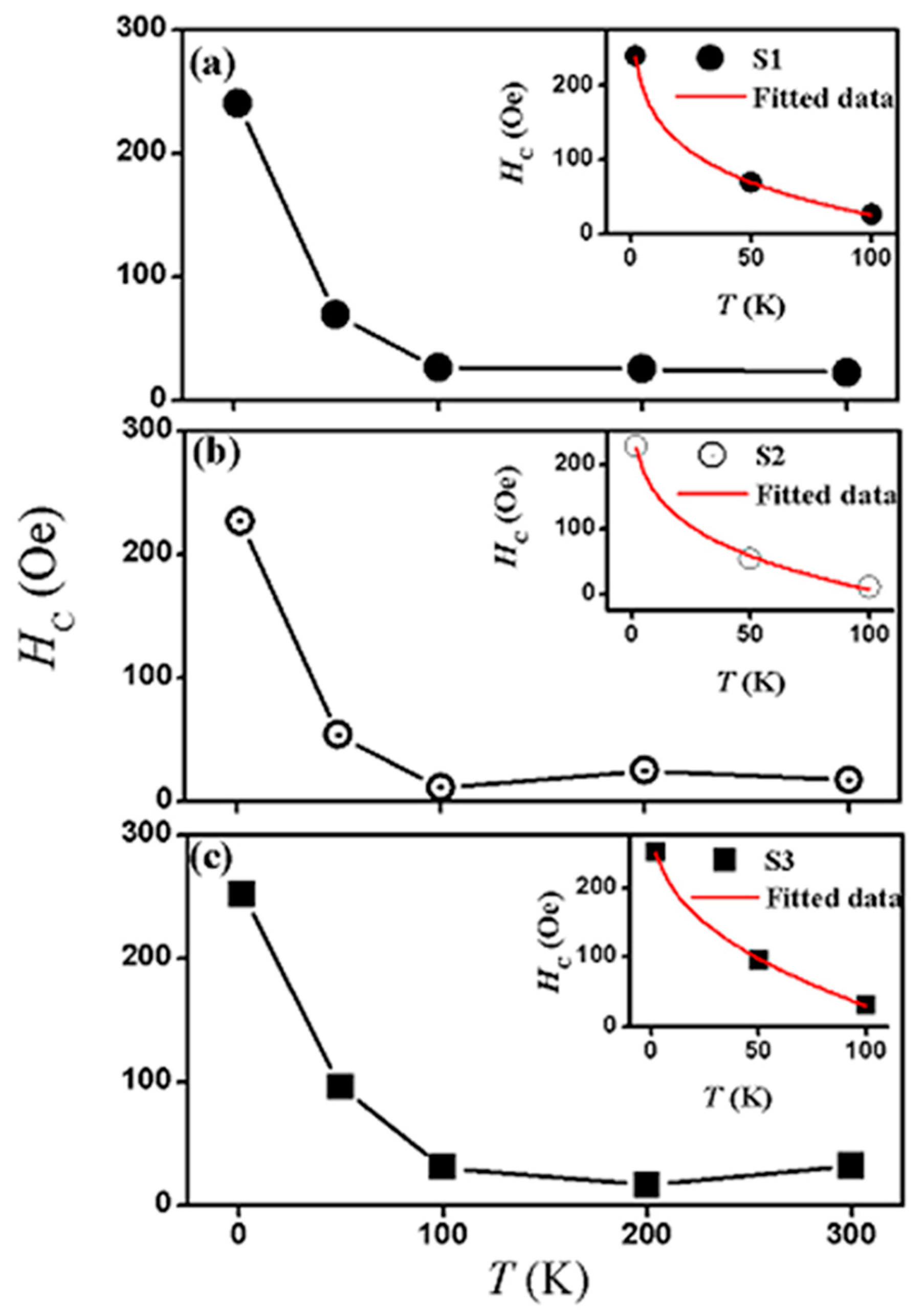
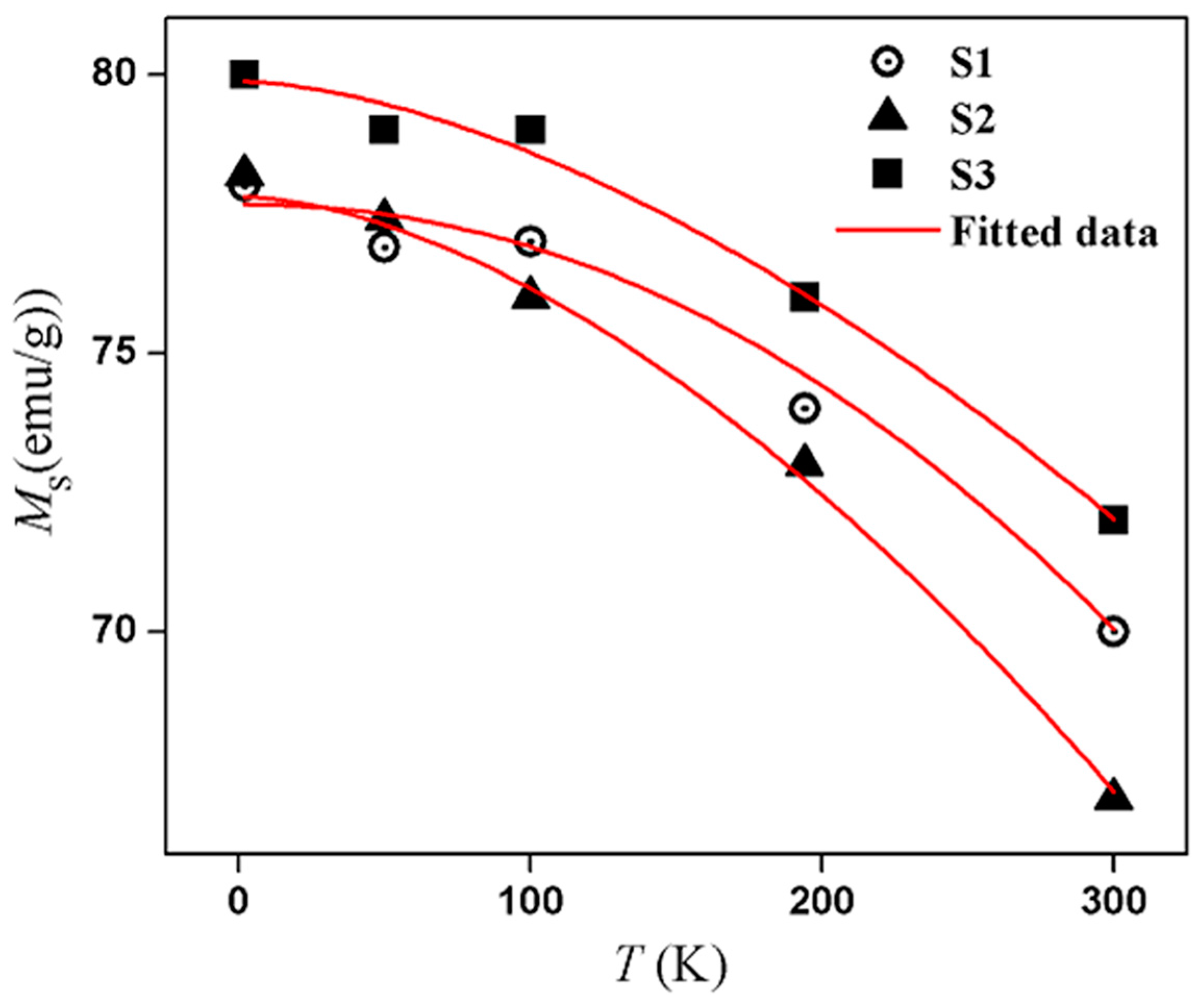
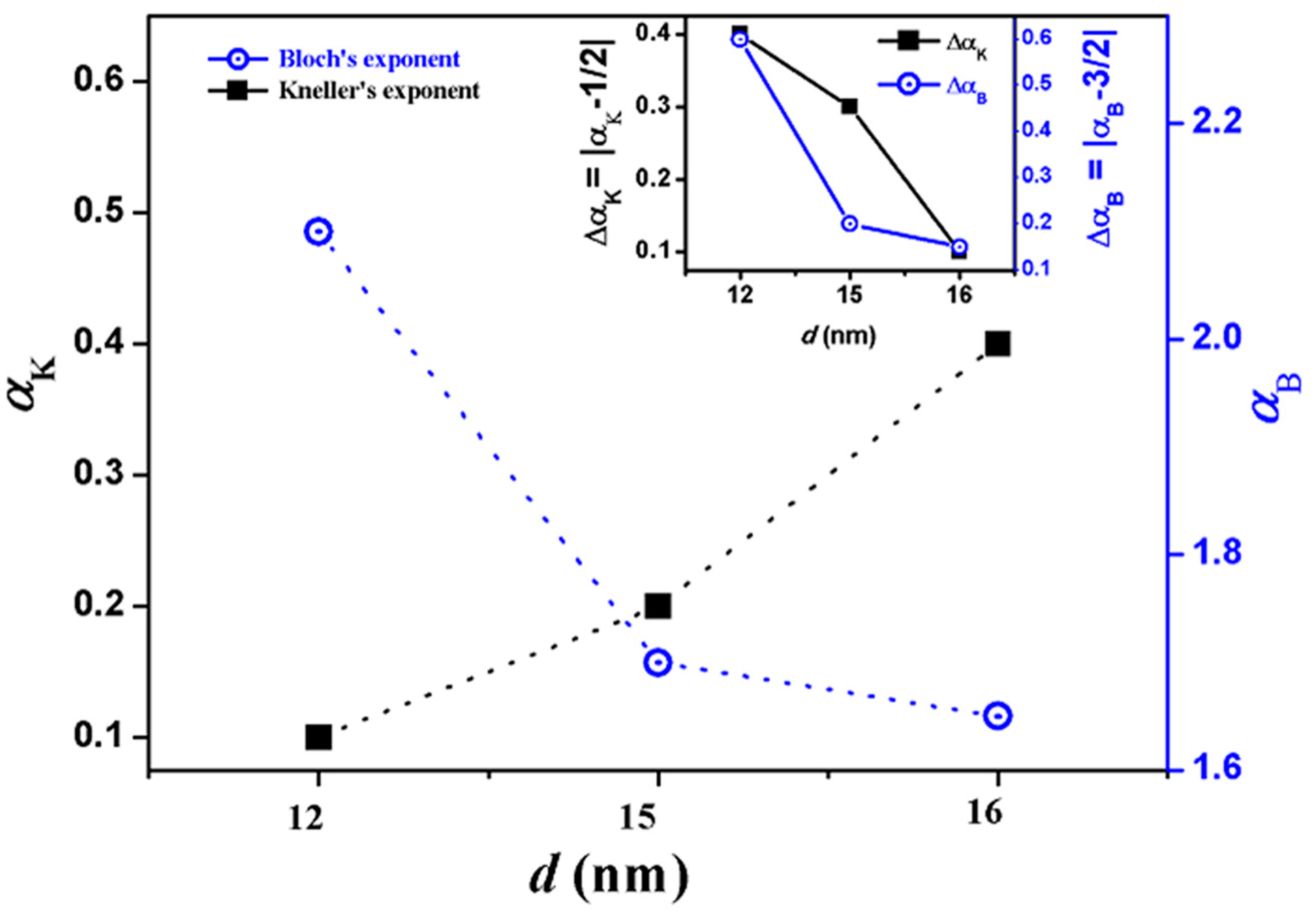
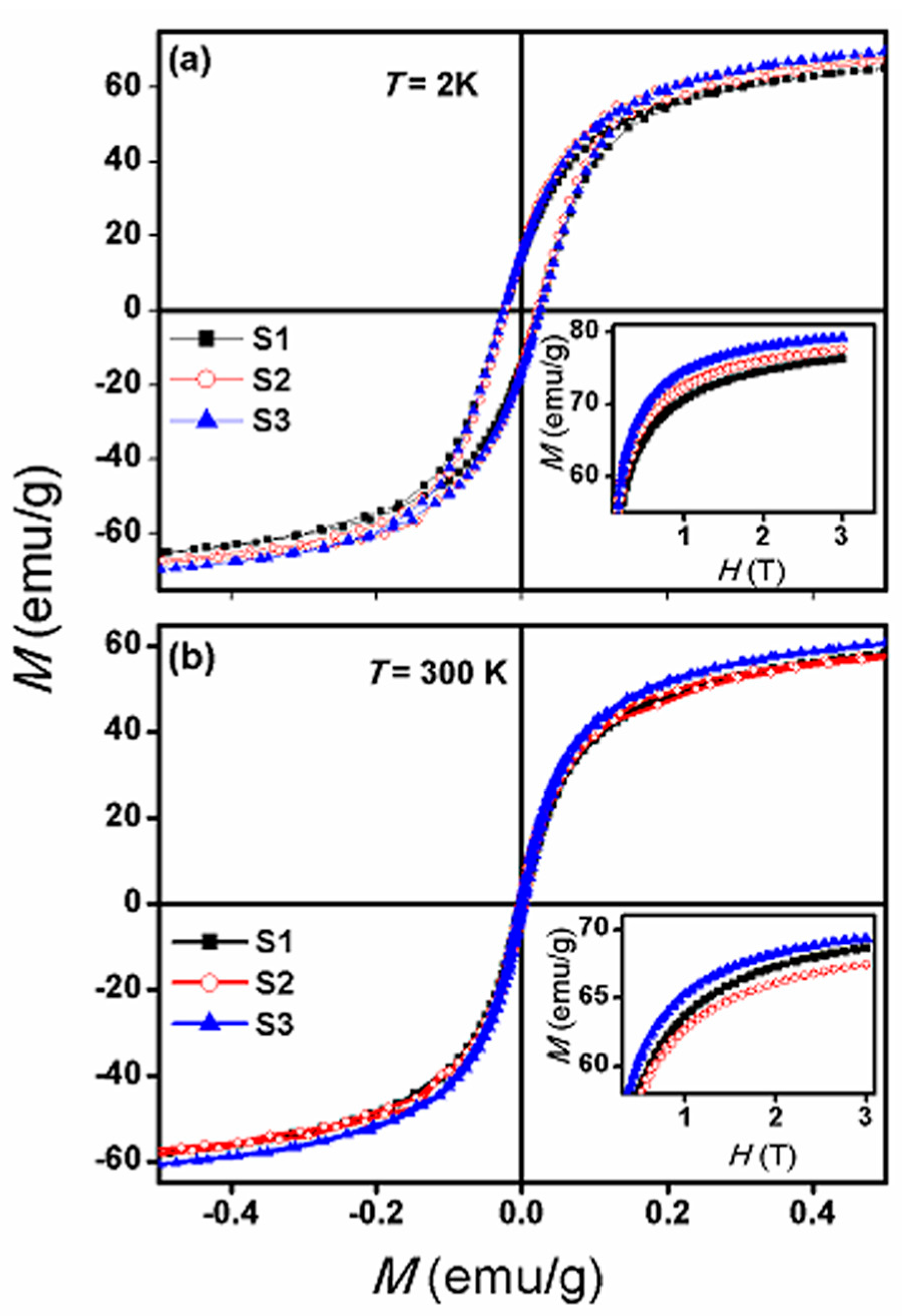
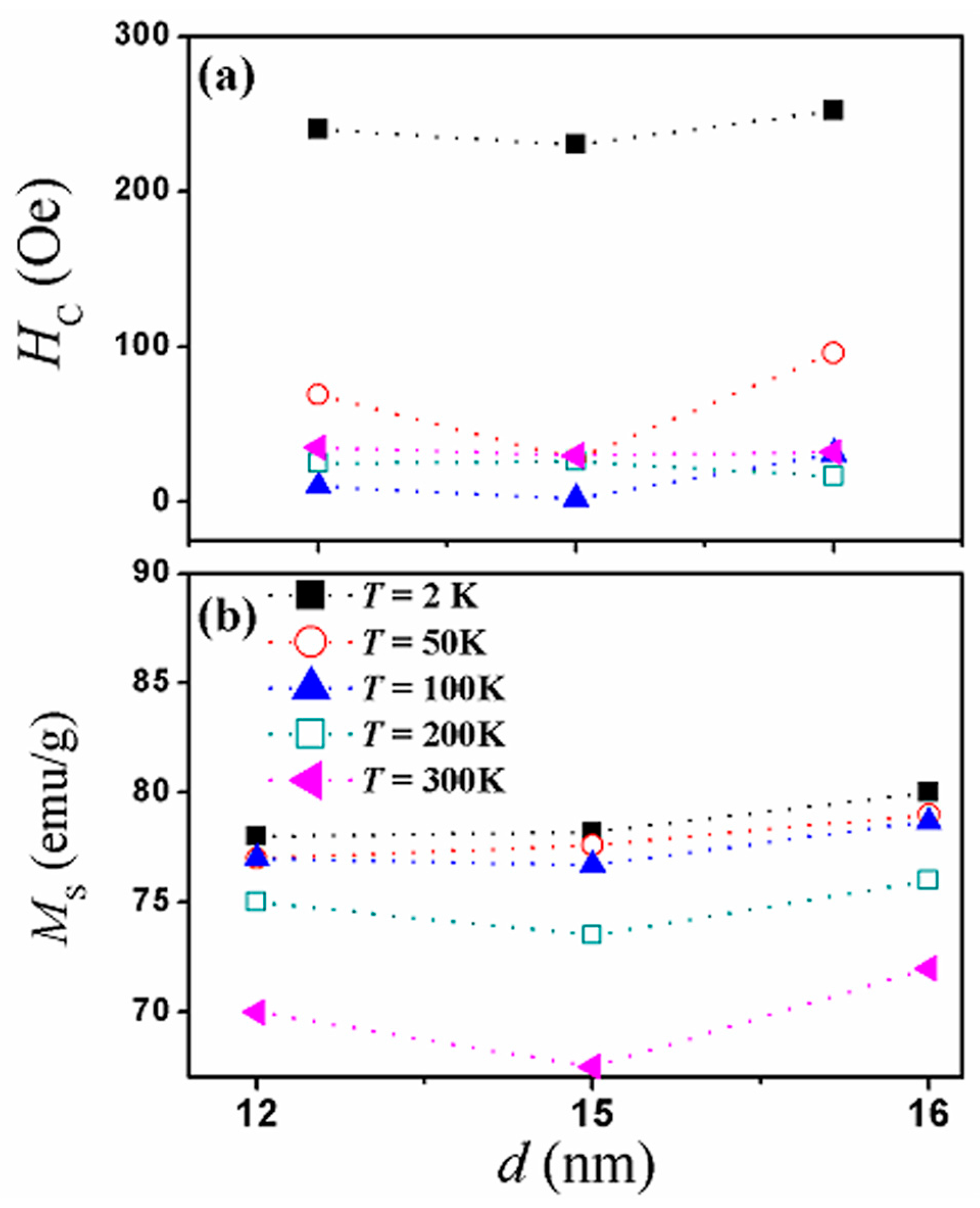
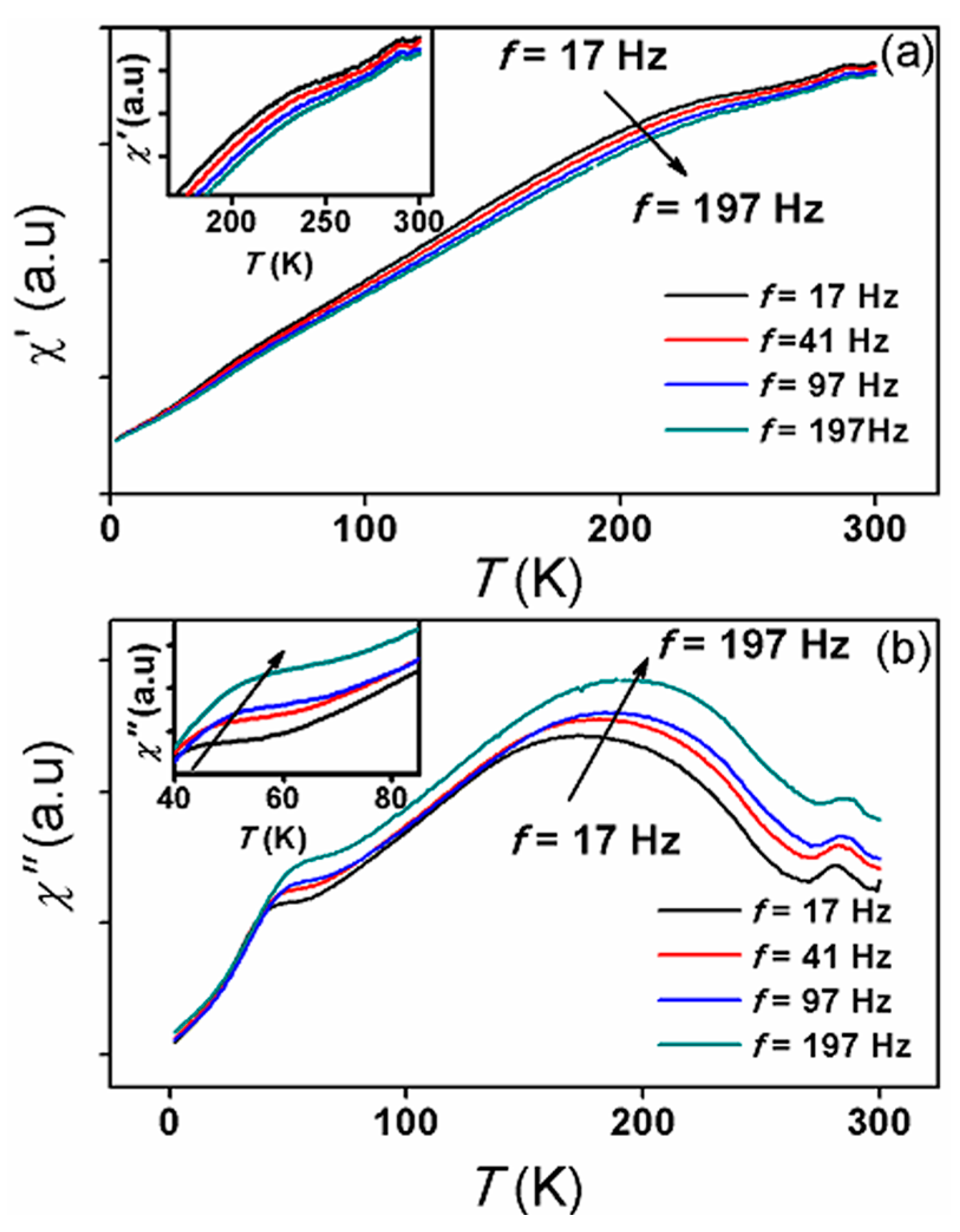
3.3. Magnetic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gu, H.; Xu, K.; Xu, C.; Xu, B. Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem. Comm. 2006, 6, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Roger, J.; Pons, J.N.; Massart, R.; Halbreich, A.; Bacri, J.C. Some biomedical applications of ferrofluids. Eur. Phys. J. AP 1999, 5, 321–325. [Google Scholar] [CrossRef]
- Wunderbaldinger, P.; Josephson, L.; Weissleder, R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug. Chem. 2002, 13, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Yamazaki, N.; Tomitaka, A.; Yamada, T.; Takemura, Y. Hyperthermia using antibody—Conjugated magnetic nanoparticles and its enhanced effect with cryptotanshinone. Nanomaterials 2014, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Nairan, A.; Khan, U.; Iqbal, M.; Khan, M.; Javed, K.; Riaz, S.; Naseem, S.; Han, X. Structural and magnetic response in bimetallic core/shell magnetic nanoparticles. Nanomaterials 2016, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Todo, S.; Chikazumi, S. Magnetization of Low Temperature Phase of Fe3O4. J. Phys. Soc. Jpn. 1977, 43, 47–52. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Sato, T.; Nagaoka, K.; Kobayashi, S.; Manjanna, J.; Murakami, T. Temperature dependence of magnetic hysteresis scaling for cubic Fe3O4 nanoparticles. AIP Adv. 2017, 7, 056319. [Google Scholar] [CrossRef]
- Anbarasu, M.; Anandan, M.; Chinnasamy, E.; Gopinath, V.; Balamurugan, K. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 135, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Auzans, E.; Zins, D.; Blums, E.; Massart, R. Synthesis and properties of Mn-Zn ferrite ferrofluids. J. Mater. Sci. 1999, 34, 1253–1260. [Google Scholar] [CrossRef]
- Zaitsev, V.S.; Filimonov, D.S.; Presnyakov, I.A.; Gambino, R.J.; Chu, B. Physical and chemical properties of magnetite and magnetite-polymer nanoparticles and their colloidal dispersions. J. Colloid Interface Sci. 1999, 212, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Ali, O.A.; Anitua, E.; Pedraz, J.L.; Emerich, D.F. Biomaterial-based technologies for brain anti-cancer therapeutics and imaging. Biochim. Biophys. Acta 2010, 1806, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Kateb, B.; Chiu, K.; Black, K.L.; Yamamoto, V.; Khalsa, B.; Ljubimova, J.Y.; Ding, H.; Patil, R.; Portilla-Arias, J.A.; Modo, M.; et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: What should be the policy? NeuroImage 2011, 54, S106–S124. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, M.D.; Illum, L.; Davis, S.S. Preparation of ultrafine silica- and PEG-coated magnetite Particles. Colloids Surf. A 2001, 179, 93–102. [Google Scholar] [CrossRef]
- Josephson, L.; Tung, C.H.; Moore, A.; Weissleder, R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjugates. Bioconjug. Chem. 1999, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Mikhaylova, M.; Zhang, Y.; Muhammed, M. Protective coating of superparamagnetic iron oxide nanoparticles. Chem. Mater. 2003, 15, 1617–1627. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Z. Self-assembled monolayer coatings on nanosized magnetic particles using 16-mercaptohexadecanoic acid. Langmuir 1995, 11, 4617–4622. [Google Scholar] [CrossRef]
- Bautista, M.C.; Bomati-Miguel, O.; Zhao, X.; Morales, M.P.; González-Carreño, T.; de Alejo, R.P.; Ruiz-Cabello, J.; Veintemillas-Verdaguer, S. Comparative study of ferrofluids based on dextran-coated iron oxide and metal nanoparticles for contrast agents in magnetic resonance imaging. Nanotechnology 2004, 15, S154–S159. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Aitchison, G.; Curtis, A.S.G. Cell response to dextran-derivatised iron oxide nanoparticles post internalization. Biomaterials 2004, 25, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Portet, D.; Denizot, B.; Rump, E.; Lejeune, J.J.; Jallet, P. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. J. Colloid Interface Sci. 2001, 238, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.; Kohler, N.; Zhang, M.Q. Self-Assembled coatings on individual monodisperse magnetic nanoparticles for efficient intracellular uptake. Biomed. Microdevices 2004, 6, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005, 74, 489–520. [Google Scholar] [CrossRef]
- Guimarães, A.P. Principles of Nanomagnetism; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-642-01482-6. [Google Scholar]
- Batlle, X.; Labarta, A. Finite-size effects in fine particles: Magnetic and transport properties. J. Phys. D Appl. Phys. 2002, 35, R15–R42. [Google Scholar] [CrossRef]
- Julia, A.; Paolo, A.; Paola, T.; Marco, S. Poly(ethyleneglycol)-coated Fe3O4 nanoparticles by UV-thiol-ene addition of PEG dithiol on vinyl-functionalized magnetite surface. Macromol. Chem. Phys. 2011, 212, 1629–1635. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Joshi, N.; Chattopadhyay, K.; De, G. A facile synthesis of PEG-coated magnetite (Fe3O4) nanoparticles and their prevention of the reduction of cytochrome c. ACS Appl. Mater. Interfaces 2012, 4, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Harandi, S.S.; Karimipour, A.; Afrand, M.; Akbari, M.; D’Orazio, A. An experimental study on thermal conductivity of F-MWCNTs–Fe3O4/EG hybrid nanofluid: Effects of temperature and concentration. Int. Commun. Heat Mass 2016, 76, 171–177. [Google Scholar] [CrossRef]
- Rabanel, J.-M.; Hildgen, P.; Banquy, X. Assessment of PEG on polymeric particles surface, a key step in drug carrier translation. J. Control. Release 2014, 185, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Bertino, M.F. Temperature dependent coercivity and magnetization of nickel ferrite nanoparticles. J. Magn. Magn. Mater. 2010, 322, 2199–2202. [Google Scholar] [CrossRef]
- Kneller, E.F.; Luborsky, F.E. Particle size dependence of coercivity and remanence of single-domain particles. J. Appl. Phys. 1963, 34, 656–658. [Google Scholar] [CrossRef]
- Batlle, X.; Garcia del Muro, M.; Tejada, J.; Pfeiffer, H.; Gornert, P.; Sinn, E. Magnetic study of M-type doped barium ferrite nanocrystalline powders. J. Appl. Phys. 1993, 74, 3333–3340. [Google Scholar] [CrossRef]
- Bloch, F. Zur theorie des ferromagnetismus. Z. Phys. 1931, 61, 206–219. [Google Scholar] [CrossRef]
- Della Torre, E.; Bennett, L.H.; Watson, R.E. Extension of the bloch T3/2 law to magnetic nanostructures: Bose-einstein condensation. Phys. Rev. Lett. 2005, 94, 147210. [Google Scholar] [CrossRef] [PubMed]
- Senz, V.; Röhlsberger, R.; Bansmann, J.; Leupold, O.; Meiwes-Broer, K.-H. Temperature dependence of the magnetization in Fe islands on W (110): Evidence for spin-wave quantization. New J. Phys. 2003, 5, 47. [Google Scholar] [CrossRef]
- Hendriksen, P.V.; Linderoth, S.; Lindgard, P.A. Finite-size effects in the magnetic properties of ferromagnetic clusters. J. Magn. Magn. Mater. 1992, 104–107, 1577–1579. [Google Scholar] [CrossRef]
- Hendriksen, P.V.; Linderoth, S.; Lindgard, P.A. Finite-size modifications of the magnetic properties of clusters. Phys. Rev. B 1993, 48, 7259–7273. [Google Scholar] [CrossRef]
- Linderoth, S.; Balcells, L.; Labarta, A.; Tejada, J.; Hendriksen, P.V.; Sethi, S.A. Magnetization and Mossbauer studies of ultrafine Fe-C particles. J. Magn. Magn. Mater. 1993, 124, 269–276. [Google Scholar] [CrossRef]
- Eggeman, A.S.; Petford-Long, A.K.; Dobson, P.J.; Wiggins, J.; Bromwich, T.; Dunin-Borkowski, R.; Kasama, T. Synthesis and characterisation of silica encapsulated cobalt nanoparticles and nanoparticle chains. J. Magn. Magn. Mater. 2006, 301, 336–342. [Google Scholar] [CrossRef]
- Cojocaru, S.; Naddeo, A.; Citro, R. Modification of the Bloch law in ferromagnetic nanostructure. Europhys. Lett. 2014, 106, 17001. [Google Scholar] [CrossRef]
- Ortega, D.; Vélez-Fort, E.; García, D.A.; García, R.; Litrán, R.; Barrera-Solano, C.; Ramírez-del-Solar, M.; Domínguez, M. Size and surface effects in the magnetic properties of maghemite and magnetite coated nanoparticles. Phil. Trans. R. Soc. A 2010, 368, 4407–4418. [Google Scholar] [CrossRef] [PubMed]
- Gubin, S.P. (Ed.) Magnetic Nanoparticles; Wiley-VCH: Berlin, Germany, 2009; ISBN 978-3-527-40790-3. [Google Scholar]
- Alves, C.R.; Aquino, R.; Sousa, M.H.; Rechenberg, H.R.; Goya, G.F.; Tourinho, F.A.; Depeyrot, J. Low temperature experimental investigation of finite-size and surface effects in CuFe2O4 nanoparticles of ferrofluids. J. Met. Nanocrys. Mater. 2004, 20–21, 694–699. [Google Scholar] [CrossRef]
- Mandal, K.; Mitra, S.; Kumar, P.A. Deviation from Bloch T3/2 law in ferrite nanoparticles. Europhys. Lett. 2006, 75, 618–623. [Google Scholar] [CrossRef]
- Kumar, P.A.; Mandal, K. Effect of spatial confinement on spin-wave spectrum: Low temperature deviation from Bloch T3/2 law in Co nanoparticles. arXiv, 2004; arXiv:cond-mat/0701152v1. [Google Scholar]
- Mathur, P.; Thakur, A.; Singh, M. Effect of nanoparticles on the magnetic properties of Mn-Zn soft ferrite. J. Magn. Magn. Mater. 2008, 320, 1364–1369. [Google Scholar] [CrossRef]
- Tung, L.D.; Kolesnichenko, V.; Caruntu, D.; Chou, N.H.; O’Connor, C.J.; Spinu, L. Magnetic properties of ultrafine cobalt ferrite particles. J. Appl. Phys. 2016, 93, 7486–7488. [Google Scholar] [CrossRef]
- Morup, S. Comment on “Deviation from the Bloch T3/2 law in ferrite nanoparticles” by K. Mandal et al. Europhys. Lett. 2007, 77, 27003. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.E.; Mcniff, E.J.; Foner, S. Surface spin disorder in ferrite nanoparticles (invited). J. Appl. Phys. 1997, 81, 5552–5557. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.E. Atomic-scale magnetic modeling of oxide nanoparticles. Phys. Rev. B 1999, 59, 6321–6336. [Google Scholar] [CrossRef]
- Alves, C.R.; Aquino, R.; Depeyrot, J.; Cotta, T.A.P.; Sousa, M.H.; Tourinho, F.A.; Rechenberg, H.R.; Goya, G.F. Surface spin freezing of ferrite nanoparticles evidenced by magnetization Measurements. J. Appl. Phys. 2006, 99, 08M905. [Google Scholar] [CrossRef]
- Mørup, S.; Brok, E.; Frandsen, C. Spin Structures in Magnetic Nanoparticles. J. Nanomater. 2013, 2013, 720629. [Google Scholar] [CrossRef]
- Greenwood, R.; Kendall, K. Selection of Suitable Dispersants for Aqueous Suspensions of Zirconia and Titania Powders using Acoustophoresis. J. Eur. Ceram. Soc. 1999, 19, 479–488. [Google Scholar] [CrossRef]


| Sample | Particle Diameter (nm) | Fitted Parameters at ZFC | |||
|---|---|---|---|---|---|
| From XRD | From TEM | H0 (emu/g) | TB (K) | αK | |
| S1 | 12.1 ± 0.6 | 12 ± 0.9 | 687 (±3.0) | 145 (±1.9) | 0.10 |
| S2 | 14.7 ± 0.7 | 15 ± 0.7 | 409 (±9.0) | 109 (±5.0) | 0.20 |
| S3 | 16.1 ± 0.8 | 16 ± 0.7 | 310 (±2.7) | 128 (±2.1) | 0.40 |
| Sample | Fitted Parameters at ZFC | |||
|---|---|---|---|---|
| Particle Diameter (nm) | M0 (emu/g) | To (K) | αB | |
| S1 | 12 | 77.6 (±0.2) | 905 (±15) | 2.1 |
| S2 | 15 | 77.8 (±0.19) | 964 (±15) | 1.7 |
| S3 | 16 | 83.3 (±0.1) | 1235 (±20) | 1.65 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayek, C.; Manna, K.; Bhattacharjee, G.; Murugavel, P.; Obaidat, I. Investigating Size- and Temperature-Dependent Coercivity and Saturation Magnetization in PEG Coated Fe3O4 Nanoparticles. Magnetochemistry 2017, 3, 19. https://doi.org/10.3390/magnetochemistry3020019
Nayek C, Manna K, Bhattacharjee G, Murugavel P, Obaidat I. Investigating Size- and Temperature-Dependent Coercivity and Saturation Magnetization in PEG Coated Fe3O4 Nanoparticles. Magnetochemistry. 2017; 3(2):19. https://doi.org/10.3390/magnetochemistry3020019
Chicago/Turabian StyleNayek, Chiranjib, Kaustuv Manna, Gourab Bhattacharjee, Pattukkannu Murugavel, and Ihab Obaidat. 2017. "Investigating Size- and Temperature-Dependent Coercivity and Saturation Magnetization in PEG Coated Fe3O4 Nanoparticles" Magnetochemistry 3, no. 2: 19. https://doi.org/10.3390/magnetochemistry3020019
APA StyleNayek, C., Manna, K., Bhattacharjee, G., Murugavel, P., & Obaidat, I. (2017). Investigating Size- and Temperature-Dependent Coercivity and Saturation Magnetization in PEG Coated Fe3O4 Nanoparticles. Magnetochemistry, 3(2), 19. https://doi.org/10.3390/magnetochemistry3020019





