Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices
Abstract
:1. Introduction
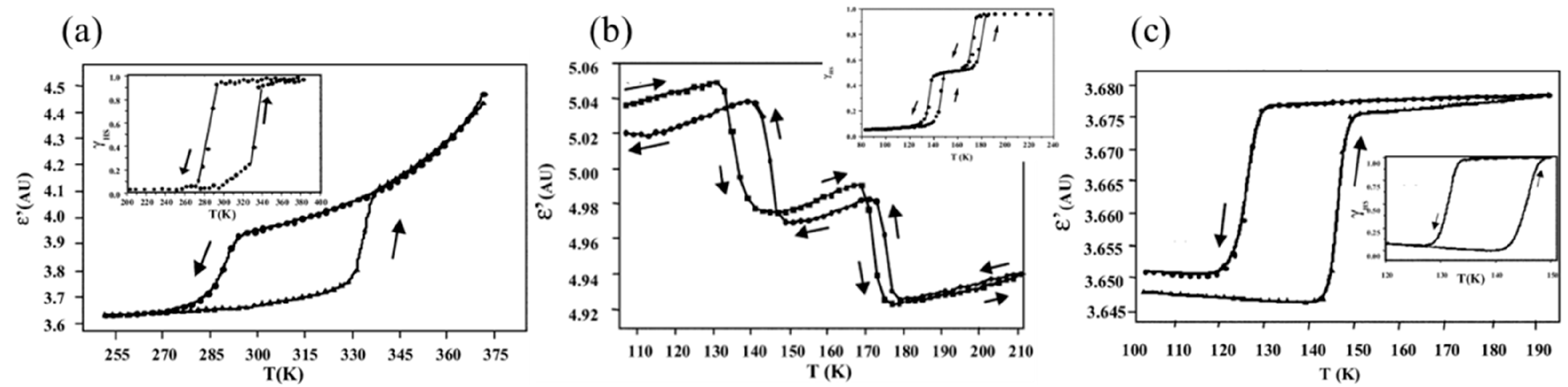
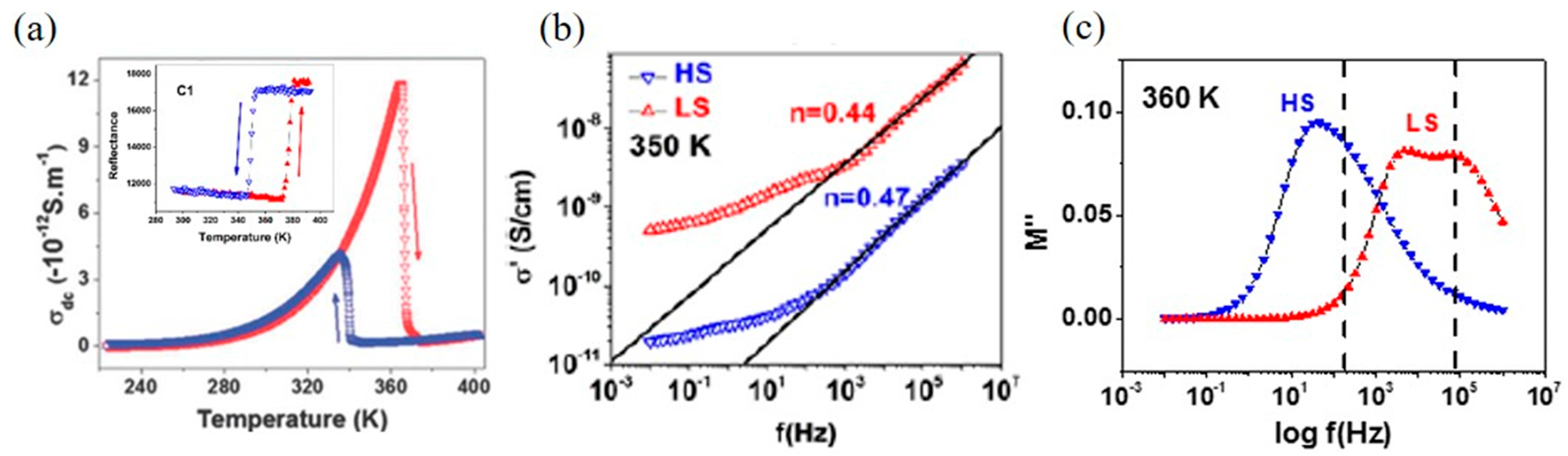
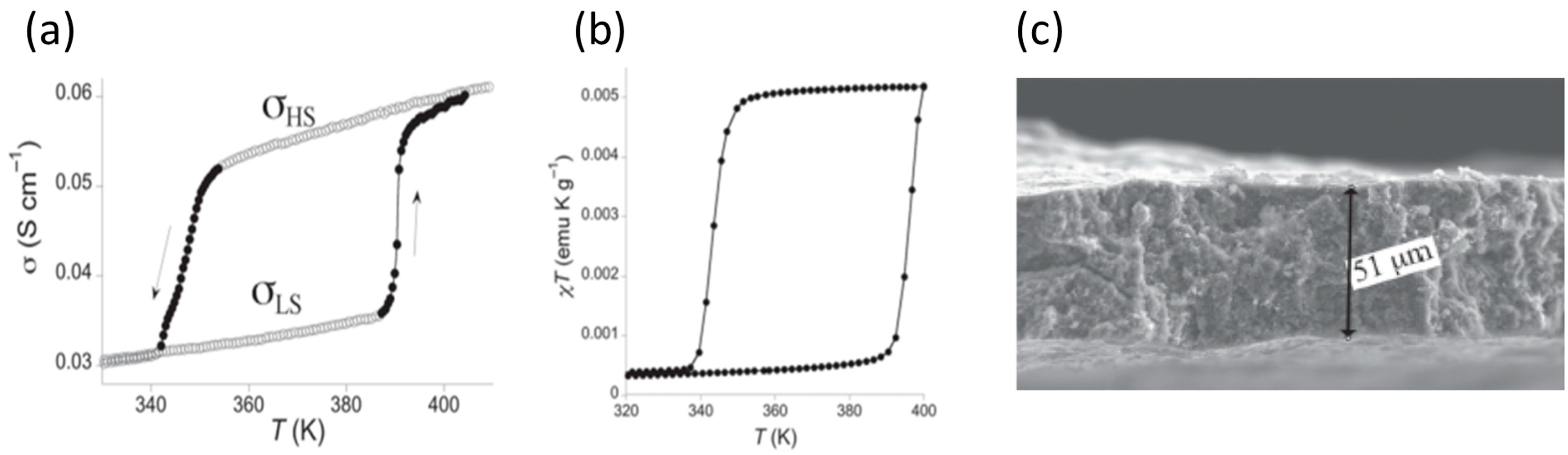
2. Macroscopic Samples
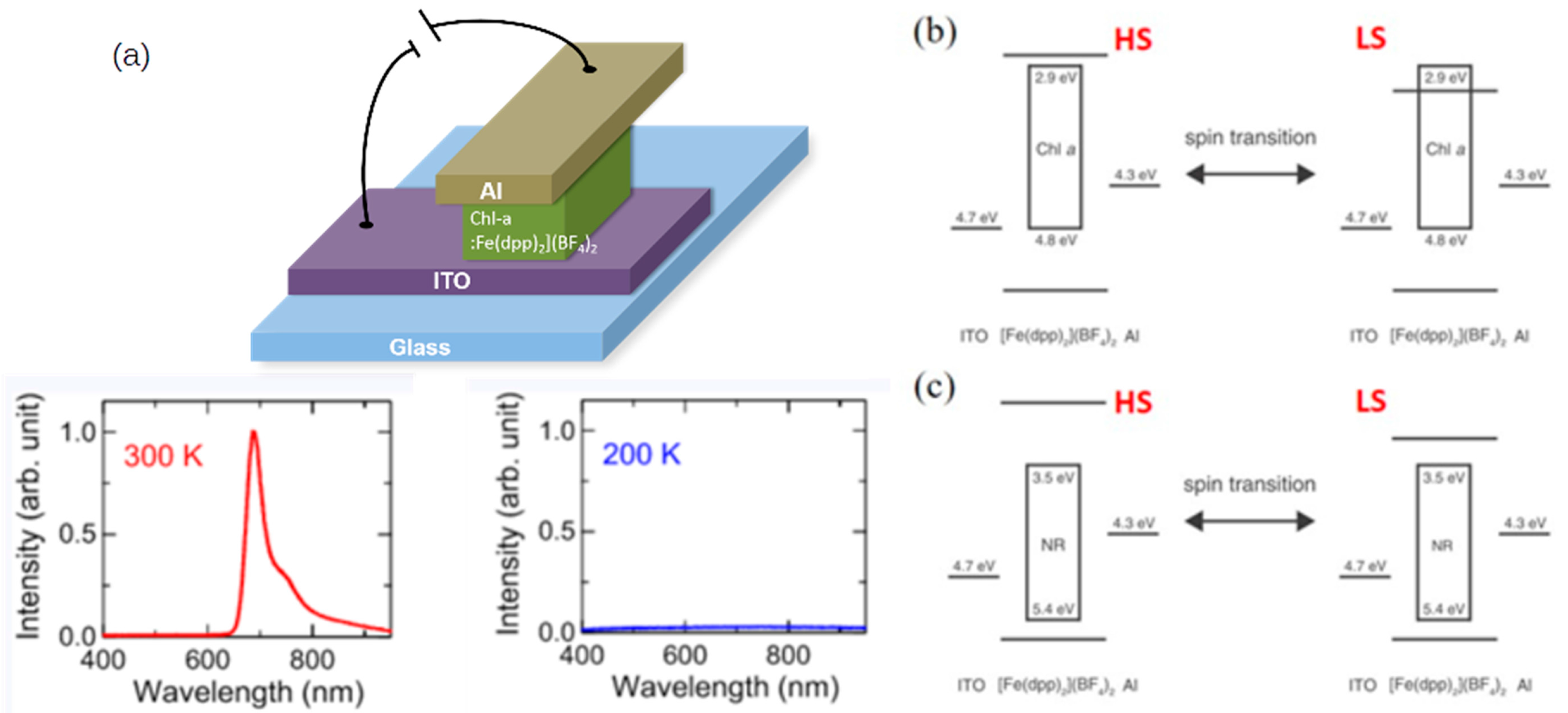
3. Micro- and Nanoscale Devices
4. Single Molecule Studies
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rohrer, H. Limits and possibilities of miniaturization. Jpn. J. Appl. Phys. 1993, 32, 1335. [Google Scholar] [CrossRef]
- Peercy, P.S. The drive to miniaturization. Nature 2000, 406, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Keyes, R.W. Fundamental limits of silicon technology. Proc. IEEE 2001, 89, 227–239. [Google Scholar] [CrossRef]
- Jan van der Molen, S.; Liljeroth, P. Charge transport through molecular switches. J. Phys. Condens. Matter 2010, 22, 133001. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, M.; Taniguchi, M. Single molecule electronics and devices. Sensors 2012, 12, 7259–7298. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; wiley-VCH: New York, NY, USA, 1993. [Google Scholar]
- De Silva, P.A.; Gunaratne, N.H.Q.; McCoy, C.P. A molecular photoionic and gate based on fluorescent signalling. Nature 1993, 364, 42–44. [Google Scholar] [CrossRef]
- Raymo, F.M. Digital processing and communication with molecular switches. Adv. Mater. 2002, 14, 401–402. [Google Scholar] [CrossRef]
- Collier, C.P.; Wong, E.W.; Belohradsky, M.; Raymo, F.M.; Stoddart, J.F.; Kuekes, P.J.; Williams, R.S.; Heath, J.R. Electronically configurable molecular-based logic gates. Science 1999, 285, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Gutlich, P.; Goodwin, H.A. Topics in Current Chemistry. Spin Crossover in Transition Metal Compounds I-III; Springer-Verlag: Berlin, Germany, 2004. [Google Scholar]
- Bousseksou, A.; Molnar, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Gutlich, P.; Hauser, A.; Spiering, H. Thermal and optical switching of iron(ii) complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Spiering, H.; Boukheddaden, K.; Linares, J.; Varret, F. Total free energy of a spin-crossover molecular system. Phys. Rev. B 2004, 70, 184106. [Google Scholar] [CrossRef]
- McCusker, J.K.; Walda, K.N.; Dunn, R.C.; Simon, J.D.; Magde, D.; Hendrickson, D.N. Sub-picosecond. Delta.S = 2 intersystem crossing in low-spin ferrous complexes. J. Am. Chem. Soc. 1992, 114, 6919–6920. [Google Scholar] [CrossRef]
- Letard, J.F.; Guionneau, P.; Goux-Capes, L. Towards spin crossover applications. In Spin Crossover in Transition Metal Compounds III; Springer-Verlag: Berlin, Germany, 2004; Volume 235, pp. 221–249. [Google Scholar]
- Kahn, O.; Martinez, C.J. Spin-transition polymers: From molecular materials toward memory devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Linares, J.; Codjovi, E.; Garcia, Y. Pressure and temperature spin crossover sensors with optical detection. Sensors 2012, 12, 4479–4492. [Google Scholar] [CrossRef] [PubMed]
- Bartual-Murgui, C.; Akou, A.; Thibault, C.; Molnar, G.; Vieu, C.; Salmon, L.; Bousseksou, A. Spin-crossover metal-organic frameworks: Promising materials for designing gas sensors. J. Mater. Chem. C 2015, 3, 1277–1285. [Google Scholar] [CrossRef]
- Salmon, L.; Molnar, G.; Zitouni, D.; Quintero, C.; Bergaud, C.; Micheau, J.C.; Bousseksou, A. A novel approach for fluorescent thermometry and thermal imaging purposes using spin crossover nanoparticles. J. Mater. Chem. 2010, 20, 5499–5503. [Google Scholar] [CrossRef]
- Matsuda, M.; Kiyoshima, K.; Uchida, R.; Kinoshita, N.; Tajima, H. Characteristics of organic light-emitting devices consisting of dye-doped spin crossover complex films. Thin Solid Films 2013, 531, 451–453. [Google Scholar] [CrossRef]
- Shepherd, H.J.; Gural‘skiy, I.A.; Quintero, C.M.; Tricard, S.; Salmon, L.; Molnár, G.; Bousseksou, A. Molecular actuators driven by cooperative spin-state switching. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E. Charge transport properties of spin crossover systems. Phys. Chem. Chem. Phys. 2014, 16, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.B.; Ksenofontov, V.; Seredyuk, M.; Gutlich, P. Multifunctionality in spin crossover materials. Coord. Chem. Rev. 2005, 249, 2661–2676. [Google Scholar] [CrossRef]
- Sato, O.; Li, Z.-Y.; Yao, Z.-S.; Kang, S.; Kanegawa, S. Multifunctional materials combining spin-crossover with conductivity and magnetic ordering. In Spin-Crossover Materials; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2013; pp. 303–319. [Google Scholar]
- Sato, O.; Kawakami, T.; Kimura, M.; Hishiya, S.; Kubo, S.; Einaga, Y. Electric-field-induced conductance switching in feco prussian blue analogues. J. Am. Chem. Soc. 2004, 126, 13176–13177. [Google Scholar] [CrossRef] [PubMed]
- Molnar, G.; Cobo, S.; Mahfoud, T.; Vertelman, E.J.M.; van Koningsbruggen, P.J.; Demont, P.; Bousseksou, A. Interplay between the charge transport phenomena and the charge-transfer phase transition in RbxMn1−xFe(CN)6(y).H2O. J. Phys. Chem. C 2009, 113, 2586–2593. [Google Scholar] [CrossRef]
- Mahfoud, T.; Molnar, G.; Bonhommeau, S.; Cobo, S.; Salmon, L.; Demont, P.; Tokoro, H.; Ohkoshi, S.I.; Boukheddaden, K.; Bousseksou, A. Electric-field-induced charge-transfer phase transition: A promising approach toward electrically switchable devices. J. Am. Chem. Soc. 2009, 131, 15049–15054. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.-I.; Nuida, T.; Matsuda, T.; Tokoro, H.; Hashimoto, K. The dielectric constant in a thermal phase transition magnetic material composed of rubidium manganese hexacyanoferrate observed by spectroscopic ellipsometry. J. Mater. Chem. 2005, 15, 3291–3295. [Google Scholar] [CrossRef]
- Ohkoshi, S.-I.; Tokoro, H.; Matsuda, T.; Takahashi, H.; Irie, H.; Hashimoto, K. Coexistence of ferroelectricity and ferromagnetism in a rubidium manganese hexacyanoferrate. Angew. Chem. Int. Ed. Engl. 2007, 46, 3238–3241. [Google Scholar] [CrossRef] [PubMed]
- Bousseksou, A.; Molnar, G.; Demont, P.; Menegotto, J. Observation of a thermal hysteresis loop in the dielectric constant of spin crossover complexes: Towards molecular memory devices. J. Mater. Chem. 2003, 13, 2069–2071. [Google Scholar] [CrossRef]
- Guillon, T.; Bonhommeau, S.; Costa, J.S.; Zwick, A.; Letard, J.-F.; Demont, P.; Molnar, G.; Bousseksou, A. On the dielectric properties of the spin crossover complex Fe(bpp)2(BF4)2. Phys. Status Solidi Appl. Mater. Sci. 2006, 203, 2974–2980. [Google Scholar] [CrossRef]
- Guillon, T.; Salmon, L.; Molnar, G.; Zein, S.; Borshch, S.; Bousseksou, A. Investigation of the two-step spin crossover complex Fe[5-NO2-sal-(1,4,7,10)] using density functional theory. J. Phys. Chem. A 2007, 111, 8223–8228. [Google Scholar] [CrossRef] [PubMed]
- Bonhommeau, S.; Guillon, T.; Daku, L.M.L.; Demont, P.; Costa, J.S.; Letard, J.F.; Molnar, G.; Bousseksou, A. Photoswitching of the dielectric constant of the spin-crossover complex [Fe(L)(CN)2].H2O. Angew. Chem. Int. Ed. Engl. 2006, 45, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Salmon, L.; Molnar, G.; Cobo, S.; Oulie, P.; Etienne, M.; Mahfoud, T.; Demont, P.; Eguchi, A.; Watanabe, H.; Tanakae, K.; et al. Re-investigation of the spin crossover phenomenon in the ferrous complex [Fe(HB(pz)3)2]. New J. Chem. 2009, 33, 1283–1289. [Google Scholar] [CrossRef]
- Rotaru, A.; Gural‘skiy, I.Y.A.; Molnar, G.; Salmon, L.; Demont, P.; Bousseksou, A. Spin state dependence of electrical conductivity of spin crossover materials. Chem. Commun. 2012, 48, 4163–4165. [Google Scholar] [CrossRef] [PubMed]
- Lefter, C.; Gural‘skiy, I.A.; Peng, H.; Molnár, G.; Salmon, L.; Rotaru, A.; Bousseksou, A.; Demont, P. Dielectric and charge transport properties of the spin crossover complex [Fe(Htrz)2(trz)](BF4). Phys. Status Solidi RRL Rapid Res. Lett. 2014, 8, 191–193. [Google Scholar] [CrossRef]
- Lefter, C.; Tricard, S.; Peng, H.; Molnár, G.; Salmon, L.; Demont, P.; Rotaru, A.; Bousseksou, A. Metal substitution effects on the charge transport and spin crossover properties of [Fe1–xZnx(Htrz)2(trz)](BF4) (trz = triazole). J. Phys. Chem. C 2015, 119, 8522–8529. [Google Scholar] [CrossRef]
- Nakano, M.; Fujita, N.; Matsubayashi, G.E.; Mori, W. Modified chesnut model for spin-crossover semiconductors [Fe(acpa)2](tcnq)n. Mol. Cryst. Liq. Cryst. 2002, 379, 365–370. [Google Scholar] [CrossRef]
- Faulmann, C.; Dorbes, S.; de Bonneval, W.G.; Molnar, G.; Bousseksou, A.; Gomez-Garcia, C.J.; Coronado, E.; Valade, L. Towards molecular conductors with a spin-crossover phenomenon: Crystal structures, magnetic properties and mossbauer spectra of [Fe(salten)mepepy][M(dmit)2] complexes. Eur. J. Inorg. Chem. 2005, 3261–3270. [Google Scholar] [CrossRef]
- Dorbes, S.; Valade, L.; Real, J.A.; Faulmann, C. Fe(sal(2)-trien)Ni(dmit)2: Towards switchable spin crossover molecular conductors. Chem. Commun. 2005, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Faulmann, C.; Dorbes, S.; Lampert, S.; Jacob, K.; de Bonneval, B.G.; Molnar, G.; Bousseksou, A.; Real, J.A.; Valade, L. Crystal structure, magnetic properties and mossbauer studies of [Fe(qsal)2][Ni(dmit)2]. Inorg. Chim. Acta 2007, 360, 3870–3878. [Google Scholar] [CrossRef]
- Takahashi, K.; Cui, H.B.; Kobayashi, H.; Einaga, Y.; Sato, O. The light-induced excited spin state trapping effect on Ni(dmit)2 salt with an Fe(III) spin-crossover cation: [Fe(qsal)2][Ni(dmit)2].2CH3CN. Chem. Lett. 2005, 34, 1240–1241. [Google Scholar] [CrossRef]
- Nihei, M.; Takahashi, N.; Nishikawa, H.; Oshio, H. Spin-crossover behavior and electrical conduction property in iron(II) complexes with tetrathiafulvalene moieties. Dalton Trans. 2011, 40, 2154–2156. [Google Scholar] [CrossRef] [PubMed]
- Fukuroi, K.; Takahashi, K.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Mori, H. Synergistic spin transition between spin crossover and spin-peierls-like singlet formation in the halogen-bonded molecular hybrid system: [Fe(iqsal)2][Ni(dmit)2]·CH3CN·H2O. Angew. Chem. Int. Ed. Engl. 2014, 53, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Djukic, B.; Lemaire, M.T. Hybrid spin-crossover conductor exhibiting unusual variable-temperature electrical conductivity. Inorg. Chem. 2009, 48, 10489–10491. [Google Scholar] [CrossRef] [PubMed]
- Faulmann, C.; Dorbes, S.; Real, J.A.; Valade, L. Electrical conductivity and spin crossover: Towards the first achievement with a metal bis dithiolene complex. J. Low Temp. Phys. 2006, 142, 261–266. [Google Scholar] [CrossRef]
- Faulmann, C.; Jacob, K.; Dorbes, S.; Lampert, S.; Malfant, I.; Doublet, M.L.; Valade, L.; Real, J.A. Electrical conductivity and spin crossover: A new achievement with a metal bis dithiolene complex. Inorg. Chem. 2007, 46, 8548–8559. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Cui, H.B.; Okano, Y.; Kobayashi, H.; Einaga, Y.; Sato, O. Electrical conductivity modulation coupled to a high-spin-low-spin conversion in the molecular system [FeIII(qsal)2][Ni(dmit)2]3.CH3CN.H2O. Inorg. Chem. 2006, 45, 5739–5741. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Cui, H.B.; Okano, Y.; Kobayashi, H.; Mori, H.; Tajima, H.; Einaga, Y.; Sato, O. Evidence of the chemical uniaxial strain effect on electrical conductivity in the spin-crossover conducting molecular system: [FeIII(qnal)2][Pd(dmit)2]5.acetone. J. Am. Chem. Soc. 2008, 130, 6688–6689. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.; Benjamin, S.M.; Steven, E.; Brooks, J.S.; Shatruk, M. Photomagnetic response in highly conductive iron(ii) spin-crossover complexes with tcnq radicals. Angew. Chem. Int. Ed. Engl. 2015, 54, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.-S.; Galán-Mascarós, J.R. Spin crossover probes confer multistability to organic conducting polymers. Adv. Mater. 2014, 26, 6785–6789. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Meng, Y.; Ni, Z.-P.; Tong, M.-L. Synergistic electrical bistability in a conductive spin crossover heterostructure. J. Mater. Chem. C 2015, 3, 945–949. [Google Scholar] [CrossRef]
- Gural‘skiy, I.Y.A.; Quintero, C.M.; Costa, J.S.; Demont, P.; Molnar, G.; Salmon, L.; Shepherd, H.J.; Bousseksou, A. Spin crossover composite materials for electrothermomechanical actuators. J. Mater. Chem. C 2014, 2, 2949–2955. [Google Scholar] [CrossRef]
- Matsuda, M.; Isozaki, H.; Tajima, H. Reproducible on-off switching of the light emission from the electroluminescent device containing a spin crossover complex. Thin Solid Films 2008, 517, 1465–1467. [Google Scholar] [CrossRef]
- Mahfoud, T.; Molnar, G.; Cobo, S.; Salmon, L.; Thibault, C.; Vieu, C.; Demont, P.; Bousseksou, A. Electrical properties and non-volatile memory effect of the [Fe(HB(pz)3)2] spin crossover complex integrated in a microelectrode device. Appl. Phys. Lett. 2011, 99, 053307. [Google Scholar] [CrossRef]
- Shi, S.; Schmerber, G.; Arabski, J.; Beaufrand, J.B.; Kim, D.J.; Boukari, S.; Bowen, M.; Kemp, N.T.; Viart, N.; Rogez, G.; et al. Study of molecular spin-crossover complex Fe(phen)2(NCS)2 thin films. Appl. Phys. Lett. 2009, 95, 043303. [Google Scholar] [CrossRef]
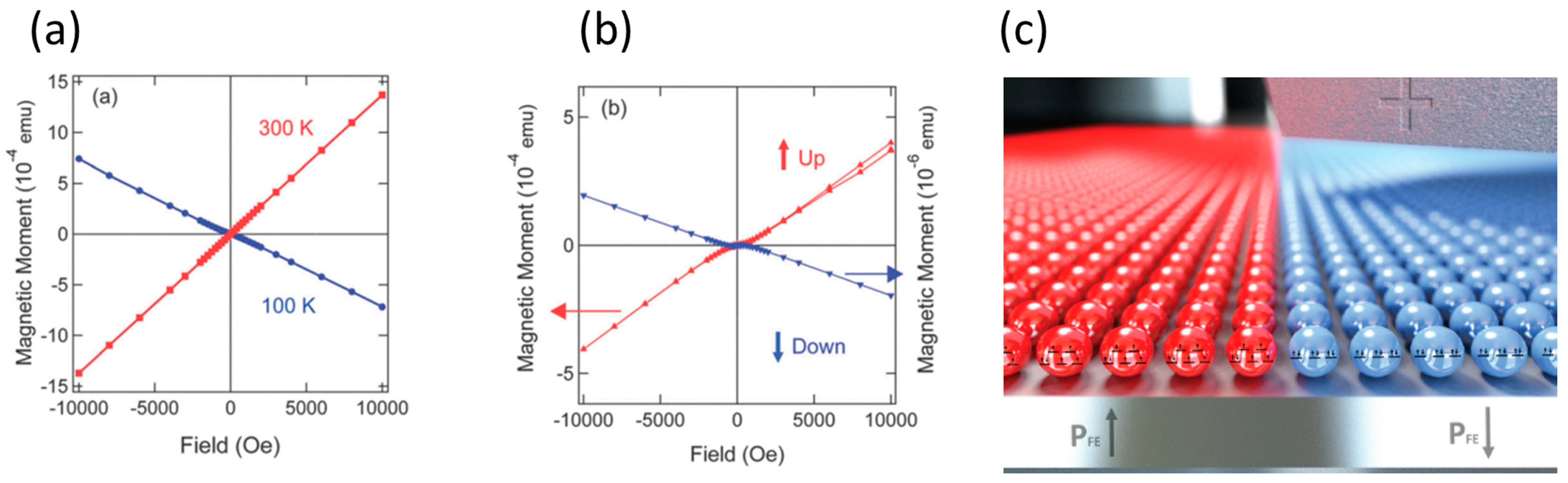
- Zhang, X.; Palamarciuc, T.; Letard, J.-F.; Rosa, P.; Lozada, E.V.; Torres, F.; Rosa, L.G.; Doudin, B.; Dowben, P.A. The spin state of a molecular adsorbate driven by the ferroelectric substrate polarization. Chem. Commun. 2014, 50, 2255–2257. [Google Scholar] [CrossRef] [PubMed]
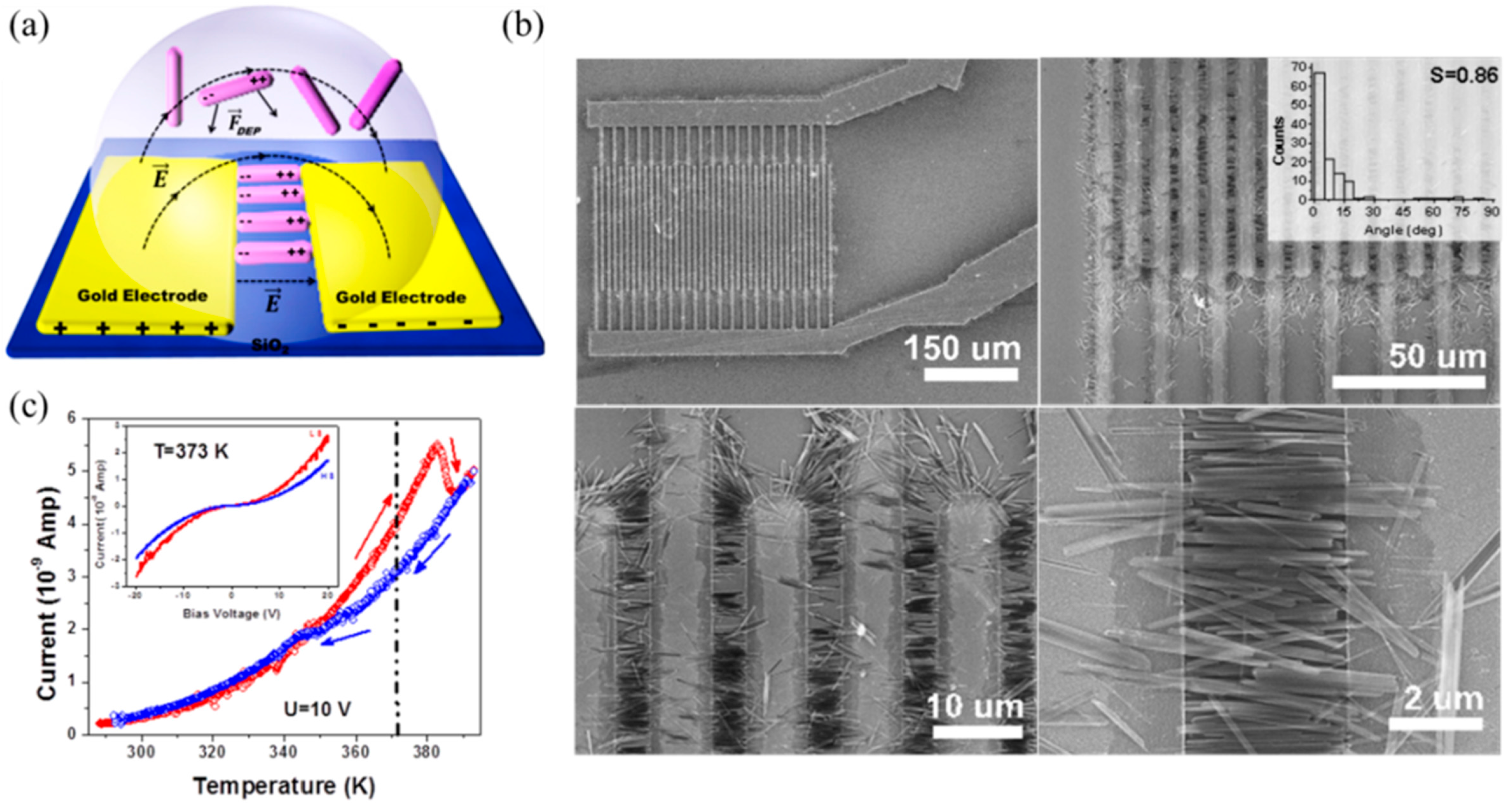
- Rotaru, A.; Dugay, J.; Tan, R.P.; Gural‘skiy, I.A.; Salmon, L.; Demont, P.; Carrey, J.; Molnar, G.; Respaud, M.; Bousseksou, A. Nano-electromanipulation of spin crossover nanorods: Towards switchable nanoelectronic devices. Adv. Mater. 2013, 25, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Dugay, J.; Giménez-Marqués, M.; Kozlova, T.; Zandbergen, H.W.; Coronado, E.; van der Zant, H.S.J. Spin switching in electronic devices based on 2d assemblies of spin-crossover nanoparticles. Adv. Mater. 2015, 27, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
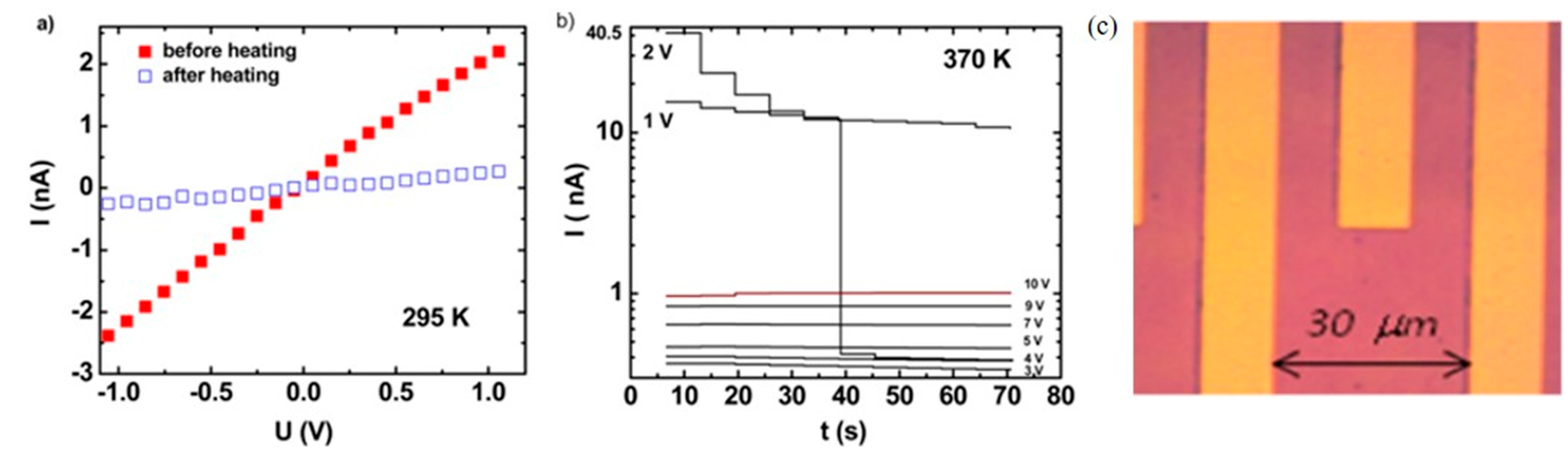
- Lefter, C.; Tan, R.; Tricard, S.; Dugay, J.; Molnár, G.; Salmon, L.; Carrey, J.; Rotaru, A.; Bousseksou, A. On the stability of spin crossover materials: From bulk samples to electronic devices. Polyhedron 2015, 102, 434–440. [Google Scholar] [CrossRef]
- Lefter, C.; Tan, R.; Dugay, J.; Tricard, S.; Molnar, G.; Salmon, L.; Carrey, J.; Rotaru, A.; Bousseksou, A. Light induced modulation of charge transport phenomena across the bistability region in [fe(htrz)2(trz)](bf4) spin crossover micro-rods. Phys. Chem. Chem. Phys. 2015, 17, 5151–5154. [Google Scholar] [CrossRef] [PubMed]
- Lefter, C.; Tan, R.; Dugay, J.; Tricard, S.; Molnar, G.; Salmon, L.; Carrey, J.; Nicolazzi, W.; Rotaru, A.; Bousseksou, A. Unidirectional electric field-induced spin-state switching in spin crossover based microelectronic devices. Chem. Phys. Lett. 2016, 644, 138–141. [Google Scholar] [CrossRef]
- Etrillard, C.; Faramarzi, V.; Dayen, J.-F.; Letard, J.-F.; Doudin, B. Photoconduction in [Fe(Htrz)2(trz)](BF4)·H2O nanocrystals. Chem. Commun. 2011, 47, 9663–9665. [Google Scholar] [CrossRef] [PubMed]
- Prins, F.; Monrabal-Capilla, M.; Osorio, E.A.; Coronado, E.; van der Zant, H.S.J. Room-temperature electrical addressing of a bistable spin-crossover molecular system. Adv. Mater. 2011, 23, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Stocker, M.; Gieb, K.; Muller, P.; Haryono, M.; Student, K.; Grohmann, A. Spin-state patterns in surface-grafted beads of iron(II) complexes. Angew. Chem. Int. Ed. Engl. 2010, 49, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, A.; Haryono, M.; Student, K.; Müller, P.; Stocker, M. Mapping the spin state in spin-crossover complex assemblies: Current-imaging tunnelling spectroscopy (cits). Eur. J. Inorg. Chem. 2013, 2013, 662–669. [Google Scholar] [CrossRef]
- Palamarciuc, T.; Oberg, J.C.; El Hallak, F.; Hirjibehedin, C.F.; Serri, M.; Heutz, S.; Letard, J.-F.; Rosa, P. Spin crossover materials evaporated under clean high vacuum and ultra-high vacuum conditions: From thin films to single molecules. J. Mater. Chem. 2012, 22, 9690–9695. [Google Scholar] [CrossRef]
- Pronschinske, A.; Chen, Y.; Lewis, G.F.; Shultz, D.A.; Calzolari, A.; Buongiorno Nardelli, M.; Dougherty, D.B. Modification of molecular spin crossover in ultrathin films. Nano Lett. 2013, 13, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Gopakumar, T.G.; Matino, F.; Naggert, H.; Bannwarth, A.; Tuczek, F.; Berndt, R. Electron-induced spin crossover of single molecules in a bilayer on gold. Angew. Chem. Int. Ed. Engl. 2012, 51, 6262–6266. [Google Scholar] [CrossRef] [PubMed]
- Miyamachi, T.; Gruber, M.; Davesne, V.; Bowen, M.; Boukari, S.; Joly, L.; Scheurer, F.; Rogez, G.; Yamada, T.K.; Ohresser, P.; et al. Robust spin crossover and memristance across a single molecule. Nat. Commun. 2012, 3, 938. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.; Davesne, V.; Bowen, M.; Boukari, S.; Beaurepaire, E.; Wulfhekel, W.; Miyamachi, T. Spin state of spin-crossover complexes: From single molecules to ultrathin films. Phys. Rev. B 2014, 89, 195415. [Google Scholar] [CrossRef]
- Gueddida, S.; Alouani, M. Spin crossover in a single Fe(phen)2(NCS)2 molecule adsorbed onto metallic substrates: An ab initio calculation. Phys. Rev. B 2013, 87, 144413. [Google Scholar] [CrossRef]
- Osorio, E.A.; Moth-Poulsen, K.; van der Zant, H.S.J.; Paaske, J.; Hedegård, P.; Flensberg, K.; Bendix, J.; Bjørnholm, T. Electrical manipulation of spin states in a single electrostatically gated transition-metal complex. Nano Lett. 2009, 10, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Meded, V.; Bagrets, A.; Fink, K.; Chandrasekar, R.; Ruben, M.; Evers, F.; Bernand-Mantel, A.; Seldenthuis, J.S.; Beukman, A.; van der Zant, H.S.J. Electrical control over the Fe(II) spin crossover in a single molecule: Theory and experiment. Phys. Rev. B 2011, 83, 245415. [Google Scholar] [CrossRef]
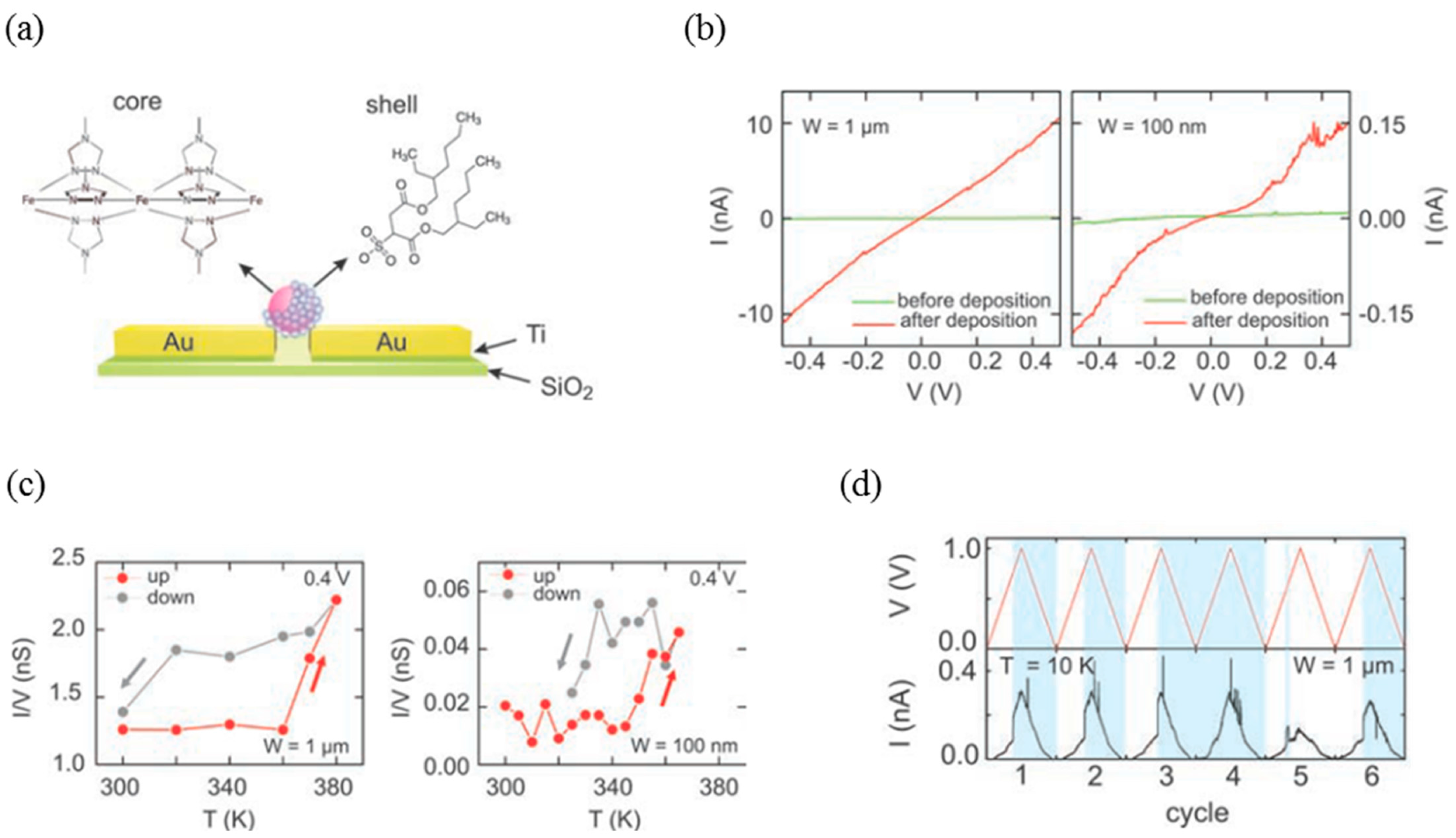
- Devid, E.J.; Martinho, P.N.; Kamalakar, M.V.; Šalitroš, I.; Prendergast, Ú.; Dayen, J.-F.; Meded, V.; Lemma, T.; González-Prieto, R.; Evers, F.; et al. Spin transition in arrays of gold nanoparticles and spin crossover molecules. ACS Nano 2015, 9, 4496–4507. [Google Scholar] [CrossRef] [PubMed]
- Harzmann, G.D.; Frisenda, R.; van der Zant, H.S.J.; Mayor, M. Single-molecule spin switch based on voltage-triggered distortion of the coordination sphere. Angew. Chem. Int. Ed. Engl. 2015, 54, 13425–13430. [Google Scholar] [CrossRef] [PubMed]
- Baadji, N.; Sanvito, S. Giant resistance change across the phase transition in spin-crossover molecules. Phys. Rev. Lett. 2012, 108, 217201. [Google Scholar] [CrossRef] [PubMed]
- Aravena, D.; Ruiz, E. Coherent transport through spin-crossover single molecules. J. Am. Chem. Soc. 2012, 134, 777–779. [Google Scholar] [CrossRef] [PubMed]

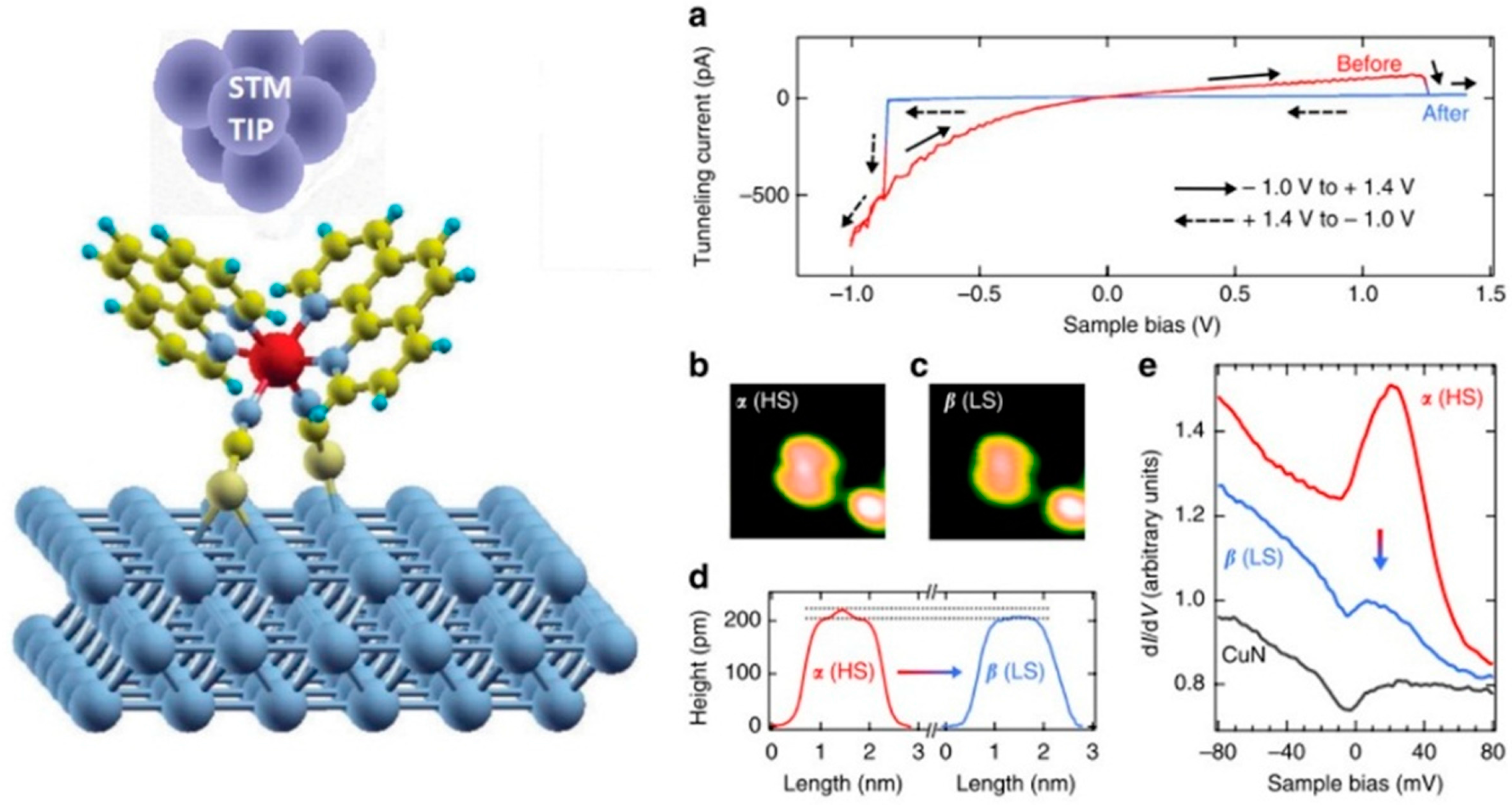
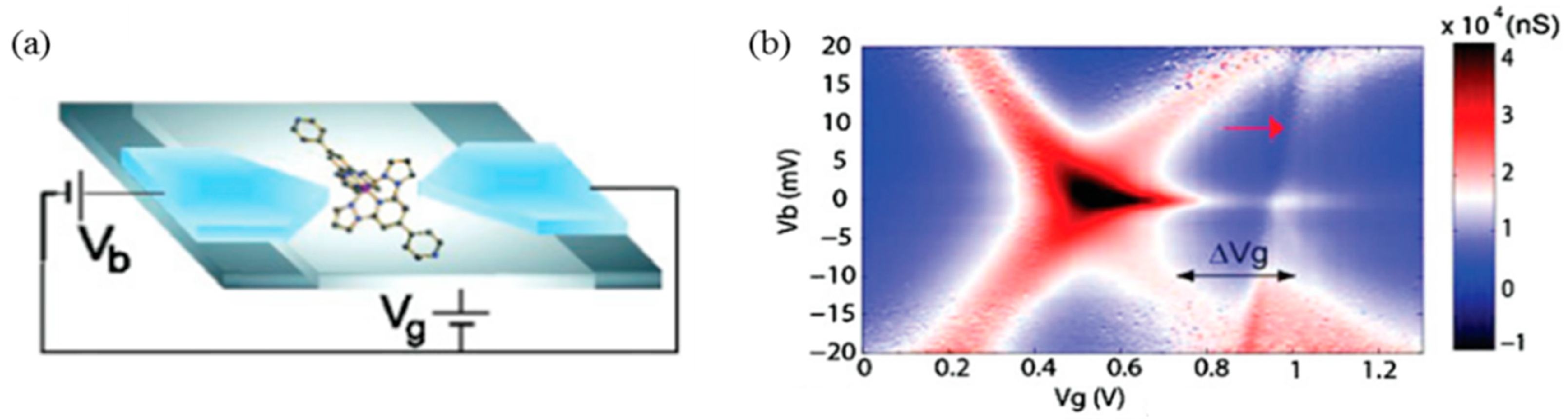

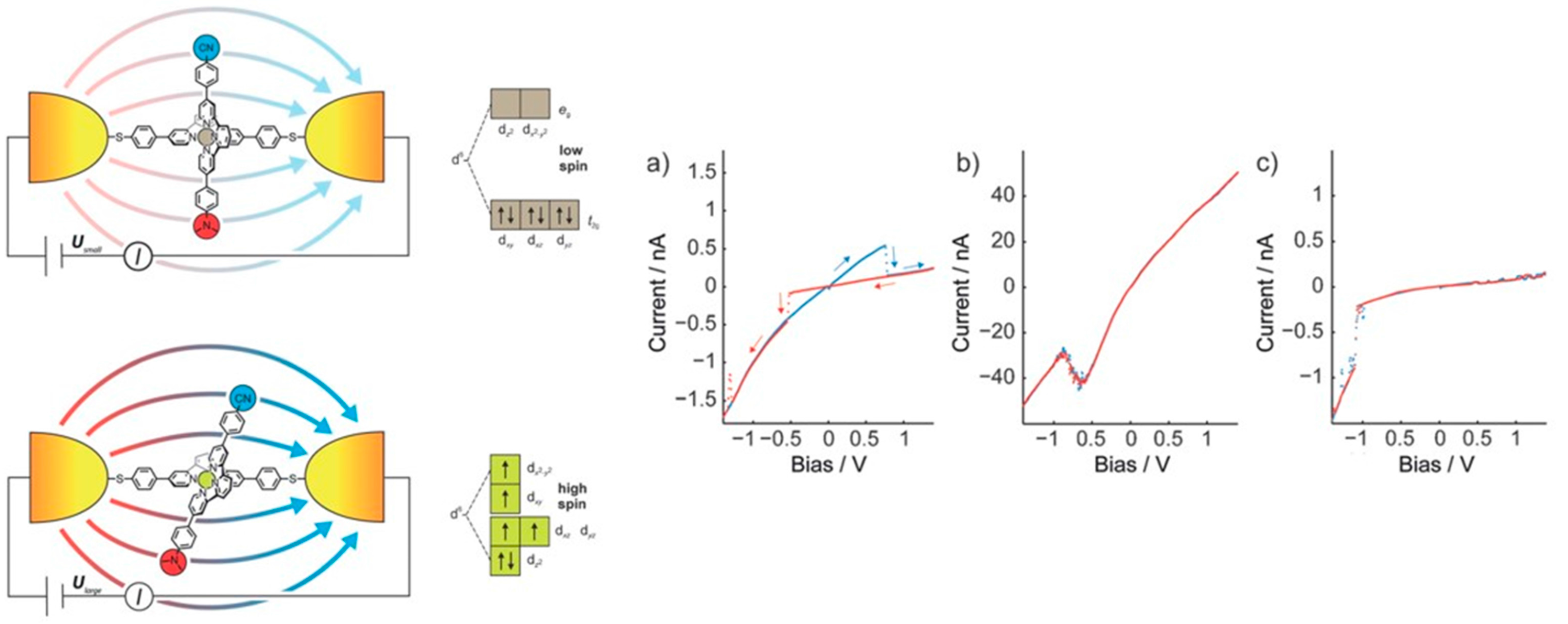
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefter, C.; Davesne, V.; Salmon, L.; Molnár, G.; Demont, P.; Rotaru, A.; Bousseksou, A. Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices. Magnetochemistry 2016, 2, 18. https://doi.org/10.3390/magnetochemistry2010018
Lefter C, Davesne V, Salmon L, Molnár G, Demont P, Rotaru A, Bousseksou A. Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices. Magnetochemistry. 2016; 2(1):18. https://doi.org/10.3390/magnetochemistry2010018
Chicago/Turabian StyleLefter, Constantin, Vincent Davesne, Lionel Salmon, Gábor Molnár, Philippe Demont, Aurelian Rotaru, and Azzedine Bousseksou. 2016. "Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices" Magnetochemistry 2, no. 1: 18. https://doi.org/10.3390/magnetochemistry2010018
APA StyleLefter, C., Davesne, V., Salmon, L., Molnár, G., Demont, P., Rotaru, A., & Bousseksou, A. (2016). Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices. Magnetochemistry, 2(1), 18. https://doi.org/10.3390/magnetochemistry2010018







