Synthesis and Antibacterial Evaluation of Silver-Coated Magnetic Iron Oxide/Activated Carbon Nanoparticles Derived from Hibiscus esculentus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
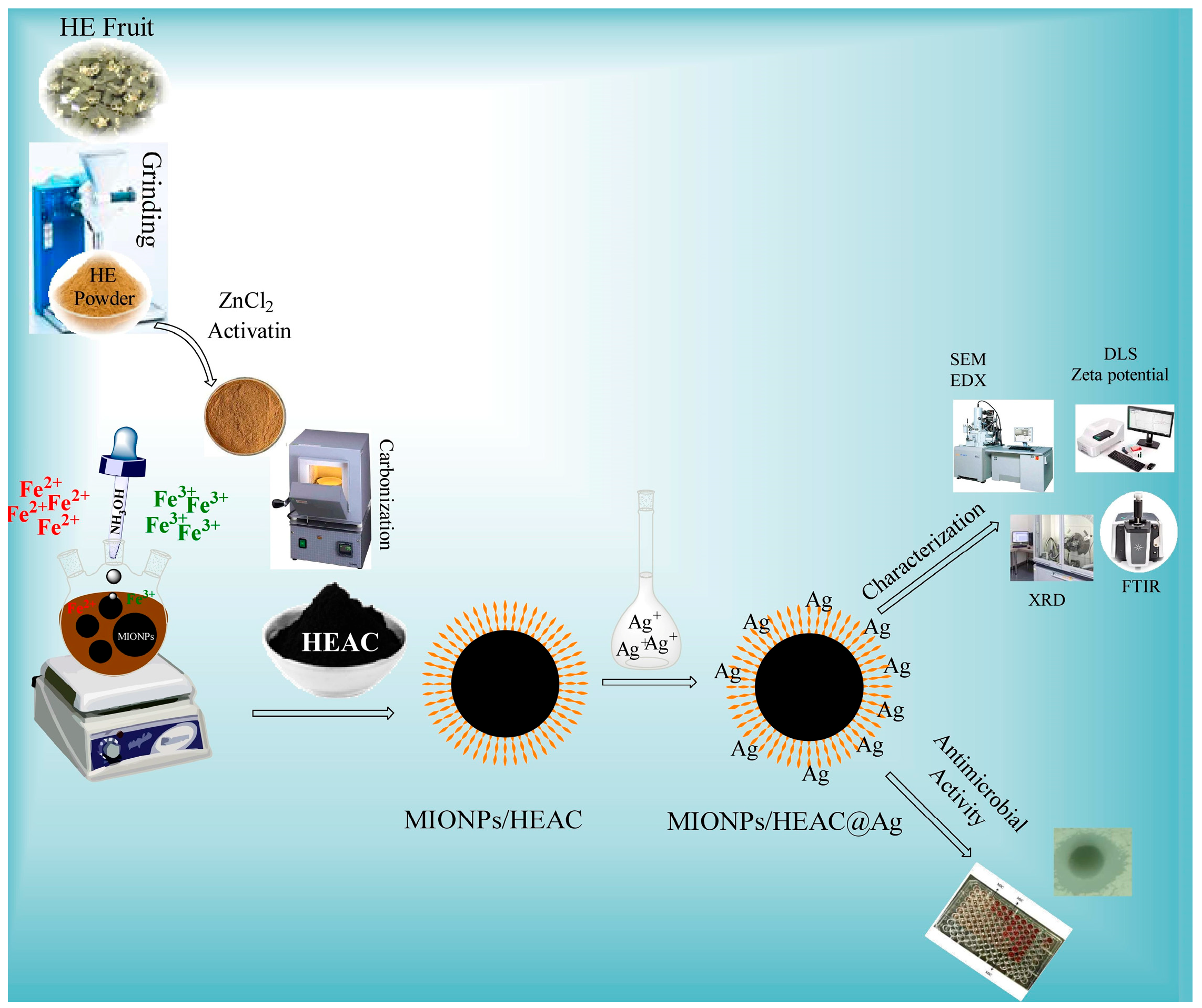
2.2. Synthesis of HEAC
2.3. Synthesis of MIONPs/HEAC
2.4. Synthesis of MIONPs/HEAC@Ag
2.5. Nanoparticle Characterization
2.6. Analysis of Antibacterial Effect of MIONPs/HEAC and MIONPs/HEAC@Ag
3. Results and Discussion
3.1. Structural Characterization
3.1.1. FTIR Analysis
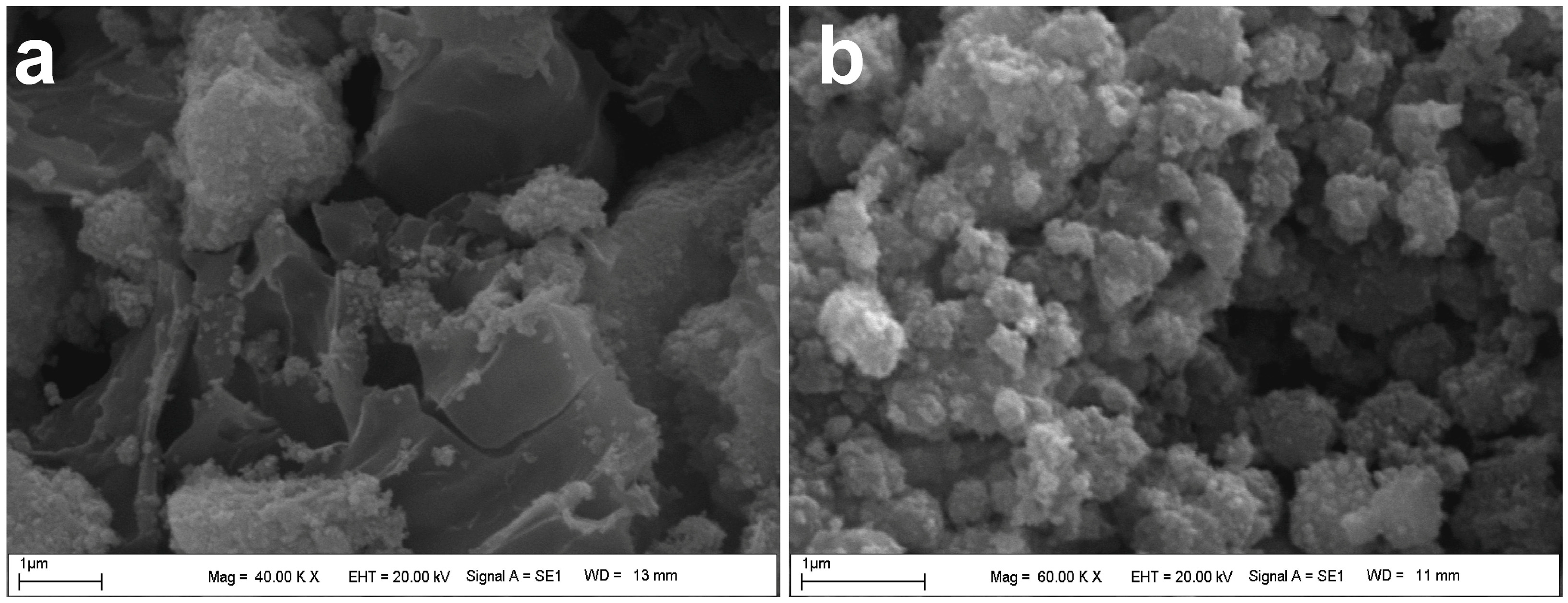
3.1.2. SEM Analysis
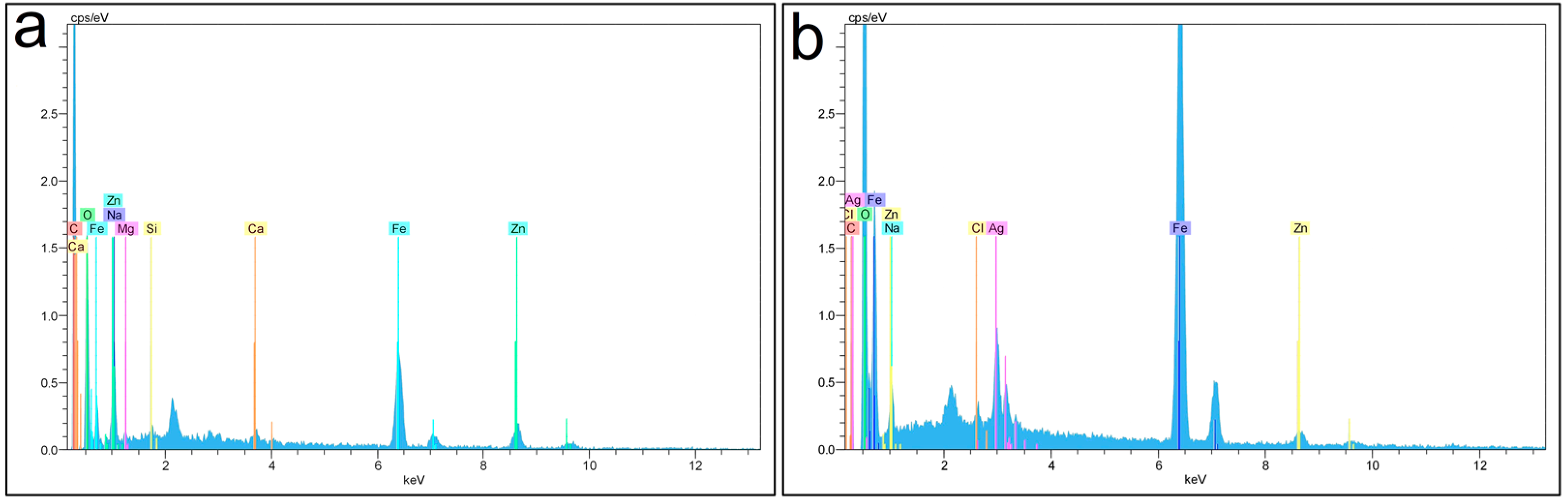
3.1.3. EDX Analysis
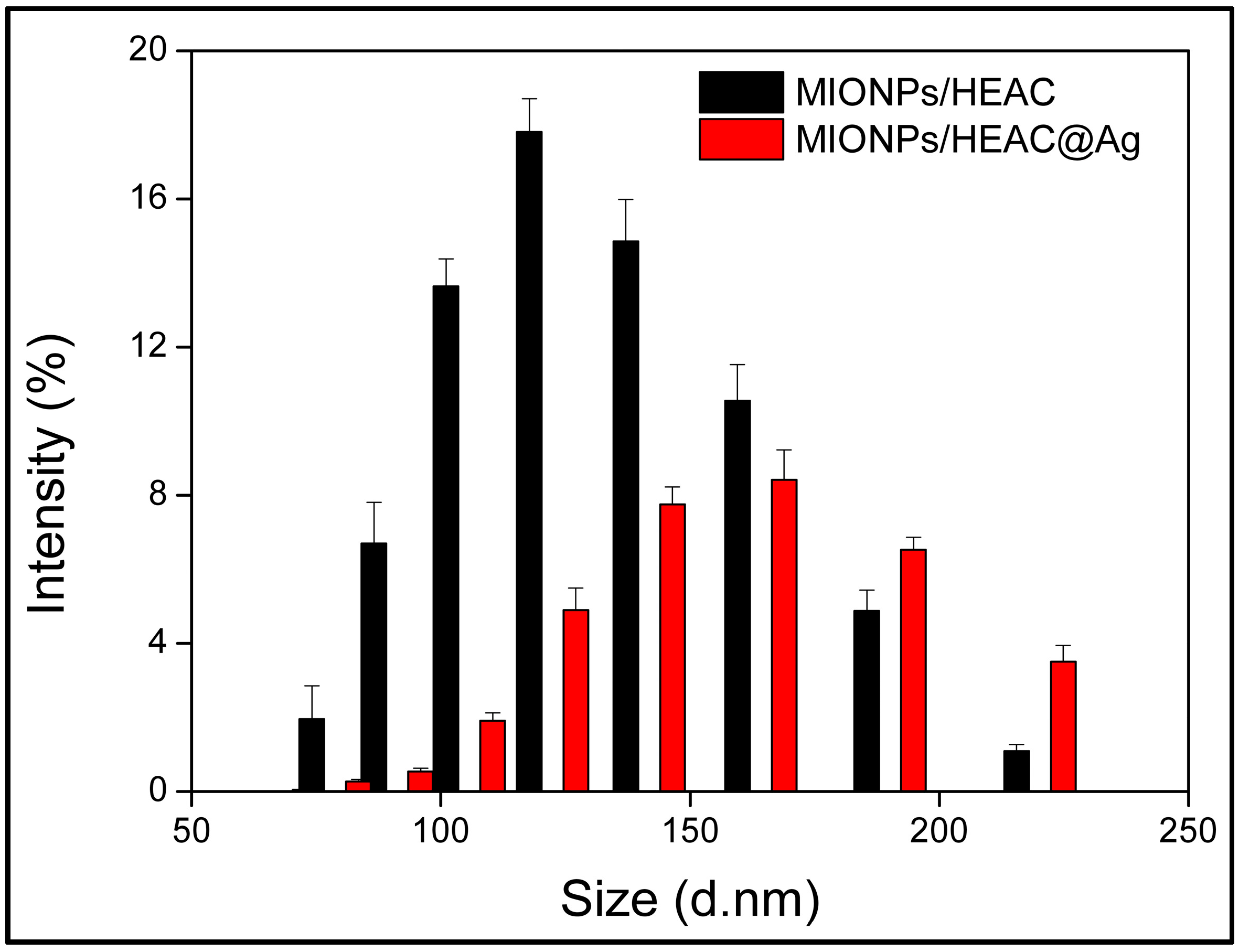
3.1.4. DLS Analysis
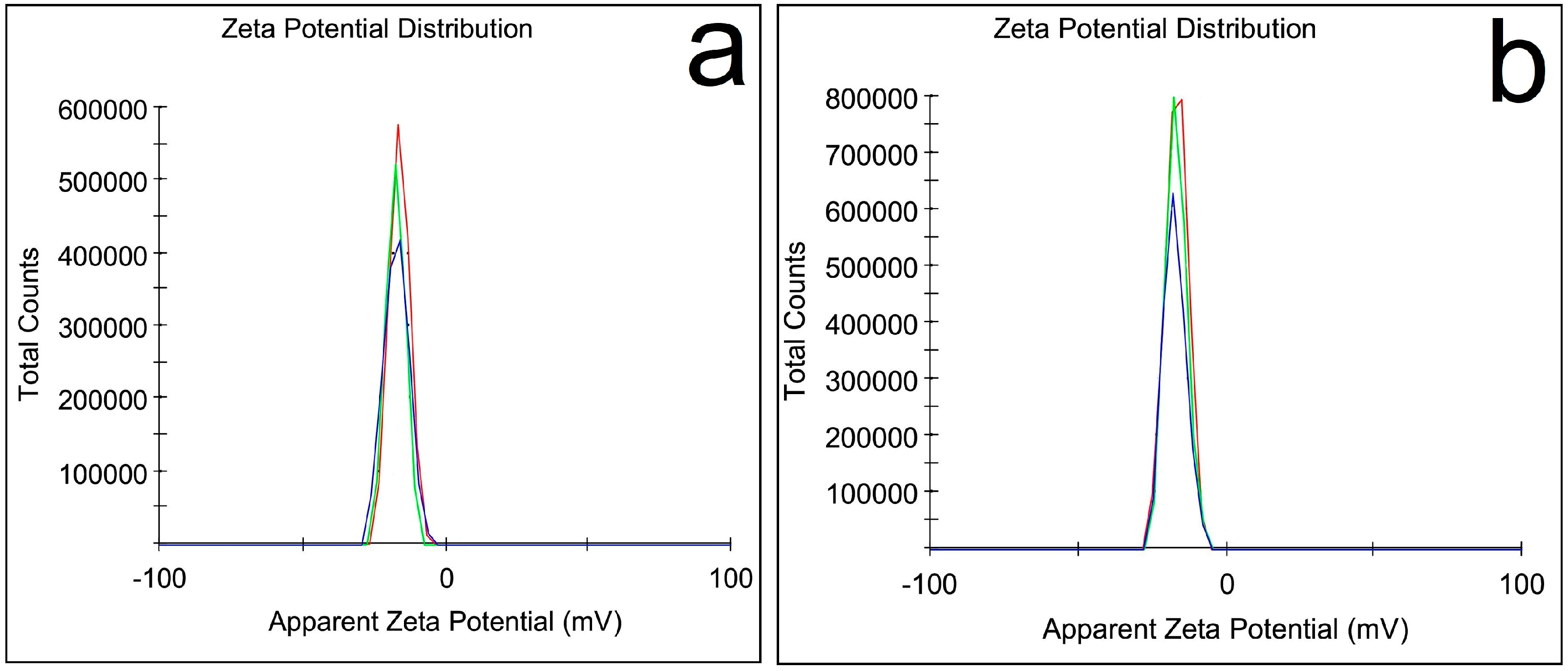
3.1.5. Zeta Potential Analysis
3.1.6. XRD Analysis
3.1.7. VSM Analysis
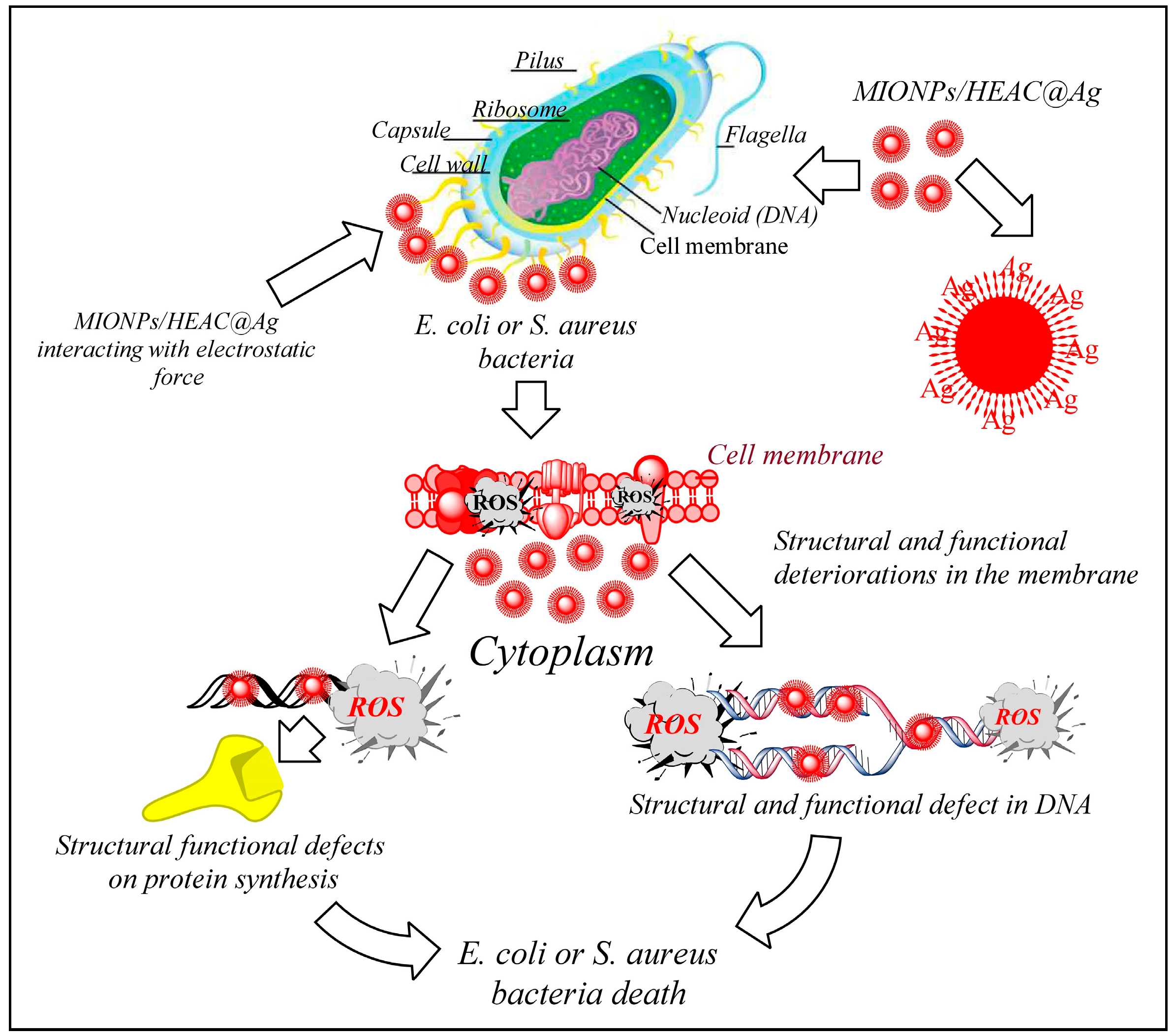
3.2. Antibacterial Properties of MIONPs/HEAC and MIONPs/HEAC@Ag
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, A.; Sengupta, P.; Chatterjee, S.; Khanam, J.; Mondal, P.K.; Romero, E.L.; Ghosal, K. Development and Evaluation of Magnetite Loaded Alginate Beads Based Nanocomposite for Enhanced Targeted Analgesic Drug Delivery. Magnetochemistry 2025, 11, 14. [Google Scholar] [CrossRef]
- Attar, S.R.; Sapkal, A.C.; Bagade, C.S.; Ghanwat, V.B.; Kamble, S.B. Biogenic CuO NPs for synthesis of coumarin derivatives in hydrotropic aqueous medium. Res. Chem. Intermed. 2023, 49, 2989–3004. [Google Scholar] [CrossRef]
- Adeeyo, A.O.; Alabi, M.A.; Oyetade, J.A.; Nkambule, T.T.; Mamba, B.B.; Oladipo, A.O.; Msagati, T.A. Magnetic Nanoparticles: Advances in Synthesis, Sensing, and Theragnostic Applications. Magnetochemistry 2025, 11, 9. [Google Scholar] [CrossRef]
- Fadeel, B. Nanomaterial characterization: Understanding nano-bio interactions. Biochem. Biophys. Res. Commun. 2022, 633, 45–51. [Google Scholar] [CrossRef]
- Ariga, K.; Fakhrullin, R. Nanoarchitectonics in Materials Science: Method for Everything in Materials Science. Materials 2023, 16, 6367. [Google Scholar] [CrossRef]
- Bataineh, S.M.; Arafa, I.M.; Abu-Zreg, S.M.; Al-Gharaibeh, M.M.; Hammouri, H.M.; Tarazi, Y.H.; Darmani, H. Synergistic effect of magnetic iron oxide nanoparticles with medicinal plant extracts against resistant bacterial strains. Magnetochemistry 2024, 10, 49. [Google Scholar] [CrossRef]
- De, A.; Singh, N.B.; Guin, M.; Barthwal, S. Water purification by green synthesized nanomaterials. Curr. Pharm. Biotechnol. 2023, 24, 101–117. [Google Scholar]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver nanoparticle-based nanocomposites for combating infectious pathogens: Recent advances and future prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef]
- Begum, S.J.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Jaremko, M. Recent advances in green synthesis, characterization, and applications of bioactive metallic nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef]
- Alburae, N.; Alshamrani, R.; Mohammed, A.E. Bioactive silver nanoparticles fabricated using Lasiurus scindicus and Panicum turgidum seed extracts: Anticancer and antibacterial efficiency. Sci. Rep. 2024, 14, 4162. [Google Scholar]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Ayala-Cortés, A.; Arias, D.M.; Longoria, A.; Cuentas-Gallegos, A.K.; Okoye, P.U. Advances in activated carbon modification, surface heteroatom configuration, reactor strategies, and regeneration methods for enhanced wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 105626. [Google Scholar] [CrossRef]
- Kang, N.; Fan, Y.; Li, D.; Jia, X.; Zhao, S. Preparation of Magnetic Nano-Catalyst Containing Schiff Base Unit and Its Application in the Chemical Fixation of CO2 into Cyclic Carbonates. Magnetochemistry 2024, 10, 33. [Google Scholar] [CrossRef]
- Doğan, Y.; Öziç, C.; Ertaş, E.; Baran, A.; Rosic, G.; Selakovic, D.; Eftekhari, A. Activated carbon-coated iron oxide magnetic nanocomposite (IONPs@CtAC) loaded with morin hydrate for drug-delivery applications. Front. Chem. 2024, 12, 1477724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Xu, R.; Wang, H.; Chen, J.Y.; Tu, Z.C. Structural properties, bioactivities, and applications of polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Ashraf, S.A. Okra (Abelmoschus esculentus) as a potential dietary medicine with nutraceutical importance for sustainable health applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef]
- Deen, G.R.; Hannan, F.A.; Henari, F.; Akhtar, S. Effects of different parts of the okra plant (Abelmoschus esculentus) on the phytosynthesis of silver nanoparticles: Evaluation of synthesis conditions, nonlinear optical and antibacterial properties. Nanomaterials 2022, 12, 4174. [Google Scholar] [CrossRef]
- Amin, M.; Shah, H.H. Effect of absorption time for the preparation of activated carbon from wasted tree leaves of quercus alba and investigating life cycle assessment. C J. Carbon Res. 2022, 8, 57. [Google Scholar] [CrossRef]
- Öziç, C.; Ertaş, E.; Baran, M.F.; Baran, A.; Ahmadian, E.; Eftekhari, A.; Yıldıztekin, M. Synthesis and characterization of activated carbon-supported magnetic nanocomposite (MNPs-OLAC) obtained from okra leaves as a nanocarrier for targeted delivery of morin hydrate. Front. Pharmacol. 2024, 15, 1482130. [Google Scholar] [CrossRef]
- Baran, A.; Ertaş, E.; Baran, M.F.; Eftekhari, A.; Gunes, Z.; Keskin, C.; Khalilov, R. Green-Synthesized characterization, antioxidant and antibacterial applications of CtAC/MNPs-Ag nanocomposites. Pharmaceuticals 2024, 17, 772. [Google Scholar] [CrossRef]
- Deivayanai, V.C.; Karishma, S.; Thamarai, P.; Saravanan, A.; Yaashikaa, P.R. Artificial neural network modeling of dye adsorption kinetics and thermodynamics with magnetic nanoparticle-activated carbon from Allium cepa peels. Chem. Pap. 2025, 79, 1775–1796. [Google Scholar] [CrossRef]
- Chishti, A.N.; Guo, F.; Aftab, A.; Ma, Z.; Liu, Y.; Chen, M.; Diao, G. Synthesis of silver doped Fe3O4/C nanoparticles and its catalytic activities for the degradation and reduction of methylene blue and 4-nitrophenol. Appl. Surf. Sci. 2021, 546, 149070. [Google Scholar] [CrossRef]
- Atunwa, B.T.; Dada, A.O.; Inyinbor, A.A.; Pal, U. Synthesis, physiochemical and spectroscopic characterization of Palm kernel shell activated carbon doped AgNPs (PKSAC@AgNPs) for adsorption of chloroquine pharma-ceutical waste. Mater. Today Proc. 2022, 65, 3538–3546. [Google Scholar] [CrossRef]
- Moazzen, M.; Khaneghah, A.M.; Shariatifar, N.; Ahmadloo, M.; Eş, I.; Baghani, A.N.; Khaniki, G.J. Multi-walled carbon nanotubes modified with iron oxide and silver nanoparticles (MWCNT-Fe3O4/Ag) as a novel adsorbent for determining PAEs in carbonated soft drinks using magnetic SPE-GC/MS method. Arab. J. Chem. 2019, 12, 476–488. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: A kinetic, equilibrium and thermodynamic study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.; Park, Y.K.; Kim, B.J.; An, K.H.; Kim, S.C.; Jung, S.C. Preparation and characterization of silver-iron bimetallic nanoparticles on activated carbon using plasma in liquid process. Nanomaterials 2021, 11, 3385. [Google Scholar] [CrossRef]
- Islam, A.M.; Mukherjee, M. Effect of temperature in synthesis of silver nanopar-ticles in triblock copolymer micellar solution. J. Exp. Nanosci. 2011, 6, 596–611. [Google Scholar] [CrossRef]
- Li, W.H.; Yue, X.P.; Guo, C.S.; Lv, J.P.; Liu, S.S.; Zhang, Y.; Xu, J. Synthesis and characterization of magnetically recyclable Ag nanoparticles immobilized on Fe3O4@C nanospheres with catalytic activity. Appl. Surf. Sci. 2015, 335, 23–28. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Razeghi, J.; Ramavandi, B. Performance of algal activated carbon/Fe3O4 magnetic composite for cationic dyes removal from aqueous solutions. Algal Res. 2019, 40, 101509. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Zheng, D.F.; Mo, Z.Y.; Dong, R.J.; Qiu, X.Q. Magnetic lignin-based carbon nanoparticles and the adsorption for removal of methyl orange. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 226–234. [Google Scholar] [CrossRef]
- Arain, M.B.; Yilmaz, E.; Hoda, N.; Kazi, T.G.; Soylak, M. Magnetic solid-phase extraction of quercetin on magnetic-activated carbon cloth (MACC). J. Iran. Chem. Soc. 2019, 16, 1365–1372. [Google Scholar] [CrossRef]
- Patil, M.P.; Singh, R.D.; Koli, P.B.; Patil, K.T.; Jagdale, B.S.; Tipare, A.R.; Kim, G.D. Antibacterial potential of silver nanoparticles synthesized using Madhuca Longifolia flower extract as a green resource. Microb. Pathog. 2018, 121, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, C.; Hou, T.; Guan, P.; Qiao, Y.; Guo, L.; Wu, H. Lasting tracking and rapid discrimination of live gram-positive bacteria by peptidoglycan-targeting carbon quantum dots. ACS Appl. Mater. Interfaces 2021, 13, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Felgueiras, H.P. Activity of specialized biomolecules against gram- positive and gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Kobylka, J.; Kuth, M.S.; Pos, K.M.; Picard, M.; Blair, J.M.; Bavro, V.N. Structure, assembly, and function of tripartite efflux and type 1 secretion systems in gram-negative bacteria. Chem. Rev. 2021, 121, 5479–5596. [Google Scholar] [CrossRef]
- Adeniji, O.O.; Ojemaye, M.O.; Okoh, A.I. Antibacterial activity of metallic nanoparticles against multidrug-resistant pathogens isolated from environmental samples: Nanoparticles/antibiotic combination therapy and cytotoxicity study. ACS Appl. Bio Mater. 2022, 5, 4814–4826. [Google Scholar] [CrossRef]
- Castrillon, E.D.C.; Correa, S.; Ávila-Torres, Y.P. Impact of Magnetic Field on ROS Generation in Cu-g-C3N4 Against E. coli Disinfection Process. Magnetochemistry 2025, 11, 28. [Google Scholar] [CrossRef]



| Microorganism | Dilution Method (µg/mL) | Disc Method Zone of Inhibition (mm) | ||||
|---|---|---|---|---|---|---|
| Antibiotic | MIONPs/HEAC | MIONPs/HEAC@Ag | Control Disc | |||
| Deionized water | MIONPs/HEAC | MIONPs/HEAC@Ag | ||||
| E. coli | 2.0 | 96 | 3.0 | 0.0 | 0.0 | 11.50 |
| S. aureus | 1.0 | 48 | 1.5 | 0.0 | 0.0 | 13.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güneş, M.; Ertaş, E.; Tumur, S.; Zulfugarova, P.; Nuriyeva, F.; Kavetskyy, T.; Kukhazh, Y.; Grozdov, P.; Šauša, O.; Smutok, O.; et al. Synthesis and Antibacterial Evaluation of Silver-Coated Magnetic Iron Oxide/Activated Carbon Nanoparticles Derived from Hibiscus esculentus. Magnetochemistry 2025, 11, 53. https://doi.org/10.3390/magnetochemistry11070053
Güneş M, Ertaş E, Tumur S, Zulfugarova P, Nuriyeva F, Kavetskyy T, Kukhazh Y, Grozdov P, Šauša O, Smutok O, et al. Synthesis and Antibacterial Evaluation of Silver-Coated Magnetic Iron Oxide/Activated Carbon Nanoparticles Derived from Hibiscus esculentus. Magnetochemistry. 2025; 11(7):53. https://doi.org/10.3390/magnetochemistry11070053
Chicago/Turabian StyleGüneş, Müslüm, Erdal Ertaş, Seyhmus Tumur, Parvin Zulfugarova, Fidan Nuriyeva, Taras Kavetskyy, Yuliia Kukhazh, Pavlo Grozdov, Ondrej Šauša, Oleh Smutok, and et al. 2025. "Synthesis and Antibacterial Evaluation of Silver-Coated Magnetic Iron Oxide/Activated Carbon Nanoparticles Derived from Hibiscus esculentus" Magnetochemistry 11, no. 7: 53. https://doi.org/10.3390/magnetochemistry11070053
APA StyleGüneş, M., Ertaş, E., Tumur, S., Zulfugarova, P., Nuriyeva, F., Kavetskyy, T., Kukhazh, Y., Grozdov, P., Šauša, O., Smutok, O., Ganbarov, D., & Kiv, A. (2025). Synthesis and Antibacterial Evaluation of Silver-Coated Magnetic Iron Oxide/Activated Carbon Nanoparticles Derived from Hibiscus esculentus. Magnetochemistry, 11(7), 53. https://doi.org/10.3390/magnetochemistry11070053







