Effect of Magnetic Field on Electrochemical Corrosion Behavior of H62 Brass Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Experiments
2.2. Surface Characterization and Composition Analysis
3. Results
3.1. Electrochemical Analysis
3.1.1. Potentiodynamic Polarization Curve Analysis
3.1.2. EIS Analysis
3.2. Characterization
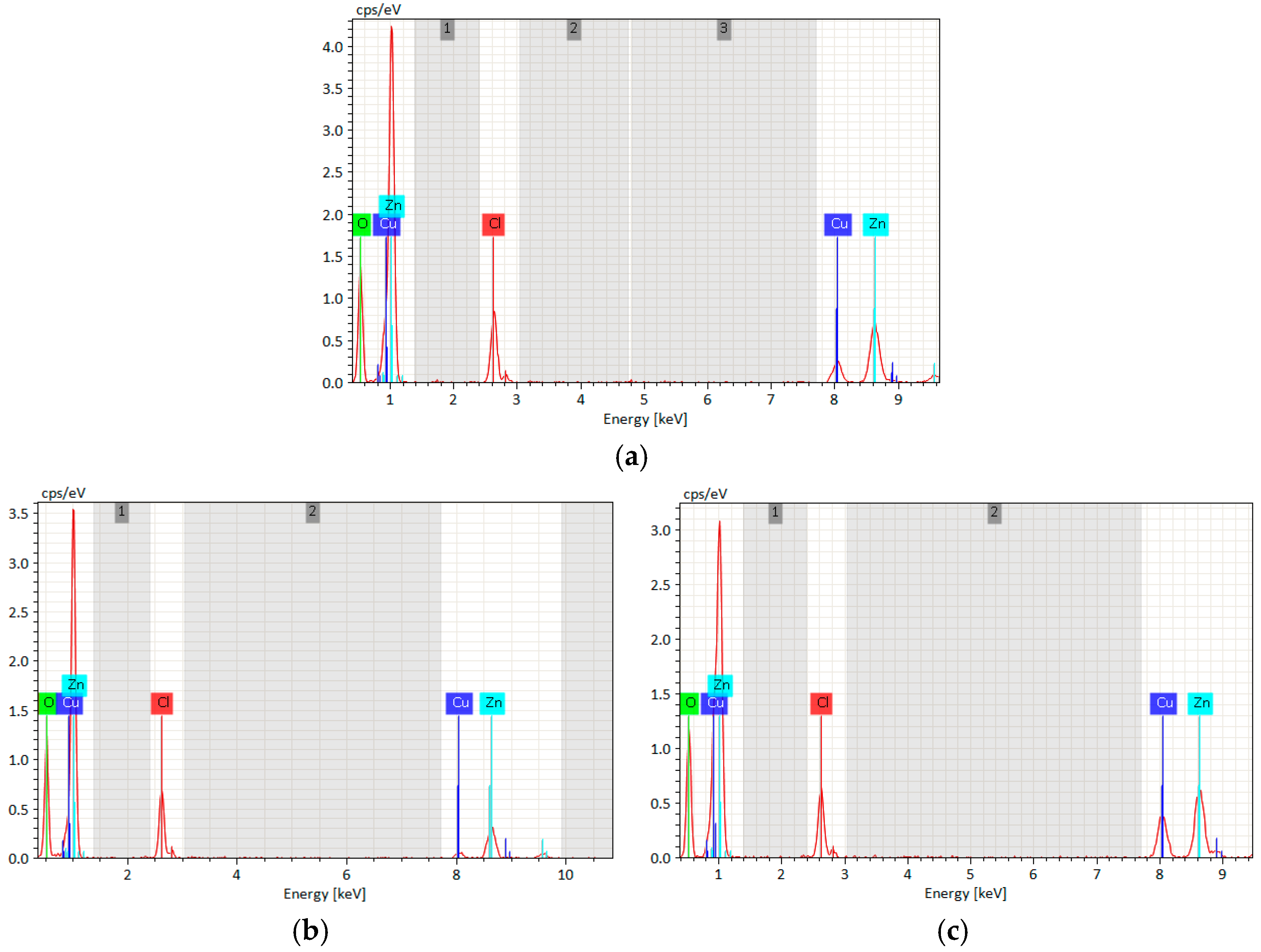
3.2.1. Low-Magnification SEM Corrosion Morphology and EDS Analysis
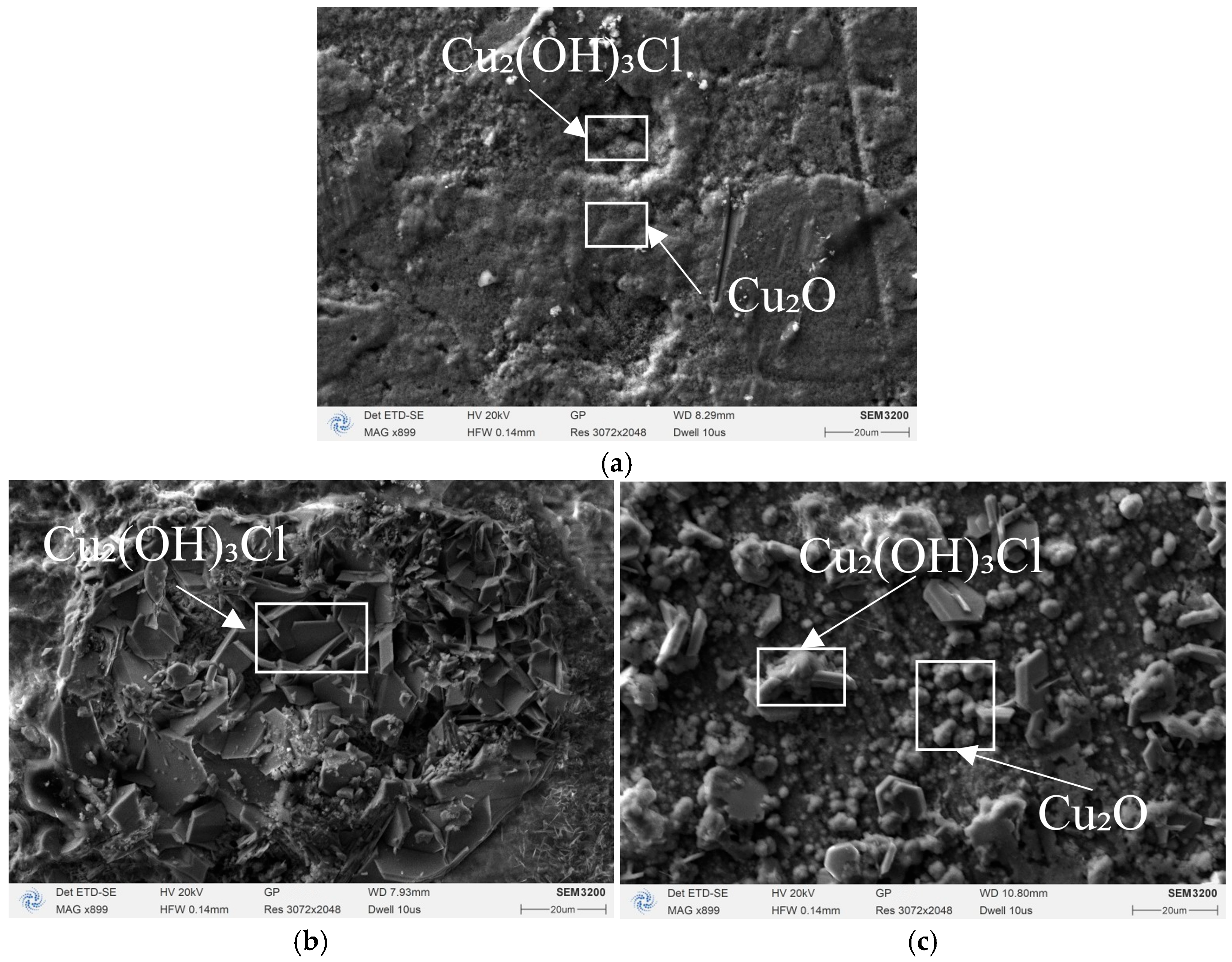
3.2.2. High-Magnification SEM Corrosion Morphology and Raman Spectroscopy Analysis
4. Discussion
5. Conclusions
- (1)
- Potentiodynamic polarization tests demonstrate that magnetic fields significantly accelerate H62 brass corrosion. Under 100 mT parallel magnetic field, corrosion current density increases from 0.49 μA/cm2 to 3.66 μA/cm2 (7.5-fold increase); under perpendicular magnetic field, it increases to 1.73 μA/cm2 (3.5-fold increase).
- (2)
- Electrochemical impedance spectroscopy analysis reveals that magnetic fields accelerate corrosion kinetics by reducing charge transfer resistance (from 3382 Ω·cm2 to 1335 Ω·cm2) and increasing double layer capacitance (from 64.9 μF·cm−2 to 176.0 μF·cm−2).
- (3)
- SEM and EDS analysis show that parallel magnetic fields promote uniform corrosion product distribution with Cl content reaching 6.22%; perpendicular magnetic fields result in “hill-like” non-uniform corrosion morphology with localized corrosion tendencies and lower Cl content (1.93%) but non-uniform distribution.
- (4)
- Raman spectroscopy confirms that magnetic fields promote the transformation of protective Cu2O films into porous Cu2(OH)3Cl, reducing the protective capability of corrosion product layers. Under parallel magnetic field, the Cu2(OH)3Cl characteristic peak (815 cm−1) intensity significantly increases while the Cu2O characteristic peak (218 cm−1) weakens.
- (5)
- Magnetic field orientation determines fundamental differences in corrosion acceleration mechanisms: parallel magnetic fields enhance mass transfer processes through Lorentz force-driven magnetohydrodynamic effects, resulting in intensified uniform corrosion; perpendicular magnetic fields alter interfacial ion distribution through magnetic gradient forces, inducing localized corrosion tendencies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MHD | magnetohydrodynamic |
| EIS | Electrochemical Impedance Spectroscopy |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive Spectroscopy |
| SCE | Saturated Calomel Electrode |
| OCP | Open Circuit Potential |
References
- Gradl, P.; Tinker, D.C.; Park, A.; Mireles, O.R.; Garcia, M.; Wilkerson, R.; Mckinney, C. Robust metal additive manufacturing process selection and development for aerospace components. J. Mater. Eng. Perform. 2022, 31, 6013–6044. [Google Scholar] [CrossRef]
- Mao, Q.; Liu, Y.; Zhao, Y. A review on copper alloys with high strength and high electrical conductivity. J. Alloys Compd. 2024, 990, 174456. [Google Scholar] [CrossRef]
- Wheeler, P. Technology for the more and all electric aircraft of the future. In Proceedings of the 2016 IEEE International Conference on Automatica (ICA-ACCA), Curico, Chile, 19–21 October 2016. [Google Scholar]
- Sarlioglu, B.; Morris, C.T. More electric aircraft: Review, challenges, and opportunities for commercial transport aircraft. IEEE Trans. Transp. Electrif. 2015, 1, 54–64. [Google Scholar] [CrossRef]
- Madonna, V.; Giangrande, P.; Galea, M. Electrical power generation in aircraft: Review, challenges, and opportunities. IEEE Trans. Transp. Electrif. 2018, 4, 646–659. [Google Scholar] [CrossRef]
- Kant, R.; Singh, H.; Nayak, M.; Bhattacharya, S. Corrosion monitoring and control in aircraft: A review. Sens. Automot. Aerosp. Appl. 2018, 39–53. [Google Scholar]
- Wang, Y.; Wang, G.; Xie, F.; Wu, M.; Zhou, Y.; Liu, F.; Cheng, L.; Du, M. Corrosion mechanism and research progress of metal pipeline corrosion under magnetic field and SRB conditions: A review. Corros. Rev. 2024, 42, 203–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Sun, X.; Li, M.; Wang, H.; Chen, X. Effect of gradient magnetic field on corrosion of carbon steel pipes in seawater pumped storage power plants. J. Appl. Electrochem. 2023, 53, 597–608. [Google Scholar] [CrossRef]
- Zhao, S.; You, Z.; Zhang, X.; Li, J. Magnetic field effects on the corrosion and electrochemical corrosion of Fe83Ga17 alloy. Mater. Charact. 2021, 174, 110994. [Google Scholar] [CrossRef]
- Liu, W.; Liang, L.; Li, Q.; Dong, Z.; Xiao, K. Insight into the acceleration effect of current-carrying condition on copper conductor atmospheric corrosion. Mater. Chem. Phys. 2024, 316, 129114. [Google Scholar] [CrossRef]
- Salinas, G.; Lozon, C.; Kuhn, A. Unconventional applications of the magnetohydrodynamic effect in electrochemical systems. Curr. Opin. Electrochem. 2023, 38, 101220. [Google Scholar] [CrossRef]
- Gregory, T.S.; Williams, K.; Ray, P.; Bauer, M. The magnetohydrodynamic effect and its associated material designs for biomedical applications: A state-of-the-art review. Adv. Funct. Mater. 2016, 26, 3942–3952. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Wang, S.; Zhang, T. Magnetic regulation of biomineralized film self-assembly on high manganese steel: Impacts on corrosion protection and underlying mechanisms. Corros. Sci. 2025, 113204. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Q.; Guo, R.; Li, Y. The influence of calcium on copper corrosion and its by-product release in drinking water. RSC Adv. 2023, 13, 19421–19431. [Google Scholar] [CrossRef] [PubMed]
- Arabul, A.Y.; Senol, S.; Oner, A.; Yilmaz, A. Perspectives and development of electrical systems in more electric aircraft. Int. J. Aerosp. Eng. 2021, 2021, 5519842. [Google Scholar] [CrossRef]
- GB/T 5231-2012; Wrought Copper and Copper Alloys—Chemical Composition and Product Forms. Standardization Administration of China: Beijing, China, 2012.
- Wang, D.; Zhang, L.; Li, H.; Chen, Y. Effect of magnetic field on the electrochemical corrosion behavior of X80 pipeline steel. Constr. Build. Mater. 2022, 350, 128897. [Google Scholar] [CrossRef]
- Waskaas, M.; Kharkats, Y.I. Effect of magnetic fields on convection in solutions containing paramagnetic ions. J. Electroanal. Chem. 2001, 502, 51–57. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Wang, X.; Zhang, M. The effect of magnetic field pretreatment on the corrosion behavior of carbon steel in static seawater. RSC Adv. 2020, 10, 2060–2066. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Zhang, W.; Li, Y. Electrochemical migration behavior of moldy printed circuit boards in a 10 mT magnetic field. RSC Adv. 2021, 11, 28178–28188. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Dong, C.; Li, X.; Xiao, K. Effects of applied magnetic field on corrosion of beryllium copper in NaCl solution. J. Mater. Sci. Technol. 2010, 26, 355–361. [Google Scholar] [CrossRef]
- Sueptitz, R.; Koza, J.; Uhlemann, M.; Gebert, A.; Schultz, L. Impact of magnetic field gradients on the free corrosion of iron. Electrochim. Acta 2010, 55, 5200–5203. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, Q.; Liu, H. Corrosion of X80 steel in a wet gas pipeline under the top-of-the-line environment. J. Electroanal. Chem. 2022, 912, 116269. [Google Scholar] [CrossRef]
- Frost, R.L. Raman spectroscopy of selected copper minerals of significance in corrosion. Spectrochim. Acta Part A 2003, 59, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.; Frost, R.; Williams, P. Raman and infrared spectroscopic study of the basic copper chloride minerals-implications for the study of the copper and brass corrosion and bronze disease. Neues Jahrb. Mineral. Abh. 2003, 178, 197–215. [Google Scholar] [CrossRef]
- He, X.; Song, R.G.; Kong, D.J. Microstructure and corrosion behaviour of laser-cladding Al-Ni-TiC-CeO2 composite coatings on S355 offshore steel. J. Alloys Compd. 2019, 770, 771–783. [Google Scholar] [CrossRef]
- Hayez, V.; Costa, V.; Guillaume, J.; Terryn, H.; Hubin, A. Micro Raman spectroscopy used for the study of corrosion products on copper alloys: Study of the chemical composition of artificial patinas used for restoration purposes. Analyst 2005, 130, 550–556. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Tromans, D.; Silva, J.C. The anodic behavior of copper in chloride solutions. Corrosion 1997, 53, 171–182. [Google Scholar] [CrossRef]
- Dunne, P.; Mazza, L.; Coey, J.M.D. Magnetic structuring of electrodeposits. Phys. Rev. Lett. 2011, 107, 024501. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Rhen, F.M.F.; Dunne, P.; McMurry, S. The magnetic concentration gradient force—Is it real? J. Solid State Electrochem. 2007, 11, 711–717. [Google Scholar] [CrossRef]
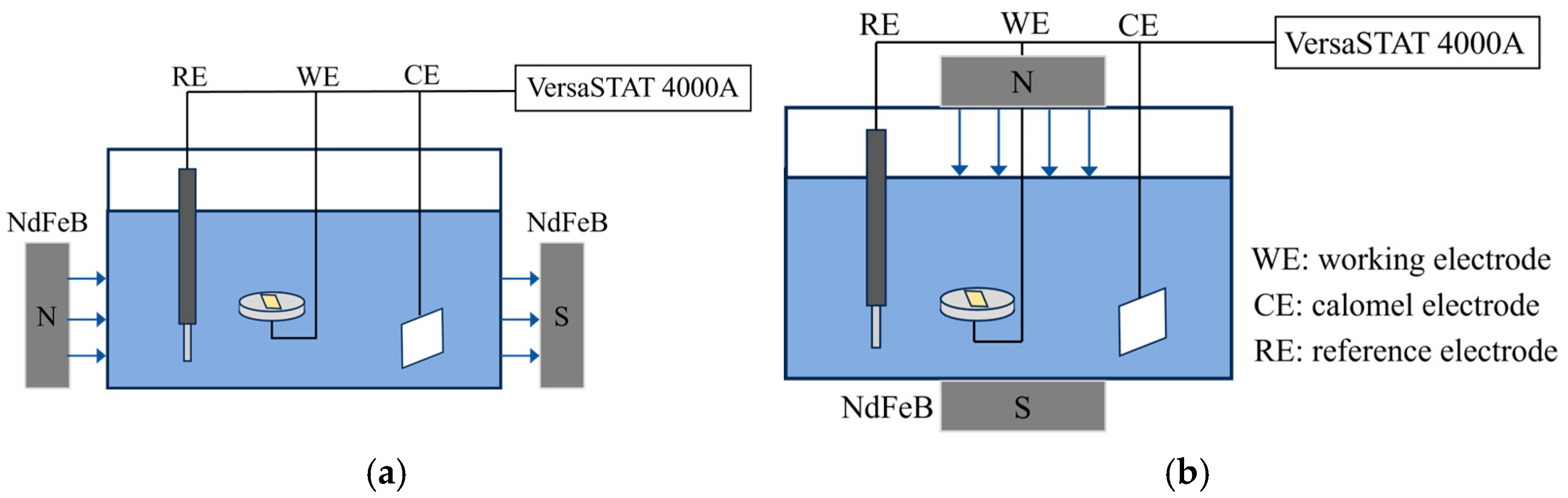
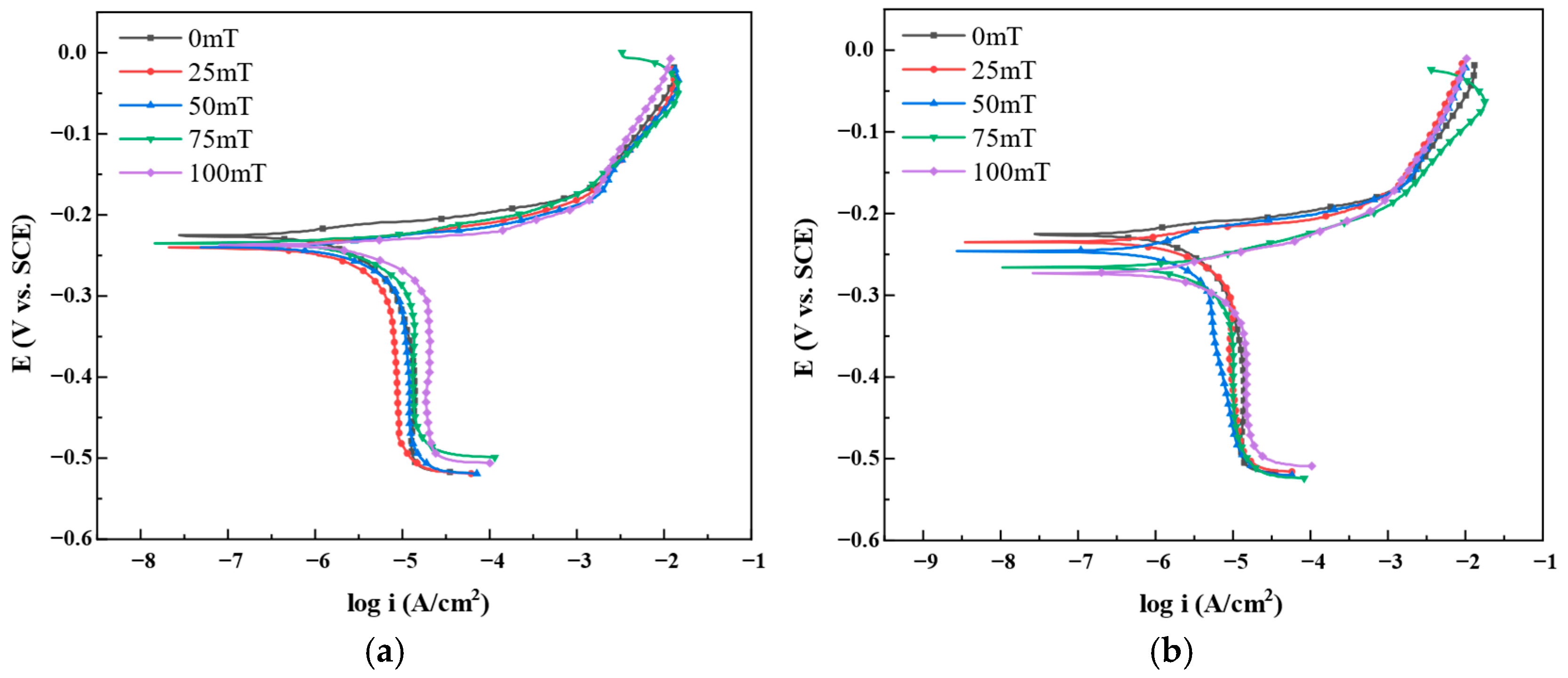
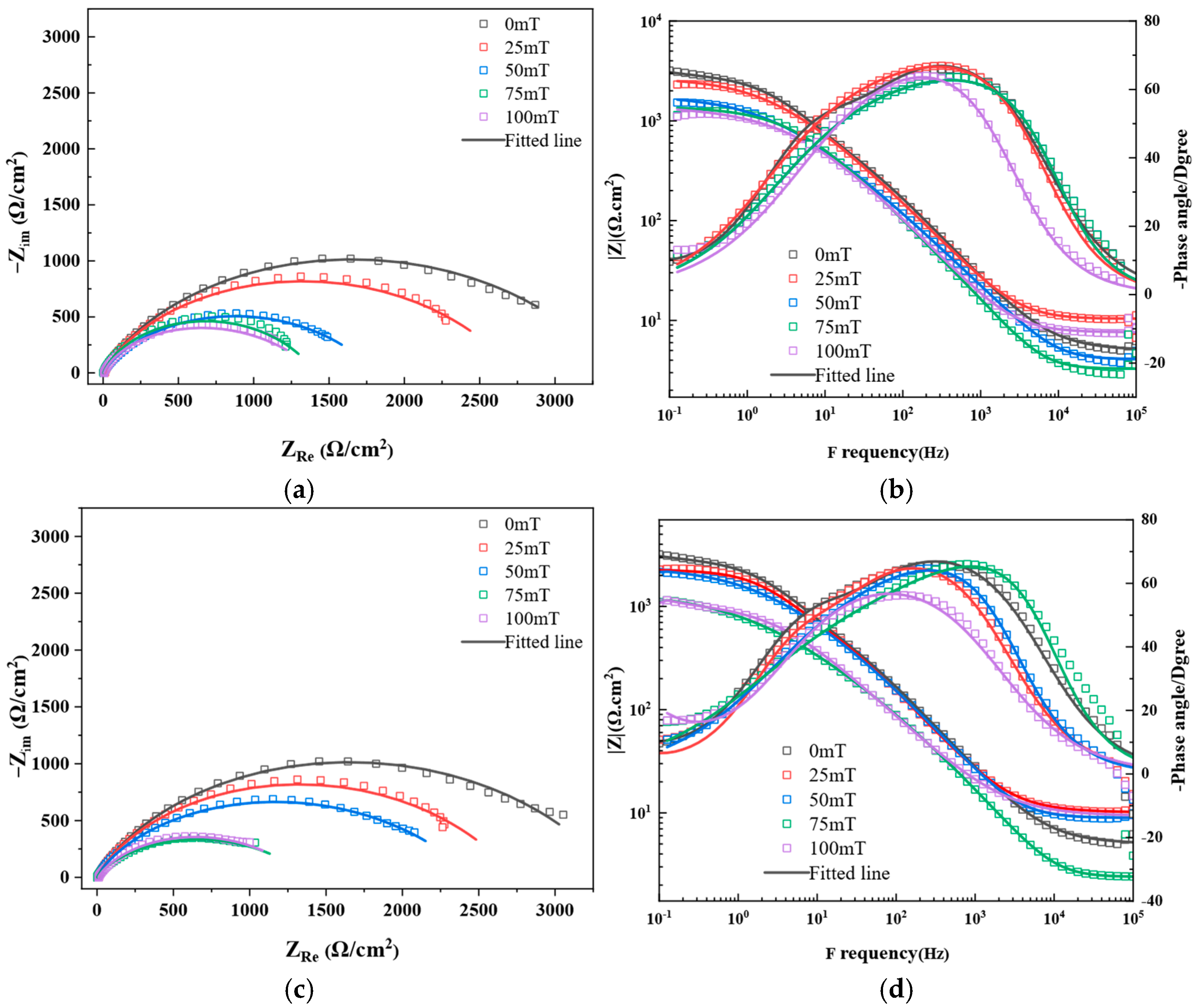


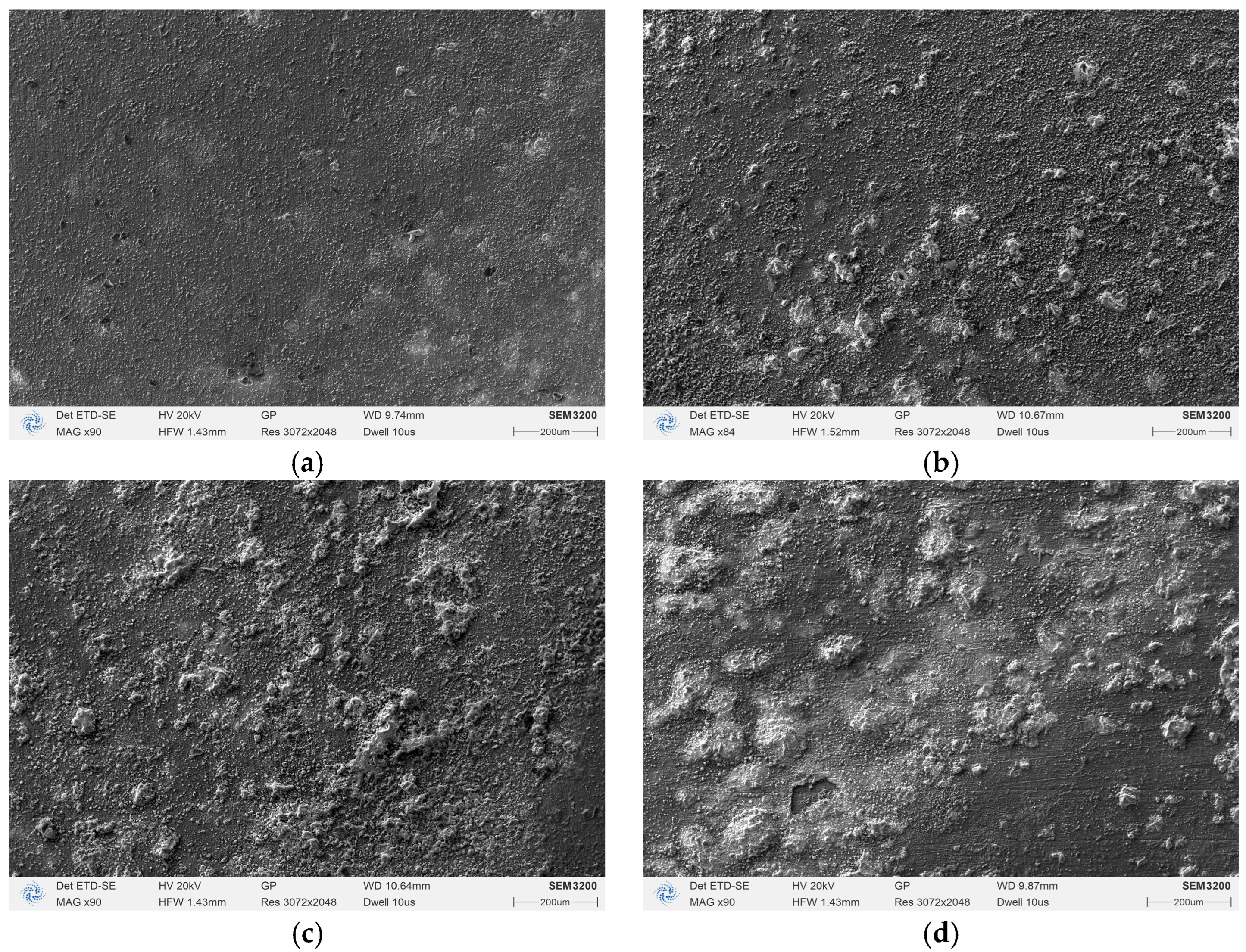


| B (mT) | Ecorr (mV) | Icorr (μA/cm2) | βc (mV/dec) | βa (mV/dec) | CR (mm/Year) | |
|---|---|---|---|---|---|---|
| 0 | −223.21 | 0.49 | 36.41 | 11.44 | 0.060 | |
| Parallel | 25 | −231.64 | 1.06 | 57.96 | 15.15 | 0.130 |
| Perpendicular | 50 | −238.82 | 1.60 | 63.46 | 13.40 | 0.196 |
| 75 | −236.51 | 2.78 | 83.03 | 24.26 | 0.341 | |
| 100 | −238.37 | 3.66 | 78.55 | 13.68 | 0.449 | |
| 25 | −230.20 | 0.83 | 49.65 | 10.64 | 0.102 | |
| 50 | −244.86 | 1.21 | 74.86 | 33.72 | 0.148 | |
| 75 | −263.47 | 1.66 | 63.00 | 20.51 | 0.204 | |
| 100 | −270.96 | 1.73 | 53.40 | 28.18 | 0.212 | |
| B (mT) | Rs (Ω·cm2) | Cf (μF·cm−2) | nf | Rf (Ω·cm2) | Cdl (μF·cm−2) | ndl | Rct (Ω·cm2) | |
|---|---|---|---|---|---|---|---|---|
| 0 | 5.34 | 4.046 | 0.964 | 18.63 | 64.9 | 0.645 | 3382 | |
| Parallel | 25 | 5.032 | 4.706 | 0.885 | 7.103 | 74.18 | 0.641 | 2992 |
| Perpendicular | 50 | 4.094 | 5.15 | 0.857 | 14.18 | 115.5 | 0.615 | 1796 |
| 75 | 3.278 | 8.754 | 0.961 | 31.96 | 76.95 | 0.664 | 1377 | |
| 100 | 7.601 | 17.58 | 0.925 | 27.04 | 119.7 | 0.538 | 1396 | |
| 25 | 10.41 | 4.778 | 0.834 | 10.89 | 69.26 | 0.637 | 2726 | |
| 50 | 8.972 | 4.875 | 0.964 | 4.778 | 93.75 | 0.581 | 2454 | |
| 75 | 2.402 | 7.363 | 0.834 | 11.08 | 234.5 | 0.545 | 1370 | |
| 100 | 9.8 | 22.18 | 0.891 | 20.44 | 176 | 0.541 | 1335 | |
| B (mT) | Cu | Zn | O | Cl | |
|---|---|---|---|---|---|
| 0 | 38.42 | 31.08 | 29.83 | 0.67 | |
| Parallel | 25 | 13.65 | 29.22 | 52.56 | 4.57 |
| Perpendicular | 50 | 9.24 | 25.85 | 59.33 | 5.58 |
| 75 | 8.38 | 22.39 | 63.32 | 5.91 | |
| 100 | 7.79 | 18.79 | 67.20 | 6.22 | |
| 25 | 29.17 | 26.30 | 42.83 | 1.70 | |
| 50 | 23.90 | 20.27 | 53.02 | 2.81 | |
| 75 | 27.17 | 10.87 | 60.04 | 1.92 | |
| 100 | 13.58 | 22.23 | 62.26 | 1.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Yu, D.; Zhao, H.; Gao, A.; Li, Y.; Qi, J. Effect of Magnetic Field on Electrochemical Corrosion Behavior of H62 Brass Alloy. Magnetochemistry 2025, 11, 92. https://doi.org/10.3390/magnetochemistry11110092
Huang H, Yu D, Zhao H, Gao A, Li Y, Qi J. Effect of Magnetic Field on Electrochemical Corrosion Behavior of H62 Brass Alloy. Magnetochemistry. 2025; 11(11):92. https://doi.org/10.3390/magnetochemistry11110092
Chicago/Turabian StyleHuang, Hexiang, Dazhao Yu, Hongjun Zhao, Aiguo Gao, Yanan Li, and Jiantao Qi. 2025. "Effect of Magnetic Field on Electrochemical Corrosion Behavior of H62 Brass Alloy" Magnetochemistry 11, no. 11: 92. https://doi.org/10.3390/magnetochemistry11110092
APA StyleHuang, H., Yu, D., Zhao, H., Gao, A., Li, Y., & Qi, J. (2025). Effect of Magnetic Field on Electrochemical Corrosion Behavior of H62 Brass Alloy. Magnetochemistry, 11(11), 92. https://doi.org/10.3390/magnetochemistry11110092







