pH and Magnetic-Responsive Carboxymethyl Chitosan/Sodium Alginate Composites for Gallic Acid Delivery
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
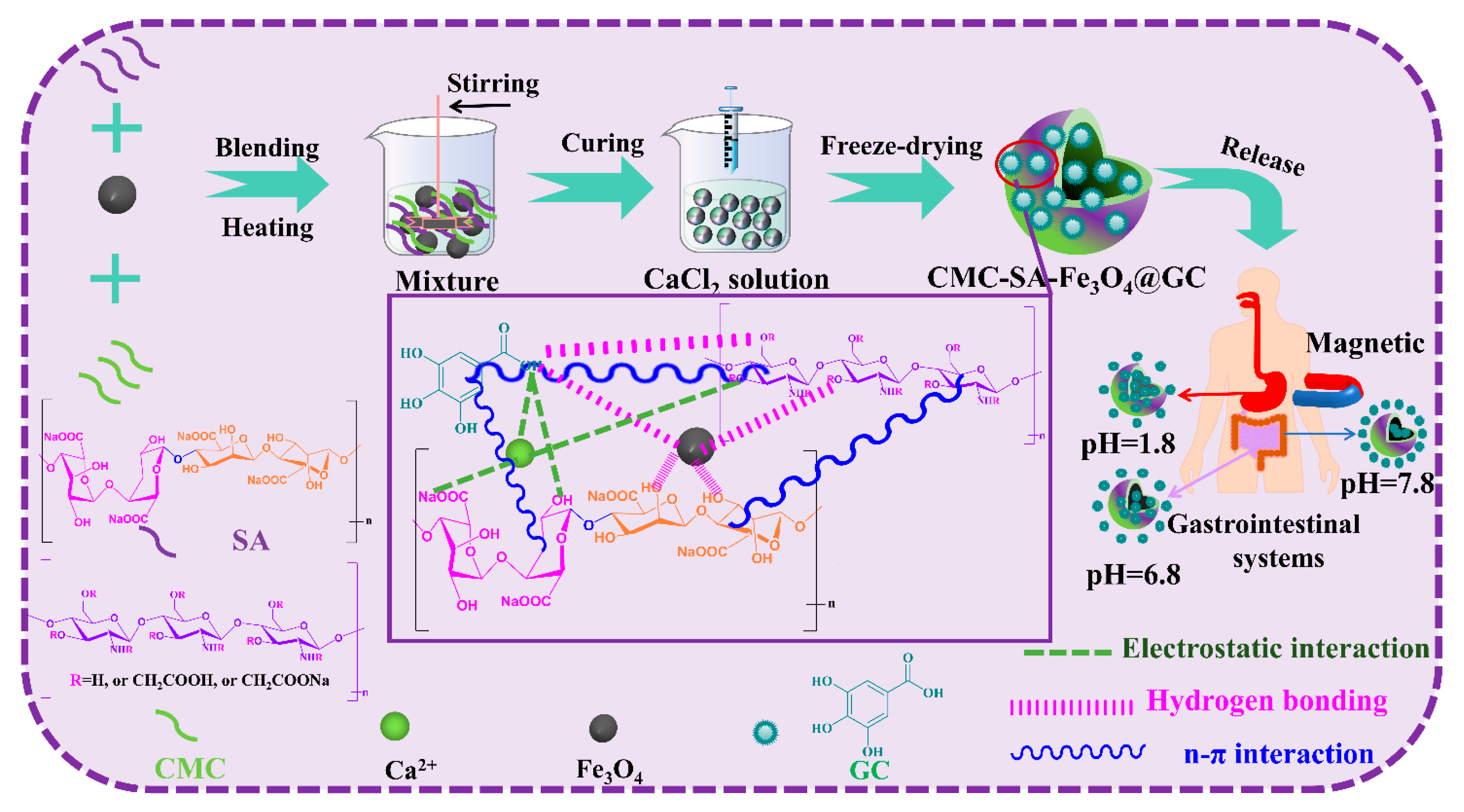
2.2. Construction of CMC-SA-Fe3O4@GA Hydrogel Beads
2.3. Characterizations
2.4. Determination of GA Loading
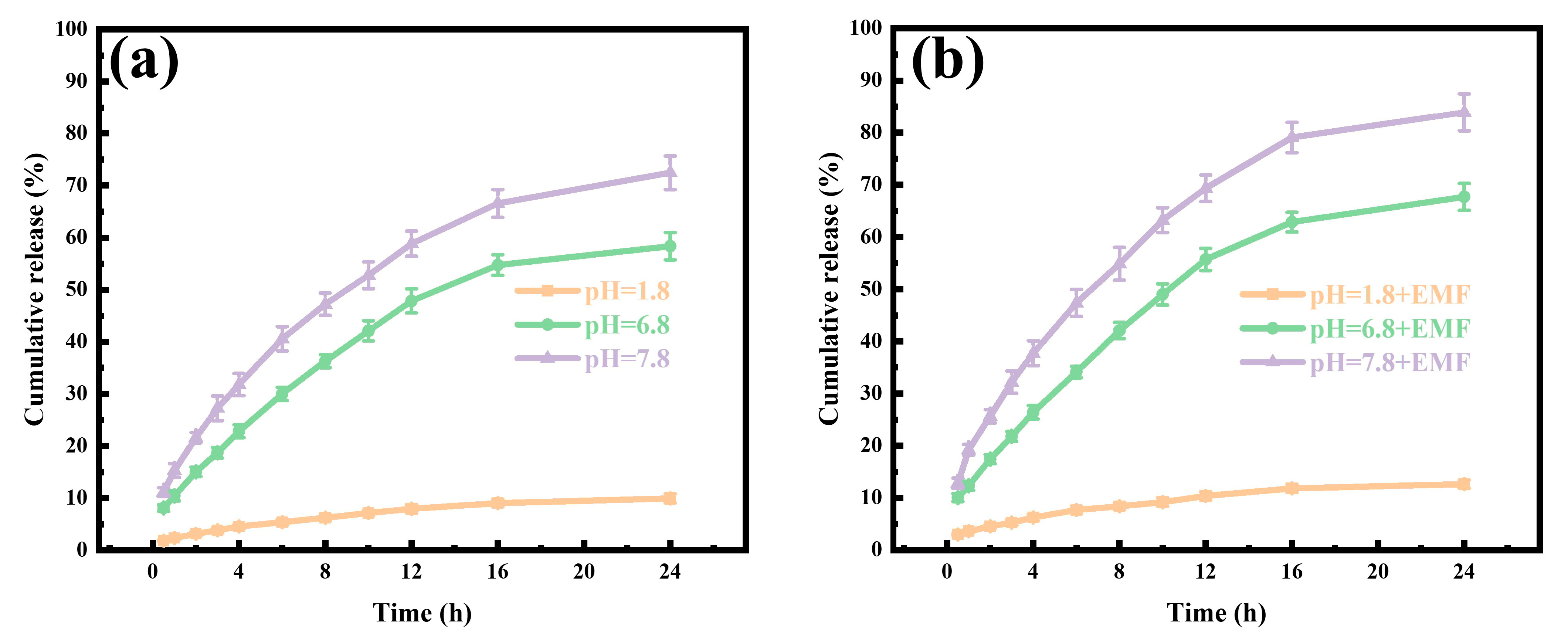
2.5. GA Release Under pH and Magnetic Response
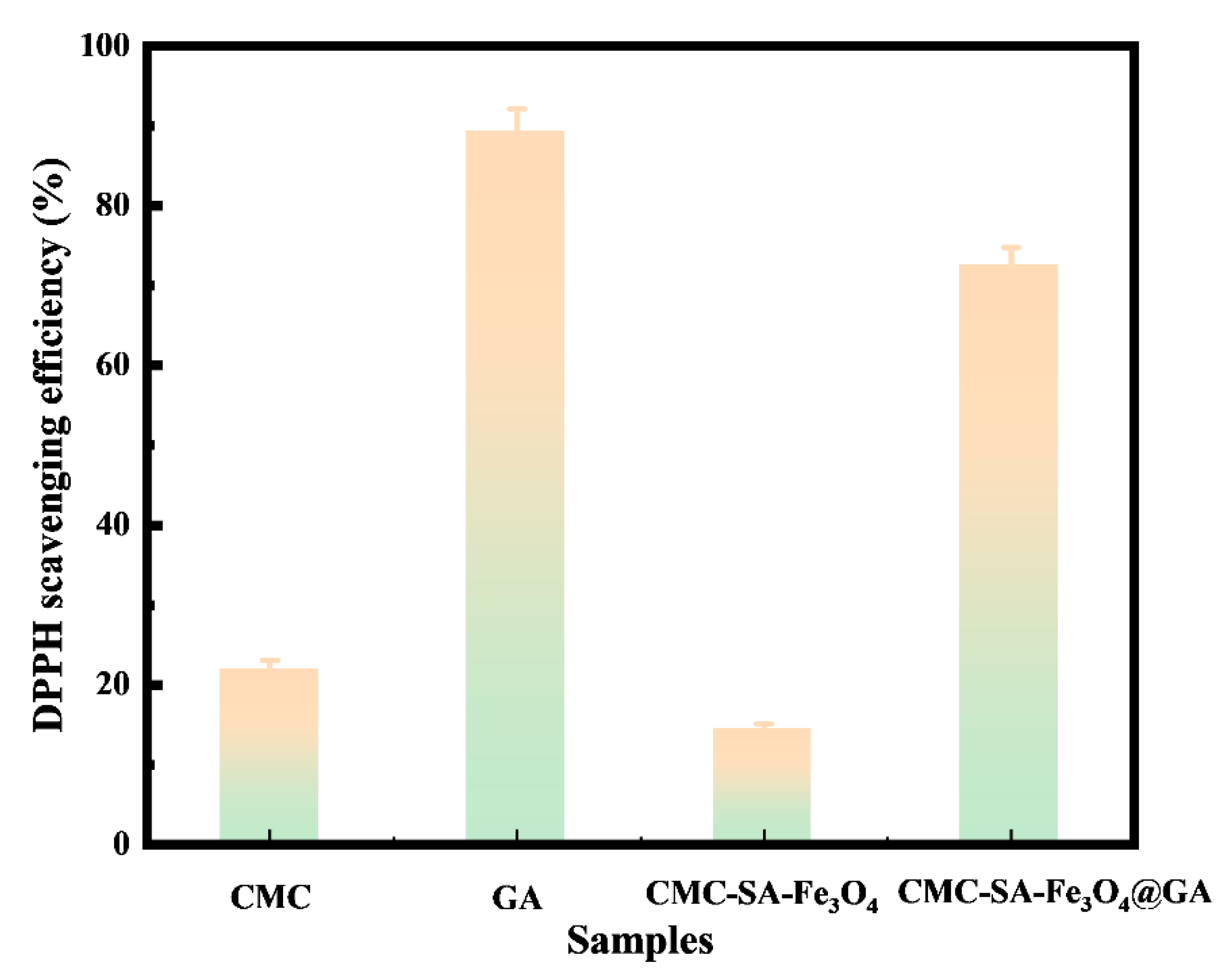
2.6. DPPH Free Radical Scavenging Activity
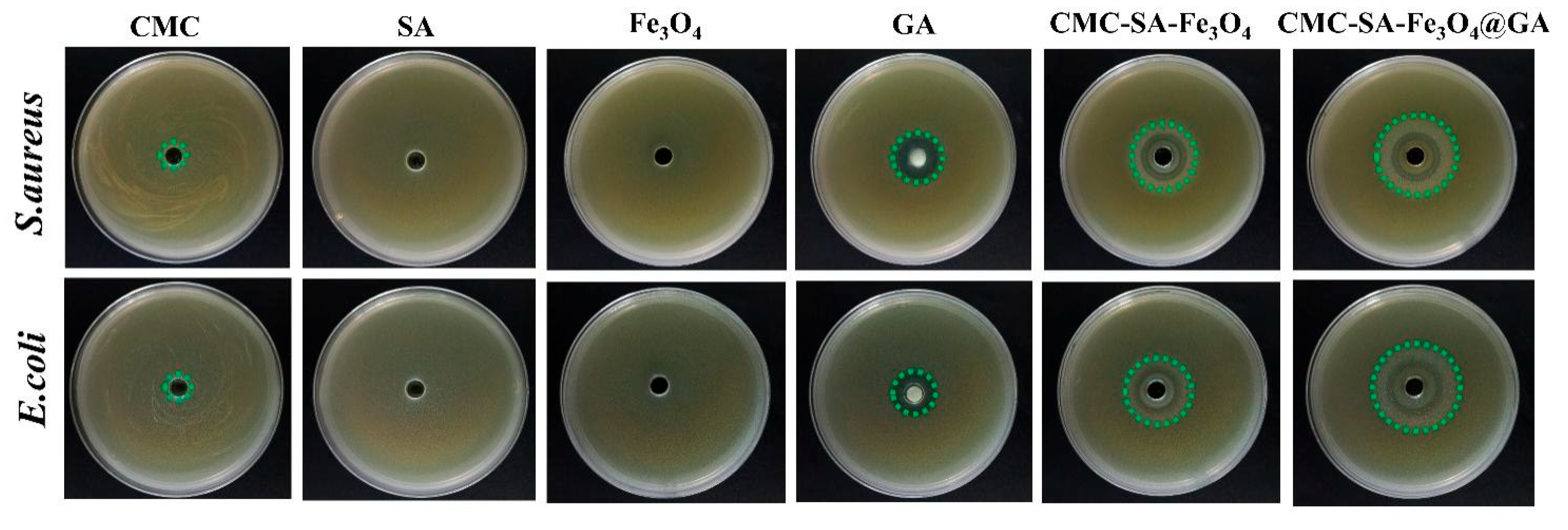
2.7. Antibacterial Activity
2.8. Cell Cytocompatibility and Toxicity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Construction of CMC-SA-Fe3O4@GA Hydrogel Beads Analysis
3.2. Characterizations Analysis
3.3. GA Loading and Release Studies
3.4. GA Release Kinetics Analysis
3.5. Antioxidant Activity Test Analysis
3.6. Antibacterial Activity Analysis
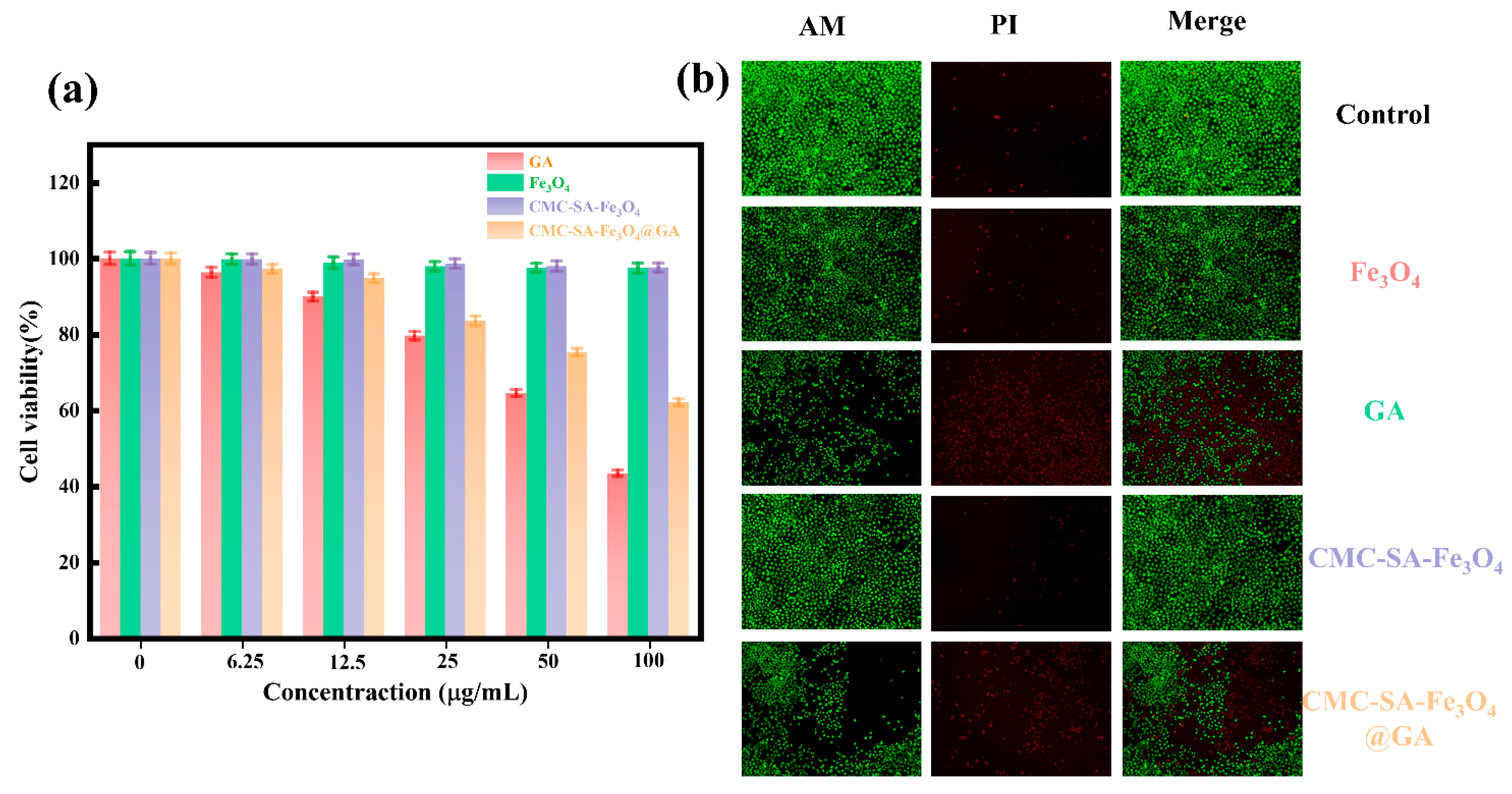
3.7. Cell Cytocompatibility and Toxicity Test Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Karthammaiah, G.N.; Venkataramanan, N.; Solomon, K.A. Synthesis, characterization, and computational studies of a gallic acid-nicotinic acid salt hydrate. J. Mol. Struct. 2025, 1326, 141119. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Li, Y.; Zhou, Z.; Wei, B.; Dong, T.; Zhao, Y.; Ye, C.; Li, J.; Cui, J. Sea buckthorn leaves and gallic acid inhibit C48/8o-induced pseudo-allergic reaction via the PLC/IP3 signaling pathway both in vitro and in vivo. Int. Immunopharmacol. 2025, 154, 114563. [Google Scholar] [CrossRef]
- Alhyari, D.; Qinna, N.A.; Sheldrake, H.M.; Kantamneni, S.; Ghanem, B.Y.; Paluch, K.J. Antioxidant, Anti-inflammatory, and oral bioavailability of novel sulfonamide derivatives of gallic acid. Antioxidants 2025, 14, 374. [Google Scholar] [CrossRef]
- Liu, K.; Luo, B.; Zhang, L.; Hou, P.; Pan, D.; Liu, T.; Zhao, C.; Li, A. Flexible and wearable sensor for in situ monitoring of gallic acid in plant leaves. Food Chem. 2024, 460, 140740. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, Q.; Yang, Y.; Li, K.; Pang, N.; Wang, J.; Yang, M.; Li, X.; Guo, J.; Liao, X. Self-assembled gallic acid-rare earth nanocomplexes against MRSA with multi-targeting antibacterial mechanisms robustly combating bacterial resistance. Chem. Eng. J. 2025, 510, 161698. [Google Scholar] [CrossRef]
- Holghoomi, R.; Kiani, M.H.; Rahdar, A.; Hashemi, S.M.; Ferreira, L.F.R.; Fathi-karkan, S. Nanoparticle-delivered gallic acid: A new frontier in cancer therapy. J. Drug Deliv. Sci. Tec. 2024, 101, 106129. [Google Scholar] [CrossRef]
- Zhang, K.q.; Lin, L.l.; Xu, H.j. Research on antioxidant performance of diglucosyl gallic acid and its application in emulsion cosmetics. Int. J. Cosmetic Sci. 2022, 44, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Sowndarya, J.; Rubini, D.; Sinsinwar, S.; Senthilkumar, M.; Nithyanand, P.; Vadivel, V. Gallic acid an agricultural byproduct modulates the biofilm matrix exopolysaccharides of the phytopathogen Ralstonia solanacearum. Curr. Microbiol. 2020, 77, 3339–3354. [Google Scholar] [CrossRef]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.; Duan, B.; Huang, L. Gallic acid: A potential anti-cancer agent. Chin. J. Integr. Med. 2022, 28, 661–671. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, B.; Fan, J.; Yuan, Q.; He, F.; Liang, H.; Chen, F.; Liu, W. Gallic acid regulates primary root elongation via modulating auxin transport and signal transduction. Front. Plant Sci. 2024, 15, 1464053. [Google Scholar] [CrossRef]
- Guo, J.; Ren, X.; Lu, L.; An, N.; Li, S.; Geng, M.; Li, G.; Shen, X.; Sun, X.; Wang, J. Microbial synthesis of gallic acid and its glucoside β-glucogallin. Biotechnol. Bioeng. 2024, 121, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Zahrani, N.A.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Majumder, S. Starch, gallic acid, their inclusion complex and their effects in diabetes and other diseases—A review. Food Sci. Nutr. 2023, 11, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Vincken, J.-P.; van Zadelhoff, A.; Hilgers, R.; Lin, Z.; de Bruijn, W.J. Presence of free gallic acid and gallate moieties reduces auto-oxidative browning of epicatechin (EC) and epicatechin gallate (ECg). Food Chem. 2023, 425, 136446. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Deshmukh, R.; Shukla, V.P.; Khunt, D.; Prajapati, B.G.; Rashid, S.; Ali, N.; Elossaily, G.M.; Suryawanshi, V.K.; Kumar, A. Recent advancements in gallic acid-based drug delivery: Applications, clinical trials, and future directions. Pharmaceutics 2024, 16, 1202. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Q.; Yan, S.; Qi, B. Hydrogel formed in situ through Schiff base reaction between gallic acid-grafted soybean protein isolate and oxidized dextran: Interactions, physicochemical properties, and digestive characteristics. Food Chem. 2025, 471, 142783. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Z.-R.; Du, S.; Chen, S.; Zhang, L.; Zhu, J. Polyphenol-sodium alginate supramolecular injectable hydrogel with antibacterial and anti-inflammatory capabilities for infected wound healing. Int. J. Biol. Macromol. 2024, 257, 128636. [Google Scholar] [CrossRef]
- Bahtiyar, C.; Cakir, N.T.; Kahveci, M.U.; Acik, G.; Altinkok, C. Fabrication of gallic acid containing poly(vinyl alcohol)/chitosan electrospun nanofibers with antioxidant and drug delivery properties. Int. J. Biol. Macromol. 2024, 281, 136055. [Google Scholar] [CrossRef]
- Graham, W.; Torbett-Dougherty, M.; Islam, A.; Soleimani, S.; Bruce-Tagoe, T.A.; Johnson, J.A. Magnetic Nanoparticles and Drug Delivery Systems for Anti-Cancer Applications: A Review. Nanomaterials 2025, 15, 285. [Google Scholar] [CrossRef]
- Fang, K.; Li, P.; Huang, X.; Wang, H.; Li, Y.; Zhu, D.; Luo, B. Recent advancements in magnetic starch-based composites for biomedical applications: A review. Carbohyd. Polym. 2025, 362, 123689. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Hui, D.; Hong, R. Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging. Nanotechnol. Rev. 2020, 9, 1265–1283. [Google Scholar] [CrossRef]
- Lesiak-Orłowska, B.; Rangam, N.V.; Sahu, N.K.; Jiricek, P.; Houdkova, J.; Atrei, A. Surface chemical and electronic properties of functionalized Fe3O4 nanoparticles influencing their cytotoxicity. Appl. Surf. Sci. 2025, 684, 161873. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Yang, T.; Feng, Y.; Yang, B.; Zhao, J.; Feng, Y. One stone two birds: Simultaneous control of eutrophication and microplastic pollution via surface functionalized nano-Fe3O4. Chem. Eng. J. 2024, 499, 156351. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, Z.; Cao, C.; Yan, M.; Liu, Z.; Cheng, X.; Wang, H.; Wang, Q.; Liu, H.; Chen, S. Multiple natural polymers in drug and gene delivery systems. Curr. Med. Chem. 2024, 31, 1691–1715. [Google Scholar] [CrossRef]
- Patel, R.; Kuwar, U.; Dhote, N.; Alexander, A.; Nakhate, K.; Jain, P.; Ajazuddin. Natural polymers as a carrier for the effective delivery of antineoplastic drugs. Curr. Drug Deliv. 2024, 21, 193–210. [Google Scholar] [CrossRef]
- Fang, K.; Li, P.; Zhang, B.; Liu, S.; Zhao, X.; Kou, L.; Xu, W.; Guo, X.; Li, J. Insights on updates in sodium alginate/MXenes composites as the designer matrix for various applications: A review. Int. J. Biol. Macromol. 2024, 269, 132032. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; He, J.; Sun, C.; Lu, W.; Zhang, Y.; Fang, Y. Doubling growth of egg-box structure during Calcium-mediated molecular assembly of alginate. J. Colloid. Interf. Sci. 2023, 634, 747–756. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, H.; Yu, Y.; Zou, X.; Shi, J.; Zhao, Y.; Ye, Y. Dissolution mechanism of sodium alginate and properties of its regenerated fiber under low temperature. Int. J. Biol. Macromol. 2020, 162, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.V.; Chan, L.W.; Ching, A.L.; Heng, P.W.S. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int. J. Pharmaceut. 2006, 309, 25–37. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H. Development and characterization of starch-sodium alginate-montmorillonite biodegradable antibacterial films. Int. J. Biol. Macromol. 2023, 233, 123462. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, L.; Huang, F.; Meng, Y.; Chen, Y.; Wang, J.; Wang, S.; Luo, Y.; Li, J.; Liang, Y. pH-sensitive chitosan/sodium alginate/calcium chloride hydrogel beads for potential oral delivery of rice bran bioactive peptides. Food Chem. 2025, 470, 142618. [Google Scholar] [CrossRef]
- Bai, W.; Chen, H.; Li, J.; Cai, W.; Kong, Y.; Zuo, X. Calcium carbonate hollow microspheres encapsulated cellulose nanofiber/sodium alginate hydrogels as a sequential delivery system. Int. J. Biol. Macromol. 2025, 309, 142839. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl cellulose-based oral delivery systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohyd. Polym. 2021, 256, 117561. [Google Scholar]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nan, W.; Zhang, R.; Shen, S.; Wu, M.; Zhong, S.; Zhang, Y.; Cui, X. Fabrication of carboxymethyl cellulose-based thermo-sensitive hydrogels and inhibition of corneal neovascularization. Int. J. Biol. Macromol. 2024, 261, 129933. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. Natural rosin modified carboxymethyl cellulose delivery system with lowered toxicity for long-term pest control. Carbohyd. Polym. 2021, 259, 117749. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, S.; Zhang, S.; Dong, S.; Hu, J.; Kang, L.; Yang, X. A double-layer hydrogel based on alginate-carboxymethyl cellulose and synthetic polymer as sustained drug delivery system. Sci. Rep. 2021, 11, 9142. [Google Scholar] [CrossRef]
- Gong, J.; Hou, L.; Ching, Y.C.; Ching, K.Y.; Dai Hai, N.; Chuah, C.H. A review of recent advances of cellulose-based intelligent-responsive hydrogels as vehicles for controllable drug delivery system. Int. J. Biol. Macromol. 2024, 264, 130525. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Zhang, Y.; Yin, J.; Yang, T.; Li, K.; Wei, L.; Li, J.; He, W. Hydrogel beads based on carboxymethyl cassava starch/alginate enriched with MgFe2O4 nanoparticles for controlling drug release. Int. J. Biol. Macromol. 2022, 220, 573–588. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.Y.; Chen, X.; Park, H.J. Calcium-alginate beads loaded with gallic acid: Preparation and characterization. LWT Food Sci. Technol. 2016, 68, 667–673. [Google Scholar] [CrossRef]
- Çakır, D.; Gümüşderelioğlu, M. Nopal mucilage-encapsulated poly (butylene adipate-co-terephthalate) nanoparticles for efficient gallic acid delivery in glioblastoma cells. J. Drug Deliv. Sci. Technol. 2025, 110, 107025. [Google Scholar] [CrossRef]
- Sripunya, A.; Chittasupho, C.; Mangmool, S.; Angerhofer, A.; Imaram, W. Gallic acid-encapsulated PAMAM dendrimers as an antioxidant delivery system for controlled release and reduced cytotoxicity against ARPE-19 cells. Bioconjugate Chem. 2024, 35, 1959–1969. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Khainskaya, K.; Hileuskaya, K.; Nikalaichuk, V.; Ladutska, A.; Akhmedov, O.; Abrekova, N.; You, L.; Shao, P.; Odonchimeg, M. Chitosan-gallic acid conjugate with enhanced functional properties and synergistic wound healing effect. Carbohyd. Res. 2025, 553, 109496. [Google Scholar] [CrossRef]
- Li, T.; Jiang, Y.; Zeng, L.; Zhang, H.; Yuan, Z.; Liu, J.; Liu, C.; Li, H.; Zhang, L.; Yang, C. Surface sizing introducing Fe3O4 nanoparticles for magnetic properties strengthening of basalt fibers. Ceram. Int. 2025, 51, 2216–2225. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Dawn, R.; Kiran, C.; Tjiu, W.W.; Aabdin, Z.; Zzaman, M.; Faizal, F.; Panatarani, C.; Joni, I.M.; Chandra, G.; Amemiya, K.; et al. Effect of spin and orbital selectivity of DNA induced SiO2 coated Fe3O4 nanoparticles: X-ray magnetic circular dichroism study. Colloid. Surface. A. 2025, 709, 136111. [Google Scholar] [CrossRef]
- Jeevakumari, J.F.; Suresh, G. Comprehensive evaluation of solvothermally synthesized Fe3O4 and PEG capped Fe3O4 nanoparticles for magnetic and biomedical applications. Inorg. Chem. Commun. 2025, 174, 113904. [Google Scholar] [CrossRef]
- Xing, X.; Liu, C.; Zheng, L. Preparation of photo-crosslinked microalgae-carboxymethyl chitosan composite hydrogels for enhanced wound healing. Carbohyd. Polym. 2025, 348, 122803. [Google Scholar] [CrossRef]
- Fei, Y.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J. Colloid. Interf. Sci. 2016, 484, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Xu, M.; Fang, C.; Li, M.; Han, H.; Du, Y. Construct of flexible Fe3O4@VO2/Ti3C2Tx composite films with absorption-dominated and tunable terahertz electromagnetic shielding. Ceram. Int. 2025, 51, 18987–18999. [Google Scholar] [CrossRef]
- Neisi, E.; Dadkhah Tehrani, A.; Shamloei, H.R. Development of cellulose nanowhisker–gallic acid antioxidant bioconjugate via covalent conjugation and supramolecular interactions: A comparative study. Int. J. Biol. Macromol. 2024, 271, 132561. [Google Scholar] [CrossRef]
- Lü, T.; Ma, R.; Ke, K.; Zhang, D.; Qi, D.; Zhao, H. Synthesis of gallic acid functionalized magnetic hydrogel beads for enhanced synergistic reduction and adsorption of aqueous chromium. Chem. Eng. J. 2021, 408, 127327. [Google Scholar] [CrossRef]
- Hao, X.; Yang, S.; Li, Y. High efficiency and selective removal of Cu (II) via regulating the pore size of graphene oxide/montmorillonite composite aerogel. J. Hazard. Mater. 2022, 424, 127680. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sánchez del Río Sáez, J.; Liu, Y.; Du, S.; Cruz, C.; Wei, G.; Wang, D.-Y. Carboxymethyl chitosan-based composite film for fire warning under high humidity conditions with wireless signal transmission. Int. J. Biol. Macromol. 2025, 316, 144540. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Chen, D.; Zhang, Z.; Xia, Y.; Zhang, K.; Ming, J.; Lei, X. Decoding the molecular interaction mechanisms of tannic acid in modulating Fe3+-sodium alginate microgel delivery systems. Food Chem. 2025, 489, 144935. [Google Scholar] [CrossRef]
- Asl, A.M.; Abdouss, M.; Kalaee, M.R.; Homami, S.S.; Pourmadadi, M. Targeted delivery of quercetin using gelatin/starch/Fe3O4 nanocarrier to suppress the growth of liver cancer HepG2 cells. Int. J. Biol. Macromol. 2024, 281, 136535. [Google Scholar] [CrossRef]
- Feng, Q.; Fan, B.; He, Y.-C. Antibacterial, antioxidant, Cr(VI) adsorption and dye adsorption effects of biochar-based silver nanoparticles-sodium alginate-tannic acid composite gel beads. Int. J. Biol. Macromol. 2024, 271, 132453. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, J.; Chen, W.; Wang, G. Electrostatically sprayed alginate-carboxymethyl chitosan microgel for colon-targeted melatonin delivery ameliorates ulcerative colitis. Food Biosci. 2025, 68, 106616. [Google Scholar] [CrossRef]
- Wu, P.; He, R.-H.; Fang, Y.; Chen, K.; Wu, M.; Zhang, W.; Lv, J.; Zhao, Y. The study of double-network carboxymethyl chitosan/sodium alginate based cryogels for rapid hemostasis in noncompressible hemorrhage. Int. J. Biol. Macromol. 2024, 266, 131399. [Google Scholar] [CrossRef] [PubMed]
- El-Basiouny, N.M.; Soliman, S.M.A.; Khalil, N.M.; Abd El-Ghany, M.N. Chitosan and alginate/Aspergillus flavus-mediated nanocomposite films for preservation of postharvest tomatoes. Int. J. Biol. Macromol. 2025, 297, 139559. [Google Scholar] [CrossRef]
- Sellappan, L.K.; Mishra, S.; Sundaresan, S.; Rathinavelan, T.; Majumdar, S. Multifunctional NiO-curcumin nanocomposite-loaded chitosan-alginate scaffolds for enhanced bone tissue regeneration and antibacterial activity. J. Drug Deliv. Sci. Tec. 2025, 110, 107114. [Google Scholar] [CrossRef]
- Sahayaraj, R.; Enoch, K.; Pati, S.S.; Somasundaram, A.A. Cobalt doped Fe3O4 nanoparticles with improved magnetic anisotropy and enhanced hyperthermic efficiency. Ceram. Int. 2025, 51, 20786–20797. [Google Scholar] [CrossRef]
- Abdolrahimi, M.; Vasilakaki, M.; Slimani, S.; Ntallis, N.; Varvaro, G.; Laureti, S.; Meneghini, C.; Trohidou, K.N.; Fiorani, D.; Peddis, D. Magnetism of nanoparticles: Effect of the organic coating. Nanomaterials 2021, 11, 1787. [Google Scholar] [CrossRef]
- Levy, L.; Gurov, A.; Radian, A. The effect of gallic acid interactions with iron-coated clay on surface redox reactivity. Water Res. 2020, 184, 116190. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Zhang, W.; Zhang, L.; Zhao, H.; Wang, S.; Huang, C. Recent advances in sustainable antimicrobial food packaging: Insights into release mechanisms, design strategies, and applications in the food industry. J. Agric. Food Chem. 2023, 71, 11806–11833. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Sanford, A.R.; Ferguson, J.S.; Filipcsei, G.; Csetneki, I.; Szilágyi, A.; Zrínyi, M. Magnetic field-responsive smart polymer composites. In Oligomers-Polymer Composites-Molecular Imprinting; Springer: Berlin/Heidelberg, Germany, 2007; pp. 137–189. [Google Scholar]
- Sulttan, S.; Rohani, S. Controlled drug release of smart magnetic self-assembled micelle, kinetics and transport mechanisms. J. Pharm. Sci. 2022, 111, 2378–2388. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Sun, T.; Yao, Q.; Zhou, D.; Mao, F. Antioxidant activity of N-carboxymethyl chitosan oligosaccharides. Bioorganic Med. Chem. Lett. 2008, 18, 5774–5776. [Google Scholar] [CrossRef]
- Milenković, D.; Đorović, J.; Petrović, V.; Avdović, E.; Marković, Z. Hydrogen atom transfer versus proton coupled electron transfer mechanism of gallic acid with different peroxy radicals. React. Kinet. Mech. Cat. 2018, 123, 215–230. [Google Scholar] [CrossRef]
- Mittal, A.; Vashistha, V.K.; Das, D.K. Free radical scavenging activity of gallic acid toward various reactive oxygen, nitrogen, and sulfur species: A DFT approach. Free Radical Res. 2023, 57, 81–90. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S.; Liu, J. Synthesis, characterization, and antibacterial activity of N, O-quaternary ammonium chitosan. Carbohyd. Res. 2011, 346, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Chen, X.G.; Li, Y.Y.; Liu, C.S.; Liu, C.G.; Cha, D.S.; Park, H.J. Antibacterial activity of oleoyl-chitosan nanoparticles: A novel antibacterial dispersion system. Carbohyd. Polym. 2008, 74, 114–120. [Google Scholar] [CrossRef]
- Chan, L.L.; Wilkinson, A.R.; Paradis, B.D.; Lai, N.J. Rapid image-based cytometry for comparison of fluorescent viability staining methods. J. Fluoresc. 2012, 22, 1301–1311. [Google Scholar] [CrossRef]





| Samples | Additions of GA (g) | Loading Capacity (LC%) | Loading Efficiency (LE%) |
|---|---|---|---|
| CMC-SA-Fe3O4@GA-1 | 0.1 | 13.3± 0.4 a | 66.5± 2.0 a |
| CMC-SA-Fe3O4@GA-2 | 0.3 | 28.6 ± 0.9 b | 47.7± 1.5 b |
| CMC-SA-Fe3O4@GA-3 | 0.5 | 31.1 ± 1.2 c | 31.1 ± 1.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, K.; Li, P.; Wang, H.; Huang, X.; Li, Y. pH and Magnetic-Responsive Carboxymethyl Chitosan/Sodium Alginate Composites for Gallic Acid Delivery. Magnetochemistry 2025, 11, 85. https://doi.org/10.3390/magnetochemistry11100085
Fang K, Li P, Wang H, Huang X, Li Y. pH and Magnetic-Responsive Carboxymethyl Chitosan/Sodium Alginate Composites for Gallic Acid Delivery. Magnetochemistry. 2025; 11(10):85. https://doi.org/10.3390/magnetochemistry11100085
Chicago/Turabian StyleFang, Kun, Pei Li, Hanbing Wang, Xiangrui Huang, and Yihan Li. 2025. "pH and Magnetic-Responsive Carboxymethyl Chitosan/Sodium Alginate Composites for Gallic Acid Delivery" Magnetochemistry 11, no. 10: 85. https://doi.org/10.3390/magnetochemistry11100085
APA StyleFang, K., Li, P., Wang, H., Huang, X., & Li, Y. (2025). pH and Magnetic-Responsive Carboxymethyl Chitosan/Sodium Alginate Composites for Gallic Acid Delivery. Magnetochemistry, 11(10), 85. https://doi.org/10.3390/magnetochemistry11100085





