Magnetic-Responsive Material-Mediated Magnetic Stimulation for Tissue Engineering
Abstract
1. Introduction
2. Magnetic-Responsive Materials
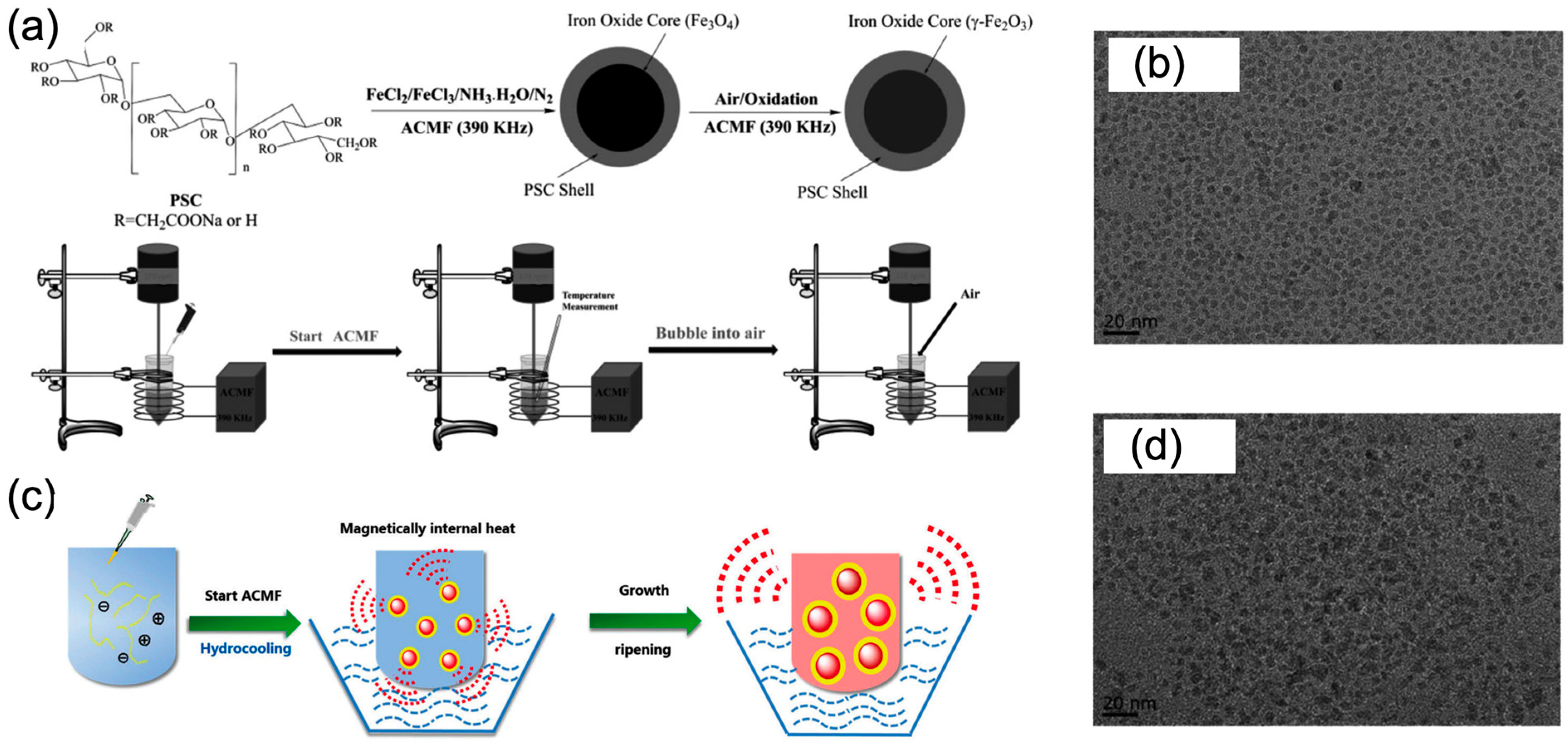
2.1. Magnetic Nanoparticles
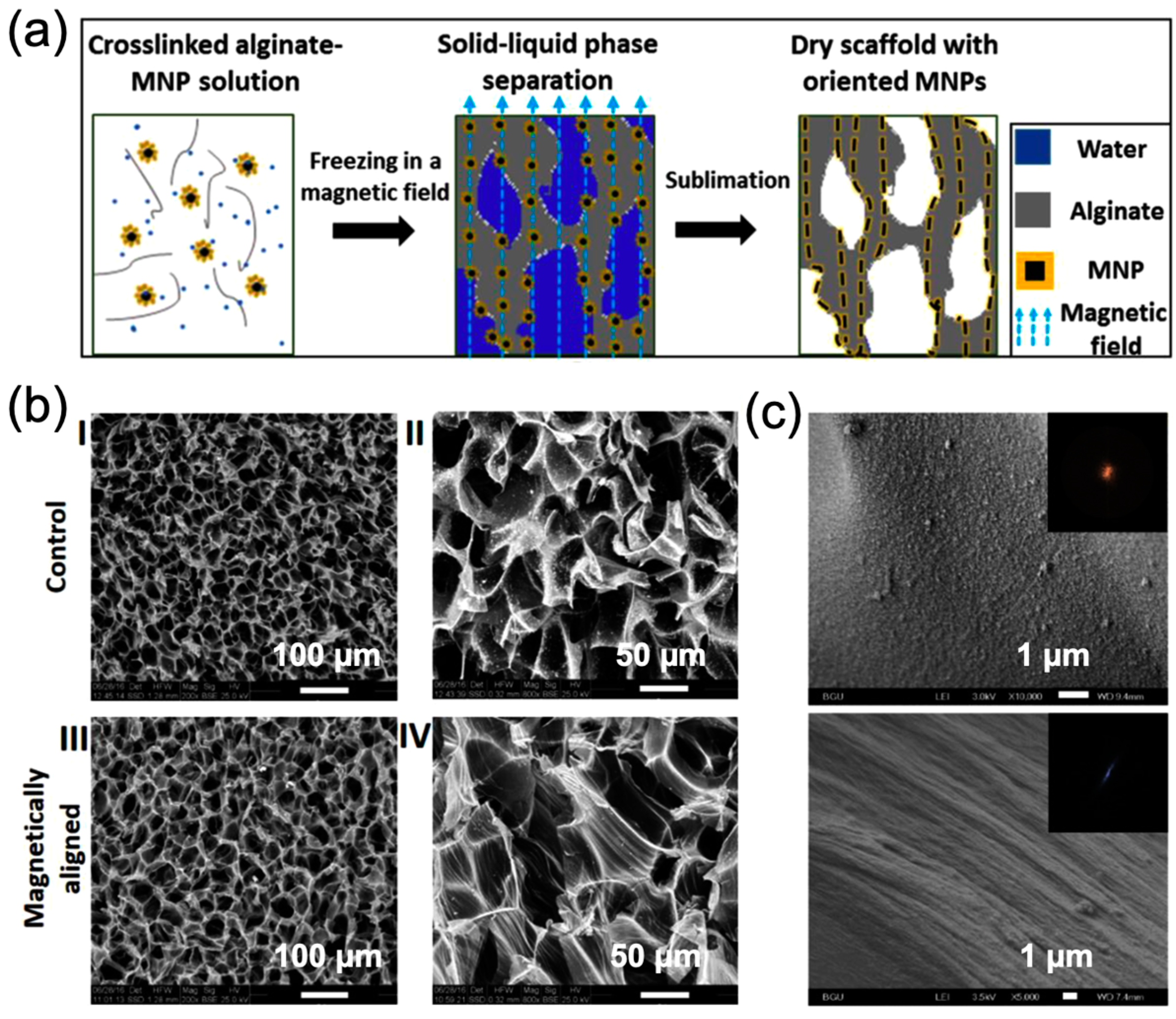
2.2. Magnetic-Responsive Polymer Scaffolds
2.3. Magnetic-Responsive Hydrogels
3. Magnetic-Responsive Effects
3.1. Magneto-Electric Effects
3.2. Magneto-Mechanical Effects
3.3. Magneto-Thermal Effects
4. Tissue Engineering
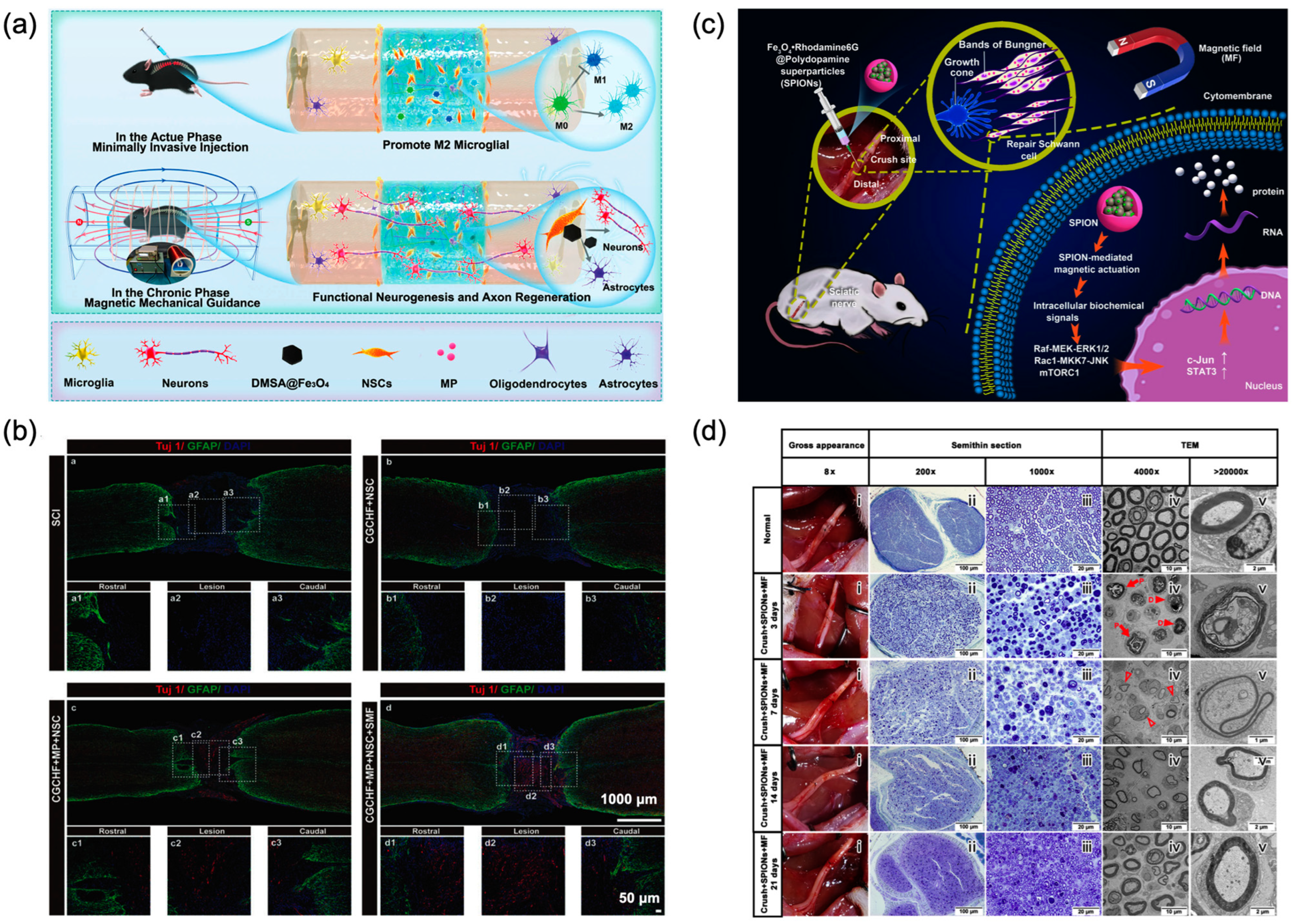
4.1. Neural Tissue Engineering
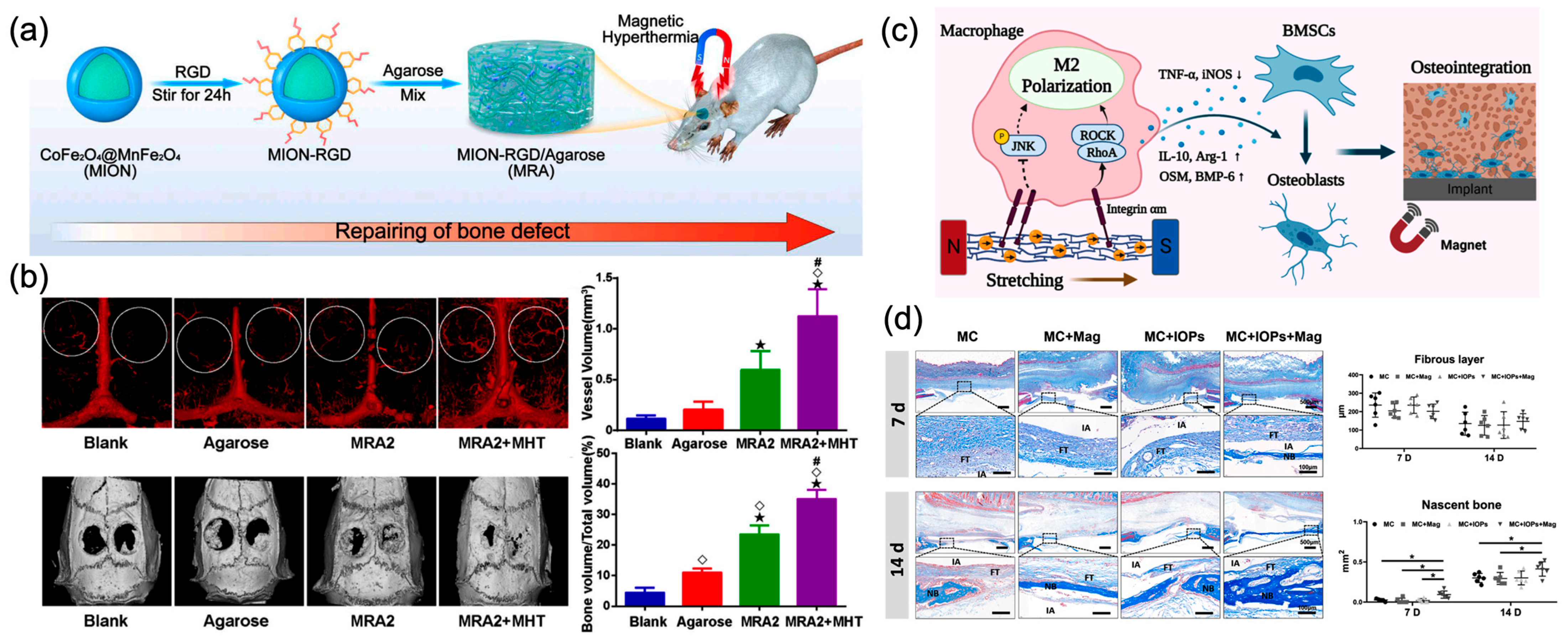
4.2. Bone Tissue Engineering
4.3. Other Tissue Engineering
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | Alternating magnetic field |
| BMSCs | Bone marrow mesenchymal stem cells |
| ECM | Extracellular matrix |
| FDA | U.S. Food and Drug Administration |
| GRN | Gigantocellular reticular nucleus |
| hBM-MSCs | human bone marrow mesenchymal stem cells |
| HSF | Heat shock factor |
| HSP | Heat shock proteins |
| MNPs | Magnetic nanoparticles |
| MSCs | Mesenchymal stem cells |
| NGF | Nerve growth factor |
| NO | Nitric oxide |
| PSC | Polydextrose-sorbitol carboxymethyl ether |
| rGO-M | reduced graphene oxide membrane |
| RMF | Rotating magnetic field |
| SMF | Static magnetic field |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
References
- Bello, S.A.; Cruz-Lebron, J.; Rodriguez-Rivera, O.A.; Nicolau, E. Bioactive scaffolds as a promising alternative for enhancing critical-size bone defect regeneration in the craniomaxillofacial region. ACS Appl. Bio Mater. 2023, 6, 4465–4503. [Google Scholar] [CrossRef] [PubMed]
- Safa, B.; Buncke, G. Autograft substitutes: Conduits and processed nerve allografts. Hand Clin. 2016, 32, 127–140. [Google Scholar] [CrossRef]
- Tupe, A.; Patole, V.; Ingavle, G.; Kavitkar, G.; Mishra Tiwari, R.; Kapare, H.; Baheti, R.; Jadhav, P. Recent advances in biomaterial-based scaffolds for guided bone tissue engineering: Challenges and future directions. Polym. Adv. Technol. 2024, 35, e6619. [Google Scholar] [CrossRef]
- Wu, H.; Feng, E.; Yin, H.; Zhang, Y.; Chen, G.; Zhu, B.; Yue, X.; Zhang, H.; Liu, Q.; Xiong, L. Biomaterials for neuroengineering: Applications and challenges. Regen. Biomater. 2025, 12, rbae137. [Google Scholar] [CrossRef]
- Yao, X.; Xue, T.; Chen, B.; Zhou, X.; Ji, Y.; Gao, Z.; Liu, B.; Yang, J.; Shen, Y.; Sun, H.; et al. Advances in biomaterial-based tissue engineering for peripheral nerve injury repair. Bioact. Mater. 2025, 46, 150–172. [Google Scholar] [CrossRef]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone tissue engineering scaffolds: Function of multi-material hierarchically structured scaffolds. Adv. Healthc. Mater. 2023, 12, 2202766. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of scaffolds from bio-based natural materials for tissue regeneration applications: A review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Zielinska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Slomski, R.; et al. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- Sultana, N.; Cole, A.; Strachan, F. Biocomposite scaffolds for tissue engineering: Materials, fabrication techniques and future directions. Materials 2024, 17, 5577. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Liao, J.; Shi, K.; Ning, L. A review on external physical stimuli with biomaterials for bone repair. Chem. Eng. J. 2024, 496, 153749. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Shi, D.; Xiao, R.; Kang, H.; Li, F.; Ling, D. Stimuli-responsive nanomaterials for wireless and precise neuromodulation. Small Methods 2025, e01275. [Google Scholar] [CrossRef]
- Ansari, M.A.A.; Dash, M.; Camci-Unal, G.; Jain, P.K.; Nukavarapu, S.; Ramakrishna, S.; Falcone, N.; Dokmeci, M.R.; Najafabadi, A.H.; Khademhosseini, A.; et al. Engineered stimuli-responsive smart grafts for bone regeneration. Curr. Opin. Biomed. Eng. 2023, 28, 100493. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; He, X.; Zhu, Y.; Xu, X.; Yang, H.; Mei, G.; Chen, S.; Ma, B.; Zhu, R. Application of bioactive materials for osteogenic function in bone tissue engineering. Small Methods 2024, 8, 2301283. [Google Scholar] [CrossRef]
- Ding, H.; Hao, L.; Mao, H. Magneto-responsive biocomposites in wound healing: From characteristics to functions. J. Mater. Chem. B 2024, 12, 7463–7479. [Google Scholar] [CrossRef]
- Ge, C.; Masalehdan, T.; Baghini, M.S.; Toro, V.D.; Signorelli, L.; Thomson, H.; Gregurec, D.; Heidari, H. Microfabrication technologies for nanoinvasive and high-Resolution magnetic neuromodulation. Adv. Sci. 2024, 11, 2404254. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.P.; Flores, M.; Madureira, S.; Zanotto, F.; Monteiro, F.J.; Laranjeira, M.S. Magnetic bone tissue engineering: Reviewing the effects of magnetic stimulation on bone regeneration and angiogenesis. Pharmaceutics 2023, 15, 1045. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, G.; Jiao, W.; Li, K.; Zhang, T.; Liu, X.; Fan, H. Magnetic nanomaterials-mediated neuromodulation. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1890. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, L.; Qu, X.; Lei, B. Advanced thermoactive nanomaterials for thermomedical tissue regeneration: Opportunities and challenges. Small Methods 2025, 9, 2400510. [Google Scholar] [CrossRef]
- Yao, J.; Yao, C.; Zhang, A.; Xu, X.; Wu, A.; Yang, F. Magnetomechanical force: An emerging paradigm for therapeutic applications. J. Mater. Chem. B 2022, 10, 7136–7147. [Google Scholar] [CrossRef]
- Martins, P.; Brito-Pereira, R.; Ribeiro, S.; Lanceros-Mendez, S.; Ribeiro, C. Magneto-electrics for biomedical applications: 130 years later, bridging materials, energy, and life. Nano Energy 2024, 126, 109569. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic nanoparticles in biology and medicine: Past, present, and future trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y. Nanoparticle-based medicines in clinical cancer therapy. Nano Today 2022, 45, 101512. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Yadav, M.D. Magnetic Nanoparticles: A comprehensive review from synthesis to biomedical frontiers. Langmuir 2024, 40, 17239–17269. [Google Scholar] [CrossRef] [PubMed]
- Stiufiuc, G.F.; Stiufiuc, R.I. Magnetic nanoparticles: Synthesis, characterization, and their use in biomedical field. Appl. Sci. 2024, 14, 1623. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent trends in preparation and biomedical applications of iron oxide nanoparticles. J. Nanobiotechnol. 2024, 22, 24. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic iron oxide nanoparticles (SPION): From fundamentals to state-of-the-art innovative applications for cancer therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Ling, D.; Lee, N.; Hyeon, T. Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.-S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Zeinali Sehrig, F.; Farkhani, S.M.; Soleymani Goloujeh, M.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Pittman, C.U.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef]
- Trifoi, A.R.; Matei, E.; Râpă, M.; Berbecaru, A.-C.; Panaitescu, C.; Banu, I.; Doukeh, R. Coprecipitation nanoarchitectonics for the synthesis of magnetite: A review of mechanism and characterization. React. Kinet. Mech. Catal. 2023, 136, 2835–2874. [Google Scholar] [CrossRef]
- Hufschmid, R.; Arami, H.; Ferguson, R.M.; Gonzales, M.; Teeman, E.; Brush, L.N.; Browning, N.D.; Krishnan, K.M. Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale 2015, 7, 11142–11154. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.-P.; Gómez-Pastora, J.; et al. Magnetic nanoparticles: A review on synthesis, characterization, functionalization, and biomedical applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.A.; Bigham, A. In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite as a magnetic drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 774–779. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion synthesis of superparamagnetic nanoparticles for bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef]
- Fatmawati, T.; Shiddiq, M.; Armynah, B.; Tahir, D. Synthesis methods of Fe3O4 nanoparticles for biomedical applications. Chem. Eng. Technol. 2023, 46, 2356–2366. [Google Scholar] [CrossRef]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, T.; Philip, J. A review on synthesis, capping and applications of superparamagnetic magnetic nanoparticles. Adv. Colloid Interface Sci. 2024, 334, 103314. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, H.; Xu, B. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef]
- Dik, G.; Ulu, A.; Ates, B. Synthesis and biomedical applications of polymer-functionalized magnetic nanoparticles. Nanofabrication 2023, 8, 1–33. [Google Scholar] [CrossRef]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic functionalized nanoparticles for biomedical, drug delivery and imaging applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Li, Y.; He, H.; Gu, N. The story of ferumoxytol: Synthesis production, current clinical applications, and therapeutic potential. Adv. Healthc. Mater. 2024, 13, 2302773. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Zhang, X.; Liu, F.; Liu, Y.; Ji, M.; Xiong, F.; Gu, N. An efficient synthesis of ferumoxytol induced by alternating-current magnetic field. Mater. Lett. 2016, 170, 93–96. [Google Scholar] [CrossRef]
- Chen, B.; Sun, J.; Fan, F.; Zhang, X.; Qin, Z.; Wang, P.; Li, Y.; Zhang, X.; Liu, F.; Liu, Y.; et al. Ferumoxytol of ultrahigh magnetization produced by hydrocooling and magnetically internal heating co-precipitation. Nanoscale 2018, 10, 7369–7376. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef]
- Sathiya, K.; Ganesamoorthi, S.; Mohan, S.; Shanmugavadivu, A.; Selvamurugan, N. Natural polymers-based surface engineering of bone scaffolds—A review. Int. J. Biol. Macromol. 2024, 282, 136840. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Zhao, P.; Peng, Z.; Geng, Z.; Zhang, J.; Zhang, Z.; Shen, R.; Li, X.; Wang, X.; et al. 3D bioprinted tissue-engineered bone with enhanced mechanical strength and bioactivities: Accelerating bone defect repair through sequential immunomodulatory properties. Adv. Healthc. Mater. 2024, 13, 2401919. [Google Scholar] [CrossRef]
- El-Bahrawy, N.R.; Elgharbawy, H.; Elmekawy, A.; Salem, M.; Morsy, R. Development of porous hydroxyapatite/PVA/gelatin/alginate hybrid flexible scaffolds with improved mechanical properties for bone tissue engineering. Mater. Chem. Phys. 2024, 319, 129332. [Google Scholar] [CrossRef]
- Lee, W.; Xu, C.; Fu, H.; Ploch, M.; D’Souza, S.; Lustig, S.; Long, X.; Hong, Y.; Dai, G. 3D bioprinting highly elastic PEG-PCL-DA hydrogel for soft tissue fabrication and biomechanical stimulation. Adv. Funct. Mater. 2024, 34, 2313942. [Google Scholar] [CrossRef]
- Xie, R.; Cao, Y.; Sun, R.; Wang, R.; Morgan, A.; Kim, J.; Callens, S.J.P.; Xie, K.; Zou, J.; Lin, J.; et al. Magnetically driven formation of 3D freestanding soft bioscaffolds. Sci. Adv. 2024, 10, 1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, C.; Shi, Z.; Liu, H.; Han, X.; Chen, Z.; Li, S.; Wang, Z.; Huang, J. A soft receiving platform for coaxial bioprinting cell-laden microtubes with uniform wall thickness. Appl. Mater. Today 2025, 42, 102599. [Google Scholar] [CrossRef]
- Li, Z.; Xue, L.; Wang, P.; Ren, X.; Zhang, Y.; Wang, C.; Sun, J. Biological scaffolds assembled with magnetic nanoparticles for bone tissue engineering: A review. Materials 2023, 16, 1429. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, C.J.; Wright, C.J. The fabrication of iron oxide nanoparticle-nanofiber composites by electrospinning and their applications in tissue engineering. Biotechnol. J. 2017, 12, 1600693. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.H.; Nakhaei, M.; Gharib, A.; Mirbagheri, M.S.; Moradi, A.; Jirofti, N. Investigation of background, novelty and recent advance of iron (II,III) oxide- loaded on 3D polymer based scaffolds as regenerative implant for bone tissue engineering: A review. Int. J. Biol. Macromol. 2024, 259, 128959. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ye, Q.; Wang, L.; Chen, H.; Xu, C.; Wang, P.; Sun, J. Endowing improved osteogenic activities with collagen membrane by incorporating biocompatible iron oxide nanoparticles. Front. Bioeng. Biotechnol. 2023, 11, 1259904. [Google Scholar] [CrossRef]
- Yang, W.; Zhong, Y.; Feng, P.; Gao, C.; Peng, S.; Zhao, Z.; Shuai, C. Disperse magnetic sources constructed with functionalized Fe3O4 nanoparticles in poly-l-lactic acid scaffolds. Polym. Test. 2019, 76, 33–42. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef]
- Margolis, G.; Polyak, B.; Cohen, S. Magnetic induction of multiscale anisotropy in macroporous alginate scaffolds. Nano Lett. 2018, 18, 7314–7322. [Google Scholar] [CrossRef]
- Taghizadeh, S.; Tayebi, L.; Akbarzadeh, M.; Lohrasbi, P.; Savardashtaki, A. Magnetic hydrogel applications in articular cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2024, 112, 260–275. [Google Scholar] [CrossRef]
- Paltanea, G.; Manescu, V.; Antoniac, I.; Antoniac, A.; Nemoianu, I.V.; Robu, A.; Dura, H. A review of biomimetic and biodegradable magnetic scaffolds for bone tissue engineering and oncology. Int. J. Mol. Sci. 2023, 24, 4312. [Google Scholar] [CrossRef] [PubMed]
- Pamula, E.; Bacakova, L.; Filova, E.; Buczynska, J.; Dobrzynski, P.; Noskova, L.; Grausova, L. The influence of pore size on colonization of poly(L-lactide-glycolide) scaffolds with human osteoblast-like MG 63 cells in vitro. J. Mater. Sci. Mater. Med. 2008, 19, 425–435. [Google Scholar] [CrossRef]
- Ge, J.; Zhai, M.; Zhang, Y.; Bian, J.; Wu, J. Biocompatible Fe3O4 subchitosan scaffolds with high magnetism. Int. J. Biol. Macromol. 2019, 128, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Guedes, D.G.; Guedes, G.G.; da Silva, J.d.O.; da Silva, A.L.; Luna, C.B.B.; Damasceno, B.P.G.d.L.; Costa, A.C.F.d.M. Development of scaffolds with chitosan magnetically activated with cobalt nanoferrite: A study on physical-chemical, mechanical, cytotoxic and antimicrobial Behavior. Pharmaceuticals 2024, 17, 1332. [Google Scholar] [CrossRef]
- Safavi, A.S.; Karbasi, S. A new path in bone tissue engineering: Polymer-based 3D-printed magnetic scaffolds (a comprehensive review of in vitro and in vivo studies). J. Biomater. Sci. Polym. Ed. 2025, 36, 1321–1341. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Farrukh, A.; Nayab, S. Shape memory hydrogels for biomedical applications. Gels 2024, 10, 270. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- Shi, W.; Huang, J.; Fang, R.; Liu, M. Imparting functionality to the hydrogel by magnetic-field-induced nano-assembly and macro-response. ACS Appl. Mater. Interfaces 2020, 12, 5177–5194. [Google Scholar] [CrossRef] [PubMed]
- Fragal, E.H.; Fragal, V.H.; Silva, E.P.; Paulino, A.T.; da Silva Filho, E.C.; Mauricio, M.R.; Silva, R.; Rubira, A.F.; Muniz, E.C. Magnetic-responsive polysaccharide hydrogels as smart biomaterials: Synthesis, properties, and biomedical applications. Carbohydr. Polym. 2022, 292, 119665. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.-E.; Song, H.-R.; Kim, Y.-M.; Song, S.-C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef]
- Ahmad, H.; Sultana, M.S.; Alam, M.A.; Rahman, M.M.; Tauer, K.; Gafur, M.A.; Sharafat, M.K. Evaluating a simple blending approach to prepare magnetic and stimuli-responsive composite hydrogel particles for application in biomedical field. Express Polym. Lett. 2016, 10, 664–678. [Google Scholar] [CrossRef]
- Li, B.; Jia, D.; Zhou, Y.; Hu, Q.; Cai, W. In situ hybridization to chitosan/magnetite nanocomposite induced by the magnetic field. J. Magn. Magn. Mater. 2006, 306, 223–227. [Google Scholar] [CrossRef]
- Hu, X.; Nian, G.; Liang, X.; Wu, L.; Yin, T.; Lu, H.; Qu, S.; Yang, W. Adhesive tough magnetic hydrogels with high Fe3O4 content. ACS Appl. Mater. Interfaces 2019, 11, 10292–10300. [Google Scholar] [CrossRef]
- Goudu, S.R.; Yasa, I.C.; Hu, X.; Ceylan, H.; Hu, W.; Sitti, M. Biodegradable untethered magnetic hydrogel milli-grippers. Adv. Funct. Mater. 2020, 30, 2004975. [Google Scholar] [CrossRef]
- Fan, F.; Sun, J.; Chen, B.; Li, Y.; Hu, K.; Wang, P.; Ma, M.; Gu, N. Rotating magnetic field-controlled fabrication of magnetic hydrogel with spatially disk-like microstructures. Sci. China Mater. 2018, 61, 1112–1122. [Google Scholar] [CrossRef]
- Dai, C.F.; Khoruzhenko, O.; Zhang, C.; Zhu, Q.L.; Jiao, D.; Du, M.; Breu, J.; Zhao, P.; Zheng, Q.; Wu, Z.L. Magneto-orientation of magnetic double stacks for patterned anisotropic hydrogels with multiple responses and modulable motions. Angew. Chem. Int. Ed. 2022, 61, e202207272. [Google Scholar] [CrossRef]
- Gila-Vilchez, C.; Manas-Torres, M.C.; Contreras-Montoya, R.; Alaminos, M.; Duran, J.D.G.; Alvarez de Cienfuegos, L.; Lopez-Lopez, M.T. Anisotropic magnetic hydrogels: Design, structure and mechanical properties. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377, 20180217. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Sun, J.; Guo, Z.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A novel magnetic hydrogel with aligned magnetic colloidal assemblies showing controllable enhancement of magneto-thermal effect in the presence of alternating magnetic field. Adv. Mater. 2015, 27, 2507–2514. [Google Scholar] [CrossRef]
- Xue, L.; Sun, J. Magnetic hydrogels with ordered structure for biomedical applications. Front. Chem. 2022, 10, 1040492. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I.P. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Wang, Z.; Li, G.; Cai, Z.W.; Wu, J.Z.; Wang, L.; Deng, L.F.; Cai, M.; Cui, W.G. Injectable microfluidic hydrogel microspheres for cell and drug delivery. Adv. Funct. Mater. 2021, 31, 2103339. [Google Scholar] [CrossRef]
- Xue, L.; Wang, L.; Ding, X.; Sun, D.; Gu, N.; Sun, J. Soft-template regulation of magnetic microsphere topology from a microfluidic device. Chem. Eng. Sci. 2025, 305, 121106. [Google Scholar] [CrossRef]
- Nguyen, T.; Gao, J.; Wang, P.; Nagesetti, A.; Andrews, P.; Masood, S.; Vriesman, Z.; Liang, P.; Khizroev, S.; Jin, X. In vivo wireless brain stimulation via non-invasive and targeted delivery of magneto-electric nanoparticles. Neurotherapeutics 2021, 18, 2091–2106. [Google Scholar] [CrossRef]
- Smith, I.T.; Zhang, E.; Yildirim, Y.A.; Campos, M.A.; Abdel-Mottaleb, M.; Yildirim, B.; Ramezani, Z.; Andre, V.L.; Scott-Vandeusen, A.; Liang, P. Nanomedicine and nanobiotechnology applications of magneto-electric nanoparticles. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1849. [Google Scholar] [CrossRef]
- Yoo, K.; Jeon, B.-G.; Chun, S.H.; Patil, D.R.; Lim, Y.-j.; Noh, S.-h.; Gil, J.; Cheon, J.; Kim, K.H. Quantitative measurements of size-dependent magneto-electric coupling in Fe3O4 nanoparticles. Nano Lett. 2016, 16, 7408–7413. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.S.; Listyawan, M.A.; Ryu, J. Core-shell magnetoelectric nanoparticles: Materials, synthesis, magnetoelectricity, and applications. Actuators 2022, 11, 380. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, E.; Toledo, D.; Smith, I.T.; Navarrete, B.; Furman, N.; Hernandez, A.F.; Telusma, M.; McDaniel, D.; Liang, P.; et al. Colossal magneto-electric effect in core-shell magneto-electric nanoparticles. Nano Lett. 2020, 20, 5765–5772. [Google Scholar] [CrossRef]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Zeng, M.; Zhang, Y.; Wei, D.; Sun, J.; Fan, H. Functional material-mediated wireless physical stimulation for neuro-modulation and regeneration. J. Mater. Chem. B 2023, 11, 9056–9083. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Akbaba, G.E.; Sharma, N.; Das, R.; Vinikoor, T.; Liu, Y.; Le, D.Q.; Angadi, K.; Nguyen, T.D. Electrically active biomaterials for stimulation and regeneration in tissue engineering. J. Biomed. Mater. Res. Part A 2025, 113, e37871. [Google Scholar] [CrossRef]
- Luo, R.; Liang, Y.; Yang, J.; Feng, H.; Chen, Y.; Jiang, X.; Zhang, Z.; Liu, J.; Bai, Y.; Xue, J.; et al. Reshaping the endogenous electric field to boost wound repair via electrogenerative dressing. Adv. Mater. 2023, 35, e2208395. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Zhang, Z.; Liu, H.; Li, L.; Wang, A.; Ouyang, J.; Xie, T.; Zhang, L.; Xue, J.; et al. Electrical stimulation system based on electroactive biomaterials for bone tissue engineering. Mater. Today 2023, 68, 177–203. [Google Scholar] [CrossRef]
- Sun, J.; Xie, W.; Wu, Y.; Li, Z.; Li, Y. Accelerated bone healing via electrical stimulation. Adv. Sci. 2024, 12, 2404190. [Google Scholar] [CrossRef]
- Mortensen, P.; Gao, H.; Smith, G.; Simitev, R.D. Action potential propagation and block in a model of atrial tissue with myocyte-fibroblast coupling. Math. Med. Biol. 2021, 38, 106–131. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, F.; Huang, J.-J.; Huang, J.-J.; Li, Z.-A.; Kong, Y.; Zhang, Z.-J. Microfluidic hollow fiber with improved stiffness repairs peripheral nerve injury through non-invasive electromagnetic induction and controlled release of NGF. Chem. Eng. J. 2021, 426, 131826. [Google Scholar] [CrossRef]
- Liu, Q.; Telezhkin, V.; Jiang, W.; Gu, Y.; Wang, Y.; Hong, W.; Tian, W.; Yarova, P.; Zhang, G.; Lee, S.M.; et al. Electric field stimulation boosts neuronal differentiation of neural stem cells for spinal cord injury treatment via PI3K/Akt/GSK-3β/β-catenin activation. Cell Biosci. 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Yushan, M.; Yusufu, A. Enhanced nerve regeneration by bionic conductive nerve scaffold under electrical stimulation. Front. Neurosci. 2022, 16, 810676. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, A.A.; Ivanova, A.V.; Semkina, A.S.; Lazareva, P.A.; Abakumov, M.A. Magneto-mechanical approach in biomedicine: Benefits, challenges, and future perspectives. Int. J. Mol. Sci. 2022, 23, 11134. [Google Scholar] [CrossRef]
- Li, W.; Tian, W.; Wu, Y.; Guo, S. A novel magnetic manipulation promotes directional growth of periodontal ligament stem cells. Tissue Eng. Part A 2023, 29, 620–632. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Y.; Wei, J.; Da, Z.; Chen, W.; Shu, X.; Luo, T.; Duan, Y.; Yang, R.; Ding, C.; et al. Alginate/GelMA microparticles via oil-free interface shearing for untethered magnetic microbots. Biomater. Sci. 2024, 12, 5562–5572. [Google Scholar] [CrossRef]
- Han, Y.; Fan, F.; Wang, P.; Liu, D.; Hu, F.; Zhu, P.; Sun, J. A Moire interference pattern formation of magnetic nanoparticles by rotational magnetic field controlled interfacial self-assembly. Appl. Phys. A Mater. Sci. Process. 2022, 128, 457. [Google Scholar] [CrossRef]
- Carrey, J.; Connord, V.; Respaud, M. Ultrasound generation and high-frequency motion of magnetic nanoparticles in an alternating magnetic field: Toward intracellular ultrasound therapy? Appl. Phys. Lett. 2013, 102, 232404. [Google Scholar] [CrossRef]
- Suzuki, S.; Satoh, A. The behavior and heat generation effect of a magnetic rod-like particle suspension in an alternating and a rotating magnetic field. Mol. Phys. 2023, 121, e2151523. [Google Scholar] [CrossRef]
- An, J.; Hong, H.; Won, M.; Rha, H.; Ding, Q.; Kang, N.; Kang, H.; Kim, J.S. Mechanical stimuli-driven cancer therapeutics. Chem. Soc. Rev. 2023, 52, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, J.; Sun, L.; Zhang, S.; Gong, D.; Li, X.; Yue, S.; Feng, L.; Zhang, D. Facile fabrication of magnetic microrobots based on spirulina templates for targeted delivery and synergistic chemo-photothermal therapy. ACS Appl. Mater. Interfaces 2019, 11, 4745–4756. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Martinac, B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001, 81, 685–740. [Google Scholar] [CrossRef]
- Sensenig, R.; Sapir, Y.; MacDonald, C.; Cohen, S.; Polyak, B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine 2012, 7, 1425–1442. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D.; Li, M.; Ma, M.; Gu, N. Adaptive materials based on iron oxide nanoparticles for bone regeneration. Chemphyschem 2018, 19, 1965–1979. [Google Scholar] [CrossRef]
- Collier, C.; Muzzio, N.; Thevi Guntnur, R.; Gomez, A.; Redondo, C.; Zurbano, R.; Schuller, I.K.; Monton, C.; Morales, R.; Romero, G. Wireless force-inducing neuronal stimulation mediated by high magnetic moment microdiscs. Adv. Healthc. Mater. 2022, 11, 2101826. [Google Scholar] [CrossRef]
- Lee, J.U.; Shin, W.; Lim, Y.; Kim, J.; Kim, W.R.; Kim, H.; Lee, J.H.; Cheon, J. Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals. Nat. Mater. 2021, 20, 1029–1036. [Google Scholar] [CrossRef]
- Xue, L.; Ye, Q.; Wu, L.; Li, D.; Bao, S.; Lu, Q.; Liu, S.; Sung, D.; Sheng, Z.; Zhang, Z.; et al. Magneto-mechanical effect of magnetic microhydrogel for improvement of magnetic neuro-stimulation. Nano Res. 2023, 16, 7393–7404. [Google Scholar] [CrossRef]
- Tay, A.; Sohrabi, A.; Poole, K.; Seidlits, S.; Di Carlo, D. A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv. Mater. 2018, 30, 1800927. [Google Scholar] [CrossRef]
- Ma, C.; Izumiya, M.; Nobuoka, H.; Ueno, R.; Mimura, M.; Ueda, K.; Ishida, H.; Tomotsune, D.; Johkura, K.; Yue, F.; et al. Three-dimensional modeling with osteoblast-like cells under external magnetic field conditions using magnetic nano-ferrite particles for the development of cell-derived artificial bone. Nanomaterials 2024, 14, 251. [Google Scholar] [CrossRef]
- Ito, A.; Takizawa, Y.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. Tissue engineering using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004, 10, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia-a promising tumour therapy? Nanotechnology 2014, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, G.; Sol-Fernández, S.D.; Flavián-Lázaro, A.C.; Lewinska, A.; Wnuk, M.; Tortiglione, C.; Moros, M. Remote magneto–thermal modulation of reactive oxygen species balance enhances tissue regeneration in vivo. Adv. Funct. Mater. 2024, 34, 2405282. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Xiao, Z.; Liu, X.; Wu, C.; Wu, K.; Liu, A.; Wei, D.; Sun, J.; Zhou, L.; et al. Magneto-electric nanoparticles incorporated biomimetic matrix for wireless electrical stimulation and nerve regeneration. Adv. Healthc. Mater. 2021, 10, 2100695. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, Q.; Wang, W.; Zhang, S.; Dong, M.; Hu, S.; Yin, A.; Meng, L.; Wang, A.; Yu, X.; et al. Reduced graphene oxide-mediated magneto-electric effect drives neural differentiation of mesenchymal stem cells. Sci. China Mater. 2023, 66, 2504–2512. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Wu, Z.; Kang, Y.; Xie, S.; Cai, Z.; Shan, X.; Li, Q. Pulsed electromagnetic field-assisted reduced graphene oxide composite 3D printed nerve scaffold promotes sciatic nerve regeneration in rats. Biofabrication 2024, 16, 035013. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Lin, Y.-H.; Liu, H.-C.; Hsu, R.-S.; Chiang, M.-R.; Wang, L.-W.; Chou, T.-C.; Lu, T.-T.; Lee, I.C.; Chu, L.-A.; et al. In situ magneto-electric generation of nitric oxide and electric stimulus for nerve therapy by wireless chargeable molybdenum carbide octahedrons. Nano Today 2023, 51, 101935. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, M.; Ren, J.; Han, S.; Zhou, X.; Zhang, D.; Guo, X.; Feng, H.; Ye, L.; Feng, S.; et al. Magnetic nanoparticles and methylprednisolone based physico-chemical bifunctional neural stem cells delivery system for spinal cord injury repair. Adv. Sci. 2024, 11, 2308993. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Lu, L.; Liu, Y. SPIONs mediated magnetic actuation promotes nerve regeneration by inducing and maintaining repair-supportive phenotypes in Schwann cells. J. Nanobiotechnol. 2022, 20, 159. [Google Scholar] [CrossRef]
- Rosenfeld, D.; Field, H.; Kim, Y.J.; Pang, K.K.L.; Nagao, K.; Koehler, F.; Anikeeva, P. Magneto-thermal modulation of calcium-dependent nerve growth. Adv. Funct. Mater. 2022, 32, 0224558. [Google Scholar] [CrossRef]
- Qi, F.; Gao, X.; Shuai, Y.; Peng, S.; Deng, Y.; Yang, S.; Yang, Y.; Shuai, C. Magnetic-driven wireless electrical stimulation in a scaffold. Compos. Part B Eng. 2022, 237, 109864. [Google Scholar] [CrossRef]
- Mushtaq, F.; Torlakcik, H.; Vallmajo-Martin, Q.; Siringil, E.C.; Zhang, J.; Röhrig, C.; Shen, Y.; Yu, Y.; Chen, X.-Z.; Müller, R.; et al. Magneto-electric 3D scaffolds for enhanced bone cell proliferation. Appl. Mater. Today 2019, 16, 290–300. [Google Scholar] [CrossRef]
- Wang, L.; Hu, P.; Jiang, H.; Zhao, J.; Tang, J.; Jiang, D.; Wang, J.; Shi, J.; Jia, W. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magneto-thermal composite-induced bone regeneration. Nano Today 2022, 43, 101401. [Google Scholar] [CrossRef]
- Estévez, M.; Cicuéndez, M.; Colilla, M.; Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Magnetic colloidal nanoformulations to remotely trigger mechanotransduction for osteogenic differentiation. J. Colloid Interface Sci. 2024, 664, 454–468. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Zheng, L.; Cai, H.; Yang, X.; Xue, Y.; Wan, Q.; Chen, J.; Li, Y. Magnetic scaffold constructing by micro-injection for bone tissue engineering under static magnetic field. J. Mater. Res. Technol. 2024, 29, 3554–3565. [Google Scholar] [CrossRef]
- Shao, J.; Li, J.; Weng, L.; Cheng, K.; Weng, W.; Sun, Q.; Wu, M.; Lin, J. Remote activation of M2 macrophage polarization via magneto-mechanical stimulation to promote osteointegration. ACS Biomater. Sci. Eng. 2023, 9, 2483–2494. [Google Scholar] [CrossRef]
- Kryuchkova, A.; Savin, A.; Kiseleva, A.; Dukhinova, M.; Krivoshapkina, E.; Krivoshapkin, P. Magnetothermal spider silk-based scaffolds for cartilage regeneration. Int. J. Biol. Macromol. 2023, 253, 127246. [Google Scholar] [CrossRef]
- Najafi, P.; Tamjid, E.; Abdolmaleki, P.; Behmanesh, M. Thermomagneto-responsive injectable hydrogel for chondrogenic differentiation of mesenchymal stem cells. Biomater. Adv. 2025, 168, 214115. [Google Scholar] [CrossRef]
- Son, B.; Kim, H.D.; Kim, M.; Kim, J.A.; Lee, J.; Shin, H.; Hwang, N.S.; Park, T.H. Physical stimuli-induced chondrogenic differentiation of mesenchymal stem cells using magnetic nanoparticles. Adv. Healthc. Mater. 2015, 4, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.; Silva, J.C.; Ferreira, F.C.; Portugal, C.A.M. Heparinized acellular hydrogels for magnetically induced wound healing applications. ACS Appl. Mater. Interfaces 2024, 16, 9908–9924. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Liu, X.; Jia, J.; Peng, B.; Xu, N.; Zhang, Q.; Wang, S.; Li, L.; Liu, M.; Huang, Y.; et al. Magnetic field-directed deep thermal therapy via double-layered microneedle patch for promoting tissue regeneration in infected diabetic skin wounds. Adv. Funct. Mater. 2024, 34, 2306357. [Google Scholar] [CrossRef]
- Zhang, E.; Abdel-Mottaleb, M.; Liang, P.; Navarrete, B.; Yildirim, Y.A.; Campos, M.A.; Smith, I.; Wang, P.; Yildirim, B.; Yang, L. Magnetic-field-synchronized wireless modulation of neural activity by magneto-electric nanoparticles. Brain Stimul. 2022, 15, 1451–1462. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E.; Dhawan, G.; Kapoor, R.; Dhawan, V.; Manes, P.K.; Calabrese, V. Nitric oxide and hormesis. Nitric Oxide Biol. Chem. 2023, 133, 1–17. [Google Scholar] [CrossRef]
- Del Sol-Fernandez, S.; Martinez-Vicente, P.; Gomollon-Zueco, P.; Castro-Hinojosa, C.; Gutierrez, L.; Fratila, R.M.; Moros, M. Magnetogenetics: Remote activation of cellular functions triggered by magnetic switches. Nanoscale 2022, 14, 2091–2118. [Google Scholar] [CrossRef]
- Abdel Fattah, A.R.; Kolaitis, N.; Van Daele, K.; Daza, B.; Rustandi, A.G.; Ranga, A. Targeted mechanical stimulation via magnetic nanoparticles guides in vitro tissue development. Nat. Commun. 2023, 14, 5281. [Google Scholar] [CrossRef]
- Bao, S.; Lu, Y.; Zhang, J.; Xue, L.; Zhang, Y.; Wang, P.; Zhang, F.; Gu, N.; Sun, J. Rapid improvement of heart repair in rats after myocardial infarction by precise magnetic stimulation on the vagus nerve with an injectable magnetic hydrogel. Nanoscale 2023, 15, 3532–3541. [Google Scholar] [CrossRef]
- Li, J.; Zhou, T.; Wang, P.; Yin, R.; Zhang, S.; Cao, Y.; Zong, L.; Xiao, M.; Zhang, Y.; Liu, W.; et al. Magnetic stimulation of gigantocellular reticular nucleus with iron oxide nanoparticles combined treadmill training enhanced locomotor recovery by reorganizing cortico-reticulo-spinal circuit. Int. J. Nanomed. 2024, 19, 7473–7492. [Google Scholar] [CrossRef]
- Yi, H.; Rehman, F.U.; Zhao, C.; Liu, B.; He, N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Gu, N. Magnetic responsive scaffolds and magnetic fields in bone repair and regeneration. Front. Mater. Sci. 2014, 8, 20–31. [Google Scholar] [CrossRef]
- Bai, Y.; Li, X.; Wu, K.; Heng, B.C.; Zhang, X.; Deng, X. Biophysical stimuli for promoting bone repair and regeneration. Med. Rev. 2025, 5, 1–22. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, F.; Guo, Y.; Deng, X.; Liu, W. Metabolic mechanism of osteogenic differentiation of bone marrow mesenchymal stem cell regulated by magneto-electric microenvironment. Small Struct. 2024, 6, 2400466. [Google Scholar] [CrossRef]
- Shan, C.; Xu, Y.; Li, S. Investigation of the photothermal performance of the composite scaffold containing light-heat-sensitive nanomaterial SiO2@Fe3O4. Appl. Sci. 2024, 14, 4911. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Zhu, Y.; Chen, F.; Chen, Z.; Guo, L.; Wu, P.; Li, G.; Zhang, C.; Wei, R.; et al. Mild photothermal-stimulation based on injectable and photocurable hydrogels orchestrates immunomodulation and osteogenesis for high-performance bone regeneration. Small 2023, 19, 2300111. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Li, Z.; Gao, F.; Zhang, Q.; Bianco, A.; Liu, H.; Ge, S.; Ma, B. Materials-mediated in situ physical cues for bone regeneration. Adv. Funct. Mater. 2024, 34, 2306534. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Zhang, G. Biomimicry in biomedical research. Organogenesis 2012, 8, 101–102. [Google Scholar] [CrossRef]
- Brady, M.A.; Talvard, L.; Vella, A.; Ethier, C.R. Bio-inspired design of a magnetically active trilayered scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 1298–1302. [Google Scholar] [CrossRef]
- Ioannidis, K.; Dimopoulos, A.; Decoene, I.; Guilliams, M.; Svitina, H.; Storozhuk, L.; de Oliveira-Silva, R.; Basov, S.; Thanh, N.T.K.; Mourdikoudis, S.; et al. 4D biofabrication of magnetically augmented callus assembloid implants enables rapid endochondral ossification via activation of mechanosensitive pathways. Adv. Sci. 2025, 12, e2413680. [Google Scholar] [CrossRef]
- Perea, H.; Aigner, J.; Hopfner, U.; Wintermantel, E. Direct magnetic tubular cell seeding: A novel approach for vascular tissue engineering. Cells Tissues Organs 2006, 183, 156–165. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Magnan, C.; Pantel, A.; Sotto, A.; Lavigne, J.-P. Polymicrobial biofilm organization of staphylococcus aureus and pseudomonas aeruginosa in a chronic wound environment. Int. J. Mol. Sci. 2022, 23, 10761. [Google Scholar] [CrossRef]
- Lalegani Dezaki, M.; Bodaghi, M. Sustainable 4D printing of magneto-electroactive shape memory polymer composites. Int. J. Adv. Manuf. Technol. 2023, 126, 35–48. [Google Scholar] [CrossRef]
- Mirasadi, K.; Yousefi, M.A.; Jin, L.; Rahmatabadi, D.; Baniassadi, M.; Liao, W.-H.; Bodaghi, M.; Baghani, M. 4D printing of magnetically responsive shape memory polymers: Toward sustainable solutions in soft robotics, wearables, and biomedical devices. Adv. Sci. 2025, e13091. [Google Scholar] [CrossRef]


| Tissue Engineering | Magnetic Effects | Magnetic Materials | Magnetic Field | Application Objects | Results After Magnetic Stimulation | Reference |
|---|---|---|---|---|---|---|
| Nerve repair | Magneto-electric | polypyrrole hydrogel | 20 Hz | Rats with 5 mm sciatic nerve defects | More rapid nerve regeneration and functional recovery | [113] |
| Magneto-electric | Fe3O4@BaTiO3 | 13 mT, 60 Hz | Rats with spinal cord injury | Improve recovery of spinal cord injury | [136] | |
| Magneto-electric | reduced graphene oxide | 400 rpm, RMF | Mesenchymal stem cells | Drive neural differentiation | [137] | |
| Magneto-electric | reduced graphene oxide | 2 mT, 50 Hz | Rats with 10 mm sciatic nerve defects | Comparable to that of autograft | [138] | |
| Magneto-electric | MoCx-Cu | 1 MHz, 3.2 kW | Mice with traumatic brain injury | Angiogenesis, neurogenesis, and functional recovery | [139] | |
| Magneto-mechanical | DMSA@Fe3O4 | 1 mT, SMF | Mice with spinal cord injury | Regulates neural stem cells differentiation and alleviates inflammatory response | [140] | |
| Magneto-mechanical | Fe3O4 | 16.0 T/m | Rats with sciatic nerve crush | Promotes peripheral nerve regeneration by inducing and maintaining repair phenotypes in Schwann cells | [141] | |
| Magneto-thermal | Fe3O4 | 152 kHz, 35 kA m−1 | Dorsal root ganglion | Promotes axonal growth | [142] | |
| Bone repair | Magneto-electric | CoFe2O4@BaCO3 | 100 Oe, 1400 Hz | Mouse bone marrow mesenchymal stem cells | Promote cell proliferation, differentiation and osteogenesis-related gene expression | [143] |
| Magneto-electric | CoFe2O4@BiFeO3 | 13 mT, 1.1 kHz | Human-derived MG63 osteoblast cells | Increase in cell proliferation | [144] | |
| Magneto-thermal | CoFe2O4@MnFe2O4 | 1.35 kA/m, AMF | Rats with skull defects | Enhance osteogenesis and angiogenesis | [145] | |
| Magneto-mechanical | Fe3O4 | 250 mT, 1 Hz | Human bone marrow mesenchymal stem cells | Trigger osteogenic differentiation | [146] | |
| Magneto-mechanical | Fe3O4 | 25–30 mT, SMF | Bone marrow mesenchymal stem cells | Promote the proliferation and adhesion | [147] | |
| Magneto-mechanical | Fe3O4 | 3000 Oe, SMF | Mice with skull defects | Enhance the repair of cranial defect via immunomodulatory | [148] | |
| Cartilage repair | Magneto-thermal | Mn0.9Zn0.1Fe2O4 | 100 Oe, AMF | Human skin postnatal fibroblasts | Promote cell adhesion | [149] |
| Magneto-mechanical | Fe3O4 | 20 mT, SMF | Bone marrow mesenchymal stem cells | Induce chondrogenic differentiation and cartilage regeneration | [150] | |
| Magneto-mechanical | Fe3O4 | 0.25 mT, SMF/60 Hz, RMF | Human bone marrow mesenchymal stem cells | Facilitate the chondrogenic differentiation | [151] | |
| Angiogenesis | Magneto-mechanical | Fe3O4 | 0.08 T, SMF | Mesenchymal stem/stromal cells | Increase the expression of angiogenic cytokines | [152] |
| Wound healing | Magneto-thermal | Fe3O4 | 3 kA/m, 60 kHz | Diabetic mice with full-thickness skin defect | Eliminate bacteria and ROS to promote wound healing | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Gui, L.; Yan, D.; Xia, X.; Xie, Z.; Xue, L. Magnetic-Responsive Material-Mediated Magnetic Stimulation for Tissue Engineering. Magnetochemistry 2025, 11, 82. https://doi.org/10.3390/magnetochemistry11100082
Gu J, Gui L, Yan D, Xia X, Xie Z, Xue L. Magnetic-Responsive Material-Mediated Magnetic Stimulation for Tissue Engineering. Magnetochemistry. 2025; 11(10):82. https://doi.org/10.3390/magnetochemistry11100082
Chicago/Turabian StyleGu, Jiayu, Lijuan Gui, Dixin Yan, Xunrong Xia, Zhuoli Xie, and Le Xue. 2025. "Magnetic-Responsive Material-Mediated Magnetic Stimulation for Tissue Engineering" Magnetochemistry 11, no. 10: 82. https://doi.org/10.3390/magnetochemistry11100082
APA StyleGu, J., Gui, L., Yan, D., Xia, X., Xie, Z., & Xue, L. (2025). Magnetic-Responsive Material-Mediated Magnetic Stimulation for Tissue Engineering. Magnetochemistry, 11(10), 82. https://doi.org/10.3390/magnetochemistry11100082





