Copper and Zinc Accumulation in Young Leaves of Eruca sativa (L.) Grown in Soilless Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Conditions
2.2. Plant Growth and Photosynthesis
2.3. Plant Stress Indicators
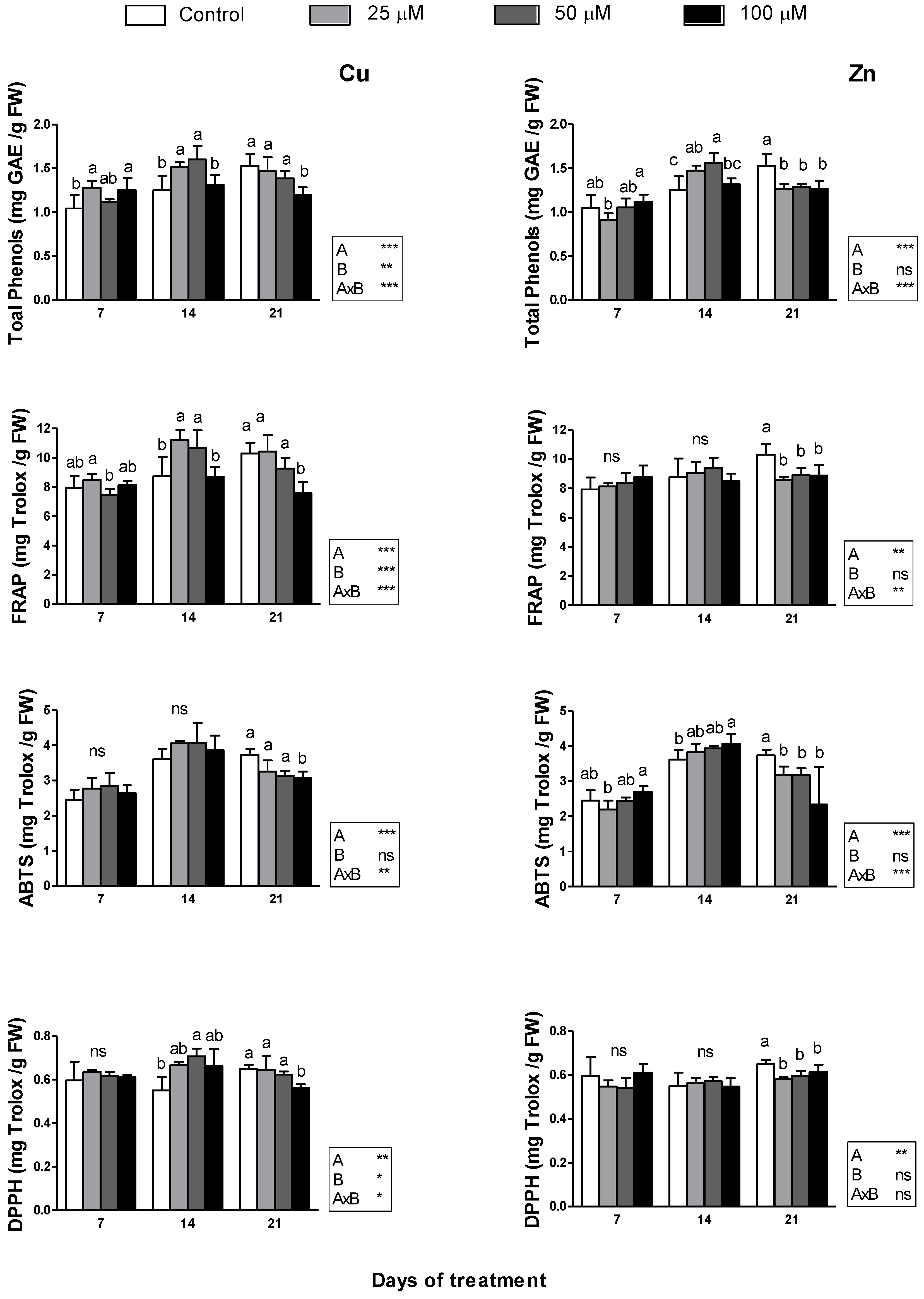
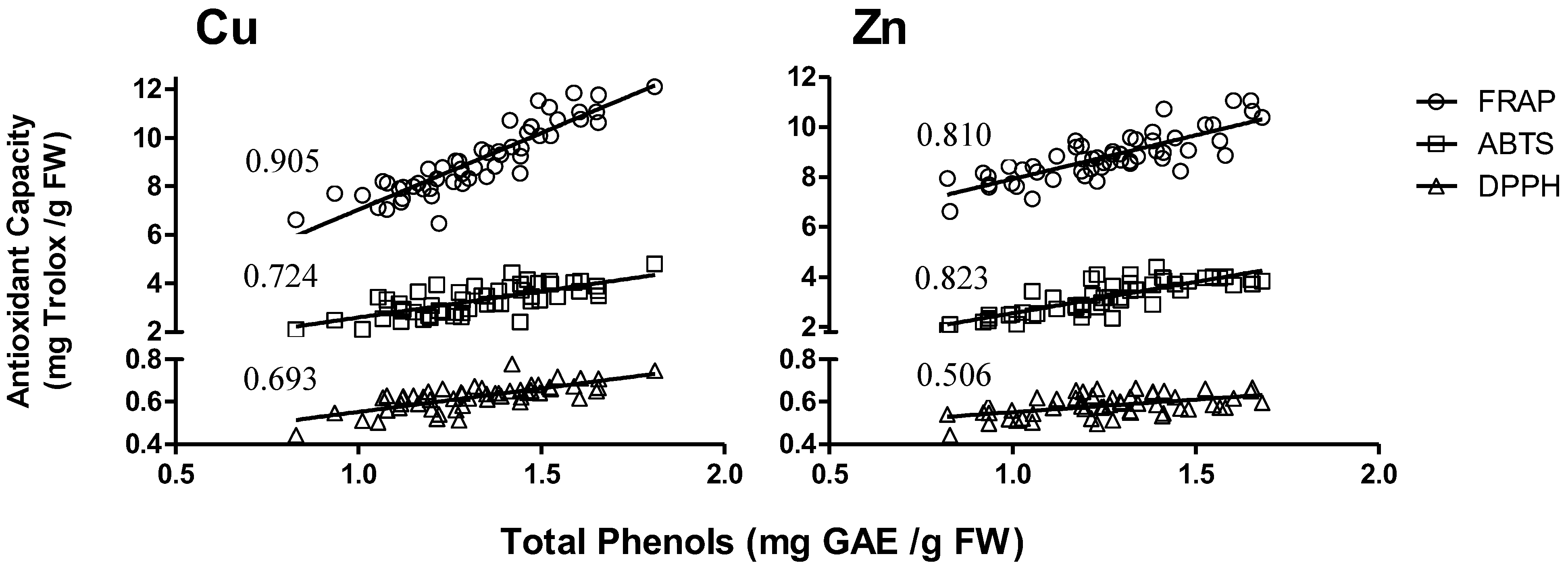
2.4. Antioxidant Activity and Concentration of Total Phenols
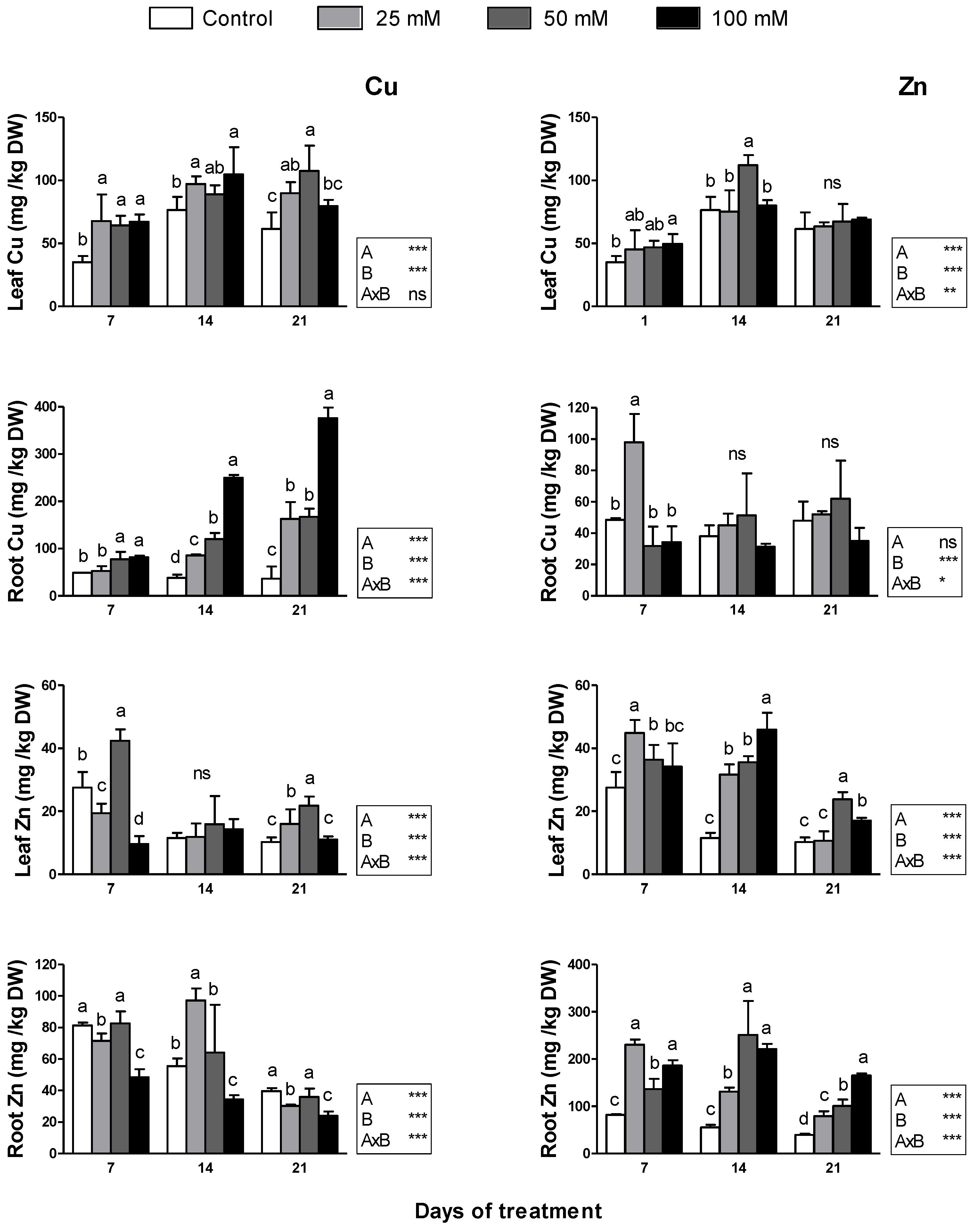
2.5. Mineral Concentration
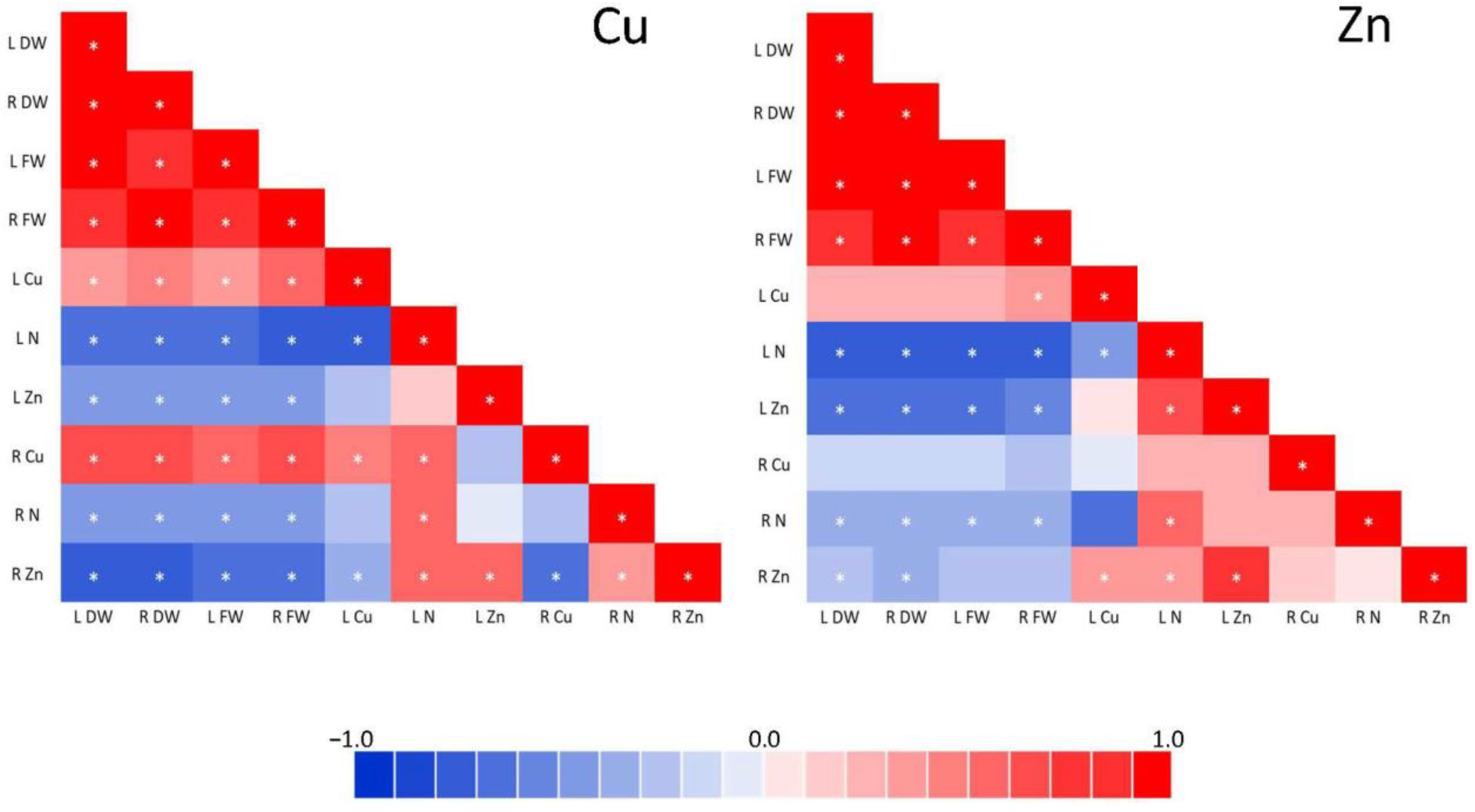
2.6. Statistical Analysis
3. Results
3.1. Plant Growth
3.2. Photosynthesis
3.3. Plant Stress Indicators
3.4. Antioxidant Activity and Total Phenol Concentration
3.5. Mineral Concentration
4. Discussion
4.1. Plant Growth
4.2. Photosynthesis
4.3. Plant Stress Indicators
4.4. Antioxidant Activity and Total Phenol Content
4.5. Mineral Concentration
4.6. Dietary Intake
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. Hindawi J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Nazir, R.; Khan, M.; Masab, M.; Rehman, H.U.; Rauf, N.U.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M.; et al. Accumulation of Heavy Metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water Collected from Tanda Dam kohat. J. Pharm. Sci. Res. 2015, 7, 89–97. [Google Scholar]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Manzoor, J.; Sharma, M.; Wani, K.A. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018, 41, 1744–1763. [Google Scholar] [CrossRef]
- Bae, B.; Park, H.; Kang, S. Quantitative estimation of synergistic toxicity of Cu and Zn on growth of Arabidopsis thaliana by isobolographic method. Toxics 2022, 10, 195. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Garcia, L.; Welchen, E.; Gonzalez, D.H. Mitochondria and copper homeostasis in plants. Mitochondrion 2014, 19, 269–274. [Google Scholar] [CrossRef]
- Jeyalakshmi, S.; Radha, R. A review on diagnosis of nutrient deficiency symptoms in plant leaf image using digital image processing. ICTACT J. Image Video Process. 2017, 7, 1515–1524. [Google Scholar] [CrossRef]
- Barrameda-Medina, Y.; Montesinos-Pereira, D.; Romero, L.; Ruiz, J.M.; Blasco, B. Comparative study of the toxic effect of Zn in Lactuca sativa and Brassica oleracea plants: I. Growth, distribution, and accumulation of Zn, and metabolism of carboxylates. Environ. Exp. Bot. 2014, 107, 98–104. [Google Scholar] [CrossRef]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of High and Low Levels of Plant-Beneficial Heavy Metal Ions on Plant Growth and Development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Maggini, R.; Incrocci, L.; Pardossi, A.; Tzortzakis, N. Copper Tolerance and Accumulation on Pelargonium graveolens L’Hér. Grown Hydroponic Culture. Plants 2021, 10, 1663. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Liščáková, P.; Nawaz, A.; Molnárová, M. Reciprocal effects of copper and zinc in plants. Int. J. Environ. Sci. Technol. 2022, 19, 9297–9312. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar] [PubMed]
- D’Imperio, M.; Montesano, F.F.; Serio, F.; Santovito, E.; Parente, A. Mineral composition and bioaccessibility in rocket and purslane after Zn biofortification process. Foods 2022, 11, 484. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry–A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Pignata, G.; Ertani, A.; Casale, M.; Niñirola, D.; Egea-Gilabert, C.; Fernández, J.A.; Nicola, S. Understanding the Postharvest Phytochemical Composition Fates of Packaged Watercress (Nasturtium officinale R. Br.) Grown in a Floating System and Treated with Bacillus subtilis as PGPR. Plants 2022, 11, 589. [Google Scholar] [CrossRef]
- Thapa, U.; Nandi, S.; Rai, R.; Upadhyay, A. Effect of nitrogen levels and harvest timing on growth, yield and quality of lettuce under floating hydroponic system. J. Plant Nutr. 2022, 45, 2563–2577. [Google Scholar] [CrossRef]
- Karnoutsos, P.; Karagiovanidis, M.; Bantis, F.; Chatzistathis, T.; Koukounaras, A.; Ntinas, G.K. Controlled root-zone temperature effect on baby leaf vegetables yield and quality in a floating system under mild and extreme weather conditions. J. Sci. Food Agric. 2021, 101, 3933–3941. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Tzionis, A.; Prasad, M.; Tzortzakis, N. Alternative soilless media using olive-mill and paper waste for growing ornamental plants. Environ. Sci. Pollut. Res. 2018, 25, 35915–35927. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Economakis, C.D. Shredded maize stems as an alternative substrate medium: Effect on growth, flowering and yield of tomato in soilless culture. J. Veg. Sci. 2005, 11, 57–70. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Athinodorou, F.; Vassiliou, R.; Papadaki, A.; Tzortzakis, N. Deployment of olive-stone waste as a substitute growing medium component for Brassica seedling production in nurseries. Environ. Sci. Pollut. Res. 2019, 26, 35461–35472. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of water stress on lavender and sage biomass production, essential oil composition and biocidal properties against Tetranychus urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Tzanakaki, K.; Economakis, C.D. Effect of origanum oil and vinegar on the maintenance of postharvest quality of tomato. Food Nutr. Sci. 2011, 2, 974–982. [Google Scholar] [CrossRef][Green Version]
- Calori, A.H.; Moraes, L.A.S.; Purquerio, L.F.V.; Factor, T.L.; Júnior, S.L.; Tivelli, S.W. Electric conductivity and space between plants on baby leaf production in NFT hydroponic system inside greenhouse. Acta Hortic. 2015, 1107, 303–310. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and iron agronomic biofortification of brassicaceae microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Chemosphere copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, M.R.; Roqueiro, G.; Tapia, R.; Crespo, D.C.; Bargiela, M.F.; Young, B.J. Chronic toxicity, bioavailability and bioaccumulation of Zn, Cu and Pb in Lactuca sativa exposed to waste from an abandoned gold mine. Chemosphere 2022, 307, 135855. [Google Scholar] [CrossRef] [PubMed]
- Cuba-Díaz, M.; Marín, C.; Castel, K.; Machuca, Á.; Rifo, S. Effect of copper(II) ions on morpho-physiological and biochemical variables in Colobanthus quitensis. J. Soil Sci. Plant Nutr. 2017, 17, 429–440. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-Metal-Induced Reactive Oxygen Species: Phytotoxicity and Physicochemical Changes in Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D., Ed.; Springer: Cham, Switherland, 2014; Volume 232. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A Role for Zinc in Plant Defense Against Pathogens and Herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha Murtaza, G.; Dumat, C.; Shahid, M. Copper Uptake Essentiality Toxicity Detoxification and Risk Assessment in Soil-Plant Environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, R.; Pirzad, A.; Sepehr, E.; Anwar, S.; Khan, S. Physiological and biochemical responses of Phragmites australis to wastewater for different time duration. Acta Physiol. Plant. 2022, 44, 130. [Google Scholar] [CrossRef]
- Lešková, A.; Javot, H.; Giehl, R.F.J. Metal crossroads in plants: Modulation of nutrient acquisition and root development by essential trace metals. J. Exp. Bot. 2022, 73, 1751–1765. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for zinc. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for copper. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- Söderqvist, K.; Rosberg, A.K.; Boqvist, S.; Alsanius, B.; Mogren, L.; Vågsholm, I. Season and Species: Two Possible Hurdles for Reducing the Food Safety Risk of Escherichia coli O157 Contamination of Leafy Vegetables. J. Food Prot. 2019, 82, 247–255. [Google Scholar] [CrossRef] [PubMed]

| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Leaf FW (g) | Root FW (g) | Leaf DW (g) | Root DW (g) | ||||
| GRAND MEAN | 23.53 | 4.245 | 2.285 | 0.3220 | ||||
| Time (days of treatment) | ||||||||
| 7 | 12.48 | 2.893 | c | 1.048 | 0.2147 | |||
| 14 | 23.92 | 4.546 | b | 2.226 | 0.3216 | |||
| 21 | 34.19 | 5.296 | a | 3.582 | 0.4292 | |||
| Concentration (µM) | ||||||||
| 4.00 (Control) | 25.42 | 3.942 | c | 2.396 | 0.3038 | |||
| 25 | 23.89 | 4.394 | ab | 2.221 | 0.3090 | |||
| 50 | 19.82 | 3.993 | bc | 1.917 | 0.2933 | |||
| 100 | 25.01 | 4.651 | a | 2.608 | 0.3811 | |||
| Time by Concentration | ||||||||
| 7, Control | 13.20 | ab | 2.708 | 1.076 | ab | 0.1788 | b | |
| 7, 25 µM | 10.94 | b | 2.627 | 0.920 | b | 0.1702 | b | |
| 7, 50 µM | 11.36 | b | 3.026 | 1.014 | b | 0.2466 | a | |
| 7, 100 µM | 14.44 | a | 3.210 | 1.183 | a | 0.2632 | a | |
| 14, Control | 25.91 | a | 4.015 | 2.345 | a | 0.3094 | b | |
| 14, 25 µM | 25.05 | a | 4.761 | 2.214 | a | 0.3034 | b | |
| 14, 50 µM | 20.77 | b | 4.065 | 1.819 | b | 0.2666 | b | |
| 14, 100 µM | 23.95 | ab | 5.344 | 2.524 | a | 0.4068 | a | |
| 21, Control | 37.14 | a | 5.103 | 3.767 | a | 0.4233 | a | |
| 21, 25 µM | 35.67 | a | 5.793 | 3.530 | a | 0.4534 | a | |
| 21, 50 µM | 27.33 | b | 4.887 | 2.917 | b | 0.3668 | b | |
| 21, 100 µM | 36.63 | a | 5.400 | 4.116 | a | 0.4734 | a | |
| MAIN EFFECTS | ||||||||
| A: Time | *** | *** | *** | *** | ||||
| B: Concentration | *** | ** | *** | *** | ||||
| INTERACTION | ||||||||
| A × B | * | ns | * | ** | ||||
| (B) | ||||||||
| Zn | Leaf FW (g) | Root FW (g) | Leaf DW (g) | Root DW (g) | ||||
| GRAND MEAN | 26.41 | 4.173 | 2.462 | 0.3086 | ||||
| Time (days of treatment) | ||||||||
| 7 | 14.36 | 2.881 | c | 1.140 | 0.1924 | |||
| 14 | 25.22 | 4.264 | b | 2.185 | 0.2899 | |||
| 21 | 39.67 | 5.375 | a | 4.060 | 0.4434 | |||
| Concentration (µM) | ||||||||
| 4.10 (Control) | 25.42 | 3.942 | bc | 2.396 | 0.3038 | |||
| 25 | 29.23 | 4.624 | a | 2.777 | 0.3456 | |||
| 50 | 21.94 | 3.756 | c | 2.008 | 0.2767 | |||
| 100 | 29.07 | 4.373 | ab | 2.665 | 0.3038 | |||
| Time by Concentration | ||||||||
| 7, Control | 13.20 | b | 2.708 | 1.076 | a | 0.1788 | b | |
| 7, 25 µM | 16.04 | a | 3.047 | 1.220 | a | 0.2200 | a | |
| 7, 50 µM | 13.89 | ab | 2.727 | 1.120 | a | 0.1934 | a | |
| 7, 100 µM | 14.33 | ab | 3.041 | 1.145 | a | 0.1774 | b | |
| 14, Control | 25.91 | ab | 4.015 | 2.345 | ab | 0.3094 | a | |
| 14, 25 µM | 29.09 | a | 4.833 | 2.500 | a | 0.3168 | a | |
| 14, 50 µM | 21.86 | b | 3.868 | 1.871 | b | 0.2634 | a | |
| 14, 100 µM | 23.99 | b | 4.342 | 2.023 | ab | 0.2700 | a | |
| 21, Control | 37.14 | ab | 5.103 | 3.767 | bc | 0.4233 | bc | |
| 21, 25 µM | 42.56 | ab | 5.991 | 4.613 | ab | 0.5000 | a | |
| 21, 50 µM | 30.06 | b | 4.673 | 3.034 | c | 0.3734 | c | |
| 21, 100 µM | 45.84 | a | 5.734 | 4.828 | a | 0.4768 | ab | |
| MAIN EFFECTS | ||||||||
| A: Time | *** | *** | *** | *** | ||||
| B: Concentration | *** | ** | *** | ** | ||||
| INTERACTION | ||||||||
| A × B | *** | ns | *** | * | ||||
| (A) | ||||||
|---|---|---|---|---|---|---|
| Cu | Fv/Fm | Stomatal Resistance (s/cm) | Total Chl (µg/g FW) | |||
| GRAND MEAN | 0.832 | 1.052 | 176 | |||
| Time (days of treatment) | ||||||
| 7 | 0.825 | b | 0.868 | 204 | a | |
| 14 | 0.846 | a | 1.310 | 191 | a | |
| 21 | 0.826 | b | 0.978 | 133 | a | |
| Concentration (µM) | ||||||
| 4.00 (Control) | 0.835 | a | 1.288 | 165 | b | |
| 25 | 0.830 | a | 0.959 | 176 | ab | |
| 50 | 0.834 | a | 0.915 | 167 | b | |
| 100 | 0.829 | a | 1.044 | 196 | a | |
| Time by Concentration | ||||||
| 7, Control | 0.823 | 0.947 | a | 188 | ||
| 7, 25 µM | 0.820 | 0.773 | a | 195 | ||
| 7, 50 µM | 0.827 | 0.815 | a | 198 | ||
| 7, 100 µM | 0.828 | 0.935 | a | 233 | ||
| 14, Control | 0.854 | 1.925 | a | 184 | ||
| 14, 25 µM | 0.841 | 1.227 | ab | 209 | ||
| 14, 50 µM | 0.848 | 1.252 | ab | 173 | ||
| 14, 100 µM | 0.841 | 0.837 | b | 199 | ||
| 21, Control | 0.828 | 0.993 | b | 123 | ||
| 21, 25 µM | 0.829 | 0.878 | b | 124 | ||
| 21, 50 µM | 0.829 | 0.678 | b | 129 | ||
| 21, 100 µM | 0.819 | 1.360 | a | 156 | ||
| MAIN EFFECTS | ||||||
| A: Time | *** | * | *** | |||
| B: Concentration | ns | ns | * | |||
| INTERACTION | ||||||
| A × B | ns | * | ns | |||
| (B) | ||||||
| Zn | Fv/Fm | Stomatal Resistance (s/cm) | Total Chl (µg/g FW) | |||
| GRAND MEAN | 0.831 | 1.395 | 177 | |||
| Time (days of treatment) | ||||||
| 7 | 0.832 | ab | 0.928 | b | 189 | |
| 14 | 0.835 | a | 2.259 | a | 203 | |
| 21 | 0.824 | b | 0.996 | b | 138 | |
| Concentration (µM) | ||||||
| 4.10 (Control) | 0.835 | 1.288 | b | 165 | ||
| 25 | 0.832 | 1.793 | a | 189 | ||
| 50 | 0.826 | 1.000 | b | 175 | ||
| 100 | 0.829 | 1.497 | ab | 178 | ||
| Time by Concentration | ||||||
| 7, Control | 0.823 | 0.947 | 188 | bc | ||
| 7, 25 µM | 0.840 | 1.318 | 148 | cd | ||
| 7, 50 µM | 0.831 | 0.600 | 200 | ab | ||
| 7, 100 µM | 0.836 | 0.848 | 222 | a | ||
| 14, Control | 0.854 | 1.925 | 184 | bc | ||
| 14, 25 µM | 0.827 | 2.777 | 243 | a | ||
| 14, 50 µM | 0.828 | 1.637 | 177 | bc | ||
| 14, 100 µM | 0.832 | 2.698 | 207 | ab | ||
| 21, Control | 0.828 | 0.993 | 123 | b | ||
| 21, 25 µM | 0.831 | 1.283 | 177 | a | ||
| 21, 50 µM | 0.819 | 0.763 | 148 | ab | ||
| 21, 100 µM | 0.820 | 0.944 | 106 | b | ||
| MAIN EFFECTS | ||||||
| A: Time | ns | *** | *** | |||
| B: Concentration | ns | ** | ns | |||
| INTERACTION | ||||||
| A × B | ns | ns | *** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysargyris, A.; Maggini, R.; Incrocci, L.; Pardossi, A.; Tzortzakis, N. Copper and Zinc Accumulation in Young Leaves of Eruca sativa (L.) Grown in Soilless Culture. Horticulturae 2023, 9, 976. https://doi.org/10.3390/horticulturae9090976
Chrysargyris A, Maggini R, Incrocci L, Pardossi A, Tzortzakis N. Copper and Zinc Accumulation in Young Leaves of Eruca sativa (L.) Grown in Soilless Culture. Horticulturae. 2023; 9(9):976. https://doi.org/10.3390/horticulturae9090976
Chicago/Turabian StyleChrysargyris, Antonios, Rita Maggini, Luca Incrocci, Alberto Pardossi, and Nikolaos Tzortzakis. 2023. "Copper and Zinc Accumulation in Young Leaves of Eruca sativa (L.) Grown in Soilless Culture" Horticulturae 9, no. 9: 976. https://doi.org/10.3390/horticulturae9090976
APA StyleChrysargyris, A., Maggini, R., Incrocci, L., Pardossi, A., & Tzortzakis, N. (2023). Copper and Zinc Accumulation in Young Leaves of Eruca sativa (L.) Grown in Soilless Culture. Horticulturae, 9(9), 976. https://doi.org/10.3390/horticulturae9090976











