Effect of Ozone Stresses on Growth and Secondary Plant Metabolism of Brassica campestris L. ssp. chinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Setup
2.2. Ozone Gas Treatments
2.3. Determination of Plant Growth Variables
2.4. Determination of Photopigments
2.5. Determination of GLSs
2.6. Statistical Calculations
3. Results
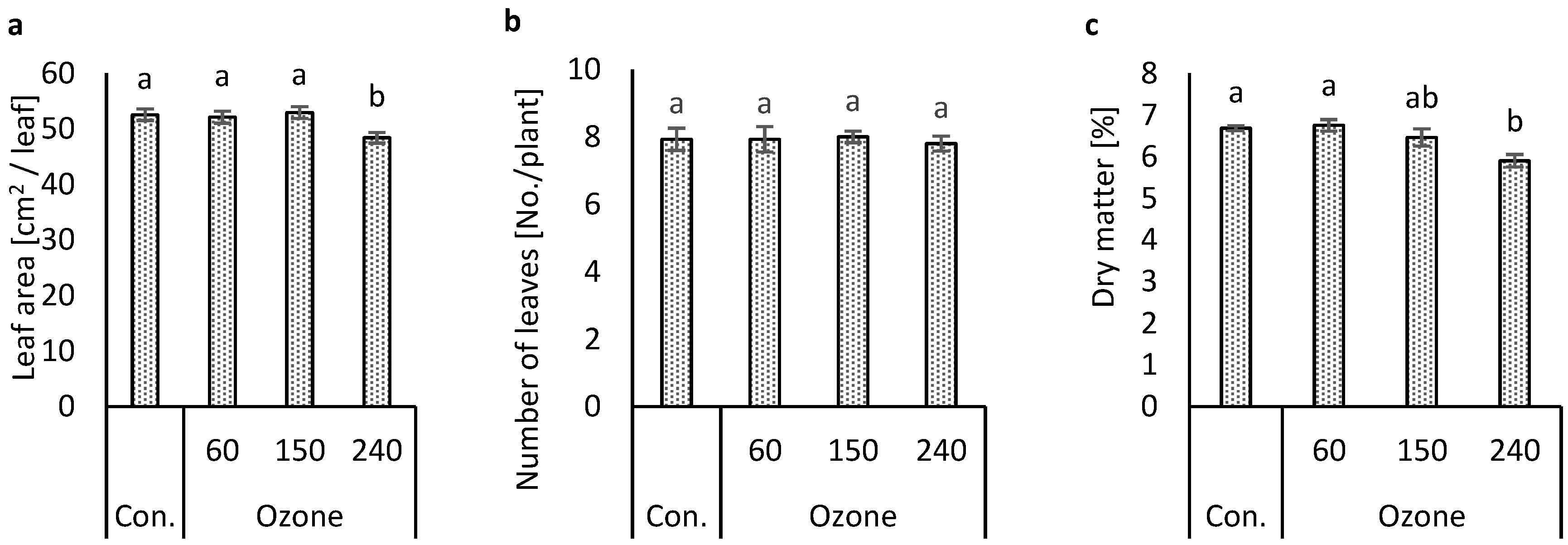
3.1. Effects of Ozone Concentrations on Growth Variables
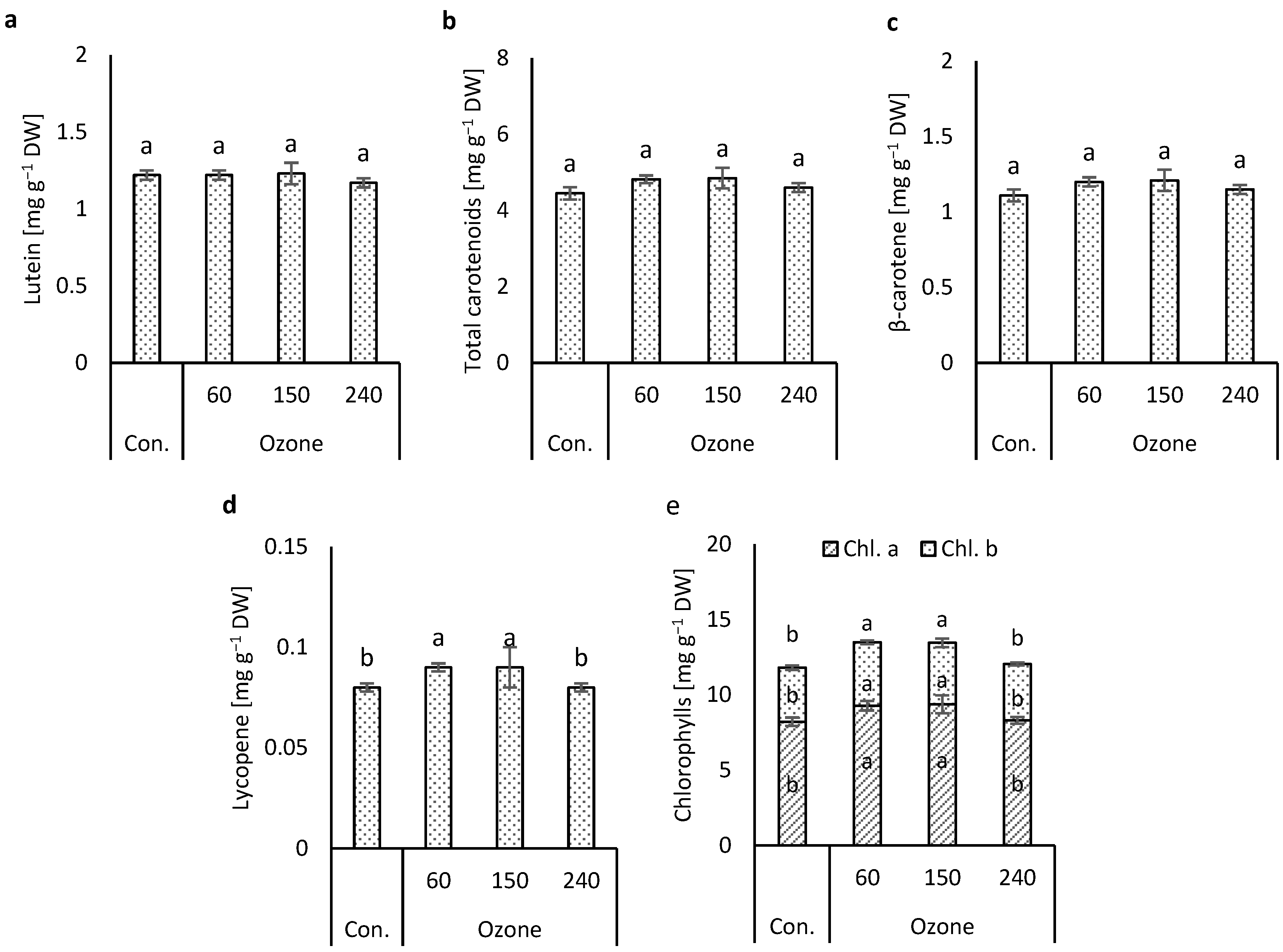
3.2. Effects of Ozone Concentrations on Photopigments
3.3. Effects of Ozone Concentrations on Glucosinolate Profile
4. Discussion
4.1. Plant Growth Variables Affected by Ozone Concentration Treatments
4.2. Photopigments Effected by Ozone Concentration Treatments
4.3. Glucosinolate Profile Affected by Ozone Concentration Treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unger, N. Global climate forcing by criteria air pollutants. Annu. Rev. Environ. Resour. 2012, 37, 1–24. [Google Scholar] [CrossRef]
- Bastl, K.; Kmenta, M.; Berger, U.W. Defining pollen seasons: Background and recommendations. Curr. Allergy Asthma Rep. 2018, 18, 73. [Google Scholar] [CrossRef]
- Hazari, M.S.; Stratford, K.M.; Krantz, Q.T.; King, C.; Krug, J.; Farraj, A.K.; Gilmour, M.I. Comparative cardiopulmonary effects of particulate matter-and ozone-enhanced smog atmospheres in mice. Environ. Sci. Technol. 2018, 52, 3071–3080. [Google Scholar] [CrossRef]
- Pandey, A.K.; Ghosh, A.; Agrawal, M.; Agrawal, S. Effect of elevated ozone and varying levels of soil nitrogen in two wheat (Triticum aestivum L.) cultivars: Growth, gas-exchange, antioxidant status, grain yield and quality. Ecotoxicol. Environ. Saf. 2018, 158, 59–68. [Google Scholar] [CrossRef]
- Singh, A.A.; Fatima, A.; Mishra, A.K.; Chaudhary, N.; Mukherjee, A.; Agrawal, M.; Agrawal, S.B. Assessment of ozone toxicity among 14 Indian wheat cultivars under field conditions: Growth and productivity. Environ. Monit. Assess. 2018, 190, 1–14. [Google Scholar] [CrossRef]
- Oksanen, E.; Pandey, V.; Pandey, A.; Keski-Saari, S.; Kontunen-Soppela, S.; Sharma, C. Impacts of increasing ozone on Indian plants. Environ. Pollut. 2013, 177, 189–200. [Google Scholar] [CrossRef]
- Leisner, C.P.; Ainsworth, E.A. Quantifying the effects of ozone on plant reproductive growth and development. Glob. Chang. Biol. 2012, 18, 606–616. [Google Scholar] [CrossRef]
- Booker, F.; Muntifering, R.; McGrath, M.; Burkey, K.; Decoteau, D.; Fiscus, E.; Manning, W.; Krupa, S.; Chappelka, A.; Grantz, D. The ozone component of global change: Potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. J. Integr. Plant Biol. 2009, 51, 337–351. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Lelieveld, J. Human impacts on atmospheric chemistry. Annu. Rev. Earth Planet. Sci. 2001, 29, 17–45. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Clemitshaw, K.C. Ozone and other secondary photochemical pollutants: Chemical processes governing their formation in the planetary boundary layer. Atmos. Environ. 2000, 34, 2499–2527. [Google Scholar] [CrossRef]
- Kley, D.; Kleinmann, M.; Sanderman, H.; Krupa, S. Photochemical oxidants: State of the science. Environ. Pollut. 1999, 100, 19–42. [Google Scholar] [CrossRef]
- Stathopoulou, E.; Mihalakakou, G.; Santamouris, M.; Bagiorgas, H. On the impact of temperature on tropospheric ozone concentration levels in urban environments. J. Earth Syst. Sci. 2008, 117, 227–236. [Google Scholar] [CrossRef]
- Jeon, W.-B.; Lee, S.-H.; Lee, H.; Park, C.; Kim, D.-H.; Park, S.-Y. A study on high ozone formation mechanism associated with change of NOx/VOCs ratio at a rural area in the Korean Peninsula. Atmos. Environ. 2014, 89, 10–21. [Google Scholar] [CrossRef]
- Ashmore, M. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005, 28, 949–964. [Google Scholar] [CrossRef]
- Kangasjärvi, J.; Jaspers, P.; Kollist, H. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2005, 28, 1021–1036. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Morris, K. Senescence and cell death in Brassica napus and Arabidopsis. Program. Cell Death Anim. Plants 2000, 52, 163–174. [Google Scholar]
- Pell, E.J.; Schlagnhaufer, C.D.; Arteca, R.N. Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant. 1997, 100, 264–273. [Google Scholar] [CrossRef]
- Han, Y.J.; Gharibeshghi, A.; Mewis, I.; Förster, N.; Beck, W.; Ulrichs, C. Plant responses to ozone: Effects of different ozone exposure durations on plant growth and biochemical quality of Brassica campestris L. ssp. chinensis. Sci. Hortic. 2020, 262, 108921. [Google Scholar] [CrossRef]
- Pääkkönen, E.; Günthardt-Goerg, M.; Holopainen, T. Responses of Leaf Processes in a Sensitive Birch (Betula pendula Roth.) Clone to Ozone Combined with Drought. Ann. Bot. 1998, 82, 49–59. [Google Scholar] [CrossRef]
- Heck, W.W.; Taylor, O.C.; Tingey, D.T. Assessment of Crop Loss from Air Pollutants; Elsevier Applied Science: London, UK; New York, NY, USA, 1988. [Google Scholar]
- Schenone, G.; Botteschi, G.; Fumagalli, I.; Montinaro, F. Effects of ambient air pollution in open-top chambers on bean (Phaseolus vulgaris L.) I. Effects on growth and yield. New Phytol. 1992, 122, 689–697. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Liu, W.; Zhao, J.; Yu, S.; Jia, H.; Zhang, C.; Li, Y. Incorporating in vitro bioaccessibility into human health risk assessment of heavy metals and metalloid (As) in soil and pak choi (Brassica chinensis L.) from greenhouse vegetable production fields in a megacity in Northwest China. Food Chem. 2022, 373, 131488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, S.; Chen, Y.; Xu, H.; Li, Y.; Zhang, Y.; Luan, F. Ozone sensitivity of four Pakchoi cultivars with different leaf colors: Physiological and biochemical mechanisms. Photosynthetica 2017, 55, 478–490. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Black, V.; Stewart, C.; Roberts, J.; Black, C. Ozone affects gas exchange, growth and reproductive development in Brassica campestris (Wisconsin Fast Plants). New Phytol. 2007, 176, 150–163. [Google Scholar] [CrossRef]
- De Bock, M.; de Beeck, M.O.; De Temmerman, L.; Guisez, Y.; Ceulemans, R.; Vandermeiren, K. Ozone dose–response relationships for spring oilseed rape and broccoli. Atmos. Environ. 2011, 45, 1759–1765. [Google Scholar] [CrossRef]
- Khaling, E.; Papazian, S.; Poelman, E.H.; Holopainen, J.K.; Albrectsen, B.R.; Blande, J.D. Ozone affects growth and development of Pieris brassicae on the wild host plant Brassica nigra. Environ. Pollut. 2015, 199, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Gielen, B.; Vandermeiren, K.; Horemans, N.; D’haese, D.; Serneels, R.; Valcke, R. Chlorophyll a fluorescence imaging of ozone-stressed Brassica napus L. plants differing in glucosinolate concentrations. Plant Biol. 2006, 8, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Himanen, S.J.; Nissinen, A.; Auriola, S.; Poppy, G.M.; Stewart, C.N.; Holopainen, J.K.; Nerg, A.-M. Constitutive and herbivore-inducible glucosinolate concentrations in oilseed rape (Brassica napus) leaves are not affected by Bt Cry1Ac insertion but change under elevated atmospheric CO2 and O3. Planta 2008, 227, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K. Hydroponics; Hakusa: Tokyo, Japan, 1978. [Google Scholar]
- Hörmann, V.; Brenske, K.-R.; Ulrichs, C. Assessment of filtration efficiency and physiological responses of selected plant species to indoor air pollutants (toluene and 2-ethylhexanol) under chamber conditions. Environ. Sci. Pollut. Res. 2018, 25, 447–458. [Google Scholar] [CrossRef]
- Martins, L.L.; Mourato, M.P.; Cardoso, A.I.; Pinto, A.P.; Mota, A.M.; de Lurdes, S.; Gonçalves, M.; de Varennes, A. Oxidative stress induced by cadmium in Nicotiana tabacum L.: Effects on growth parameters, oxidative damage and antioxidant responses in different plant parts. Acta Physiol. Plant. 2011, 33, 1375–1383. [Google Scholar] [CrossRef]
- Maas, E. Salt tolerance of plants. Appl. Agric. Res. 1986, 1, 12–25. [Google Scholar]
- AirNow. Air Quality Guide for Ozone. Available online: https://www.airnow.gov (accessed on 29 June 2016).
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Geneva, Switzerland, 2006; Available online: https://apps.who.int/iris/bitstream/handle/10665/69477/WHO_SDE_PHE_OEH_06.02_eng.pdf?sequence=1 (accessed on 17 June 2012).
- Goodwin, T.W.; Britton, G. Distribution and analysis of carotenoids. In Plant Pigment; Academic Press: London, UK, 1988; pp. 61–132. [Google Scholar]
- Mewis, I.; Appel, H.M.; Hom, A.; Raina, R.; Schultz, J.C. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 2005, 138, 1149–1162. [Google Scholar] [CrossRef]
- SAS. Institute Inc. SAS/STAT 15.1 User’s Guide, SAS 9.4 and SAS Viya 3.4 Programming Documentation. Available online: https://documentation.sas.com/doc/en/pgmsascdc/9.4_3.4/statug/titlepage.htm (accessed on 30 March 2021).
- Menéndez, A.I.; Gundel, P.E.; Lores, L.M.; Martínez-Ghersa, M.A. Assessing the impacts of intra-and interspecific competition between Triticum aestivum and Trifolium repens on the species’ responses to ozone. Botany 2017, 95, 923–932. [Google Scholar] [CrossRef]
- Pääkkönen, E.; Paasisalo, S.; Holopainen, T.; Kärenlamp, L. Growth and stomatal responses of birch (Betula pendula Roth.) clones to ozone in open-air and chamber fumigations. New Phytol. 1993, 125, 615–623. [Google Scholar] [CrossRef]
- Feng, Z.; Pang, J.; Kobayashi, K.; Zhu, J.; Ort, D.R. Differential responses in two varieties of winter wheat to elevated ozone concentration under fully open-air field conditions. Glob. Chang. Biol. 2011, 17, 580–591. [Google Scholar] [CrossRef]
- Ferdinand, J.; Fredericksen, T.; Kouterick, K.; Skelly, J. Leaf morphology and ozone sensitivity of two open pollinated genotypes of black cherry (Prunus serotina) seedlings. Environ. Pollut. 2000, 108, 297–302. [Google Scholar] [CrossRef]
- Saunier, A.; Blande, J.D. The effect of elevated ozone on floral chemistry of Brassicaceae species. Environ. Pollut. 2019, 255, 113257. [Google Scholar] [CrossRef]
- Wang, D.; Karnosky, D.F.; Bormann, F.H. Effects of ambient ozone on the productivity of Populus tremuloides Michx. grown under field conditions. Can. J. For. Res. 1986, 16, 47–55. [Google Scholar] [CrossRef]
- Ottosson, S.; Wallin, G.; Skärby, L.; Karlsson, P.-E.; Medin, E.-L.; Räntfors, M.; Pleijel, H.; Selldén, G. Four years of ozone exposure at high or low phosphorus reduced biomass in Norway spruce. Trees 2003, 17, 299–307. [Google Scholar] [CrossRef]
- Karlsson, P.; Medin, E.; Selldén, G.; Wallin, G.; Ottosson, S.; Pleijel, H.; Skärby, L. Impact of ozone and reduced water supply on the biomass accumulation of Norway spruce saplings. Environ. Pollut. 2002, 119, 237–244. [Google Scholar] [CrossRef]
- Roberts, H.R.; Dodd, I.C.; Hayes, F.; Ashworth, K. Chronic tropospheric ozone exposure reduces seed yield and quality in spring and winter oilseed rape. Agric. For. Meteorol. 2022, 316, 108859. [Google Scholar] [CrossRef]
- Skärby, L.; Troeng, E.; Boström, C.-Å. Ozone uptake and effects on transpiration, net photosynthesis, and dark respiration in Scots pine. For. Sci. 1987, 33, 801–808. [Google Scholar]
- Zhang, W.; Wang, G.; Liu, X.; Feng, Z. Effects of elevated O3 exposure on seed yield, N concentration and photosynthesis of nine soybean cultivars (Glycine max (L.) Merr.) in Northeast China. Plant Sci. 2014, 226, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Reig-Armiñana, J.; Calatayud, V.; Cerveró, J.; Garcıa-Breijo, F.; Ibars, A.; Sanz, M. Effects of ozone on the foliar histology of the mastic plant (Pistacia lentiscus L.). Environ. Pollut. 2004, 132, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Greitner, C.S.; Pell, E.J.; Winner, W.E. Analysis of aspen foliage exposed to multiple stresses: Ozone, nitrogen deficiency and drought. New Phytol. 1994, 127, 579–589. [Google Scholar] [CrossRef]
- Singh, P.; Agrawal, M.; Agrawal, S.B. Evaluation of physiological, growth and yield responses of a tropical oil crop (Brassica campestris L. var. Kranti) under ambient ozone pollution at varying NPK levels. Environ. Pollut. 2009, 157, 871–880. [Google Scholar] [CrossRef]
- Betzelberger, A.M.; Gillespie, K.M.; Mcgrath, J.M.; Koester, R.P.; Nelson, R.L.; Ainsworth, E.A. Effects of chronic elevated ozone concentration on antioxidant capacity, photosynthesis and seed yield of 10 soybean cultivars. Plant Cell Environ. 2010, 33, 1569–1581. [Google Scholar] [CrossRef]
- Burkey, K.O.; Carter Jr, T.E. Foliar resistance to ozone injury in the genetic base of US and Canadian soybean and prediction of resistance in descendent cultivars using coefficient of parentage. Field Crops Res. 2009, 111, 207–217. [Google Scholar] [CrossRef]
- Vandermeiren, K.; Black, C.; Pleijel, H.; De Temmerman, L. Impact of rising tropospheric ozone on potato: Effects on photosynthesis, growth, productivity and yield quality. Plant Cell Environ. 2005, 28, 982–996. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D. Differential response of photosynthetic pigments, rubisco activity and rubisco protein of Arabidopsis thaliana exposed to UVB and ozone. Photochem. Photobiol. 1995, 62, 727–735. [Google Scholar] [CrossRef]
- Eckardt, N.; Pell, E. O3-induced degradation of Rubisco protein and loss of Rubisco mRNA in relation to leaf age in Solanum tuberosum L. New Phytol. 1994, 127, 741–748. [Google Scholar] [CrossRef]
- Skärby, L.; Ro-Poulsen, H.; Wellburn, F.A.; Sheppard, L.J. Impacts of ozone on forests: A European perspective. New Phytol. 1998, 139, 109–122. [Google Scholar] [CrossRef]
- Coleman, M.; Dickson, R.; Isebrands, J.; Karnosky, D. Root growth and physiology of potted and field-grown trembling aspen exposed to tropospheric ozone. Tree Physiol. 1996, 16, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Saitanis, C.; Riga-Karandinos, A.; Karandinos, M. Effects of ozone on chlorophyll and quantum yield of tobacco (Nicotiana tabacum L.) varieties. Chemosphere 2001, 42, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Krizek, D.T.; Agrawal, S.B.; Kramer, G.F.; Lee, E.H.; Mirecki, R.M.; Rowland, R.A. Influence of inverse day/night temperature on ozone sensitivity and selected morphological and physiological responses of cucumber. J. Am. Soc. Hortic. Sci. 1993, 118, 649–654. [Google Scholar] [CrossRef]
- Han, Y.J.; Gharibeshghi, A.; Mewis, I.; Förster, N.; Beck, W.; Ulrichs, C. Effect of different durations of moderate ozone exposure on secondary metabolites of Brassica campestris L. ssp. chinensis. J. Hortic. Sci. Biotechnol. 2021, 96, 110–120. [Google Scholar] [CrossRef]
- Landolt, W.; Günthardt-Goerg, M.; Pfenninger, I.; Einig, W.; Hampp, R.; Maurer, S.; Matyssek, R. Effect of fertilization on ozone-induced changes in the metabolism of birch (Betula pendula) leaves. New Phytol. 1997, 137, 389–397. [Google Scholar] [CrossRef]
- Maurer, S.; Matyssek, R.; GuÈnthardt-Goerg, M.S.; Landolt, W.; Einig, W. Nutrition and the ozone sensitivity of birch (Betula pendula). Trees 1997, 12, 1–10. [Google Scholar] [CrossRef]
- Singh, A.A.; Ghosh, A.; Agrawal, M.; Agrawal, S.B. Secondary metabolites responses of plants exposed to ozone: An update. Environ. Sci. Pollut. Res. 2023, 30, 88281–88312. [Google Scholar] [CrossRef]
- Thwe, A.A.; Vercambre, G.; Gautier, H.; Pagès, L.; Jourdan, C.; Gay, F.; Kasemsap, P. Dynamic shoot and root growth at different developmental stages of tomato (Solanum lycopersicum Mill.) under acute ozone stress. Sci. Hortic. 2013, 150, 317–325. [Google Scholar] [CrossRef]
- Lee, J.G.; Kwak, M.J.; Jeong, S.G.; Woo, S.Y. Indivisual and interactive effects of elevated ozone and temperature on plant responses. Horticulturae 2020, 8, 211. [Google Scholar] [CrossRef]
- Baier, M.; Kandlbinder, A.; Golldack, D.; Dietz, K.J. Oxidative stress and ozone: Perception, signalling and response. Plant Cell Environ. 2005, 28, 1012–1020. [Google Scholar] [CrossRef]
- Rao, M.V.; Davis, K.R. The physiology of ozone induced cell death. Planta 2001, 213, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.J.; Van Dam, N.M.; Loon, J.v. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Vanstone, V.; Davies, K.; Kirkegaard, J.; Rathjen, A. Reduced susceptibility of Brassica napus to Pratylenchus neglectus in plants with elevated root levels of 2-phenylethyl glucosinolate. J. Nematol. 1999, 31, 291. [Google Scholar]
- Koritsas, V.; Lewis, J.; Fenwick, G. Glucosinolate responses of oilseed rape, mustard and kale to mechanical wounding and infestation by cabbage stem flea beetle (Psylliodes chrysocephala). Ann. Appl. Biol. 1991, 118, 209–221. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef]
- Mikkelsen, M.D.; Petersen, B.L.; Glawischnig, E.; Jensen, A.B.; Andreasson, E.; Halkier, B.A. Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol. 2003, 131, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Brown, P.; Figuth, A.; Pedersen, D.; Gershenzon, J.; Mitchell-Olds, T. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 2001, 126, 811–825. [Google Scholar] [CrossRef]
- Vandermeiren, K.; De Bock, M.; Horemans, N.; Guisez, Y.; Ceulemans, R.; De Temmerman, L. Ozone effects on yield quality of spring oilseed rape and broccoli. Atmos. Environ. 2012, 47, 76–83. [Google Scholar] [CrossRef]
- Mithen, R.F. Glucosinolates and their degradation products. Advances in Botanical Research. 2001, 35, 213–232. [Google Scholar]
- Bradburne, R.P.; Mithen, R. Glucosinolate genetics and the attraction of the aphid parasitoid Diaeretiella rapae to Brassica. Proc. R. Soc. London. Ser. B Biol. Sci. 2000, 267, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D. Control of soil-borne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- Cole, R. Abiotic induction of changes to glucosinolate profiles in Brassica species and increased resistance to the specialist aphid Brevicoryne brassicae. In Proceedings of the 9th International Symposium on Insect-Plant Relationships; Springer Netherlands: Dordrecht, The Netherlands, 1996; pp. 228–230. [Google Scholar]
- Kiddle, G.A.; Doughty, K.J.; Wallsgrove, R.M. Salicylic acid-induced accumulation of glucosinolates in oilseed rape (Brassica napus L.) leaves. J. Exp. Bot. 1994, 45, 1343–1346. [Google Scholar] [CrossRef]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Tapio Palva, E. Altering glucosinolate profiles modulates disease resistance in plants. Plant J. 2006, 46, 758–767. [Google Scholar] [CrossRef]
- Loivamäki, M.; Holopainen, J.K.; Nerg, A.-M. Chemical changes induced by methyl jasmonate in oilseed rape grown in the laboratory and in the field. J. Agric. Food Chem. 2004, 52, 7607–7613. [Google Scholar] [CrossRef]
- Doughty, K.J.; Kiddle, G.A.; Pye, B.J.; Wallsgrove, R.M.; Pickett, J.A. Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry 1995, 38, 347–350. [Google Scholar] [CrossRef]
- Cipollini, D.; Enright, S.; Traw, M.; Bergelson, J. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol. Ecol. 2004, 13, 1643–1653. [Google Scholar] [CrossRef]
| Photopigment. | Formula |
|---|---|
| Lutein | (E445 × Volume)/Fresh weight (g) |
| Total carotenoids | (E450 × Volume × 4)/Fresh weight (g) |
| β-carotene | (E453 × Volume)/Fresh weight (g) |
| Lycopene | (E505 × Volume)/Fresh weight (g) |
| Chlorophyll a | {(10.1 × E663) − (10.1 × E645) × Volume}/Fresh weight (g) |
| Chlorophyll b | {(16.4 × E645) − (2.57 × E663) × Volume}/Fresh weight (g) |
| Parameter | Optimized Condition |
|---|---|
| Column | 2.7 µm, 2.1 × 100 mm, Agilent, USA |
| Oven temperature | 25 °C |
| Injection | 10 µL |
| Flow rate | 0.4 mL min−1 |
| Gradient program | 0–2 min: 0–0.5% B 2–15 min: 0.5–100% B 15–16 min: 100% B 16–17 min: 100–0.5% B 17–21 min: 0.5% B |
| Eluents | A: ultra-pure waterB: acetonitrile 100% |
| Molecular Formula | Compound Identity | Compound Class | Retention Time [min] |
|---|---|---|---|
| C11H19NO10S2 | Progoitrin (2-Hydroxy-3-Butenylglucosinolate) | Aliphatic | 2.21 |
| C11H19NO9S2 | Gluconapin (3-Butenylglucosinolate) | Aliphatic | 6.89 |
| C11H20NO9S3− | Glucoibervirin (3-Methylthiopropylglucosinolate) | Aliphatic | 7.82 |
| C12H21NO10S2 | Gluconapoleiferin (2-Hydroxy-4-Pentenylglucosinolate) | Aliphatic | 6.12 |
| C12H21NO9S2 | Glucobrassicanapin (4-Pentenylglucosinolate) | Aliphatic | 8.49 |
| C12H22NO9S3− | Glucoerucin (4-Methylthiobutylglucosinolate) | Aliphatic | 8.79 |
| C13H25NO10S3 | Glucoalyssin (5-Methylsulfinylpentylglucosinolate) | Aliphatic | 6.38 |
| C15H20NO9S2− | Gluconasturtiin (2-Phenylethylglucosinolate) | Aromatic | 10.20 |
| C16H20N2O10S2 | 4-Hydroxyglucobrassicin (4-Hydroxy-3-Indolylmethylglucosinolate) | Indole | 7.38 |
| C16H20N2O9S2 | Glucobrassicin (3-Indolylmethylglucosinolate) | Indole | 9.42 |
| C17H22N2O10S2 | 4-Methoxyglucobrassicin (4-Methoxy-3-Indolylmethylglucosinolate) | Indole | 10.30 |
| C17H22N2O10S2 | Neoglucobrassicin (1-Methoxy-3-Indolylmethylglucosinolate) | Indole | 11.76 |
| Aliphatic GLS | ||||||||||||||||||||||||
| 2H3B | 2H4P | 5MGS | 3BGS | 3MGS | 4PGS | 4MGS | Total | |||||||||||||||||
| Con. | 1.65 ± 0.01 a | 0.54 ± 0.05 a | 0.04 ± 0.00 a | 1.51 ± 0.05 a | 0.13 ± 0.01 a | 4.96 ± 0.10 a | 0.06 ± 0.01 c | 8.89 ± 0.19 a | ||||||||||||||||
| 60 | 1.28 ± 0.01 b | 0.41 ± 0.01 b | 0.04 ± 0.00 a | 1.15 ± 0.02 b | 0.14 ± 0.01 a | 3.75 ± 0.06 b | 0.19 ± 0.00 b | 6.94 ± 0.10 b | ||||||||||||||||
| 150 | 1.11 ± 0.02 c | 0.32 ± 0.01 bc | 0.02 ± 0.00 c | 1.12 ± 0.02 b | 0.12 ± 0.00 a | 3.37 ± 0.05 c | 0.26 ± 0.01 a | 6.43 ± 0.11 b | ||||||||||||||||
| 240 | 1.14 ± 0.01 c | 0.22 ± 0.00 c | 0.03 ± 0.00 b | 1.52 ± 0.01 a | 0.14 ± 0.00 a | 3.40 ± 0.02 c | 0.24 ± 0.00 a | 6.69 ± 0.03 b | ||||||||||||||||
| Indole GLS | Aromatic GLS | |||||||||||||||||||||||
| 4H3I | 3IGS | 4M3I | 1M3I | Total | 2PGS | |||||||||||||||||||
| Con. | 0.01 ± 0.00 a | 0.07 ± 0.00 c | 0.67 ± 0.04 b | 0.23 ± 0.03 a | 0.98 ± 0.07 b | 1.11 ± 0.01 d | ||||||||||||||||||
| 60 | 0.03 ± 0.00 a | 0.06 ± 0.00 c | 0.56 ± 0.02 b | 0.20 ± 0.00 a | 0.84 ± 0.02 b | 1.53 ± 0.03 c | ||||||||||||||||||
| 150 | 0.01 ± 0.01 a | 0.11 ± 0.00 b | 0.64 ± 0.02 b | 0.25 ± 0.01 a | 1.02 ± 0.03 b | 2.22 ± 0.06 a | ||||||||||||||||||
| 240 | 0.01 ± 0.00 a | 0.23 ± 0.00 a | 1.27 ± 0.02 a | 0.20 ± 0.00 a | 1.71 ± 0.02 a | 1.96 ± 0.01 b | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.J.; Beck, W.; Mewis, I.; Förster, N.; Ulrichs, C. Effect of Ozone Stresses on Growth and Secondary Plant Metabolism of Brassica campestris L. ssp. chinensis. Horticulturae 2023, 9, 966. https://doi.org/10.3390/horticulturae9090966
Han YJ, Beck W, Mewis I, Förster N, Ulrichs C. Effect of Ozone Stresses on Growth and Secondary Plant Metabolism of Brassica campestris L. ssp. chinensis. Horticulturae. 2023; 9(9):966. https://doi.org/10.3390/horticulturae9090966
Chicago/Turabian StyleHan, Young Jong, Winston Beck, Inga Mewis, Nadja Förster, and Christian Ulrichs. 2023. "Effect of Ozone Stresses on Growth and Secondary Plant Metabolism of Brassica campestris L. ssp. chinensis" Horticulturae 9, no. 9: 966. https://doi.org/10.3390/horticulturae9090966
APA StyleHan, Y. J., Beck, W., Mewis, I., Förster, N., & Ulrichs, C. (2023). Effect of Ozone Stresses on Growth and Secondary Plant Metabolism of Brassica campestris L. ssp. chinensis. Horticulturae, 9(9), 966. https://doi.org/10.3390/horticulturae9090966





