The Effects of Foliar Salicylic Acid and Zinc Treatments on Proline, Carotenoid, and Chlorophyll Content and Anti-Oxidant Enzyme Activity in Galanthus elwesii Hook
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Characteristics
2.2. Plant Material and Experimental Design
2.3. Chemicals
2.4. Bradford Protein Assay
2.5. Estimation of Proline Content
2.6. Estimation of Chlorophyll and Carotenoid Content
2.7. Oxidative Stress Markers—Assay of Lipid Peroxidation
2.8. Determination of Activities of Reactive Oxygen Species (ROS) Scavenging Enzymes
2.8.1. Ascorbate Peroxidase (APX) Activity
2.8.2. Catalase (CAT) Activity
2.9. Statistical Analysis
3. Results and Discussion
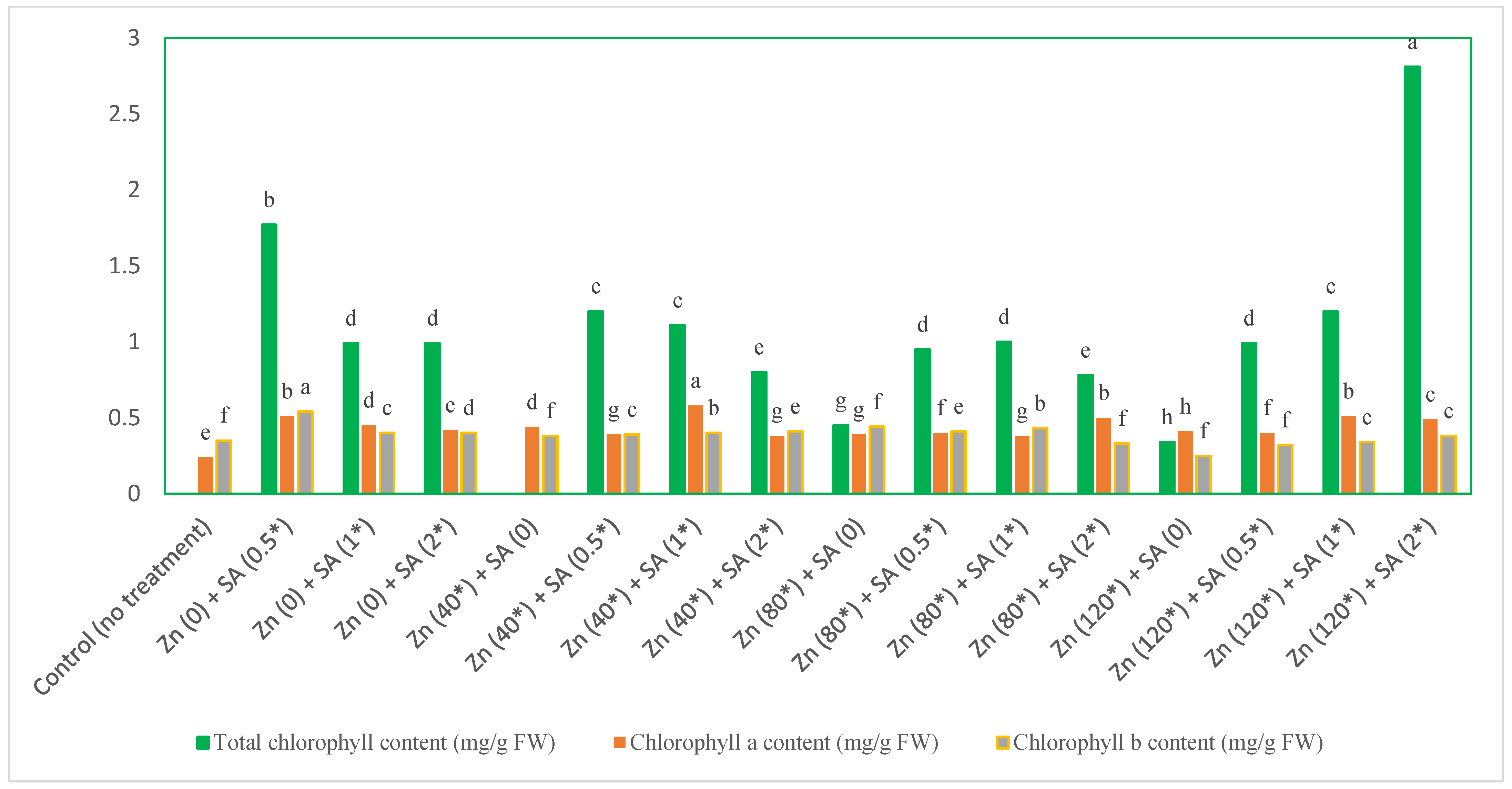
3.1. Soluble Protein (SP), Proline, Chlorophyll, and Carotenoid Content
3.2. Lipid Peroxidation and Antioxidant Enzyme Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Semerdjieva, I.; Sidjimova, B.; Yankova-Tsvetkova, E.; Kostova, M.; Zheljazkov, V.D. Study on Galanthus species in the Bulgarian flora. Heliyon 2019, 5, e03021. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.K.; Low, L.E.; Siew, W.S.; Yap, W.H.; Khaw, K.Y.; Ming, L.C.; Mocan, A.; Goh, B.H.; Goh, P.H. Biological activities of snowdrop (Galanthus spp., Family Amaryllidaceae). Front. Pharmacol. 2021, 11, 552453. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kaya, G.I.; Onur, M.A.; Unver-Somer, N. Chemo-profiling of some Turkish Galanthus L. (Amaryllidaceae) species and their anticholinesterase activity. S. Afr. J. Bot. 2021, 136, 65–69. [Google Scholar] [CrossRef]
- Ay, E.B.; Açıkgöz, M.A.; Kocaman, B.; Mesci, S.; Kocaman, B.; Yıldırım, T. Zinc and phosphorus fertilization in Galanthus elwesii Hook: Changes in the total alkaloid, flavonoid, and phenolic content, and evaluation of anti-cancer, anti-microbial, and antioxidant activities. Sci. Hortic. 2023, 317, 112034. [Google Scholar] [CrossRef]
- Trujillo, L.; Bedoya, J.; Cortés, N.; Osorio, E.H.; Gallego, J.-C.; Leiva, H.; Castro, D.; Osorio, E. Cytotoxic Activity of Amaryllidaceae Plants against Cancer Cells: Biotechnological, In Vitro, and In Silico Approaches. Molecules 2023, 28, 2601. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.L.; Biscaro, B.; Piazza, F.; Tesco, G. BACE1 Protein endocytosis and trafficking are differentially regulated by ubiquitination at lysine 501 and the di-leucine motif in the carboxyl terminus. J. Biol. Chem. 2012, 287, 42867–42880. [Google Scholar] [CrossRef]
- Ghane, S.; Attar, U.; Yadav, P.; Lekhak, M. Antioxidant, anti-diabetic, acetylcholinesterase inhibitory potential and estimation of alkaloids (lycorine and galanthamine) from Crinum species: An important source of anticancer and anti-Alzheimer drug. Ind. Crop. Prod. 2018, 125, 168–177. [Google Scholar] [CrossRef]
- Pesaresi, A.; Lamba, D.; Vezenkov, L.; Tsekova, D.; Lozanov, V. Kinetic and structural studies on the inhibition of acetylcholinesterase and butyrylcholinesterase by a series of multitarget-directed galantamine-peptide derivatives. Chem. Biol. Interact. 2022, 365, 110092. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H.; et al. Lycorine, a non-nucleoside RNA dependent RNA polymerase inhibitor, as potential treatment for emerging coronavirus infections. Phytomedicine 2020, 86, 153440. [Google Scholar] [CrossRef]
- Kaur, H.; Chahal, S.; Jha, P.; Lekhak, M.M.; Shekhawat, M.S.; Naidoo, D.; Arencibia, A.D.; Ochatt, S.J.; Kumar, V. Harnessing plant biotechnology-based strategies for in vitro galanthamine (GAL) biosynthesis: A potent drug against Alzheimer’s disease. Plant Cell, Tissue Organ Cult. (PCTOC) 2022, 149, 81–103. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Khalid, N.; Islam, W.; Sanaullah, T.; Anwar, M.; Khan, S.; Ye, W.; Lou, Y. Zinc finger protein transcription factors: Integrated line of action for plant antimicrobial activity. Microb. Pathog. 2019, 132, 141–149. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.A.; Huang, C.-C.; Fierke, C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. (Eds.) Redox State as a Central Regulator of Plant-Cell Stress Responses; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; p. 386. [Google Scholar]
- Natasha, N.; Shahid, M.; Bibi, I.; Iqbal, J.; Khalid, S.; Murtaza, B.; Bakhat, H.F.; Farooq, A.B.U.; Amjad, M.; Hammad, H.M.; et al. Zinc in soil-plant-human system: A data-analysis review. Sci. Total Environ. 2021, 808, 152024. [Google Scholar] [CrossRef] [PubMed]
- Fongfon, S.; Prom-U-Thai, C.; Pusadee, T.; Jamjod, S. Responses of purple rice genotypes to nitrogen and zinc fertilizer application on grain yield, nitrogen, zinc, and anthocyanin concentration. Plants 2021, 10, 1717. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Açikgöz, M.A.; Kara, M.; Aygün, A.; Özcan, M.M.; Ay, E.B. Effects of methyl jasmonate and salicylic acid on the production of camphor and phenolic compounds in cell suspension culture of endemic Turkish yarrow(Achillea gypsicola) species. Turk. J. Agric. For. 2019, 43, 351–359. [Google Scholar] [CrossRef]
- Howlader, P.; Bose, S.K.; Jia, X.; Zhang, C.; Wang, W.; Yin, H. Oligogalacturonides induce resistance in Arabidopsis thaliana by triggering salicylic acid and jasmonic acid pathways against Pst DC3000. Int. J. Biol. Macromol. 2020, 164, 4054–4064. [Google Scholar] [CrossRef]
- Khokhar, S.; Taggar, G.K.; Grewal, S.K. Alteration in the developmental physiology of Maruca vitrata (Fabricius) on jasmonic acid and salicylic acid treated pigeonpea. Arthropod-Plant Interact. 2023, 17, 389–400. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ros regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted role of salicylic acid in combating cold stress in plants: A review. J. Plant Growth Regul. 2020, 40, 464–485. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Evaluation of phytochemical compositions and biological properties of achillea gypsicola at different phenological stages. Chem. Biodivers. 2019, 16, e1900373. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crop. Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Determination of essential oil compositions and antimicrobial activity of Achillea gypsicola Hub.-Mor. at different plant parts and phenological stages. J. Essent. Oil Res. 2020, 32, 331–347. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Effects of sorbitol on the production of phenolic compounds and terpenoids in the cell suspension cultures of Ocimum basilicum L. Biologia 2020, 76, 395–409. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef]
- Huhta, A. Decorative or Outrageous—The significance of the Common Reed (Phragmites australis) on water quality. Comments Turku Univ. Appl. Sci. 2009, 48, 1–33. [Google Scholar]
- Sharma, S.; Katoch, V.; Kumar, S.; Chatterjee, S. Functional relationship of vegetable colors and bioactive compounds: Implications in human health. J. Nutr. Biochem. 2021, 92, 108615. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Pal, A.; Chopra, D.S. Enzymes. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 335–358. [Google Scholar]
- Xu, C.; Zhang, J.; Mihai, D.M.; Washington, I. Light-harvesting chlorophyll pigments enable mammalian mitochondria to capture photonic energy and produce ATP. J. Cell Sci. 2013, 127, 388–399. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Rocha-Pimienta, J.; Martillanes, S.; Garrido, M.; Espino, J.; Delgado-Adámez, J. Chlorophyll Pigments of Olive Leaves and Green Tea Extracts Differentially Affect Their Antioxidant and Anticancer Properties. Molecules 2023, 28, 2779. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of Natural Chlorophylls as Agents for Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Kalra, S.J.S.; Naraian, R. Environmental perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Appl. Water Sci. 2013, 4, 193–202. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef]
- Latvala, A.; Önür, M.A.; Gözler, T.; Linden, A.; Kivçak, B.; Hesse, M. Alkaloids of Galanthus elwesii. Phytochemistry 1995, 39, 1229–1240. [Google Scholar] [CrossRef]
- Berkov, S.; Sidjimova, B.; Evstatieva, L.; Popov, S. Intraspecific variability in the alkaloid metabolism of Galanthus elwesii. Phytochemistry 2004, 65, 579–586. [Google Scholar] [CrossRef]
- Berkov, S.; Reyes-Chilpa, R.; Codina, C.; Viladomat, F.; Bastida, J. Revised NMR data for Incartine: An Alkaloid from Galanthus elwesii. Molecules 2007, 12, 1430–1435. [Google Scholar] [CrossRef]
- Berkov, S.; Bastida, J.; Sidjimova, B.; Viladomat, F.; Codina, C. Phytochemical differentiation of Galanthus nivalis and Galanthus elwesii (Amaryllidaceae): A case study. Biochem. Syst. Ecol. 2008, 36, 638–645. [Google Scholar] [CrossRef]
- Berkov, S.; Bastida, J.; Sidjimova, B.; Viladomat, F.; Codina, C. Alkaloid Diversity in Galanthus elwesii and Galanthus nivalis. Chem. Biodivers. 2011, 8, 115–130. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coban, G.; Kaya, G.; Onur, M.; Unver-Somer, N. Alkaloid profiling, anticholinesterase activity and molecular modeling study of Galanthus elwesii. S. Afr. J. Bot. 2017, 113, 119–127. [Google Scholar] [CrossRef]
- Bulduk, I.; Karafakıoğlu, Y.S. Evaluation of Galantamine, Phenolics, Flavonoids and Antioxidant Content of Galanthus Species in Turkey. Int. J. Biochem. Res. Rev. 2019, 25, 1–12. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Zengin, G.; Sinan, K.I.; Yıldıztugay, E.; Lobine, D.; Ouelbani, R.; Bensari, S.; Ak, G.; Yılmaz, M.A.; Gallo, M.; et al. A comprehensive evaluation of the chemical profiles and biological properties of six geophytes from Turkey: Sources of bioactive compounds for novel nutraceuticals. Food Res. Int. 2020, 140, 110068. [Google Scholar] [CrossRef]
- El Tahchy, A.; Bordage, S.; Ptak, A.; Dupire, F.; Barre, E.; Guillou, C.; Henry, M.; Chapleur, Y.; Laurain-Mattar, D. Effects of sucrose and plant growth regulators on acetylcholinesterase inhibitory activity of alkaloids accumulated in shoot cultures of Amaryllidaceae. Plant Cell, Tissue Organ Cult. (PCTOC) 2011, 106, 381–390. [Google Scholar] [CrossRef]
- Ločárek, M.; Nováková, J.; Klouček, P.; Hošt’álková, A.; Kokoška, L.; Gábrlová, L.; Šafratová, M.; Opletal, L.; Cahlíková, L. Antifungal and Antibacterial Activity of Extracts and Alkaloids of Selected Amaryllidaceae Species. Nat. Prod. Commun. 2015, 10, 1537–1540. [Google Scholar] [CrossRef]
- Ay, E.B.; Gul, M.; Acikgoz, M.A.; Yarilgac, T.; Kara, S.M. Assessment of Antioxidant Activity of Giant Snowdrop (Galanthus elwesii Hook) Extracts with Their Total Phenol and Flavonoid Contents. Indian J. Pharm. Educ. Res. 2018, 52, s128–s132. [Google Scholar] [CrossRef]
- Ay, E.B.; Açıkgöz, M.A.; Kocaman, B.; Güler, S.K. Effect of jasmonic and salicylic acids foliar spray on the galanthamine and lycorine content and biological characteristics in Galanthus elwesii Hook. Phytochem. Lett. 2023, 57, 140–150. [Google Scholar] [CrossRef]
- Henriksen, A.; Selmer-Olsen, H.R. Automatic methods for determining nitrate and nitrite in water and soil extracts. Analyst 1970, 95, 514–518. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 595–617. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Witham, F.H.; Blaydes, D.F.; Devlin, R.M. Experiments in Plant Physiology; Van Nostrend Reinhold Company: New York, NY, USA, 1971. [Google Scholar]
- Heath, R.L.; Packer, L. Reprint of: Photoperoxidation in Isolated Chloroplasts I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 2022, 726, 109248. [Google Scholar] [CrossRef] [PubMed]
- Uarrota, V.G.; Moresco, R.; Schmidt, E.C.; Bouzon, Z.L.; Nunes, E.d.C.; Neubert, E.d.O.; Peruch, L.A.M.; Rocha, M.; Maraschin, M. The role of ascorbate peroxidase, guaiacol peroxidase, and polysaccharides in cassava (Manihot esculenta Crantz) roots under postharvest physiological deterioration. Food Chem. 2016, 197, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Derakhshani, Z.; Hassani, A.; Sadaghiani, M.H.R.; Hassanpouraghdam, M.B.; Khalifani, B.H.; Dalkani, M. Effect of Zinc Application on Growth and Some Biochemical Characteristics of Costmary (Chrysanthemum balsamita L.). Commun. Soil Sci. Plant Anal. 2011, 42, 2493–2503. [Google Scholar] [CrossRef]
- Arough, Y.K.; Sharifi, R.S.; Sedghi, M.; Barmaki, M. Effect of Zinc and Bio Fertilizers on Antioxidant Enzymes Activity, Chlorophyll Content, Soluble Sugars and Proline in Triticale Under Salinity Condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 116–124. [Google Scholar] [CrossRef][Green Version]
- Behtash, F.; Abedini, F.; Ahmadi, H.; Mosavi, S.B.; Aghaee, A.; Morshedloo, M.R.; Lorenzo, J.M. Zinc Application Mitigates Copper Toxicity by Regulating Cu Uptake, Activity of Antioxidant Enzymes, and Improving Physiological Characteristics in Summer Squash. Antioxidants 2022, 11, 1688. [Google Scholar] [CrossRef]
- Salimi, A.; Oraghi Ardebili, Z.; Salehibakhsh, M. Potential benefits of foliar treatment of chitosan and Zinc in tomato. Iran. J. Plant Physiol. 2019, 9, 2703–2708. [Google Scholar]
- Paschke, M.W.; Valdecantos, A.; Redente, E.F. Manganese toxicity thresholds for restoration grass species. Environ. Pollut. 2005, 135, 313–322. [Google Scholar] [CrossRef]
- Emrahi, R.; Morshedloo, M.R.; Ahmadi, H.; Javanmard, A.; Maggi, F. Intraspecific divergence in phytochemical characteristics and drought tolerance of two carvacrol-rich Origanum vulgare subspecies: Subsp. hirtum and subsp. gracile. Ind. Crop. Prod. 2021, 168, 113557. [Google Scholar] [CrossRef]
- Kukreti, N.; Chitme, H.R.; Varshney, V.K.; Abdel-Wahab, B.A.; Khateeb, M.M.; Habeeb, M.S. Antioxidant Properties Mediate Nephroprotective and Hepatoprotective Activity of Essential Oil and Hydro-Alcoholic Extract of the High-Altitude Plant Skimmia anquetilia. Antioxidants 2023, 12, 1167. [Google Scholar] [CrossRef]
- Ulusu, F.; Şahin, A. Changes in cytotoxic capacity, phenolic profile, total phenols and flavonoids of Nigella damascena L. seed extracts under different liquid fertilization. S. Afr. J. Bot. 2022, 150, 500–510. [Google Scholar] [CrossRef]
- Aery, N.C.; Sarkar, S. Responses of Zn and Cd treatment in soybean and fenugreek. NBU J. Plant Sci. 2012, 6, 71–76. [Google Scholar] [CrossRef]
- Chakhchar, A.; Lamaoui, M.; Aissam, S.; Ferradous, A.; Wahbi, S.; El Mousadik, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; El Modafar, C. Physiological and Biochemical Mechanisms of Drought Stress Tolerance in the Argan Tree. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: San Diego, CA, USA, 2018; pp. 311–322. [Google Scholar] [CrossRef]
- Khan, I.; Seleiman, M.F.; Chattha, M.U.; Jalal, R.S.; Mahmood, F.; Hassan, F.A.S.; Izzet, W.; Alhammad, B.A.; Ali, E.F.; Roy, R.; et al. Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12303. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of Salicylic Acid and Citric Acid for Alleviation of Cadmium Toxicity to Brassica juncea. J. Plant Growth Regul. 2019, 39, 641–655. [Google Scholar] [CrossRef]
- Bazihizina, N.; Taiti, C.; Marti, L.; Rodrigo-Moreno, A.; Spinelli, F.; Giordano, C.; Caparrotta, S.; Gori, M.; Azzarello, E.; Mancuso, S. Zn2+-induced changes at the root level account for the increased tolerance of acclimated tobacco plants. J. Exp. Bot. 2014, 65, 4931–4942. [Google Scholar] [CrossRef] [PubMed]
- Glińska, S.; Gapińska, M.; Michlewska, S.; Skiba, E.; Kubicki, J. Analysis of Triticum aestivum seedling response to the excess of zinc. Protoplasma 2015, 253, 367–377. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, G.; Kumar, A.; Meena, V.; Kaur, J.; Pandey, A.K. Overlapping transcriptional expression response of wheat zinc-induced facilitator-like transporters emphasize important role during Fe and Zn stress. BMC Mol. Biol. 2019, 20, 1–17. [Google Scholar] [CrossRef]
- Aydin, S.S.; Gökçe, E.; Büyük, I.; Aras, S. Characterization of stress induced by copper and zinc on cucumber (Cucumis sativus L.) seedlings by means of molecular and population parameters. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 746, 49–55. [Google Scholar] [CrossRef]
- Marichali, A.; Dallali, S.; Ouerghemmi, S.; Sebei, H.; Hosni, K. Germination, morpho-physiological and biochemical responses of coriander (Coriandrum sativum L.) to zinc excess. Ind. Crop. Prod. 2014, 55, 248–257. [Google Scholar] [CrossRef]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; Pueyo, J. Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: Role of plant growth promoting bacteria. S. Afr. J. Bot. 2019, 124, 573–582. [Google Scholar] [CrossRef]
| Treatments | Soluble Protein Content (µg/g FW) | Proline Content (µg/g FW) | Carotenoid Content (mg/g FW) |
|---|---|---|---|
| Control (no treatment) | 1.84 ± 0.50 e | 200.3 ± 0.11 m | 0.14 ± 0.01 f |
| Zn (0) + SA (0.5 *) | 2.01 ± 0.30 c | 2480 ± 0.78 a | 0.57 ± 0.08 a |
| Zn (0) + SA (1 *) | 1.88 ± 0.71 e | 1251 ± 2.44 c | 0.24 ± 0.20 c |
| Zn (0) + SA (2 *) | 2.16 ± 0.24 a | 1587 ± 0.56 b | 0.19 ± 0.06 d |
| Zn (40 *) + SA (0) | 2.03 ± 0.20 c | 381.2 ± 0.30 l | 0.13 ± 0.02 f |
| Zn (40 *) + SA (0.5 *) | 1.72 ± 0.18 f | 1120 ± 0.45 d | 0.24 ± 0.05 c |
| Zn (40 *) + SA (1 *) | 2.05 ± 0.57 c | 1100 ± 0.28 d | 0.36 ± 0.18 b |
| Zn (40 *) + SA (2 *) | 1.95 ± 0.30 d | 803.6 ± 0.15 h | 0.14 ± 0.15 e |
| Zn (80 *) + SA (0) | 1.96 ± 0.25 d | 176.5 ± 0.44 n | 0.13 ± 0.04 f |
| Zn (80 *) + SA (0.5 *) | 1.97 ± 0.25 d | 1043 ± 0.34 e | 0.15 ± 0.04 e |
| Zn (80 *) + SA (1 *) | 2.17 ± 0.60 a | 975.8 ± 2.35 f | 0.31 ± 0.30 b |
| Zn (80 *) + SA (2 *) | 2.11 ± 0.34 b | 592.7 ± 1.87 k | 0.12 ± 0.10 f |
| Zn (120 *) + SA (0) | 1.95 ± 0.18 d | 126.2 ± 0.42 j | 0.12 ± 0.02 f |
| Zn (120 *) + SA (0.5 *) | 1.96 ± 0.40 d | 720.2 ± 0.28 i | 0.12 ± 0.08 f |
| Zn (120 *) + SA (1 *) | 1.95 ± 0.62 d | 880.5 ± 0.63 g | 0.26 ± 0.03 c |
| Zn (120 *) + SA (2 *) | 2.03 ± 0.20 c | 394.3 ± 0.70 l | 0.24 ± 0.10 c |
| Treatments | Malondialdehyde Content (nmol/g FW) | Ascorbate Peroxidase Activity (EU/mg Protein) | Catalase Activity (EU/mg Protein) |
|---|---|---|---|
| Control (no treatment) | 1.05± 0.10 h | 0.16 ± 0.62 n | 11.76 ± 0.15 m |
| Zn (0) + SA (0.5 *) | 1.30 ± 0.14 f | 0.56 ± 0.50 k | 29.86 ± 1.30 f |
| Zn (0) + SA (1 *) | 1.31 ± 0.18 f | 0.85 ± 0.38 h | 73.41 ± 2.70 c |
| Zn (0) + SA (2 *) | 1.40 ± 0.20 e | 0.27 ± 0.40 m | 21.48 ± 1.65 h |
| Zn (40 *) + SA (0) | 1.38 ± 0.10 e | 0.93 ± 0.60 g | 14.12 ± 1.45 l |
| Zn (40 *) + SA (0.5 *) | 1.36 ± 0.13 e | 3.52 ± 0.18 b | 36.79 ± 1.42 e |
| Zn (40 *) + SA (1 *) | 1.25 ± 0.25 g | 0.40 ± 0.80 l | 19.61 ± 0.80 h |
| Zn (40 *) + SA (2 *) | 1.84 ± 0.28 a | 1.62 ± 0.42 f | 24.77 ± 0.74 g |
| Zn (80 *) + SA (0) | 1.56 ± 0.26 c | 1.01 ± 0.36 g | 154.6 ± 4.10 a |
| Zn (80 *) + SA (0.5 *) | 1.53 ± 0.38 c | 1.87 ± 0.40 e | 18.27 ± 0.72 k |
| Zn (80 *) + SA (1*) | 1.51 ± 0.17 d | 2.13 ± 0.91 d | 15.54 ± 0.90 l |
| Zn (80 *) + SA (2*) | 1.28 ± 0.35 f | 0.59 ± 0.25 k | 15.53 ± 1.50 l |
| Zn (120 *) + SA (0) | 1.31 ± 0.27 f | 3.99 ± 0.58 a | 108.0 ± 3.72 b |
| Zn (120 *) + SA (0.5 *) | 1.64 ± 0.30 b | 2.86 ± 0.48 c | 28.36 ± 0.44 f |
| Zn (120 *) + SA (1 *) | 1.29 ± 0.42 f | 1.54 ± 0.60 f | 55.77 ± 0.75 d |
| Zn (120 *) + SA (2 *) | 1.27 ± 0.18 g | 0.81 ± 0.70 h | 9.500 ± 0.56 n |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kırgeç, Y.; Batı-Ay, E.; Açıkgöz, M.A. The Effects of Foliar Salicylic Acid and Zinc Treatments on Proline, Carotenoid, and Chlorophyll Content and Anti-Oxidant Enzyme Activity in Galanthus elwesii Hook. Horticulturae 2023, 9, 1041. https://doi.org/10.3390/horticulturae9091041
Kırgeç Y, Batı-Ay E, Açıkgöz MA. The Effects of Foliar Salicylic Acid and Zinc Treatments on Proline, Carotenoid, and Chlorophyll Content and Anti-Oxidant Enzyme Activity in Galanthus elwesii Hook. Horticulturae. 2023; 9(9):1041. https://doi.org/10.3390/horticulturae9091041
Chicago/Turabian StyleKırgeç, Yasemin, Ebru Batı-Ay, and Muhammed Akif Açıkgöz. 2023. "The Effects of Foliar Salicylic Acid and Zinc Treatments on Proline, Carotenoid, and Chlorophyll Content and Anti-Oxidant Enzyme Activity in Galanthus elwesii Hook" Horticulturae 9, no. 9: 1041. https://doi.org/10.3390/horticulturae9091041
APA StyleKırgeç, Y., Batı-Ay, E., & Açıkgöz, M. A. (2023). The Effects of Foliar Salicylic Acid and Zinc Treatments on Proline, Carotenoid, and Chlorophyll Content and Anti-Oxidant Enzyme Activity in Galanthus elwesii Hook. Horticulturae, 9(9), 1041. https://doi.org/10.3390/horticulturae9091041







