Identification and Expression Analysis of Adenylate Kinase Gene Family in Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Acquisition of ADK Gene Sequence in Potato
2.3. Physicochemical Properties and Subcellular Localization Prediction of Potato ADK Protein
2.4. Amino Acid Sequence Analysis and Phylogenetic Tree Construction of ADK Protein
2.5. Analysis of ADK Motifs and Gene Structure in Potato
2.6. Analysis of Location on Chromosomes, Gene Duplication
2.7. Collinearity of ADK Family Genes in Potato
2.8. Expression Pattern Analysis of ADK Genes in Potato
2.9. Verification of Expression of ADK Genes in Potato
3. Results
3.1. Identification of ADK Gene Family Members in Potato
3.2. Physicochemical Properties and Subcellular Localization Prediction of Potato ADK Protein
3.3. Phylogenetic Analysis of ADK Genes
3.4. Analysis of Motifs and Gene Structures of StADK
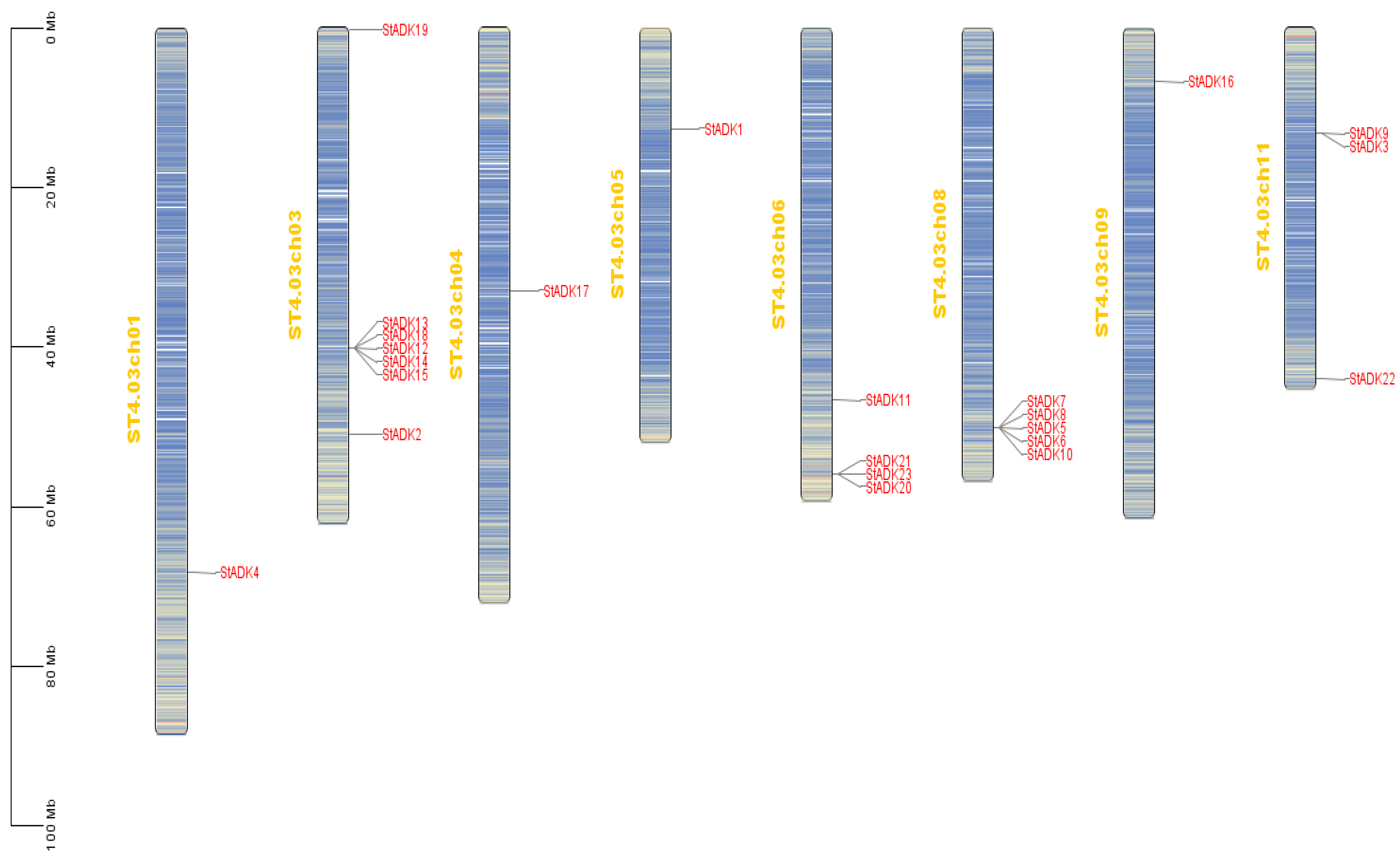
3.5. Location of Chromosomes and ADK Family Genes in Potato
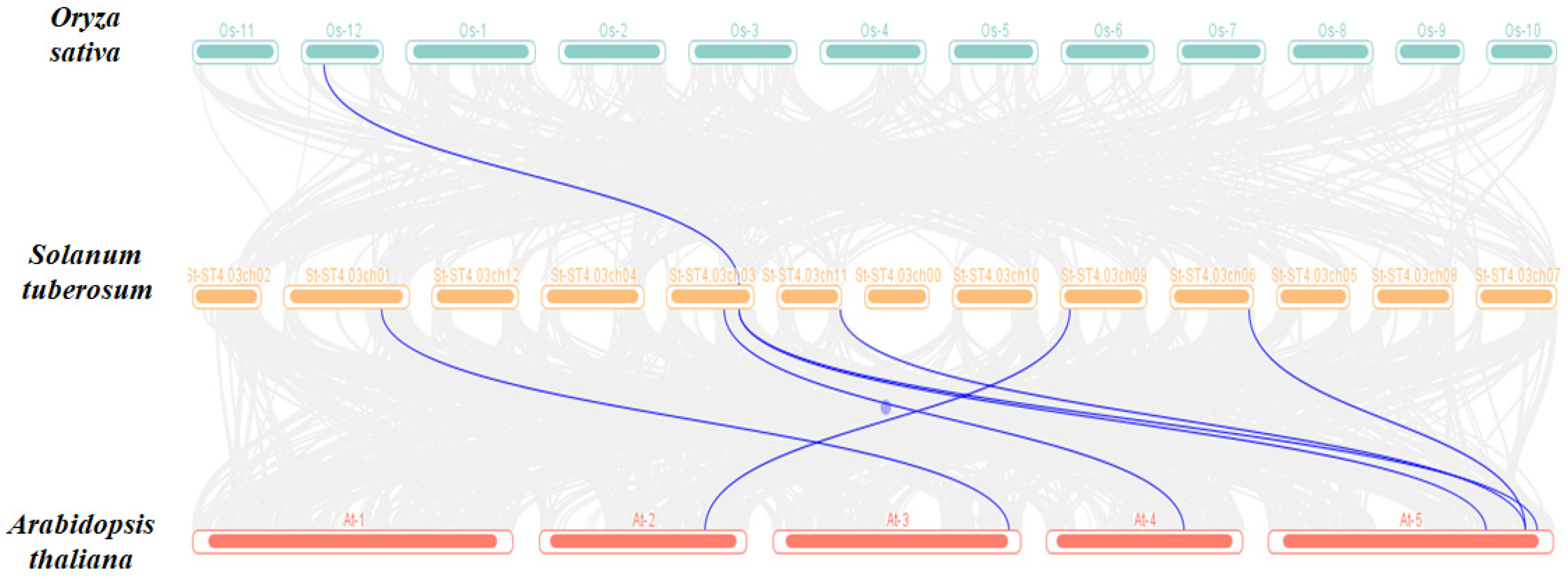
3.6. Collinearity of ADK Family Genes in Potato
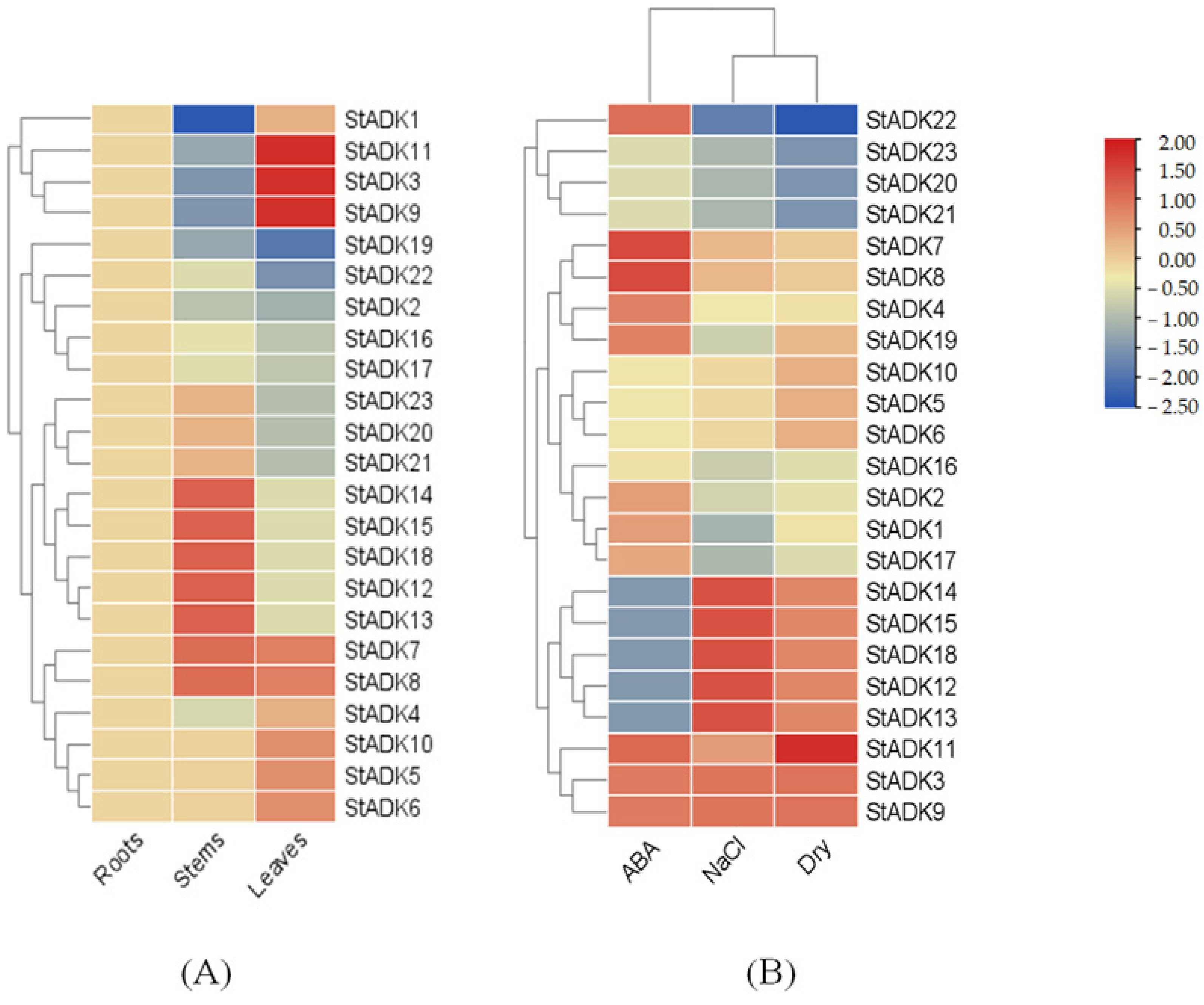
3.7. Expression Pattern Analysis of ADK Genes in Potato
3.8. Verification of Expression of ADK Genes in Potato
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atkinson, D.E. The Energy Charge of the Adenylate Pool as a Regulatory Parameter. Interaction with Feedbac k Modifiers. Bioc Hemistry 1968, 7, 4030–4034. [Google Scholar]
- Ching, T.M.; Crane, J.M.; Stamp, D.L. Adenylate Energy Pool and Energy Charge in Maturing Rape Seeds. Plant Physiol. 1974, 54, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Pradet, A.; Raymond, P. Adenine Nucleotide Ratios and Adenylate Energy Charge in Energy Metabolism. Annu. Rev. Plant Physiol. 1983, 34, 199–224. [Google Scholar] [CrossRef]
- Park, J.; Gupta, R.S. Adenosine Meta Bolism, Adenosine Kinase, and Evolution; Springer: New York, NY, USA, 2012. [Google Scholar]
- Arora, K.; Brooks, C.L. Large Scale allosteric conformational transitions of adenylate kinase appear to involve a population-shift mechanism. Proc. Natl. Acad. Sci. USA 2007, 104, 18496–18501. [Google Scholar] [CrossRef] [PubMed]
- Birkenhead, K.; Walker, D.; Foyer, C. The Intracellular Distribution of Adenylate Kinase in the Leaves of Spinach, Wheat and Barley. Planta 1982, 156, 171–175. [Google Scholar] [CrossRef]
- Yang, L.; Cao, H.; Zhang, X.; Gui, L.; Chen, Q.; Qian, G.; Xiao, J.; Li, Z. Genome-wide identification and expression analysis of tomato ADK gene family during development and stress. Int. J. Mol. Sci. 2021, 22, 7708. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Xu, J.; Wei, Z.; Wei, J.; Min, X. Identification and analysis of ADK gene family members in alfalfa (Medicago sativa). Caoye Kexue 2022, 39, 1803–1814. [Google Scholar]
- Regierer, B.; Fernie, A.R.; Springer, F.; Perez-Melis, A.; Leisse, A.; Koehl, K.; Willmitzer, L.; Geigenberger, P.; Kossmann, J. Starch Content and Yield Increase as a Result of Altering Adenylate Pools in Transgenic Plants. Nat Biotechnol. 2002, 20, 1256–1260. [Google Scholar] [CrossRef]
- Single, B.; Leist, M.; Nicotera, P. Simultaneous Release of Adenylate Kinase and Cytochrome C in Cell Death. Cell Death Differ. 1998, 5, 1001–1003. [Google Scholar] [CrossRef]
- Weretigk, E.A.; Alexander, K.J.; Drebensteat, M.; Ssnider, J.D.; Summers, P.S.; Mofratt, B.A. Maitaning Mtlyation Actvties Durng Salt Stess, the Ivovement of Adenosine Kinase 1. Plant Physiol. 2001, 125, 856–865. [Google Scholar]
- Hawkes, J.G. The potato, evolution, biodiversity and genetic resources. Am. Potato J. 1990, 67, 733–735. [Google Scholar]
- Chen, W.; Cai, R.; Lin, L. Strategic conception of boosting potato staple food normalization. Guizhou Agric. Sci. 2016, 44, 182–185. [Google Scholar]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 7, 1807. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. Tbtools: An Integrative Toolkit Developed for Interac tive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. Meme Suite: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009, 37, 202–220. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Wang, J. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [PubMed]
- Igamberdiev, A.U.; Kleczkowski, L.A. Equlibration of adenylates in the mitochondrial intermembrane space maintains respiration and regulates cytosolic metabolism. J. Exp. Bot. 2006, 57, 2133–2141. [Google Scholar] [CrossRef]
- Carrari, F.; Coll-Garcia, D.; Schauer, N.; Lytovchenko, A.; Palacios-Rojas, N.; Balbo, I.; Rosso, M.; Fernie, A.R. Deficiency of a plastidial adenylate kinase in Arabidopsis results in elevated photosynthetic amino acid biosynthesis and enhanced growth. Plant Physiol. 2005, 137, 70–82. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Z.; Liu, C.; Li, L.; Xu, D. Bioinformatics analysis of rice ADK gene family. Mol. Plant Breed. 2023, 1–10. Available online: https://www.cnki.com.cn/Article/CJFDTotal-FZZW20230523008.htm (accessed on 6 August 2023).
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Ences 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Tang, C.; Zhu, X.; Qiao, X.; Gao, H.; Li, Q.; Wang, P.; Wu, J.; Zhang, S. Characterization of the Pectin Methyl Esterase Gene Famiy and its Function in Cntrlling Pllen Tube Growth in Pear (Pyrus Bretschneiden). Genomics 2020, 112, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- De Grassi, A.; Lanave, C.; Saccone, C. Genome duplication and gene-family evolution: The case of three OXPHOS gene families. Gene 2008, 421, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hampp, R.; Goller, M.; Ziegler, H. Adenylate levels, energy charge, and phosphorylation potential during dark-light and light-dark transition in chloroplasts, mitochondria, and cytosol of mesophyll protoplasts from Avena sativa L. Plant Physiol. 1982, 69, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Lilley, R.M.; Heldt, H.W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982, 70, 971–977. [Google Scholar] [CrossRef]
- Wang, X.; Xue, S.; Chen, S.; Ma, C.; Xu, S. Evolutionary origin, gradual accumulation and functional divergence of heat shock factor gene family with plant evolution. Front. Plant Sci. 2018, 9, 71. [Google Scholar] [CrossRef]
- Rose, A.B. Intron-Mediated Regulation of Gene Expression; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Moore, R.C.; Purugganan, M.D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 15682–15687. [Google Scholar] [CrossRef]
- Kong, H.; Landherr, L.L.; Frohlich, M.W.; Leebens-Mack, J.; Ma, H.; DePamphilis, C.W. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: Evidence for multiple mechanisms of rapid gene birth. Plant J. 2007, 50, 873–885. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Peterson, T.A.; Nlieman, R.H.; Clark, R.A. Nucleotide metabolism in salt-stressed Zea mays L. Root tips: I. Adenine and uridine nucleotides. Plant Physiol. 1987, 85, 984–989. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, S.; Boone, B.; Levy, S. Microarray analysis of genes affected by salt stress in tomato. Afr. J. Environ. Sci. Technol. 2007, 1, 14–26. [Google Scholar]
- Raveneau, M.P.; Benamar, A.; Macherel, D. Water content, adenylate kinase, and mitochondria drive adenylate balance in dehydrating and imbibing seeds. J. Exp. Bot. 2017, 68, 3501–3512. [Google Scholar] [CrossRef]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Bita, N. Linkages of Farmers Operations with Rhizoctonia Root Rot Spread in Bean Crops on a Regional Basis. J. Phytopathol. 2013, 161, 814–822. [Google Scholar]
- Bita, N.; Mozhgan, V. How variable characteristics of bean cropping systems affect Fusarium and Rhizoctonia root rot epidemics? Arch. Phytopathol. Plant Prot. 2019, 52, 1–15. [Google Scholar]






| ID | Sequence ID | Number of Amino Acid | Molecular Weight | pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Localization Prediction |
|---|---|---|---|---|---|---|---|---|
| PGSC0003DMT400003152 | StADK1 | 242 | 26,522.51 | 6.97 | 42.79 | 89.42 | −0.348 | Cytoplasmic |
| PGSC0003DMT400039275 | StADK2 | 244 | 26,614.83 | 8.57 | 44.31 | 91.93 | −0.282 | Cytoplasmic |
| PGSC0003DMT400036202 | StADK3 | 297 | 32,605.37 | 7.06 | 46.96 | 91.92 | −0.285 | Cytoplasmic |
| PGSC0003DMT400004310 | StADK4 | 209 | 22,984.22 | 5.61 | 34.54 | 84.83 | −0.28 | Mitochondrial |
| PGSC0003DMT400062218 | StADK5 | 189 | 21,725.05 | 8.66 | 45.55 | 74.71 | −0.579 | Mitochondrial |
| PGSC0003DMT400062217 | StADK6 | 216 | 24,492.22 | 8.76 | 45.36 | 78.43 | −0.59 | Mitochondrial |
| PGSC0003DMT400062219 | StADK7 | 216 | 24,492.22 | 8.76 | 45.36 | 78.43 | −0.59 | Mitochondrial |
| PGSC0003DMT400062220 | StADK8 | 216 | 24,492.22 | 8.76 | 45.36 | 78.43 | −0.59 | Mitochondrial |
| PGSC0003DMT400036200 | StADK9 | 297 | 32,821.92 | 8.2 | 47.56 | 97.17 | −0.178 | Mitochondrial |
| PGSC0003DMT400062216 | StADK10 | 213 | 24,141.59 | 8.8 | 51.05 | 70.85 | −0.702 | Mitochondrial |
| PGSC0003DMT400067159 | StADK11 | 297 | 33,695.81 | 8.69 | 57.32 | 84.01 | −0.32 | Mitochondrial |
| PGSC0003DMT400049088 | StADK12 | 236 | 26,728.79 | 8.99 | 47.1 | 84.66 | −0.358 | Mitochondrial |
| PGSC0003DMT400049089 | StADK13 | 236 | 26,728.79 | 8.99 | 47.1 | 84.66 | −0.358 | Mitochondrial |
| PGSC0003DMT400049087 | StADK14 | 237 | 26,815.87 | 8.99 | 46.95 | 84.3 | −0.359 | Mitochondrial |
| PGSC0003DMT400049085 | StADK15 | 316 | 35,929.9 | 8.48 | 33.94 | 76.84 | −0.551 | Cytoplasmic |
| PGSC0003DMT400071737 | StADK16 | 288 | 31,268.74 | 6.03 | 44.87 | 97.85 | −0.157 | Mitochondrial |
| PGSC0003DMT400074574 | StADK17 | 270 | 30,145.76 | 6.9 | 42.92 | 94.96 | −0.288 | Mitochondrial |
| PGSC0003DMT400049086 | StADK18 | 203 | 23,120.1 | 9.18 | 57 | 90.74 | −0.141 | Cytoplasmic |
| PGSC0003DMT400034809 | StADK19 | 283 | 31,788.09 | 6.09 | 45.4 | 90.95 | −0.407 | Mitochondrial |
| PGSC0003DMT400019372 | StADK20 | 177 | 19,962.22 | 4.34 | 58.15 | 85.88 | −0.289 | Nuclear |
| PGSC0003DMT400019370 | StADK21 | 183 | 20,694.09 | 4.35 | 57.89 | 85.19 | −0.283 | Nuclear |
| PGSC0003DMT400070488 | StADK22 | 177 | 20,075.48 | 4.63 | 65.56 | 86.95 | −0.424 | Nuclear |
| PGSC0003DMT400019371 | StADK23 | 138 | 15,559.51 | 4.51 | 57.68 | 86.88 | −0.078 | Nuclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lyu, C.; Song, J.; Lu, Y.; Zeng, F.; Lu, L.; Li, L. Identification and Expression Analysis of Adenylate Kinase Gene Family in Potato. Horticulturae 2023, 9, 1025. https://doi.org/10.3390/horticulturae9091025
Li X, Lyu C, Song J, Lu Y, Zeng F, Lu L, Li L. Identification and Expression Analysis of Adenylate Kinase Gene Family in Potato. Horticulturae. 2023; 9(9):1025. https://doi.org/10.3390/horticulturae9091025
Chicago/Turabian StyleLi, Xiang, Chengcheng Lyu, Jun Song, Yifei Lu, Fuchun Zeng, Liming Lu, and Liqin Li. 2023. "Identification and Expression Analysis of Adenylate Kinase Gene Family in Potato" Horticulturae 9, no. 9: 1025. https://doi.org/10.3390/horticulturae9091025
APA StyleLi, X., Lyu, C., Song, J., Lu, Y., Zeng, F., Lu, L., & Li, L. (2023). Identification and Expression Analysis of Adenylate Kinase Gene Family in Potato. Horticulturae, 9(9), 1025. https://doi.org/10.3390/horticulturae9091025





