Phenotypic Variations in Banana Cultivars in the Utilization and Tolerance to Different Magnesium Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Banana Genotypes and Magnesium Treatment
2.2. Collection of Samples for Measurements
2.3. Photosynthesis-Related Parameters
2.4. Photosynthetic Pigments, Membrane Lipid Peroxidation, and Antioxidant Enzymes Activities
2.5. Root and Shoot Dry Weight Morphological Traits
2.6. Starch and Sucrose Content of Leaves
2.7. Mineral Nutrients of Leaves
2.8. Statistical Analysis
3. Results
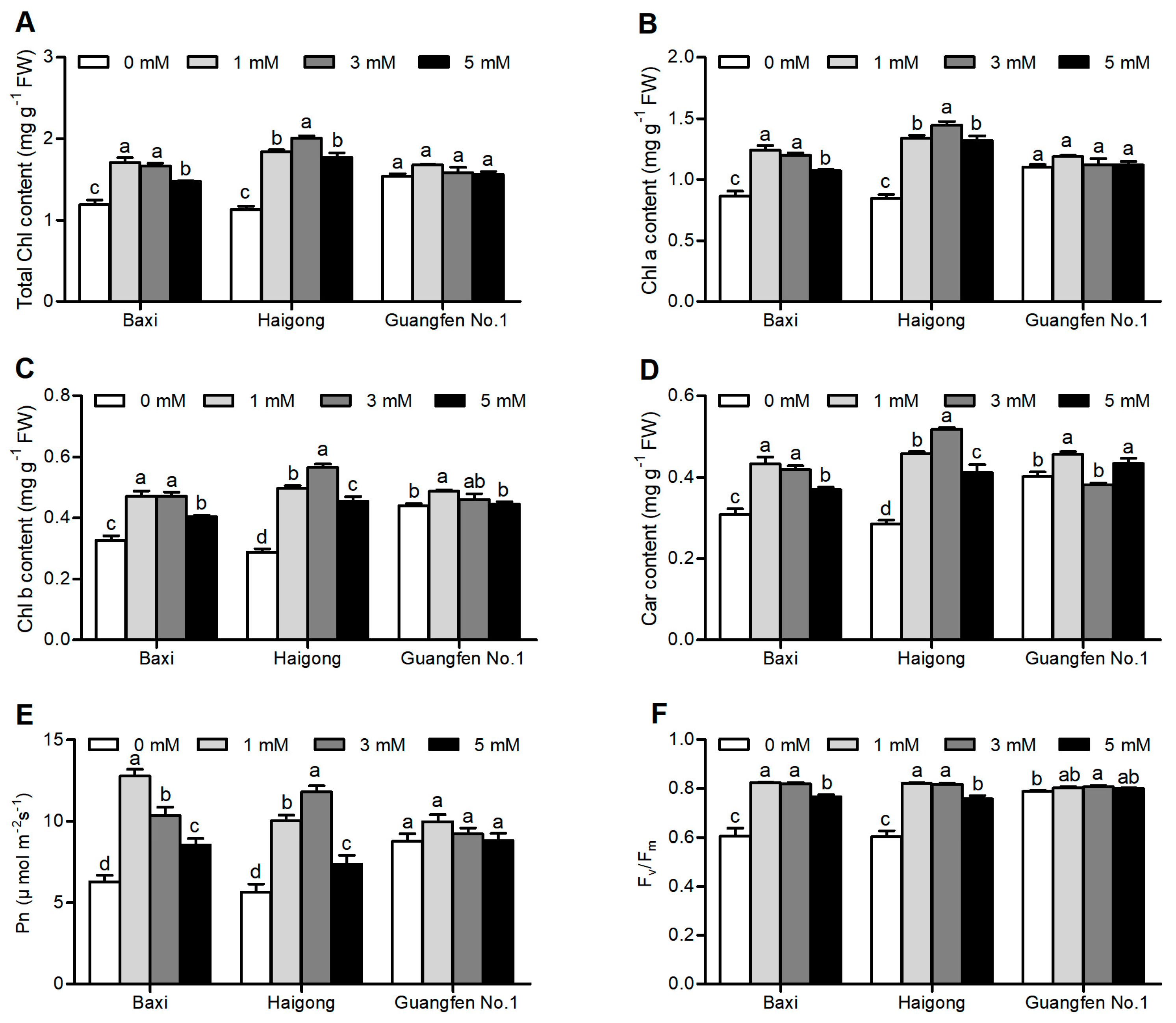
3.1. Photosynthetic Pigments and Photosynthesis of Leaves of Banana Genotypes
3.2. MDA Content and Antioxidant Enzyme Activity
3.3. Sucrose and Starch Content of Leaves
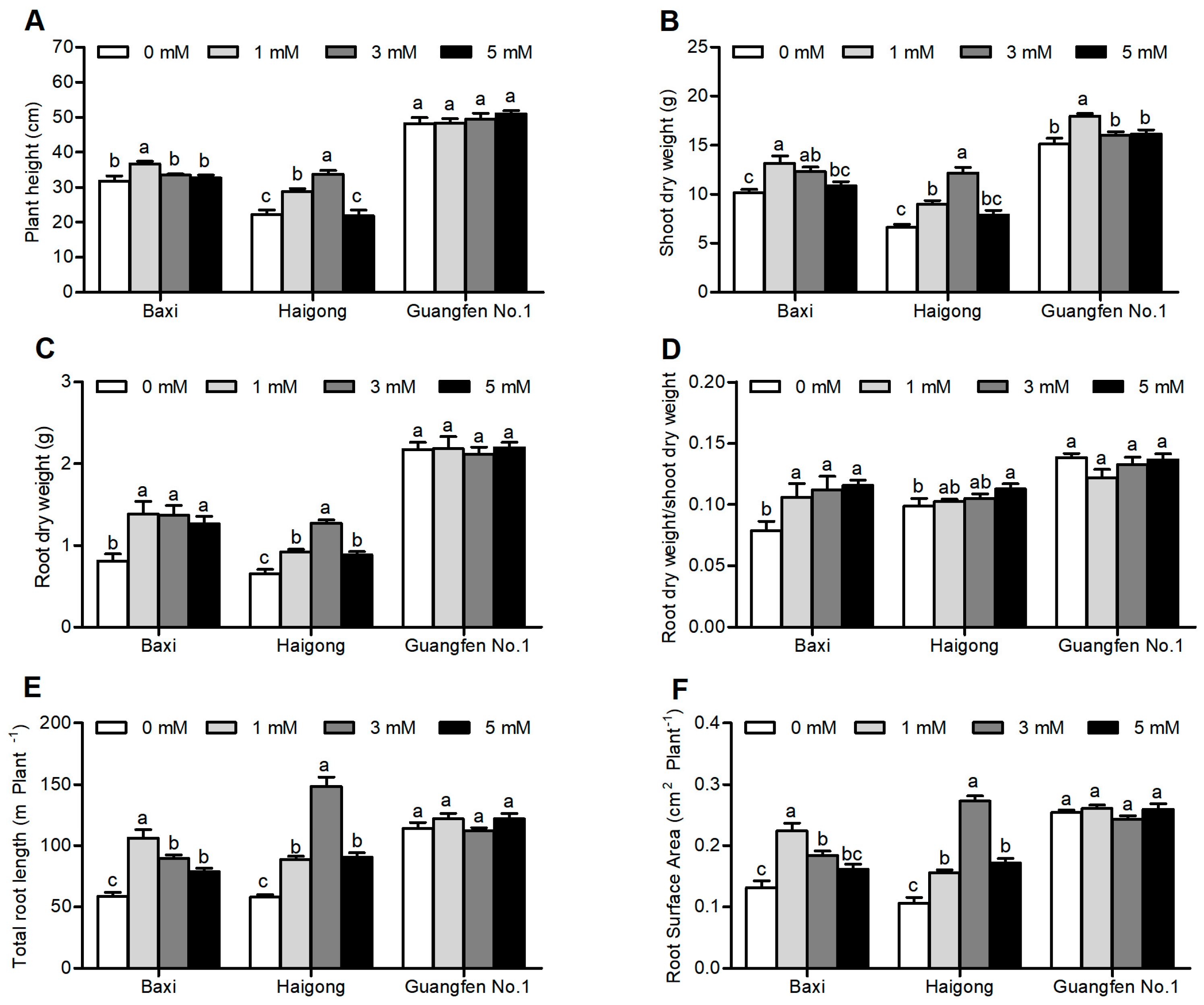
3.4. Biomass Accumulation and Changes in Root Morphology
3.5. Mineral Nutrients of Banana Leaf
4. Discussion
4.1. Optimum Mg Varied among Banana Cultivars and Altered the Mineral Nutrients of Banana Leaf
4.2. Mg Deficiency Reduced Root Growth by Impairing Photosynthate Translocation
4.3. Mg Deficiency Reduces Photosynthetic Pigment Content and Photosynthesis in Leaf of Banana
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Marschner, H.; Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Portis, J.A. Regulation of Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase Activity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 415–437. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef]
- Boxler-Baldoma, C.; Lütz, C.; Heumann, H.G.; Siefermann-Harms, D. Structural changes in the vascular bundles of light-exposed and shaded spruce needles suffering from Mg deficiency and ozone pollution. J. Plant Physiol. 2006, 163, 195–205. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Yang, Y. Magnesium nutrition on accumulation and transport of amino acids in tea plants. J. Sci. Food Agric. 2012, 92, 1375–1383. [Google Scholar] [CrossRef]
- Li, C.-P.; Qi, Y.-P.; Zhang, J.; Yang, L.-T.; Wang, D.-H.; Ye, X.; Lai, N.-W.; Tan, L.-L.; Lin, D.; Chen, L.-S. Magnesium-deficiency-induced alterations of gas exchange, major metabolites and key enzymes differ among roots, and lower and upper leaves of Citrus sinensis seedlings. Tree Physiol. 2017, 37, 1564–1581. [Google Scholar] [CrossRef]
- Huang, J.H.; Xu, J.; Ye, X.; Luo, T.Y.; Ren, L.H.; Fan, G.C.; Qi, Y.P.; Li, Q.; Ferrarezi, R.S.; Chen, L.S. Magnesium deficiency affects secondary lignification of the vascular system in Citrus sinensis seedlings. Trees 2018, 33, 171–182. [Google Scholar] [CrossRef]
- Cai, Y.-T.; Zhang, H.; Qi, Y.-P.; Ye, X.; Huang, Z.-R.; Guo, J.-X.; Chen, L.-S.; Yang, L.-T. Responses of reactive oxygen species and methylglyoxal metabolisms to magnesium-deficiency differ greatly among the roots, upper and lower leaves of Citrus sinensis. BMC Plant Biol. 2019, 19, 76. [Google Scholar] [CrossRef]
- Jezek, M.; Geilfus, C.M.; Bayer, A.; Mühling, K.H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 781. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Y.; Kutman, U.B.; Mengutay, M.; Cakmak, I. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil 2016, 406, 145–156. [Google Scholar] [CrossRef]
- Shaul, O.; Hilgemann, D.W.; de-Almeida-Engler, J.; Van Montagu, M.; Inzé, D.; Galili, G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 2009, 18, 3973–3980. [Google Scholar] [CrossRef]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary Ecology of Plant Adaptation to Serpentine Soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- He, H.; Jin, X.; Ma, H.; Deng, Y.; Huang, J.Q.; Yin, L.Y. Changes of plant biomass partitioning, tissue nutrients and carbohydrates status in magnesium-defcient banana seedlings and remedy potential byfoliar application of magnesium. Sci. Hortic. 2020, 268, 109377. [Google Scholar] [CrossRef]
- Farhat, N.; Rabhi, M.; Krol, M.; Barhoumi, Z.; Ivanov, A.G.; Mccarthy, A.; Abdelly, C.; Smaoui, A.; Hüner, N.P.A. Starch and sugar accumulation in Sulla carnosa leaves upon Mg2+ starvation. Acta Physiol. Plant. 2014, 36, 2157–2165. [Google Scholar] [CrossRef]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg defificiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef]
- Fischer, E.S.; Lohaus, G.; Heineke, D.; Heldt, H.W. Magnesium defificiency results in accumulation of carbohydrates and amino acids in source and sink leaves of spinach. Physiol. Plant. 1998, 102, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Kobayashi, N.I.; Hermans, C.; Ichihashi, Y.; Shibata, A. Short-Term Magnesium Deficiency Triggers Nutrient Retranslocation in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 563. [Google Scholar] [CrossRef]
- Tränkner, M.; Jákli, B.; Tavakol, E.; Geilfus, C.M.; Cakmak, I.; Dittert, K.; Senbayram, M. Magnesium defciency decreases biomasswater-use efciency and increases leaf water-use efciency andoxidative stress in barley plants. Plant Soil 2016, 406, 409–423. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, E.; Yu, X.; Chen, Y.; Yang, Q.; Cao, Y.; Li, X.; Wang, Y.; Fu, A.; Xu, M. Molecular characterization of magnesium chelatase in soybean [Glycine max (L.) Merr.]. Front. Plant Sci. 2018, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Hong, X.; Hu, K.K.; Wang, Y.; Wang, X.X.; Du, S.Y.; Li, Y.; Hu, D.D. Impaired Magnesium Protoporphyrin IX Methyltransferase (ChlM) Impedes Chlorophyll Synthesis and Plant Growth in Rice. Front. Plant Sci. 2017, 8, 1694. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Saghaiesh, S.P.; Souri, M.K.; Moghaddam, M. Efects of diferent magnesium levels on some morphophysiological characteristics and nutrient elements uptake in Khatouni melons (Cucumis melo var. inodorus). J. Plant Nutr. 2019, 42, 27–39. [Google Scholar] [CrossRef]
- Perrier, X.; De Langhe, E.; Donohue, M.; Lentfer, C.; Vrydaghs, L.; Bakry, F.; Carreel, F.; Hippolyte, I.; Horry, J.P.; Jenny, C.; et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Rai, R. Translating the “Banana Genome” to Delineate Stress Resistance, Dwarfing, Parthenocarpy and Mechanisms of Fruit Ripening. Front. Plant Sci. 2016, 7, 1543. [Google Scholar] [CrossRef]
- Mazumdar, P.; Lau, S.E.; Singh, P.; Takhtgahi, H.M.; Harikrishna, J.A. Impact of sea-salt on morpho-physiological and biochemical responses in banana (Musa acuminata cv. Berangan). Physiol. Mol. Biol. Plants 2019, 25, 713–726. [Google Scholar] [CrossRef]
- Robinson, J.; Sauco, V.G. Bananas and Plantains, 2nd ed.; CABI: Cambridge, MA, USA, 2010; Volume 2. [Google Scholar]
- Silva, J.; Silva, I.; Pereira, R. Phosphorus fertilization in banana ‘Prata an’ (AAB) cultivated in two latosols. Rev. Ceres 2011, 58, 238–242. [Google Scholar] [CrossRef]
- Hermans, C.; Bourgis, F.M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef]
- He, H.S.; Khan, S.; Deng, Y.; Hu, H.; Yin, L.Y.; Huang, J.Q. Supplemental Foliar-Applied Magnesium Reverted Photosynthetic Inhibition and Improved Biomass Partitioning in Magnesium-Deficient Banana. Horticulturae 2022, 8, 1050. [Google Scholar] [CrossRef]
- Camak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soyabean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Giannopotitis, C.N.; Ries, S.K. Superoxide dismutases I: Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascobbate-specific peroxidase in Spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996, 112, 1631–1640. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zhao, J.Y.; Wu, S.M.; Fan, X.W.; Luo, X.L.; Chen, B.S. Characters related to higher starch accumulation in cassava storage roots. Sci. Rep. 2016, 6, 19823. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural-Chemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Meireles da Silva, D.; Brandão, I.R.; Alves, J.D.; de Santos, M.O.; de Souza, K.R.D.; de Silveira, H.R.O. Physiological and biochemical impacts of magnesium-deficiency in two cultivars of coffee. Plant Soil 2014, 382, 133–150. [Google Scholar] [CrossRef]
- Riga, P.; Anza, M. Effect of Magnesium Deficiency on Pepper Growth Parameters: Implications for Determination of Magnesium-Critical Value. J. Plant Nutr. 2003, 26, 1581–1593. [Google Scholar] [CrossRef]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Cakmak, I.; Christine, H.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffffering form phosphorus, potassium and magnesium defificiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Venkatesan, S.; Jayaganesh, S. Characterisation of magnesium toxicity, its influence on amino acid synthesis pathway and biochemical parameters of tea. Res. J. Phytochem. 2010, 4, 67–77. [Google Scholar] [CrossRef]
- Niu, Y.; Chai, R.; Liu, L.; Jin, G.; Liu, M.; Tang, C.; Zhang, Y. Magnesium availability regulates the development of root hairs in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2014, 37, 2795–2813. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cong, Y.; Hussain, N.; Wang, Y.; Liu, Z.; Jiang, L.; Liang, Z.; Chen, K. The remodeling of seedling development in response to long-term magnesium toxicity and regulation by ABA-DELLA signaling in Arabidopsis. Plant Cell Physiol. 2014, 55, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Liao, L.L.; Liu, S.; Nie, M.M.; Li, J.; Zhang, L.D.; Ma, J.F.; Chen, Z.C. Magnesium Deficiency Triggers SGR–Mediated Chlorophyll Degradation for Magnesium Remobilization. Plant Physiol. 2019, 181, 262–275. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, L.-S. Magnesium deficiency-induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photo-oxidative damage. J. Plant Nutr. Soil Sci. 2012, 175, 784–793. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]


| Variety | Mg Treatment (mM) | Mg (mg g−1 DW) | N (mg g−1 DW) | P (mg g−1 DW) | K (mg g−1 DW) | Ca (mg g−1 DW) | Zn (mg g−1 DW) |
|---|---|---|---|---|---|---|---|
| Baxi | 0 | 1.39 ± 0.15 d | 35.74 ± 1.30 a | 5.22 ± 0.18 a | 36.28 ± 0.93 a | 10.79 ± 0.55 a | 19.84 ± 0.49 a |
| 1 | 2.44 ± 0.40 c | 32.49 ± 1.15 b | 4.25 ± 0.25 c | 31.44 ± 0.72 c | 9.26 ± 0.56 b | 18.06 ± 0.48 b | |
| 3 | 3.71 ± 0.24 b | 31.73 ± 1.20 b | 4.68 ± 0.09 b | 33.49 ± 0.51 b | 8.18 ± 0.31 c | 16.63 ± 1.33 b | |
| 5 | 4.35 ± 0.35 a | 36.23 ± 1.69 a | 4.67 ± 0.26 b | 34.51 ± 0.50 b | 8.69 ± 0.27 b,c | 16.81 ± 0.69 b | |
| Haigong | 0 | 1.15 ± 0.16 d | 36.30 ± 1.17 a | 5.23 ± 0.16 a | 32.57 ± 0.94 a | 13.53 ± 0.29 a | 32.70 ± 2.21 a |
| 1 | 1.73 ± 0.14 c | 33.19 ± 0.84 b | 4.67 ± 0.19 b | 29.80 ± 0.74 b | 11.04 ± 0.33 b | 24.64 ± 3.44 b | |
| 3 | 2.20 ± 0.18 b | 30.77 ± 1.09 c | 3.67 ± 0.28 c | 26.30 ± 0.74 c | 7.66 ± 0.64 c | 16.44 ± 1.28 c | |
| 5 | 2.87 ± 0.09 a | 32.93 ± 0.57 b | 4.59 ± 0.18 b | 29.62 ± 1.42 b | 11.72 ± 0.29 b | 22.41 ± 0.88 b | |
| Guangfen No.1 | 0 | 1.52 ± 0.16 c | 35.79 ± 1.27 a | 4.05 ± 0.10 a | 31.97 ± 1.45 a | 8.37 ± 0.28 a | 19.72 ± 0.57 a |
| 1 | 2.69 ± 0.21 b | 32.65 ± 1.21 b | 3.91 ± 0.09 a | 28.90 ± 1.00 b | 7.51 ± 0.36 b | 19.12 ± 0.19 a,b | |
| 3 | 3.62 ± 0.22 a | 33.89 ± 0.97 a,b | 4.14 ± 0.14 a | 30.11 ± 0.43 b | 8.36 ± 0.15 a | 17.92 ± 0.31 c | |
| 5 | 3.66 ± 0.12 a | 35.64 ± 1.11 a | 4.12 ± 0.12 a | 32.45 ± 0.74 a | 7.70 ± 0.40 b | 18.92 ± 0.34 b | |
| Variety (Var) | 7.10 ** | 8.72 n,s | 2.75 ** | 120.52 ** | 54.57 ** | 264.30 ** | |
| Treatment (Trt) | 27.26 ** | 96.60 ** | 2.29 ** | 84.40 ** | 36.26 ** | 235.87 ** | |
| Var × Trt | 2.23 ** | 26.52 * | 2.92 ** | 36.07 ** | 31.28 ** | 196.47 ** | |
| Error | 1.15 | 32.33 | 0.79 | 19.45 | 3.72 | 44.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Wu, X.; Khan, S.; Tan, J.; Wu, J.; Sun, Z. Phenotypic Variations in Banana Cultivars in the Utilization and Tolerance to Different Magnesium Levels. Horticulturae 2023, 9, 1017. https://doi.org/10.3390/horticulturae9091017
He H, Wu X, Khan S, Tan J, Wu J, Sun Z. Phenotypic Variations in Banana Cultivars in the Utilization and Tolerance to Different Magnesium Levels. Horticulturae. 2023; 9(9):1017. https://doi.org/10.3390/horticulturae9091017
Chicago/Turabian StyleHe, Hongsu, Xinran Wu, Shahbaz Khan, Jiahao Tan, Jinxu Wu, and Zhihua Sun. 2023. "Phenotypic Variations in Banana Cultivars in the Utilization and Tolerance to Different Magnesium Levels" Horticulturae 9, no. 9: 1017. https://doi.org/10.3390/horticulturae9091017
APA StyleHe, H., Wu, X., Khan, S., Tan, J., Wu, J., & Sun, Z. (2023). Phenotypic Variations in Banana Cultivars in the Utilization and Tolerance to Different Magnesium Levels. Horticulturae, 9(9), 1017. https://doi.org/10.3390/horticulturae9091017






