Abstract
Organic production is expected to play a major role in reducing the impact of agricultural practices on the environment. Soil is considered a major component of the organic production process, and organic practices aim at increasing its health and fertility. However, the control of soil-borne pests, particularly plant-parasitic nematodes, can be difficult in organic horticultural crops due to the rules allowed in this farming system. Applying a holistic approach that fosters and exploits the activity of the soil microbiome to control plant-parasitic nematodes has been at the basis of the analysis of the available scientific knowledge carried out for this review article. This review thus focuses on the multifunctional capacity of microorganisms, including that of bacteria and fungi not normally considered biocontrol agents, and the need to also better understand their relations with the plant and other environmental and agronomic factors. The implementation of the “multi-biotics” concept, applying prebiotics, probiotics and postbiotics, which supports an integrated agroecological strategy for the protection of organic horticultural crops, is proposed as an efficient practice that should be further studied to be adapted under different crops and pedo-climatic conditions.
1. Introduction
According to the International Federation of Organic Agriculture Movements (IFOAM), organic agriculture is a production system that maintains the health of soils, ecosystems and people. The European Union (EU) has established legal provisions to regulate organic production, and these provisions place a strong emphasis on the idea of soil health in organic farming [1]. Moreover, the EU has embarked on a strong policy to support the development of organic farming (Green Deal and Farm to Fork strategies) included within the “EU soil strategy for 2030” [2], which sets a vision and objectives to achieve healthy soils by 2050, including a new Soil Health Law expected to be enacted by 2023. Indeed, the use of chemical fertilisers and pesticides has been shown to reduce the soil’s natural fertility and microbial richness, while soils managed under organic farming practices have been shown to have greater microbial activity than those cultivated using conventional methods [3].
The soil life web, composed of the microbiome, mesofauna and macrofauna, plays a significant role in soil functions in agroecosystems [4], affecting soil fertility and promoting plant growth and disease suppression [5]. Interactions between soil fauna and microorganisms are crucial for regulating soil processes and the impact of soil-borne diseases [6]. Soil functional biodiversity shall thus be promoted, particularly in organic crops, to reduce the risks of damage from soil-borne pests, including plant-parasitic nematodes (PPNs).
Several practices allowed in organic farming improve soil biodiversity (e.g., use of organic amendments and fertilisers), but they could also provoke some undesired effects. Some trophic groups of soil nematodes, e.g., bacterial and fungal feeders, can support the availability of nutrients to plants [7], particularly under organic farming [8]. The abundance of these trophic groups tends to rise in organic managed soils, as is the case of bacteria feeders after the addition of organic matter, in the form of green manure or organic fertilisers and amendments [9]. Nevertheless, different organic fertilisers and amendments are expected to differentially affect nematode communities because of their distinct physical characteristics and chemical composition [10]. For this reason, a comparison of nematodes communities present in organic farming systems with those of conventional systems emerged showed that PPNs were also abundant and numerically greater in organically managed soil than in conventional soil [11]. However, host crops and tillage practices were shown to impact the nematode community structure and function to an extent similar to or even greater than the application of mineral fertilisers and pesticides. Moreover, PPNs had a greater medium-term influence on organic managed soil, particularly on species with wide host spectra like Meloidogyne spp. and Pratylenchus spp. [12], which, together with Heterodera spp. and Globodera spp., are the genera responsible for significant losses in horticultural crops [13].
Organic farming methods include practices aimed at boosting soil biological fertility. These are somewhat limited in the case of multiannual fruit tree crops, but can still be applied by implementing management strategies such as living mulches and cover crops in the orchard soil [14]. Moreover, in light of growing environmental and climatic concerns [15], using plant-beneficial microorganisms and microbial-based pesticides (biopesticides) or organic biostimulants is becoming a practice that can also be helpful for the control of PPNs in organic farming [16,17]. Nevertheless, knowledge about the potential of these practices on PPN control is scattered.
As a part of this study, a science mapping analysis of published papers was used to better understand recent trends in nematode control research. The following keywords and strings were used to retrieve the relevant publication from the SCOPUS database, which was consulted on 28 July 2023: nematode AND plant-parasitic OR “organic farming” OR PGPR OR rhizobia OR “mycorrhizal fungi” OR amendment OR manure OR Pochonia OR Purpureocillium in the combined fields of “title”, “abstract” and “keywords”. The software VOSviewer, version1.6.19 (available at www.vosviewer.com (accessed on 28 July 2023) with default settings, was used to create a bibliometric map based on the retrieved publications. The performed analysis (Figure 1), based on 3400 publications, showed that the most important cluster is connected with root-knot nematodes, i.e., with pests related to horticultural crops. A high number of papers dealt with ecological aspects (i.e., diversity) and, to a lower extent, with microbial inocula or amendments, two major tools of any control strategy applicable to organic productions. Interestingly, entomopathogenic nematodes resulted to be a major cluster, likely due to their useful application in the control of several pests in organic farming.
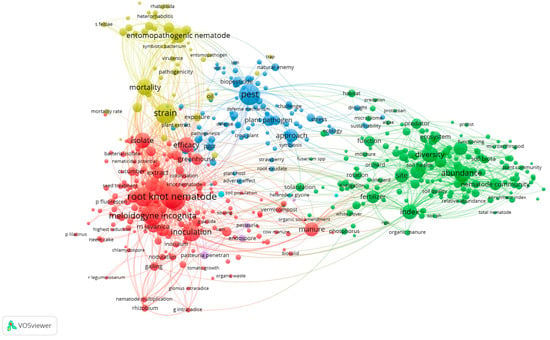
Figure 1.
The terms clustering map based on the analysis of publications concerned with plant-parasitic nematode studies retrieved from Scopus database from the period 2000–2023. Red, green, blue and yellow colours represent the terms belonging to different clusters. The dot size of each term is based on the number of times it occurs. The connecting lines indicate co-occurrence links between terms.
This review is thus addressing these aspects, focusing on the use of microbial-based products and the factors affecting their efficacy (summarised on Figure 2), also taking into account the relevant legal framework in the EU, particularly considering organic horticultural systems.
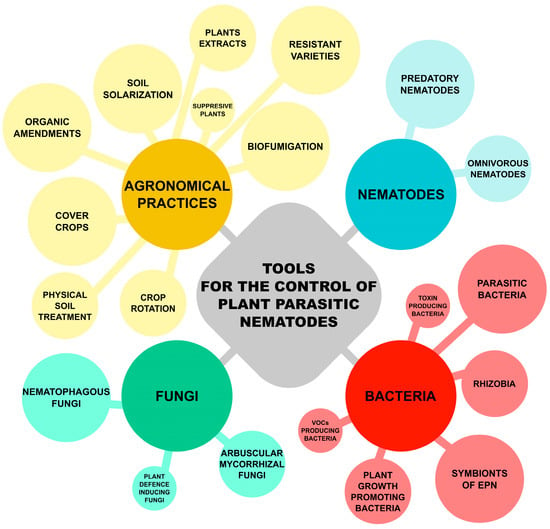
Figure 2.
Different tools for the control of PPNs, which could be used in organic farming of horticultural crops.
2. Biocontrol of Plant-Parasitic Nematodes
In recent decades, microorganisms have been considered an alternative to chemical pesticides for the biological control of PPNs [18]. In this regard, both bacteria and fungi have demonstrated potential applications, but due to the constraints and requirements deriving from the registration procedure [16], their use is still limited. Moreover, there is a frequent knowledge gap about the interactions of microbial-based products with native soil microorganisms and the plant, and the difficulties in developing optimal formulations and application methods can significantly affect the efficacy of these products [19]. These aspects are crucial to assure the effective control of PPNs and thus are also discussed.
2.1. Bacteria
Many bacterial species show some control or suppression activity against different PPNs. Species such as Pasteuria penetrans directly parasitise nematodes, while other genera, including plant-growth promoters like Bacillus, Agrobacterium, Azotobacter and Pseudomonas, produce toxins that can kill nematodes [20]. Among Bacillus species, B. cereus [21], B. firmus [22], B. thuringiensis [23], B. licheniformis [24] or B. nematocida [25] have been found to efficiently control PPNs. Considering that Bacillus species are included in several formulations available on the EU market for biocontrol, and that some of them also demonstrate plant growth promotion, the assessment of their potential positive effect on PPN reduction could be beneficial to organic horticultural crops. In the EU, among the 28 Bacillus strains currently listed in the pesticides database, the strain B. firmus I-158 is registered for its nematicidal activity, and thus can be used in organic productions.
Pseudomonas fluorescens, a rhizobacterium frequently showing plant-growth-promotion effects, was successful in controlling a variety of PPN species, including M. javanica on tomato [26] and M. incognita on tomato and herbs [27,28]. The J2111 strain of Burkholderia arboris reduced the galling index and egg mass of M. incognita per plant in both pot and field trials with tobacco, ensuring a greater yield than untreated plants [29].
Nitrogen-fixing bacteria, an important component of agronomical practices in organic horticulture to increase soil fertility and provide nitrogen through cover/inter crops or living mulches, have also shown potential to control PPNs, particularly root-knot nematodes. Among free living species, Azotobacter chroococcum and Azospirillum brasilense were able to limit root-knot nematodes, though less effectively than P. fluorescens [30]. An analysis of several reports about the impact of symbiotic nitrogen-fixing bacteria on PPNs showed that neutral, positive and negative effects can occur when both rhizobia and root-knot nematodes are inoculated to legume species [31]. Indeed, even though reduced nodule numbers and often reduced gall numbers were observed as a result of the interaction between the two organisms, this did not always occur, and sometimes opposite effects were described. Similarly to other soil interactions, the nematode–plant–rhizobia interaction can be affected by the soil characteristics and nematode population density [32]. However, the interactions between PPNs and rhizobia on a legume root system also depend on plant and microbial genetic factors [33], which could account for the different outcomes observed in the pot/field trials. The complexity of the interaction, and its impact on organic production, was demonstrated by a recent analysis of the rhizosphere and root endophytic microbiota of tomato plants parasitised by Meloidogyne spp. [34]. A significant modification of the root endophytic microbiota was observed, with 15 out of 17 orders present in the endophytic community showing higher relative abundance in healthy than in nematode-parasitised roots. The other two orders, Rhizobiales and Betaproteobacteriales, were enriched in nematode-parasitised tomato roots. This resulted in a significant enrichment of the key gene/enzyme related to biological nitrogen fixation along all stages of nematode parasitism in the roots, and led the authors to suggest that these bacteria might be suitable biomarker taxa to differentiate healthy plants from nematode-parasitised ones. Interestingly, the addition of 13 kinds of nitrogen sources (both mineral and organic) to the soil modified the N-fixing bacteria population, but only the organic fertilisers reduced root-knot nematode galling, providing some hints for the development of a PPN control complex strategy suitable for organic production [34].
However, there are many limitations that pose challenges to the application of bacterial strains to control PPNs. These include their virulence [35], the requirements of the production process, particularly in case of obligate bacterial parasites (e.g., Pasteuria penetrans) when large-scale production is limited by in vivo cultivation [36] as well as other ecological and agronomical factors that affect the persistence and fate of applied biocontrol agents [37]. For these reasons, a suitable alternative to bacterial formulations could be the use of their metabolites present in cell-free substrates, exploiting a post-biotic approach to control PPNs in organic horticulture [17]. The hatching of M. incognita was inhibited in vitro by rhizobia culture filtrates [38]. Culture filtrates obtained from Paenibacillus polymyxa KM2501-1 revealed severe toxicity to J2 juveniles of M. incognita, causing mortality up to 87% within 72 h in laboratory experiments. Its application to tomato plants under greenhouse conditions showed a higher efficacy of the culture filtrate of this strain on decreasing the root gall index of M. incognita compared to the bacterial suspensions [39]. A comprehensive characterization of its composition revealed the presence of 11 volatile organic compounds (VOCs), with furfural acetone being the most active compound against M. incognita in a contact assay [39]. The reproductive toxicity of furfural acetone to M. incognita was demonstrated by further field studies with tomato showing a nematode-control efficacy similar to that of commercial nematicides [40]. VOCs produced by Bacillus atrophaeus GBSC56 stimulated the induction of systematic resistance against M. incognita in tomato, which could be an interesting control method in organic horticulture [41]. Similar potential effects against M. incognita were shown with volatile organic compounds derived from B. altitudinis AMCC 1040 [42] and many other species (Burkholderia, Dyella, Pantoea and Pseudomonas), which were successfully tested under laboratory conditions [43]. Bacterial fermentation products could also be an interesting alternative for the organic management of potato cyst nematodes—Globodora rostochiensis—[44].
2.2. Fungi
Several fungal species can be employed to minimise PPN damage. They can be classified according to their major activity into nematophagous fungi and multifunctional fungi such as root symbiotic species.
2.2.1. Nematophagous Fungi
The generic group of fungi named nematophagous is composed of diverse species that have the ability to transform from saprophytic behaviour to parasitic behaviour towards other organisms, including nematodes, and species that feed exclusively on their hosts (e.g., the endoparasites Catenaria sp. and Myzocytiopsis sp.). They use a variety of mechanisms which allow them to be classified according to the mode of attacking nematodes (visualised on Figure 3) into (i) nematode-trapping fungi (NTF) using adhesive or mechanical hyphal structures, (ii) endoparasites using their spores, (iii) egg-parasitic fungi invading nematode eggs with their hyphal tips, (iv) toxin-producing fungi and (v) producers of special attack devices [45,46].
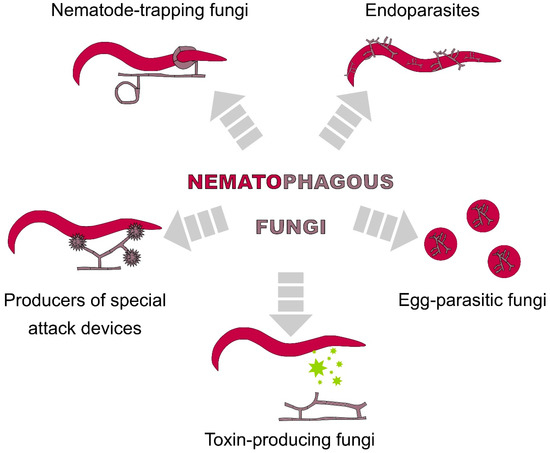
Figure 3.
Nematophagous fungi and their feeding strategies.
More than 200 species of NTF have been identified globally, and they are capable of growing certain mycelial structures or a “trap” to collect nematodes and subsequently extract nutrients from them [47]. NFT species like Arthrobotrys oligospora or Drechslerella dactyloides are a promising source of PPN biocontrol agents [45,47,48]. However, even though their nematicidal activity has been widely studied in laboratory conditions, it is still difficult to obtain consistent efficacy under field conditions [49], as, similarly to other biocontrol species, their activity in soil is significantly impacted by the formulation type (see Section 3.2). The impact of the formulation was also determined with the endoparasite Hirsutella rhossiliensis, which was quite sensitive to biotic inhibition when formulated as pelletised hyphae, but was insensitive to biotic inhibition when formulated as parasitised nematodes [50].
Exploitation of the native NFT soil population is another approach that has been tested to control PPNs [51]. A. dactyloides and Nematoctonus leiosporus were two species more often present with higher population densities in organically maintained plots than in conventional fields [52]. Nevertheless, no differences in the overall density between conventional and organic fields were observed in the case of A. haptotyla and A. thaumasia. Moreover, the suppression of M. javanica was positively correlated with the total microbial biomass rather than management system or population density, which suggests a scenario of a complex trophic web with the soil and its biota as background [48].
2.2.2. Multifunctional Fungi with PPN Control Capacity
PPNs can be parasitised by a variety of fungal species expressing multifunctional features that have lately been registered for biocontrol as well as as microbial biostimulants (i.e., to improve plant nutrition). Among biocontrol agents, Purpureocillium lilacinum (formerly Paecilomyces lilacinus) has been shown to successfully parasitise several nematode life stages [53]. Two strains are currently approved as low-risk substances for use against Meloidogyne spp. on several horticultural crops in European Union [54]. Interestingly, recent reports highlight the multifunctionality of this species, showing plant-growth-promotion effects [55] or soil-borne pathogen reduction [56].
Pochonia chlamydosporia is a another multitrophic biocontrol agent that can colonise plant roots and parasitises several genera of cyst and root-knot nematodes, including Heterodera spp., Globodera spp., Meloidogyne spp., Nacobbus spp. and Rotylenchulus spp. [57], through the production of extracellular enzymes [58] and appressoria [59]. Even though some commercial products based on P. chlamydosporia are available [60], they are not yet registered in the EU. The control activity of P. chlamydosporia has been reported for both conventional and organic production methods [61]. Interestingly, the addition of chitosan, a polymer used for microencapsulation of fertilisers and pesticides [62], registered in the EU as an elicitor of plant resistance against pathogenic fungi and bacteria and allowed in organic farming, boosted the conidiation, parasitic ability and root colonization of P. chlamydosporia, also promoting plant development [63]. Nevertheless, the effect has been found to depend on the soil type and dosage applied [64].
Clonostachys rosea, a well-known fungal species with excellent biocontrol ability, particularly against soil-borne plant pathogens, has also shown potential control capacity against Pratylenchus or Heterodera species through several mechanisms, including parasitism, induction of plant defence reactions or production of secondary metabolites and enzymes with antibiosis properties [65]. The strain J1446 is currently registered in the EU, making it worthy to verify its effect on PPNs in organic horticultural crops. Trichoderma harzianum, another biocontrol agent normally applied to control soil-borne pathogens with several strains registered in the EU, effectively controlled M. javanica [66].
Entomopathogenic Metarhizium anisopliae and Beauveria bassiana are fungal species normally used for the biocontrol of arthropods, with few strains registered for this purpose at the EU or country level, and show typical multifunctional activities, including PPN control [16]. Metarhizium anisopliae was able to parasitise M. hapla eggs, preventing egg hatching and killing juveniles [67]. Beauveria bassiana 08F04, isolated from cyst surfaces, has shown potential as a nematode-controlling agent by reducing the amount of females of Heterodera filipjevi, a cereal cyst nematode [68].
Syncephalastrum racemosum, a fungus used for the biotechnological production of enzymes [69], has shown to lower the populations of root-knot nematodes via direct parasitism and the synthesis of secondary metabolites [70]. Under field conditions, a certain amount of heterogeneity in its biocontrol effect has been documented [71]. However, compared to untreated plants, the mixture of S. racemosum and P. lilacinum boosted the production of cucumbers while also being efficient against Meloidogyne spp. [72].
Considering fungi improving nutrient uptake capacity, arbuscular mycorrhizal fungi (AMF) have long been exploited as biofertilisers, notably to improve phosphorous uptake [37]. They have been shown to be able to reduce PPN damage in the field [73], in nurseries [74] and under controlled conditions [75], primarily for the genera Heterodera, Meloidogyne, Pratylenchus and Radopholus [76,77]. The mechanisms at the base of this activity are not only attributable to mycorrhiza-induced growth promotion in the host plant, but also to an increased plant tolerance or/and resistance against nematodes [78,79]. The effect of root mycorrhization was also found to be important in reducing the infestation by nematode species that are vectors of viruses: an isolate of Rhizophagus intraradices was found to reduce Xiphinema index (vector of the Grapevine Fanleaf Virus—GFLV) gall formation on the grapevine rootstock SO4 and nematode reproduction in soil [78]. The root colonization by R. intraradices was especially helpful under strong nematode pressure [80], also reducing the severity of fanleaf degenerative disease. Mycorrhization contributed to reducing the population of Nacobbus aberrans in both grafted and ungrafted tomato plants [81]. The colonization of pepper roots by Rhizoglomus fasciculatum decreased the amount of M. incognita galls and egg masses in the root system, allowing the plant to develop and yield similarly to uninfected plants [53]. Several AMF species, including Claroideoglomus claroideum, Diversispora eburnean, Dentiscutata heterogama, Funneliformis mossease and Rhizophagus intraradices, significantly reduced the number of Heterodera glycines cysts and the egg hatching and population size of juveniles in soybean [82].
Better knowledge about soil conditions in terms of physico-chemical characteristics and biodiversity status to exploit AMF to minimise PPN damage would help to clarify the ecological mechanisms underlaying co-occurrence patterns between AMF and PPNs, and offer guidance for improved organic crop management [76]. Indeed, it is noteworthy that variations in the potassium, phosphorus and moisture content at the rhizospheric soil revealed negative co-occurrence patterns between AMF and PPNs [83]. Moreover, a better understanding would allow better exploitation of the possible mechanisms of action of AMF, which include enhanced plant tolerance, direct competition for nutrients and space, induced systemic resistance and altered rhizosphere interactions [76].
2.3. Entomopathogenic Nematodes
Entomopathogenic nematodes (EPNs) have been demonstrated to effectively control various PPN species, particularly Meloidogyne spp., through several mechanisms of action, including a reduction in eggs and egg masses or the infection of second-stage juveniles [84,85]. Because many species are commercially available and do not need registration as biocontrol agents, the application of EPNs in organic crops is considered a sustainable practice [86]. However, their effectiveness, like other biocontrol agents, might fluctuate depending on the PPN species or application technique [87], and in certain cases has not been proved [88].
Exploiting the symbiotic bacteria hosted by EPNs might increase the advantages of their use [89]. Indeed, it was demonstrated that Meloidogyne species are less likely to penetrate host roots when EPN symbiotic bacteria and/or their metabolites are applied [90]. More important, the reduction in the gall index obtained with them was equivalent to that observed after chemical treatment under certain conditions [90], but populations of diverse kinds of root-knot nematodes responded differently to the bacterial suspensions [88].
The application of postbiotics derived from cultures of EPNs and/or their symbiotic bacteria can be an approach similar to the bacteria cell-free cultures mentioned above, favouring commercial production and use because of their cheaper manufacturing, simpler formulation and longer shelf life. Application of the cell-free supernatant was the most effective treatment to control two root-knot nematodes species among several alternatives (juveniles of Heterorhabditis bacteriophora and Steinernema spp., cell-free supernatants of their symbiotic bacteria and infected insect larvae) [84]. In greenhouse experiments, the application of suspensions of Pseudomonas oryzihabitans (associated with Steinernema abassi) at a concentration of 103 or 106 cell/mL resulted in a decrease by 22% and 82%, respectively, in the number of Meloidogyne spp. females in tomato roots, and reduced egg masses [91]. It is noteworthy that the lack of efficacy after application of the bacterial suspension was overcome with the application of its supernatant [88]. Studies with Photorhabdus luminescens, a naturally occurring symbiont of Heterorhabditis belonging to a bacterial order well known for producing a broad spectrum of biologically active compounds, revealed that the crude extract of this species had a toxic effect on M. incognita. [92]. The substances causing the toxicity were isolated from the supernatant of the bacterial culture and three were identified (trans-cinnamic acid, (4E)-5-phenylpent-4-enoic acid and indole), proving their effectiveness against M. incognita and Tylenchulus semipenetrans. All these compounds were found to selectively control several PPNs, with little effects on non-target nematodes such as Caenorhabditis, Steinernema or Heterorhabditis [93].
2.4. Combination of Bioinocula
Combining different bioinoculants to promote a more extensive colonization of the rhizosphere and expression of PPN suppression, eventually exploiting different mechanisms of action, can be a solution for overcoming the limited or inconsistent performance of individual microbial inoculants [94]. However, the combined microorganisms can be potentially antagonistic with each other or require different ecological conditions to fully express their activity, reducing the potential synergistic effect expected from dual or consortia inoculation [70].
Nevertheless, several studies have reported a higher efficacy in controlling PPNs, particularly root-knot nematode species, with dual inoculation in comparison to the single bioinoculum. The approaches tested by researchers included several possible combinations of bacteria and fungi, with or without specific biopesticide characteristics (e.g., different PGPR, N-fixing or biocontrol bacteria, or fungi with biopesticide potential or the ability to improve plant performance like AMF). For example, when considering the application of bacteria species, Rhizobium and Pseudomonas putida or P. alcaligenes were found to be the most effective combinations to reduce the galling and multiplication of M. javanica [95]. A consortium composed of B. subtilis FMCH002 and Bacillus paralicheniformis FMCH001 efficiently interfered with different stages of several Meloidogyne species, particularly impairing giant-cell development, as a result of multiple modes of action [96].
Several studies have reported positive outcomes obtained with the application of an AMF species with the bacteria. G. mosseae (currently Funneliformis. Mosseae) or Rhizophagus irregularis in combination with common PGPRs (Bacillus sp. Or pseudomonads, respectively), demonstrating that they were successful in controlling M. incognita [97]. G. mosseae was also effective when associated with rhizobacteria to reduce the impact of R. similis in bean [98], as well as when co-inoculated with a biocontrol species (Pasteuria penetrans) to reduce the final density of root-knot nematodes in tomato [99]. Effective control of M. javanica was also demonstrated by applying a natural consortium of AMF species isolated from tomato rhizospheric soils with Trichoderma harzianum, which also improved the nutrient acquisition and growth of tomato plants [100].
AMF-based biofertilisers are also applied as consortia of species or genera [101] and this technological solution has been proven to also be suitable in the case of PPN control. A consortium of Funneliformis mosseae and Rhizophagus fasciculatus resulted in being more effective in reducing the number of cysts, eggs by cyst as well as the final population of Heterodera cajani compared to the individual species [102]. A mixture of AMF species from different families of Glomeromycota significantly reduced the overall number of M. incognita galls in roots of the ornamental plant Impatiens balsamina in comparison to plants inoculated with only one species [103]. However, despite the fact that the egg masses and reproduction factors of N. aberrans decreased in mycorrhized tomato plants [75,104], no differences in the nematode’s penetration of the roots between single- or dual-strain (R. intraradices and F. mosseae) inoculated plants was observed, attributing the result to the similar physiological and morphological characteristics of the two AMF species [105].
The PPN control potential of a combined application of bacteria and/or fungi with biopesticide potential, not exclusively against nematodes, has been proved. For example, Bacillus subtilis and Paecilomyces lilacinus (currently Purpureocillium lilacinum) increased plant growth and suppressed root galls and nematodes beyond application of the individual strain [106]. A consortium composed of different biocontrol agents (P. fluorescens, P. lilacinus and Pichia guilliermondii) was highly effective against nematodes, also inducing systemic resistance in tomato plants [70], even though the addition of a cyanobacterium (Calothrix parietina) to the consortium antagonised the other biocontrol agents. A consortium formed by Bacillus species, T. harzianum and a mycorrhizal fungus (G. aggregatum) was successful in lessening the effects of PPNs on basil growth and significantly modified the quality of its aromatic oil [107].
3. Agronomical Factors Affecting the Efficacy of Microbial Inocula for PPN Control
The efficacy of microbial inocula, either strains showing biocontrol or plant nutrition and growth-promotion properties, can be modified by a number of factors that relate to the environmental conditions (particularly soil physical–chemical characteristics), the soil management practices applied by the farmer and the characteristics of the microbial formulation and its mode of use (Figure 4).
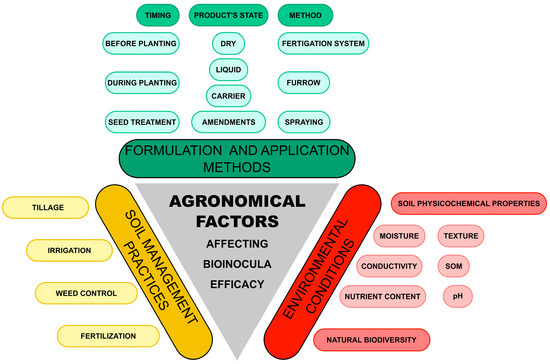
Figure 4.
Different agronomical factors, which could affect efficacy of bioinocula used for PPN control.
3.1. Environmental Conditions
The effectiveness of any biocontrol microorganism is influenced by the environmental conditions, particularly the soil physical–chemical properties. Temperature may significantly affect the bioinocula development dynamic and, consequently, its capacity to control PPNs. For example, P. chlamydosporia isolates from Portugal or Spain exhibited growth inhibition over 33 °C and below 10 °C for the mycelium [108], but strains isolated from the UK showed an optimal growth temperature of 18 °C [109]. Soil texture and the derived water and air capacity can also modify the biocontrol microorganism’s behaviour: clay soils are often less conductive compared to light soils, which are characterised by greater aeration [110]. However, P. chlamydosporia propagules were able to penetrate the soil profile and colonise deeper layers and the root system up to an around 50 cm depth, both in sandy and clay soils [110,111].
Soil organic matter content, pH, moisture and nutrient content can impact the distribution and activity of nematophagous fungi [112], as well as of other multifunctional species [113], and can thus be considered factors also affecting the efficacy of inoculated microbial formulations for PPN control. Considering particularly nematophagous fungi, species forming adhesive nets, such as A. oligospora, were found to be associated with soils with low organic matter and water content and a variable pH range [112]. On the other hand, endoparasitic fungi forming conidia (such as H. rhossiliensis) were generally associated with soils with high organic matter, low pH and higher soil moisture. The natural presence of P. lilacinum and A. oligospora has been positively associated with soil available water capacity [114]. Soil electrical conductivity, a measure of salinity, was found to impact five different nematophagous fungi, including species that are proposed as biocontrol agents [114]. Interestingly, the same study showed that several nematophagous fungi were positively associated with P and K content in soil.
However, considering the mode of action of the available formulated species, it is interesting to underline the ecological aspects characteristic of the different nematophagous fungi groups. Endoparasitic fungi (i.e., H. rhossiliensis), being antagonistic obligate symbionts and thus independent of the soil for nutrients, are better suited to soils where nematode density is high [115], a condition frequently found in organic fields. Nevertheless, potassium was found to enhance nematode infection by H. rhossiliensis [116] and its soil content was found to be associated with the presence of species also used in biocontrol formulations (A. oligospora, H. rhossiliensis and P. lilacinum) [114]. On the other hand, predatory nematophagous fungi, which can be also efficient saprophytes (e.g., A. oligospora or D. brochopaga), are able to compete with other species for the nutrients available in the soil and thus can also better survive on less-fertile soils [112,115], even though soil P content in citrus orchards was found to be positively associated with the natural presence of A. oligospora [114].
3.2. Formulation and Application Methods
The time, application method as well as the density of nematodes also affect the efficacy of microbial-based products [117]. Suitable application methods include integration into the soil using dry formulations, application of liquid or soluble (wettable powder) formulations to the furrow before or during crop planting [118], spraying [119], inoculation of seedling substrate [61], distribution through the fertigation system [120] and seed treatment [111].
Good timing of the application is also critical to ensure an optimal level of efficacy. Cucumber plants in commercial greenhouses were protected against PPNs when the inoculum was applied into the soil 1 to 2 weeks prior to transplanting [121]. An additional benefit can be obtained applying the microbial product prior to transplantation, soaking the substrate where seedlings are developing: the application of P. lilacinus and S. racemosum in this way reduced the number of M. incognita galls and eggs [72]. Inoculation at sowing and transplanting a strain of the endophytic fungus Fusarium oxysporum that inhibits M. incognita juvenile penetration and development in tomato roots resulted in slightly higher levels of biocontrol, though not significantly different when compared with single inoculation at sowing [122]. The capacity of the microorganism to grow on the seed surface and colonise the roots and rhizosphere as well as the surrounding soil can determine the efficacy of application via seed coating [123], as was also demonstrated with some industrial crops [124].
The formulation, beside required to assure long shelf life, a low dosage and application using various methods, can have a significant impact on the control efficacy of bioinocula based on both fungi and bacteria [57]. For microorganisms intended to control PPNs, the formulation of fungi as cereal kernels after solid-state fermentation or based on alginate has only been tested on a small scale [125]. However, these techniques are quite widely used for other microbial-based preparations [9] and thus could be suitable to formulate microorganisms for PPN control.
The form of the fungus inoculum used to formulate the biocontrol agent can also affect its efficacy. A contrasting effect of biotic inhibition (soil heating) was observed in laboratory and field trials for H. rhossiliensis and A. haptotyla depending on whether the species were formulated as pelletised hyphae or as parasitised nematodes [50]. H. rhossiliensis was found to be insensitive to biotic inhibition when formulated as parasitised nematodes, while the opposite occurred with A. haptotyla. The different sensitivity, and thus potential efficacy, would be related to the production process (pellets with the hyphae are dried, unlike parasitised nematodes), or to biological characteristics (e.g., the presence of a cuticle or other barriers in the parasitised nematodes or the fragmentation of the assimilative hyphae in pellets). A similar contrasting behaviour was observed for cases of different doses and types of organic amendments and the capacity of Dactylellina haptotyla and Arthrobotrys oligospora in vineyards: population density and trapping were most enhanced by a smaller quantity of alfalfa amendment in the case of the former species, while a larger quantity increased the capacity of the latter species [51]. On the other hand, a commercial formulation of Dactylaria brochopaga dramatically reduced the amount of M. incognita in soil and the number of galls on grapevine roots compared to untreated plants [126].
Nevertheless, the formulation is frequently designed by the manufacturer based on technological possibilities and the target crop. Liquid formulations of bacteria and fungi (i.e., based on conidia or spores) were successful in controlling M. incognita, M. javanica and M. hapla in tomato [127] and carrot crops [128]. Combining a liquid formulation of B. subtilis applied to seeds (10 mL kg−1 seeds) together with soil application as a bacteria-enriched vermicompost resulted in a significant reduction in the PPN population [129]. When used in liquid form, P. lilacinus and T. viride controlled M. hapla or M. incognita up to 69.5% more effectively than the untreated control [127,128], while P. lilacinus produced by liquid fermentation and formulated with talc powder reached only 48% efficacy against M. incognita in carrot fields [130]. A similar talc formulations of P. fluorescens was not effective in decreasing the population density of root-knot nematodes under field conditions [131]. However, the application of B. thuringensis as a soil drench also reduced tomato root galling caused by M. inognita by only 53% [23]. The simulating effects of the additives present in the formulation may contribute to the biocontrol activity of many Bacillus species [132]. On the other hand, solid formulations were also able to achieve a sufficient level of efficacy: regardless of the dose applied (200 or 400 kg ha−1), a solid B. firmus formulation was very effective in decreasing the number of galls in tomato seedlings [22]. A. dactyloides, and P. chlamydosporium combined in a granulated product strongly reduced the population of nematodes and their damage to greenhouse-grown plants [133].
The addition of organic amendments, also as carriers, to microbial-based bioinocula may be a smart strategy for maximizing the benefits of both types of products [19]. The application of antagonistic fungi, PGPR and animal manure resulted in a good development of plants challenged with PPNs [134]. The addition of cattle manure when applying Pseudomonas putida or P. lilacinus resulted in the greatest reduction in galling and nematode multiplication and the largest increase in plant growth. The best combination for controlling M. incognita on tomatoes was P. fluorescens and poultry manure, but high levels of control were also achieved in association with goat dung [30]. Animal manure can be a source of spores for a number of nematophagous fungi, including Arthrobotrys spp. and Monacrosporium spp., i.e., acting as a prebiotic, thus having a direct impact on PPNs [135]. Another prebiotic approach can be considered the treatment of carrot seeds or soil substrate with neem cake enriched with P. putida and P. lilacinus formulations, which resulted in a reduction in the population of M. incognita in roots and soil [130]. Applying a formulation based on B. firmus before or immediately after removing the plastic sheet used for soil solarization or combining soil solarization with a variety of organic amendments (broiler litter, cottonseed meal, feather meal or soybean oilcake) were more effective than the amendments or soil solarization alone [132].
3.3. Soil Management Practices
Soil management practices shall be also considered when planning the use of microbial-based products to control PPNs as they can interact with unpredictable effects. Those that have been found to limit PPN damage include (i) crop rotation [136]; (ii) cover crops with trap or suppressive plants [137]; (iii) application of organic amendments or fertilisers [138]; (iv) biofumigation, either as a result of green manuring [139] or as specific application of brassica meals [140]; (v) soil solarization [141]; (vi) physical soil treatments [142]; and (vii) resistant rootstock varieties [143]. All these practices are in line with organic farming principles and rules and can thus be used to reduce PPN population development in horticultural crops. The kind of interactions that occur between these practices with the application of microbial-based products can be hypothesised considering the possible mechanisms by which they can suppress nematodes [144,145]: (i) depending on the original matrix of the amendment, the enhancement and/or introduction of antagonistic microorganisms, particularly fungi (in the case of composts and animal manures); (ii) the indirect increase in plant tolerance and resistance brought on by rhizosphere bacteria like Bacillus spp. and Pseudomonas spp., or endophytic fungi (e.g., Trichoderma spp.) (in the case of plant extracts and organic fertilisers); (iii) the release of pre-existing nematicidal compounds (e.g., polythienyls from Tagetes spp. and other Asteraceae used as cover crops); (iv) production of other products with nematicidal properties during the degradation process (e.g., nitriles or isothiocyanates from Brassica species or cyanoglucoside compounds from Sudan grass when used as green manure or for biofumigation); and (v) the modification of the chemical and physical conditions of soil, making it less suitable for nematode behaviour (in the case of physical treatments).
The soil complexity and the various mechanisms and interactions between the different trophic levels, which are generally increased by organic farming management, would also account for the contrasting results found on the impact on PPN populations and damage, as in the case of kinds of manures [30] or composts [146] or other organic materials applied [53] or as a result of changes in the soil biogeochemical cycles [147].
4. Conclusions and Future Prospects
In order to attain a sufficient and reliable degree of effectiveness, PPN management in organic horticultural crops must tackle the complex soil environment considering the peculiarities of the soil management practices commonly adopted by organic farmers to maintain soil health and fertility.
The improvement in the PPN biocontrol capacity and efficacy of bioinocula can be achieved only through an increased knowledge about the soil microbiome’s role and its interactions with inoculated beneficial microorganisms. However, the interactions within the complex life web present in the soil, as affected by soil management practices, are also one of the major factors that interfere with microbial inocula as well as with the nematodes’ population. Therefore, studies are needed to better understand these interactions, which could lead to higher effectiveness and improvements in the application methods of microbial bioinocula within specific cropping systems and agronomic practices.
New strains showing PPN control capacity are continuously isolated, and this is paralleled by an increased number of registered products on the market, showing the industry interest in this approach. At the same time, policies are being developed to support this trend. Researchers should take advantage of these conditions to deepen work on the development of consortia, improved formulations and the integration of bioinocula with soil management practices. These studies are required to be able to develop effective control strategies.
Organic production is based on the concept that soil is a key production factor. The physical, chemical and biological processes in soil are strongly linked to root system function, the rhizosphere microbiome and agronomical practices. In-depth knowledge on the modes of action of the microbial control agents and of other microbial inocula would also allow their better exploitation for PPN control. A strategy to overcome some drawbacks of microbial inocula that should further be researched in organic horticultural crops include the integrated application of “multi-biotics”: prebiotics (products fostering an autochthonous soil microbiome), probiotics (beneficial microorganisms) and postbiotics (metabolic derivatives of microbial strains) [17]. The “multi-biotic” concept approach is derived from the multifunctional capacities of microorganisms, which can be exploited irrespective of the constraints derived from legal provisions (i.e., the process of the registration of plant protection products). The multifunctional and complex effect of this strategy would require interdisciplinary studies to fine tune its implementation in different organic horticultural crops.
The multifunctional properties of the different soil microorganisms applied as bioinocula can be enhanced via various soil management practices, as they are closely intertwined and consequently affect each other. New organic materials (e.g., biochar, biodigestates, extracts of humic acids, plant and algae extracts, etc.) are becoming available on the market and are allowed to be applied in organic farming. The soil microbiome’s structure can be modified by them, and it is assumed that they can impact soil nematode populations. The production process, formulation and applied doses of these materials are different and can modify their characteristics, thus requiring studies to evaluate their effect on PPN control.
PPN control strategies in organic horticultural crops should implement a complex approach to achieve satisfactory efficacy. Therefore, instead of implementing an input substitution strategy when converting to organic production (i.e., where the technical means allowed by organic farming rules are applied instead of synthetic chemical inputs), applying agroecological principles, increasing biodiversity and combining inputs with soil management practices fostering soil fertility would modify the current paradigm of organic horticulture and help in the design of a healthy agroecosystem by creating cropping systems that naturally limit the increase in pests and are conducive to an ecological equilibrium [148]. Interdisciplinary research would thus be necessary to handle the complex problem of managing plant health in organic horticulture and be able to provide farmers and advisers with useful knowledge and tools to support this paradigm shift: we believe this is the major challenge that researchers should address in future work.
Author Contributions
Investigation, writing and editing was performed by E.M.F. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work was partially supported by the project EXCALIBUR funded by the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 817946.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The European Parliament and the Council of the European Union. Regulation (EU) 2018/848 of the European Par-Liament and of the Council of 30 May 2018 on Organic Production and Lbelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. J. Eur. Union 2018, L150/1. [Google Scholar]
- 2. European Commission. Communication from the Commission to the European Parliament, the Council, the European Eco-nomic and Social Committee and the Committee of the Regions: EU Soil Strategy for 2030—Reaping the Benefits of Healthy Soils for People, Food, Nature and Climate. COM2021699 Final. 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52021DC0699 (accessed on 3 August 2023).
- Huang, R.; McGrath, S.P.; Hirsch, P.R.; Clark, I.M.; Storkey, J.; Wu, L.; Zhou, J.; Liang, Y. Plant–microbe networks in soil are weakened by century-long use of inorganic fertilizers. Microb. Biotechnol. 2019, 12, 1464–1475. [Google Scholar] [CrossRef]
- de Vries, F.T.; Thébault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Jørgensen, H.B.; Brady, M.V.; Christensen, S.; de Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef] [PubMed]
- Maron, P.-A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High Microbial Diversity Promotes Soil Ecosystem Functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef]
- Friberg, H.; Lagerlöf, J.; Rämert, B. Influence of soil fauna on fungal plant pathogens in agricultural and horticultural systems. Biocontrol Sci. Technol. 2005, 15, 641–658. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T. Nematode indicators of organic enrichment. J. Nematol. 2006, 38, 3–12. [Google Scholar]
- Malusa, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, L.R., III; Barker, K.R.; Ristaino, J.B. Influences of organic and synthetic soil fertility amendments on nematode trophic groups and community dynamics under tomatoes. Appl. Soil Ecol. 2002, 21, 233–250. [Google Scholar] [CrossRef]
- Neher, D.A. Nematode communities in organically and conventionally managed agricultural soils. J. Nematol. 1999, 31, 142–154. [Google Scholar]
- Hallmann, J.; Frankenberg, A.; Paffrath, A.; Schmidt, H. Occurrence and importance of plant-parasitic nematodes in organic farming in Germany. Nematology 2007, 9, 869–879. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Mia, M.J.; Furmanczyk, E.M.; Golian, J.; Kwiatkowska, J.; Malusá, E.; Neri, D. Living Mulch with Selected Herbs for Soil Management in Organic Apple Orchards. Horticulturae 2021, 7, 59. [Google Scholar] [CrossRef]
- Shilev, S.; Azaizeh, H.; Vassilev, N.; Georgiev, D.; Babrikova, I. Interactions in Soil-Microbe-Plant System: Adaptation to Stressed Agriculture. In Microbial Interventions in Agriculture and Environment: Volume 1: Research Trends, Priorities and Prospects; Singh, D.P., Gupta, V.K., Prabha, R., Eds.; Springer: Singapore, 2019; pp. 131–171. ISBN 9789811383915. [Google Scholar]
- Kowalska, J.; Tyburski, J.; Matysiak, K.; Tylkowski, B.; Malusá, E. Field Exploitation of Multiple Functions of Beneficial Microorganisms for Plant Nutrition and Protection: Real Possibility or Just a Hope? Front. Microbiol. 2020, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, M.; Flor-Peregrin, E.; Malusá, E.; Vassilev, N. Towards Better Understanding of the Interactions and Efficient Ap-plication of Plant Beneficial Prebiotics, Probiotics, Postbiotics and Synbiotics. Front. Plant Sci. 2020, 11, 1068. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Mahmood, I. Biological control of plant parasitic nematodes by fungi: A review. Bioresour. Technol. 1996, 58, 229–239. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Del Moral, L.F.G.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of Microbial Inoculants by Encapsulation in Natural Polysaccharides: Focus on Beneficial Properties of Carrier Additives and Derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Walia, R.K.; Sharma, S.B.; Vats, R. Bacterial Antagonists of Phytonematodes. In Biocontrol Potential and Its Exploitation in Sus-tainable Agriculture: Crop Diseases, Weeds, and Nematodes; Upadhyay, R.K., Mukerji, K.G., Chamola, B.P., Eds.; Springer: Boston, MA, USA, 2000; pp. 173–186. ISBN 978-1-4615-4209-4. [Google Scholar]
- Xiao, T.-J.; Chen, F.; Gao, C.; Zhao, Q.-Y.; Shen, Q.-R.; Ran, W. Bacillus cereus X5 Enhanced Bio-Organic Fertilizers Effectively Control Root-Knot Nematodes (Meloidogyne sp.). Pedosphere 2013, 23, 160–168. [Google Scholar] [CrossRef]
- Terefe, M.; Tefera, T.; Sakhuja, P.K. Effect of a formulation of Bacillus firmus on root-knot nematode Meloidogyne incognita infestation and the growth of tomato plants in the greenhouse and nursery. J. Invertebr. Pathol. 2009, 100, 94–99. [Google Scholar] [CrossRef]
- Zuckerman, B.M.; Dicklow, M.B.; Acosta, N. A Strain of Bacillus thuringiensis for the Control of Plant-parasitic Nematodes. Biocontrol Sci. Technol. 1993, 3, 41–46. [Google Scholar] [CrossRef]
- Du, J.; Gao, Q.; Ji, C.; Song, X.; Liu, Y.; Li, H.; Li, C.; Zhang, P.; Li, J.; Liu, X. Bacillus licheniformis JF-22 to Control Meloidogyne incognita and Its Effect on Tomato Rhizosphere Microbial Community. Front. Microbiol. 2022, 13, 863341. [Google Scholar] [CrossRef]
- Bo, T.; Kong, C.; Zou, S.; Mo, M.; Liu, Y. Bacillus nematocida B16 Enhanced the Rhizosphere Colonization of Pochonia chlamydosporia ZK7 and Controlled the Efficacy of the Root-Knot Nematode Meloidogyne incognita. Microorganisms 2022, 10, 218. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S. Systemic Resistance in Tomato Induced by Biocontrol Bacteria Against the Root-Knot Nematode, Meloidogyne javanicais Independent of Salicylic Acid Production. J. Phytopathol. 2004, 152, 48–54. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Iqbal, A.; Mahmood, I. Effects of Pseudomonas fluorescens and fertilizers on the reproduction of Meloidogyne incognita and growth of tomato. Appl. Soil Ecol. 2001, 16, 179–185. [Google Scholar] [CrossRef]
- Seenivasan, N.; Devrajan, K. Management of Meloidogyne Incognita on Medicinal Coleus by Commercial Biocontrol Formu-lations. Nematol. Mediterr. 2008, 36, 61–67. [Google Scholar]
- Zhang, R.; Ouyang, J.; Xu, X.; Li, J.; Rehman, M.; Deng, G.; Shu, J.; Zhao, D.; Chen, S.; Sayyed, R.Z.; et al. Nematicidal Activity of Burkholderia arboris J211 Against Meloidogyne incognita on Tobacco. Front. Microbiol. 2022, 13, 915546. [Google Scholar] [CrossRef]
- Siddiqui, Z.A. Effects of plant growth promoting bacteria and composed organic fertilizers on the reproduction of Meloidogyne incognita and tomato growth. Bioresour. Technol. 2004, 95, 223–227. [Google Scholar] [CrossRef]
- Costa, S.R.; Ng, J.L.P.; Mathesius, U. Interaction of Symbiotic Rhizobia and Parasitic Root-Knot Nematodes in Legume Roots: From Molecular Regulation to Field Application. Mol. Plant Microbe Interact. 2021, 34, 470–490. [Google Scholar] [CrossRef]
- Desaeger, J.; Odee, D.; Machua, J.; Esitubi, M. Interactions between Meloidogyne javanica (Treub) chitwood and rhizobia on growth of Sesbania sesban (L.) Merr. Appl. Soil Ecol. 2005, 29, 252–258. [Google Scholar] [CrossRef]
- Wood, C.W.; Pilkington, B.L.; Vaidya, P.; Biel, C.; Stinchcombe, J.R. Genetic conflict with a parasitic nematode disrupts the legume–rhizobia mutualism. Evol. Lett. 2018, 2, 233–245. [Google Scholar] [CrossRef]
- Li, Y.; Lei, S.; Cheng, Z.; Jin, L.; Zhang, T.; Liang, L.-M.; Cheng, L.; Zhang, Q.; Xu, X.; Lan, C.; et al. Microbiota and functional analyses of nitrogen-fixing bacteria in root-knot nematode parasitism of plants. Microbiome 2023, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Rae, R.; Iatsenko, I.; Witte, H.; Sommer, R.J. A subset of naturally isolated Bacillus strains show extreme virulence to the free-living nematodes Caenorhabditis elegans and Pristionchus pacificus. Environ. Microbiol. 2010, 12, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Dickson, D.W. Review of Pasteuria penetrans: Biology, Ecology, and Biological Control Potential. J. Nematol. 1998, 30, 313–340. [Google Scholar] [PubMed]
- Malusà, E.; Pinzari, F.; Canfora, L. Efficacy of Biofertilizers: Challenges to Improve Crop Production. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Singh, H.B., Prabha, R., Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 17–40. ISBN 978-81-322-2642-0. [Google Scholar]
- Khan, M.R.; Mohiddin, F.A.; Ahamad, F. Inoculant rhizobia suppressed root-knot disease, and enhanced plant productivity and nutrient uptake of some field-grown food legumes. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 166–174. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, J.; Nie, Q.; Huang, D.; Yu, C.; Zheng, L.; Cai, M.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Sci. Rep. 2017, 7, 16213. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, X.; Xue, H.; Huang, D.; Cai, M.; Huang, F.; Zheng, L.; Yu, Z.; Zhang, J. Reproductive Toxicity of Furfural Acetone in Meloidogyne incognita and Caenorhabditis elegans. Cells 2022, 11, 401. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.-Y.; Liu, X.-F.; Guan, Q.; Dou, N.-X.; Li, J.; Zhang, Q.; Gao, Y.-M.; Wang, M.; Zhou, B. Nematicidal activity of volatile organic compounds produced by Bacillus altitudinis AMCC 1040 against Meloidogyne incognita. Arch. Microbiol. 2022, 204, 521. [Google Scholar] [CrossRef]
- Diyapoglu, A.; Chang, T.-H.; Chang, P.-F.L.; Yen, J.-H.; Chiang, H.-I.; Meng, M. Fumigant Activity of Bacterial Volatile Organic Compounds against the Nematodes Caenorhabditis elegans and Meloidogyne incognita. Molecules 2022, 27, 4714. [Google Scholar] [CrossRef]
- Pulavarty, A.; Singh, A.; Smyth, D.; Mehta, J.P.; Horgan, K.; Kakouli-Duarte, T. Sustainable management of the potato cyst nematode, Globodera rostochiensis, with two microbial fermentation products. Front. Plant Sci. 2022, 13, 987059. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.-Q. Molecular Mechanisms of Nematode-Nematophagous Microbe Interactions: Basis for Biological Control of Plant-Parasitic Nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhao, Y.; Zhou, J.; Feng, H.; Jiang, D.; Zhang, K.-Q.; Yang, J. Trapping devices of nematode-trapping fungi: Formation, evolution, and genomic perspectives. Biol. Rev. 2017, 92, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Nordbring-Hertz, B.; Jansson, H.-B.; Tunlid, A. Nematophagous Fungi. In Encyclopedia of Life Sciences; Wiley-Blackwell: Hoboken, NJ, USA, 2006; ISBN 978-0-470-01590-2. [Google Scholar]
- Lopez-Llorca, L.V.; Maciá-Vicente, J.G.; Jansson, H.-B. Mode of Action and Interactions of Nematophagous Fungi. In Integrated Management and Biocontrol of Vegetable and Grain Crops Nematodes; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 51–76. ISBN 978-1-4020-6063-2. [Google Scholar]
- Jaffee, B.A. Correlations Between Most Probable Number and Activity of Nematode-Trapping Fungi. Phytopathology 2003, 93, 1599–1605. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaffee, B.A. Augmentation of Soil with the Nematophagous Fungi Hirsutella rhossiliensis and Arthrobotrys haptotyla. Phytopathology 2000, 90, 498–504. [Google Scholar] [CrossRef][Green Version]
- Jaffee, B.A. Do Organic Amendments Enhance the Nematode-Trapping Fungi Dactylellina Haptotyla and Arthrobotrys Oli-gospora? J. Nematol. 2004, 36, 267–275. [Google Scholar]
- Jaffee, B.A.; Ferris, H.; Scow, K.M. Nematode-Trapping Fungi in Organic and Conventional Cropping Systems. Phytopathology 1998, 88, 344–350. [Google Scholar] [CrossRef]
- Giri, B.; Rawat, R.; Saxena, G.; Manchanda, P.; Wu, Q.-S.; Sharma, A. Effect of Rhizoglomus fasciculatum and Paecilomyces lilacinus in the biocontrol of root-knot nematode, Meloidogyne incognita in Capsicum annuum L. Commun. Integr. Biol. 2022, 15, 75–87. [Google Scholar] [CrossRef]
- EU Pesticide Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 14 April 2023).
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, J.; Zong, Z.; Ma, Q.; Wang, Y. Evaluation of the Biocontrol Potential of Purpureocillium lilacinum QLP12 against Verticillium dahliae in Eggplant. BioMed Res. Int. 2017, 2017, 4101357. [Google Scholar] [CrossRef]
- Manzanilla-López, R.H.; Esteves, I.; Finetti-Sialer, M.M.; Hirsch, P.R.; Ward, E.; Devonshire, J.; Hidalgo-Díaz, L. Pochonia Chlamydosporia: Advances and Challenges to Improve Its Performance as a Biological Control Agent of Sedentary En-do-Parasitic Nematodes. J. Nematol. 2013, 45, 1–7. [Google Scholar]
- Yang, J.; Liang, L.; Li, J.; Zhang, K.-Q. Nematicidal enzymes from microorganisms and their applications. Appl. Microbiol. Biotechnol. 2013, 97, 7081–7095. [Google Scholar] [CrossRef]
- Lopez-Llorca, L.V.; Olivares-Bernabeu, C.; Salinas, J.; Jansson, H.-B.; Kolattukudy, P.E. Pre-penetration events in fungal parasitism of nematode eggs. Mycol. Res. 2002, 106, 499–506. [Google Scholar] [CrossRef]
- Bontempo, A.F.; Lopes, E.A.; Fernandes, R.H.; DE Freitas, L.G.; Dallemole-Giaretta, R. DOSE-RESPONSE EFFECT OF Pochonia chlamydosporia AGAINST Meloidogyne incognita ON CARROT UNDER FIELD CONDITIONS. Rev. Caatinga 2017, 30, 258–262. [Google Scholar] [CrossRef]
- Viggiano, J.R.; de Freitas, L.G.; Lopes, E.A. Use of Pochonia chlamydosporia to control Meloidogyne javanica in cucumber. Biol. Control 2014, 69, 72–77. [Google Scholar] [CrossRef]
- Tylkowski, B.; Olkiewicz, M.; Montane, X.; Nogalska, A.; Haponska, M.; Montornes, J.M.; Kowalska, J.; Malusá, E. Encapsulation Technologies in Agriculture; De Gruyter: Berlin, Germany, 2020; pp. 287–302. ISBN 978-3-11-064207-0. [Google Scholar]
- Escudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biol. 2016, 120, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibañez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan Increases Tomato Root Colonization by Pochonia chlamydosporia and Their Combination Reduces Root-Knot Nematode Damage. Front. Plant Sci. 2017, 8, 1415. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Broberg, M.; Haarith, D.; Broberg, A.; Bushley, K.E.; Durling, M.B.; Viketoft, M.; Jensen, D.F.; Dubey, M.; Karlsson, M. Natural variation of root lesion nematode antagonism in the biocontrol fungus Clonostachys rosea and identification of biocontrol factors through genome-wide association mapping. Evol. Appl. 2020, 13, 2264–2283. [Google Scholar] [CrossRef]
- Sahebani, N.; Hadavi, N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol. Biochem. 2008, 40, 2016–2020. [Google Scholar] [CrossRef]
- Sun, M.-H.; Gao, L.; Shi, Y.-X.; Li, B.-J.; Liu, X.-Z. Fungi and actinomycetes associated with Meloidogyne spp. eggs and females in China and their biocontrol potential. J. Invertebr. Pathol. 2006, 93, 22–28. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, B.; Lin, Q.; Riley, I.T.; Ding, S.; Chen, L.; Cui, J.; Yang, L.; Li, H. Colonization of Beauveria bassiana 08F04 in root-zone soil and its biocontrol of cereal cyst nematode (Heterodera filipjevi). PLoS ONE 2020, 15, e0232770. [Google Scholar] [CrossRef]
- Wonganu, B.; Pootanakit, K.; Boonyapakron, K.; Champreda, V.; Tanapongpipat, S.; Eurwilaichitr, L. Cloning, expression and characterization of a thermotolerant endoglucanase from Syncephalastrum racemosum (BCC18080) in Pichia pastoris. Protein Expr. Purif. 2008, 58, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Abo-Elyousr, K.A. Management of the root-knot nematode Meloidogyne incognita on tomato with combinations of different biocontrol organisms. Crop Prot. 2011, 30, 285–292. [Google Scholar] [CrossRef]
- Huang, W.-K.; Sun, J.-H.; Cui, J.-K.; Wang, G.-F.; Kong, L.-A.; Peng, H.; Chen, S.-L.; Peng, D.-L. Efficacy Evaluation of Fungus Syncephalastrum racemosum and Nematicide Avermectin against the Root-Knot Nematode Meloidogyne incognita on Cucumber. PLoS ONE 2014, 9, e89717. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-K.; Cui, J.-K.; Liu, S.-M.; Kong, L.-A.; Wu, Q.-S.; Peng, H.; He, W.-T.; Sun, J.-H.; Peng, D.-L. Testing various biocontrol agents against the root-knot nematode (Meloidogyne incognita) in cucumber plants identifies a combination of Syncephalastrum racemosum and Paecilomyces lilacinus as being most effective. Biol. Control 2016, 92, 31–37. [Google Scholar] [CrossRef]
- Affokpon, A.; Coyne, D.L.; Lawouin, L.; Tossou, C.; Agbèdè, R.D.; Coosemans, J. Effectiveness of native West African arbuscular mycorrhizal fungi in protecting vegetable crops against root-knot nematodes. Biol. Fertil. Soils 2011, 47, 207–217. [Google Scholar] [CrossRef]
- Castillo, P.; Nico, A.I.; Azcón-Aguilar, C.; Rincón, C.D.R.; Calvet, C.; Jiménez-Díaz, R.M. Protection of olive planting stocks against parasitism of root-knot nematodes by arbuscular mycorrhizal fungi. Plant Pathol. 2006, 55, 705–713. [Google Scholar] [CrossRef]
- Marro, N.; Lax, P.; Cabello, M.; Doucet, M.E.; Becerra, A.G. Use of the arbuscular mycorrhizal fungus Glomus intraradices as biological control agent of the nematode Nacobbus aberrans parasitizing tomato. Braz. Arch. Biol. Technol. 2014, 57, 668–674. [Google Scholar] [CrossRef]
- Schouteden, N.; De Waele, D.; Panis, B.; Vos, C.M. Arbuscular Mycorrhizal Fungi for the Biocontrol of Plant-Parasitic Nematodes: A Review of the Mechanisms Involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef]
- de Sá, C.S.B.; Campos, M.A.S. Arbuscular mycorrhizal fungi decrease Meloidogyne enterolobii infection of Guava seedlings. J. Helminthol. 2020, 94, e183. [Google Scholar] [CrossRef]
- Hao, Z.; Fayolle, L.; van Tuinen, D.; Chatagnier, O.; Li, X.; Gianinazzi, S.; Gianinazzi-Pearson, V. Local and systemic mycorrhiza-induced protection against the ectoparasitic nematode Xiphinema index involves priming of defence gene responses in grapevine. J. Exp. Bot. 2012, 63, 3657–3672. [Google Scholar] [CrossRef]
- Vos, C.; Tesfahun, A.; Panis, B.; De Waele, D.; Elsen, A. Arbuscular mycorrhizal fungi induce systemic resistance in tomato against the sedentary nematode Meloidogyne incognita and the migratory nematode Pratylenchus penetrans. Appl. Soil Ecol. 2012, 61, 1–6. [Google Scholar] [CrossRef]
- Hao, Z.; van Tuinen, D.; Fayolle, L.; Chatagnier, O.; Li, X.; Chen, B.; Gianinazzi, S.; Gianinazzi-Pearson, V. Arbuscular mycorrhiza affects grapevine fanleaf virus transmission by the nematode vector Xiphinema index. Appl. Soil Ecol. 2018, 129, 107–111. [Google Scholar] [CrossRef]
- Garita, S.A.; Bernardo, V.F.; Guimarães, M.D.A.; Arango, M.C.; Ruscitti, M.F. Mycorrhization and grafting improve growth in the tomato and reduce the population of Nacobbus aberrans. Rev. Ciênc. Agron. 2019, 50, 609–615. [Google Scholar] [CrossRef]
- Pawlowski, M.L.; Hartman, G.L. Impact of Arbuscular Mycorrhizal Species on Heterodera glycines. Plant Dis. 2020, 104, 2406–2410. [Google Scholar] [CrossRef]
- Ferreira, B.S.; Santana, M.V.; Macedo, R.S.; Silva, J.O.; Carneiro, M.A.C.; Rocha, M.R. Co-occurrence patterns between plant-parasitic nematodes and arbuscular mycorrhizal fungi are driven by environmental factors. Agric. Ecosyst. Environ. 2018, 265, 54–61. [Google Scholar] [CrossRef]
- Kepenekci, I.; Hazir, S.; Lewis, E.E. Evaluation of entomopathogenic nematodes and the supernatants of the in vitro culture medium of their mutualistic bacteria for the control of the root-knot nematodes Meloidogyne incognita and M. arenaria. Pest Manag. Sci. 2016, 72, 327–334. [Google Scholar] [CrossRef] [PubMed]
- El Aimani, A.; Houari, A.; Laasli, S.-E.; Mentag, R.; Iraqi, D.; Diria, G.; Khayi, S.; Lahlali, R.; Dababat, A.A.; Mokrini, F. Antagonistic potential of Moroccan entomopathogenic nematodes against root-knot nematodes, Meloidogyne javanica on tomato under greenhouse conditions. Sci. Rep. 2022, 12, 2915. [Google Scholar] [CrossRef]
- Lacey, L.A.; Georgis, R. Entomopathogenic Nematodes for Control of Insect Pests Above and Below Ground with Comments on Commercial Production. J. Nematol. 2012, 44, 218–225. [Google Scholar]
- Del Valle, E.E.; Lax, P.; Dueñas, J.R.; Doucet, M.E. Effects of insect cadavers infected by Heterorhabditis bacteriophora and Steinernema diaprepesi on Meloidogyne incognita parasitism in pepper and summer squash plants. Cienc. Investig. Agrar. 2013, 40, 109–118. [Google Scholar] [CrossRef][Green Version]
- Caccia, M.; Marro, N.; Dueñas, J.R.; Doucet, M.E.; Lax, P. Effect of the entomopathogenic nematode-bacterial symbiont complex on Meloidogyne hapla and Nacobbus aberrans in short-term greenhouse trials. Crop Prot. 2018, 114, 162–166. [Google Scholar] [CrossRef]
- Sharma, M.P.; Sharma, A.N.; Hussaini, S.S. Entomopathogenic nematodes, a potential microbial biopesticide: Mass production and commercialisation status—A mini review. Arch. Phytopathol. Plant Prot. 2011, 44, 855–870. [Google Scholar] [CrossRef]
- Vyas, R.V.; Patel, B.; Maghodia, A.; Patel, D.J. Significance of Metabolites of Native Xenorhabdus, a Bacterial Symbiont of Steinernema, for Suppression of Collar Rot and Root Knot Diseases of Groundnut. Indian J. Biotechnol. 2008, 7, 371–377. [Google Scholar]
- Vagelas, I.K.; Pembroke, B.; Gowen, S.R.; Davies, K.G. The control of root-knot nematodes (Meloidogyne spp.) by Pseudomonas oryzihabitans and its immunological detection on tomato roots. Nematology 2007, 9, 363–370. [Google Scholar] [CrossRef]
- Orozco, R.A.; Molnár, I.; Bode, H.; Stock, S.P. Bioprospecting for secondary metabolites in the entomopathogenic bacterium Photorhabdus luminescens subsp. sonorensis. J. Invertebr. Pathol. 2016, 141, 45–52. [Google Scholar] [CrossRef]
- Kusakabe, A.; Wang, C.; Xu, Y.-M.; Molnár, I.; Stock, S.P. Selective Toxicity of Secondary Metabolites from the Entomopathogenic Bacterium Photorhabdus luminescens sonorensis against Selected Plant Parasitic Nematodes of the Tylenchina Suborder. Microbiol. Spectr. 2022, 10, e0257721. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.L.F.; Roberts, D.P. Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant-pathogenic fungi. J. Nematol. 2002, 34, 1–8. [Google Scholar]
- Siddiqui, Z.A.; Baghel, G.; Akhtar, M.S. Biocontrol of Meloidogyne javanica by Rhizobium and plant growth-promoting rhizobacteria on lentil. World J. Microbiol. Biotechnol. 2007, 23, 435–441. [Google Scholar] [CrossRef]
- Díaz-Manzano, F.E.; Amora, D.X.; Martínez-Gómez, Á.; Moelbak, L.; Escobar, C. Biocontrol of Meloidogyne spp. in Solanum lycopersicum using a dual combination of Bacillus strains. Front. Plant Sci. 2023, 13, 1077062. [Google Scholar] [CrossRef]
- Sharma, I.P.; Sharma, A.K. Physiological and biochemical changes in tomato cultivar PT-3 with dual inoculation of mycorrhiza and PGPR against root-knot nematode. Symbiosis 2017, 71, 175–183. [Google Scholar] [CrossRef]
- Van der Veken, L.; Cabasan, M.T.N.; Elsen, A.; Swennen, R.; De Waele, D. Effect of single or dual inoculation of the arbuscular mycorrhizal fungus Glomus mosseae and root-nodulating rhizobacteria on reproduction of the burrowing nematode Radopholus similis on non-leguminous and leguminous banana intercrops. J. Plant Dis. Prot. 2021, 128, 961–971. [Google Scholar] [CrossRef]
- Flor-Peregrín, E.; Azcón, R.; Martos, V.; Verdejo-Lucas, S.; Talavera, M. Effects of dual inoculation of mycorrhiza and endophytic, rhizospheric or parasitic bacteria on the root-knot nematode disease of tomato. Biocontrol Sci. Technol. 2014, 24, 1122–1136. [Google Scholar] [CrossRef]
- Nafady, N.A.; Sultan, R.; El-Zawahry, A.M.; Mostafa, Y.S.; Alamri, S.; Mostafa, R.G.; Hashem, M.; Hassan, E.A. Effective and Promising Strategy in Management of Tomato Root-Knot Nematodes by Trichoderma harzianum and Arbuscular Mycorrhizae. Agronomy 2022, 12, 315. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Pandey, S. Can VAM Occurring in the Rhizosphere of Cowpea Be A Source of Natural Antagonist to Heterodera Cajani Pop-ulation? Indian J. Fundam. Appl. Life Sci. 2011, 1, 51–58. [Google Scholar]
- Banuelos, J.; Alarcón, A.; Larsen, J.; Cruz-Sánchez, S.; Trejo, D. Interactions between arbuscular mycorrhizal fungi and Meloidogyne incognitain the ornamental plant Impatiens balsamina. J. Soil Sci. Plant Nutr. 2014, 14, 63–74. [Google Scholar] [CrossRef]
- Lax, P.; Becerra, A.G.; Soteras, F.; Cabello, M.; Doucet, M.E. Effect of the arbuscular mycorrhizal fungus Glomus intraradices on the false root-knot nematode Nacobbus aberrans in tomato plants. Biol. Fertil. Soils 2011, 47, 591–597. [Google Scholar] [CrossRef]
- Marro, N.; Caccia, M.; Doucet, M.E.; Cabello, M.; Becerra, A.; Lax, P. Mycorrhizas reduce tomato root penetration by false root-knot nematode Nacobbus aberrans. Appl. Soil Ecol. 2018, 124, 262–265. [Google Scholar] [CrossRef]
- Gautam, A.; Siddiqui, A.; Mahmood, I. Integrated Management of Meloidogyne Incognita on Tomato. Nematol. Mediterr. 1995, 23, 245–247. [Google Scholar]
- Tiwari, S.; Pandey, S.; Chauhan, P.S.; Pandey, R. Biocontrol agents in co-inoculation manages root knot nematode [ Meloidogyne incognita (Kofoid & White) Chitwood] and enhances essential oil content in Ocimum basilicum L. Ind. Crops Prod. 2017, 97, 292–301. [Google Scholar] [CrossRef]
- dos Santos, M.C.V.; Esteves, I.; Kerry, B.; Abrantes, I. Biology, growth parameters and enzymatic activity of Pochonia chlamydosporia isolated from potato cyst and root-knot nematodes. Nematology 2013, 15, 493–504. [Google Scholar] [CrossRef]
- Kerry, B.R.; Irving, F.; Hornsey, J.C. Variation Between Strains of the Nematophagous Fungus, Verticillium Chlamydosporium Goddard. I. Factors Affecting Growth in Vitro. Nematologica 1986, 32, 461–473. [Google Scholar] [CrossRef]
- Leij, F.A.A.M.D.; Kerry, B.R.; Dennehy, J.A. Verticillium Chlamydosporium as a Biological Control Agent for Meloidogyne Incognita and M. Hapla in Pot and Micro-Plot Tests. Nematologica 1993, 39, 115–126. [Google Scholar] [CrossRef]
- Nasu, d.G.C.; Amora, D.X.; Monteiro, T.S.A.; Alves, P.S.; de Podestá, G.S.; Ferreira, F.C.; de Freitas, L.G. Pochonia chlamydosporia applied via seed treatment for nematode control in two soil types. Crop Prot. 2018, 114, 106–112. [Google Scholar] [CrossRef]
- Gray, N.F. Ecology of nematophagous fungi: Effect of soil moisture, organic matter, pH and nematode density on distribution. Soil Biol. Biochem. 1985, 17, 499–507. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Księżniak, A.; Gałązka, A.; Hetman, B.; Kopacki, M.; Skwaryło-Bednarz, B. Impact of abiotic factors on development of the community of arbuscular mycorrhizal fungi in the soil: A Review. Int. Agrophysics 2018, 32, 133–140. [Google Scholar] [CrossRef]
- Pathak, E.; Campos–Herrera, R.; El–Borai, F.E.; Duncan, L.W. Spatial relationships between entomopathogenic nematodes and nematophagous fungi in Florida citrus orchards. J. Invertebr. Pathol. 2017, 144, 37–46. [Google Scholar] [CrossRef]
- Gray, N.F. Ecology of nematophagous fungi: Effect of the soil nutrients N, P and K, and seven major metals on distribution. Plant Soil 1988, 108, 286–290. [Google Scholar] [CrossRef]
- Eayre, C.G.; Jaffe, B.A.; Zehr, E.I. Suppression of Criconemella Xenoplax by the Fungus Hirsutella Rhossiliensis. Phytopathology 1983, 73, 500. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Lu, F.; Du, L.; Wang, G. The efficacy of nematicidal strain Syncephalastrum racemosum. Ann. Microbiol. 2008, 58, 369–373. [Google Scholar] [CrossRef]
- Podestá, G.S.; Amora, D.X.; Maffia, L.A.; Nasu, G.C.; Ferraz, S.; Freitas, L.G. Effect of time between soil infestation with Pochonia chlamidosporia and planting on the efficacy of the fungus in managing Meloidogyne javanica. Crop Prot. 2016, 90, 77–83. [Google Scholar] [CrossRef]
- Bontempo, A.F.; Fernandes, R.H.; Lopes, J.; Freitas, L.G.; Lopes, E.A. Pochonia Chlamydosporia Controls Meloidogyne Incog-nita on Carrot. Australas. Plant Pathol. 2014, 43, 421–424. [Google Scholar] [CrossRef]
- Malusá, E.; Tartanus, M.; Soika, G. Monitoring and possibilities of controlling nematodes and fruit damaging pests of Rosa spp. with microbial-derived products. J. Plant Prot. Res. 2019, 59, 334–340. [Google Scholar] [CrossRef]
- Anastasiadis, I.A.; Giannakou, I.O.; Prophetou-Athanasiadou, D.A.; Gowen, S.R. The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop Prot. 2008, 27, 352–361. [Google Scholar] [CrossRef]
- Dababat, A.; Sikora, R.A. Importance of Application Time and Inoculum Density of Fusarium Ox-Ysporum 162 for Biological Control of Meloidogyne Incognita on Tomato. Nematropica 2007, 91, 267–276. [Google Scholar]
- O’callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Jaffee, B.A.; Muldoon, A.E. Susceptibility of root-knot and cyst nematodes to the nematode-trapping fungi Monacrosporium ellipsosporum and M. cionopagum. Soil Biol. Biochem. 1995, 27, 1083–1090. [Google Scholar] [CrossRef]
- Aboul-Eid, H.Z.; Noweer, E.M.A.; Ashour, N.E.; Ameen, H.H. Evaluation of a nematode bio-product Dbx-20% against root-knot nematode Meloidogyne incognita affecting grapevine under field conditions. Commun. Agric. Appl. Biol. Sci. 2006, 71, 659–668. [Google Scholar]
- Song, Z.Y.; Shen, L.; Zhong, Q.; Yin, Y.P.; Wang, Z.K. Liquid culture production of microsclerotia of Purpureocillium lilacinum for use as bionematicide. Nematology 2016, 18, 719–726. [Google Scholar] [CrossRef]
- Nagachandrabose, S. Liquid bioformulations for the management of root-knot nematode, Meloidogyne hapla that infects carrot. Crop Prot. 2018, 114, 155–161. [Google Scholar] [CrossRef]
- Rao, M.S.; Kamalnath, M.; Umamaheswari, R.; Rajinikanth, R.; Prabu, P.; Priti, K.; Grace, G.N.; Chaya, M.K.; Gopalakrishnan, C. Bacillus subtilis IIHR BS-2 enriched vermicompost controls root knot nematode and soft rot disease complex in carrot. Sci. Hortic. 2017, 218, 56–62. [Google Scholar] [CrossRef]
- Sowmya, D.S.; Rao, M.S.; Kumar, R.M.; Gavaskar, J.; Priti, K. Bio-Management of Meloidogyne Incognita and Erwinia Ca-rotovora in Carrot (Daucus Carota L.) Using Pseudomonas Putida and Paecilomyces Lilacinus. Nematol. Mediterr. 2012, 40, 189–194. [Google Scholar]
- Guru Prasad, G.R.; Ravichandra, N.G.; Narasimhamurthy, T.N.; Punith Kumar, C.H.; Yadahalli, P. Management of Meloidogyne Inognita Infecting Carrot by Using Bioagents. JBiopest 2014, 7, 144–150. [Google Scholar]
- Giannakou, I.O.; Karpouzas, D.G.; Prophetou-Athanasiadou, D. A novel non-chemical nematicide for the control of root-knot nematodes. Appl. Soil Ecol. 2004, 26, 69–79. [Google Scholar] [CrossRef]
- Stirling, G.R.; Smith, L.J.; Licastro, K.A.; Eden, L.M. Control of Root-knot Nematode with Formulations of the Nematode-Trapping FungusArthrobotrys dactyloides. Biol. Control. 1998, 11, 224–230. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Futai, K. Biocontrol of Meloidogyne incognita on tomato using antagonistic fungi, plant-growth-promoting rhizobacteria and cattle manure. Pest Manag. Sci. 2009, 65, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Saumell, C.A.; Padilha, T.; Santos, C.d.P. Nematophagous fungi in sheep faeces in Minas Gerais, Brazil. Mycol. Res. 2000, 104, 1005–1008. [Google Scholar] [CrossRef]
- Abawi, G.S.; Widmer, T.L. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Appl. Soil Ecol. 2000, 15, 37–47. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xiu, W.; Tan, B.; Li, G.; Zhao, J.; Yang, D.; Zhang, G.; Zhang, Y. Responses of Soil Microbial and Nematode Communities to Various Cover Crop Patterns in a Tea Garden of China. Int. J. Environ. Res. Public Health 2022, 19, 2695. [Google Scholar] [CrossRef]
- Pulavarty, A.; Horgan, K.; Kakouli-Duarte, T. Effect of an Alltech soil health product on entomopathogenic nematodes, root-knot nematodes and on the growth of tomato plants in the greenhouse. J. Nematol. 2020, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valdes, Y.; Viaene, N.; Moens, M. Effects of yellow mustard amendments on the soil nematode community in a potato field with focus on Globodera rostochiensis. Appl. Soil Ecol. 2012, 59, 39–47. [Google Scholar] [CrossRef]
- Fourie, H.; Ahuja, P.; Lammers, J.; Daneel, M. Brassicacea-based management strategies as an alternative to combat nematode pests: A synopsis. Crop Prot. 2016, 80, 21–41. [Google Scholar] [CrossRef]
- Oka, Y.; Shapira, N.; Fine, P. Control of root-knot nematodes in organic farming systems by organic amendments and soil solarization. Crop Prot. 2007, 26, 1556–1565. [Google Scholar] [CrossRef]
- Peruzzi, A.; Martelloni, L.; Frasconi, C.; Fontanelli, M.; Pirchio, M.; Raffaelli, M. Machines for non-chemical intra-row weed control in narrow and wide-row crops: A review. J. Agric. Eng. 2017, 48, 57–70. [Google Scholar] [CrossRef]
- Expósito, A.; Munera, M.; Giné, A.; López-Gómez, M.; Cáceres, A.; Picó, B.; Gisbert, C.; Medina, V.; Sorribas, F.J. Cucumis metuliferusis resistant to root-knot nematodeMi1.2gene (a)virulent isolates and a promising melon rootstock. Plant Pathol. 2018, 67, 1161–1167. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Caboni, P. Botanical Nematicides: A Review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- Ntalli, N.; Adamski, Z.; Doula, M.; Monokrousos, N. Nematicidal Amendments and Soil Remediation. Plants 2020, 9, 429. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, M.; Jiang, L.; Chen, X.; Griffiths, B.S.; Li, H.; Hu, F. Vermicompost increases defense against root-knot nematode (Meloidogyne incognita) in tomato plants. Appl. Soil Ecol. 2016, 105, 177–186. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Deguine, J.-P.; Penvern, S. Agroecological Crop Protection in Organic Farming: Relevance and Limits. In Organic Farming, Prototype for Sustainable Agricultures: Prototype for Sustainable Agricultures; Bellon, S., Penvern, S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 107–130. ISBN 978-94-007-7927-3. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).