1. Introduction
Horticultural crops are important for global agriculture and food production, providing various crops cultivated for human consumption, commercial applications, and industrial purposes [
1]. These crops have pivotal functions in human nutrition by providing essential vitamins, minerals, and other beneficial compounds for overall health and well-being [
1]. Additionally, horticultural crops provide an important source of income for farmers and contribute to local and national economies [
2]. However, the production of horticultural crops is sometimes limited by numerous kinds of stresses (biotic and abiotic). Biotic stresses are pests and diseases, which can cause remarkable harm to crops and reduce productivity and crop quality [
3]. Abiotic stresses, like temperature variations (both high and low), drought, salinity, and metal poisoning, can also negatively impact crop growth and yield [
4,
5]. Therefore, there is a need to develop effective strategies for managing these stresses and enhancing the productivity and sustainability of horticultural crop yield.
Melatonin has emerged as an assuring tool for enhancing stress tolerance and growth in horticultural crops [
6]. It is a versatile chemical extensively distributed in plants, where it performs important functions in regulating various physiological activities [
7]. Recent studies have narrated that the exogenous treatment of melatonin can improve stress tolerance, delay senescence, and promote growth in horticultural crops [
8,
9,
10,
11,
12]. The capabilities of melatonin in the production of horticulture crops is a promising area of study. Melatonin has been found to influence the expression of genes involved in stress response, hormone signaling, and antioxidant defense, improving stress tolerance and growth in horticultural crops [
9,
10,
11,
13]. It can also interact with other phytohormones,
viz cytokinins, auxins, and abscisic acid, to promote root growth, postpone senescence, and improve resistance [
12,
14,
15].
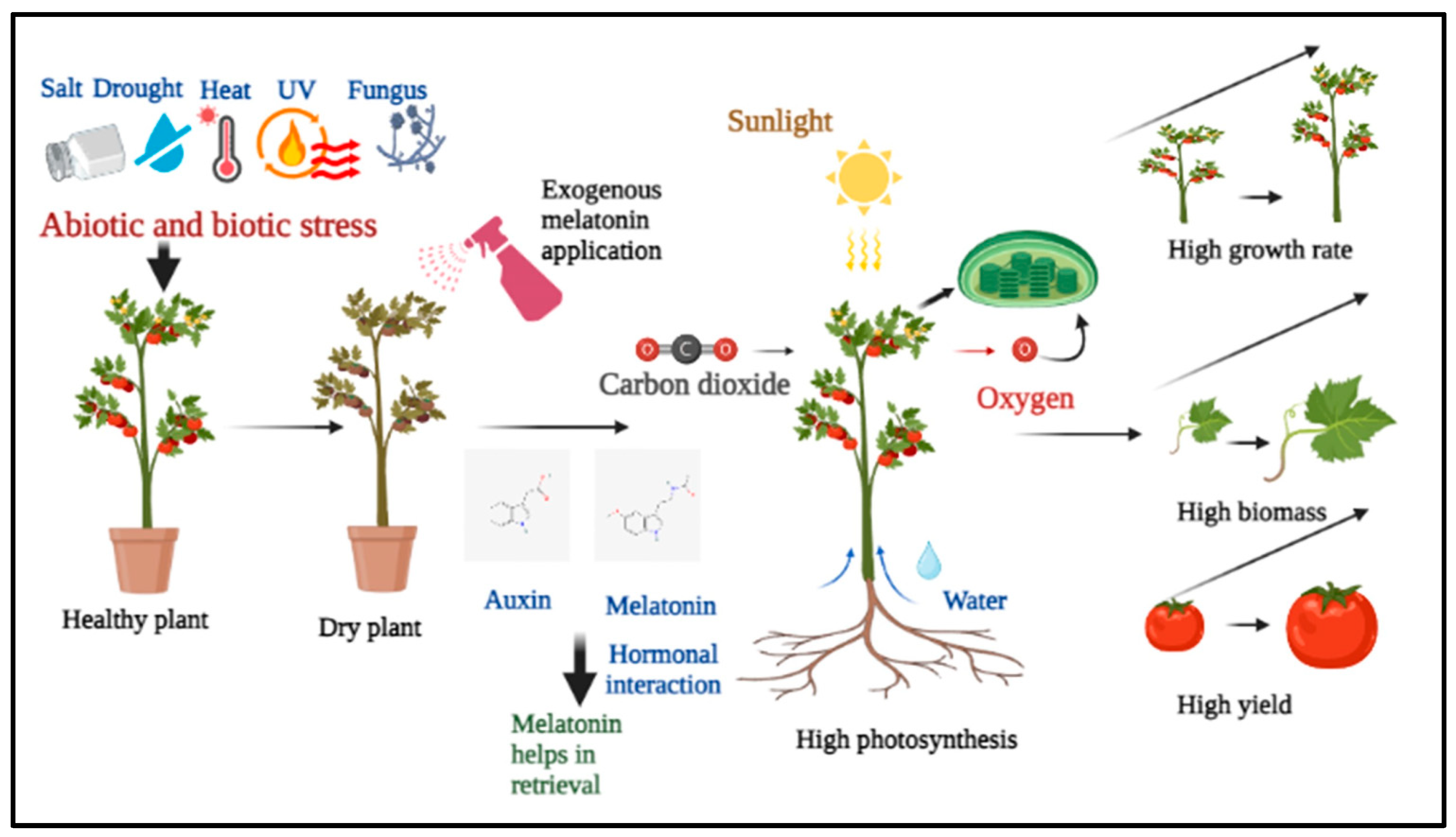
The interaction of melatonin with auxin, specifically indole-3-acetic acid (IAA), to alleviate the negative impact of moisture stress on plants [
6]. They reported that the exogenous use of melatonin increased the concentration of IAA in plants. Melatonin and IAA have similar effects on promoting photosynthetic activity in plants. Furthermore, when plants are exposed to drought stress, melatonin interacts with IAA during maturity, enhancing plant growth and yield. Their findings on the interaction of melatonin and auxin are very important (
Figure 1).
In addition, melatonin has been found to work as a broad-spectrum antioxidant, scavenging reactive oxygen species (ROS) and upregulating the activity of antioxidant enzymes to reduce oxidative damage in horticultural crops [
4,
13]. This review represents melatonin’s biosynthesis, physiological functions, mechanism of action, interaction with other phytohormones, and potential horticulture applications. The objectives are to provide an overview of the various physiological processes in horticultural plants regulated by melatonin. We have summarized the mechanisms of action and the potential applications of melatonin in crop production and stress management by identifying the gaps in knowledge and opportunity for future study in the pace of horticultural plants. The current review article intends to offer a comprehensive insight into the present state of knowledge about melatonin in horticulturally important plants and a detailed understanding of melatonin’s functions in them. It has a more up-to-date understanding of the topic, broader scope, and novel directions, which suggests unexplored areas for investigation and the research gaps compared to previous reviews on this topic.
2. Interaction of Melatonin with Other Phytohormones
Melatonin has been shown to interact with several other phytohormones in plants. Some of these interactions are:
2.1. Interaction with Auxins
Recent reports have uncovered that melatonin can enhance the activity of auxins in specific plant species, including wild-leaf mustard (
Brassica juncea) and
Arabidopsis seedlings. The application of exogenous melatonin in these plants has been shown to elevate the levels of endogenous auxins, resulting in improved root growth [
16,
17]. Melatonin increases the activity of auxin-responsive genes, leading to enhanced lateral root formation in tomato plants [
18]. Wen et al. [
18] demonstrated that a concentration of 50 µM of melatonin is optimal for stimulating adventitious root formation (ARF) in tomato plants. This effect was attributed to the role of melatonin in the regulation of auxin metabolism. Chen et al. [
16] observed that when applied exogenously in small quantities, melatonin could enhance root growth in tomato explants, while excessive amounts could inhibit it. Yadav et al. [
19] reported that the polar localization of PIN proteins is accountable for auxin transport and disruption, which directly affects IAA transport to regulate the ARF. The study by Wen et al. [
18] provided further insights into melatonin’s effects on auxin transport, signal transduction, and auxin accumulation. They concluded that melatonin modifies auxin levels, such as IAA and IBA, and regulates the expression of several auxin metabolisms genes, like PIN1, PIN3, PIN7, IAA19, and IAA24, through the nitric oxide (NO) signaling pathway. An experiment involving the overexpression of MzASMT in
Arabidopsis thaliana. Their findings suggested that melatonin does not substitute IAA in establishing apical dominance but instead collaborates closely with IAA to perform this function [
20]. The expression of nearly 320 genes involved in rhizogenesis can be increased or decreased by the external use of melatonin in cucumber [
21].
2.2. Interaction with Cytokinins
Several studies have demonstrated a synergistic relationship between melatonin and cytokinins in mitigating plant stress. In a recent study by Bychkov et al. [
22], it was found that in
Arabidopsis, melatonin not only boosted the expression of cytokinin biosynthesis genes but also augmented cytokinin levels. Similarly, cytokinin-signaling and biosynthesis genes were notably more expressive in ipt-transgenic creeping bentgrass plants (
Agrostis stolonifera) that were treated with melatonin [
23]. Melatonin suppresses the expression of some senescence-related markers in apple, suggesting its contribution in controlling senescence [
24]. Zhang et al. [
25] found that in perennial ryegrass two cytokinin biosynthesis genes (LpIPT2 and LpOG1) were activated. However, two kinds of transcription factors involved in the cytokinin metabolic pathways (A-ARRs and B-ARRs) were downregulated and upregulated, respectively. They also noted that melatonin postponed senescence in the perennial ryegrass by reducing ABA biosynthesis and increasing the production of cytokinins, which serve as senescence-delaying agents under conditions of heat stress.
2.3. Interaction with Abscisic Acid (ABA)
The crosstalk between melatonin and abscisic acid (ABA) in plants is an ongoing area of study. In grape berries, the application of exogenous melatonin increased the expression of ABA biosynthesis genes and enhanced ABA levels, which subsequently improved fruit ripening [
26]. During water stress, treatment of melatonin (at concentrations between 10–500 μm) in apple leaves led to the upregulation of ABA catabolic genes and a subsequent decrease in ABA content [
14,
27]. Research also shows that exogenous melatonin administration enhanced the expression of genes involved in ABA catabolism (two CYP707 monooxygenases), while it suppressed the key enzyme 9-cis-epoxycarotenoid dioxygenase (NCED), which is essential for ABA biosynthesis. This process results in a rapid decrease in ABA concentration during seed germination under salt stress in cucumber seedlings. The application of melatonin influences ripeness in tomato varieties [
28].
2.4. Interaction with Gibberellins (GA)
Melatonin also plays a role in plant growth and development in association with gibberellin (GA). Melatonin enhances the biosynthesis of GA, leading to improved seed germination under high salinity conditions in cucumber plants [
29]. It was noticed that exogenous melatonin application increased the expression of GA biosynthesis genes like GA20ox and GA3ox in the seedlings of cucumber under saline conditions, leading to an increase in the concentration of gibberellins like GA
3 and GA
4, resulting in an increase in seed germination [
29].
2.5. Interaction with Ethylene
The melatonin–ethylene interaction manages plant growth and development. Research has demonstrated that melatonin plays a critical role in promoting fruit ripening and enhancing fruit quality. Melatonin regulates several ripening parameters, including lycopene content, fruit softening, and flavour. It also influences ethylene signaling and the biosynthesis of enzymes and related genes, such as those responsible for the expression of ACC synthase in tomatoes. Additionally, the study revealed that the external application of melatonin could stimulate the expression of ethylene receptor genes like NR and ETR4, as well as transducing elements like EIL1, EIL3, and ERF2 [
30].
2.6. Interaction with Jasmonic Acid (JA)
It is reported that melatonin interacts with JA in regulating plant defense responses [
31]. In tomatoes and blueberries, external melatonin increased the expression of JA biosynthesis genes and increased the levels of JA, thereby improving resistance against fungal pathogens [
31,
32,
33]. In grafted watermelon, melatonin application helped in cold tolerance enhancement via the expression of JA biosynthesis genes and increasing the levels of JA [
34].
2.7. Interaction with Salicylic Acid (SA)
The interaction between melatonin and salicylic acid (SA) plays a crucial role in plant defense mechanisms. For regulating the plant defense responses, melatonin requires synergistic influence. In
Arabidopsis, exogenous melatonin enhanced the expression of SA biosynthesis genes and enhanced the levels of SA. This resulted in improved resistance against fungal pathogens [
31]. Exogenous melatonin application upregulated the SA biosynthesis pathway and endogenous SA level, improving Kiwi fruits’ chilling tolerance and alleviating oxidative stress [
35].
In a fascinating study involving watermelon plants performed [
36], an intriguing phenomenon was observed where in the use of melatonin directly to the roots activated cold-tolerance-responsive genes in other untreated organs, such as the leaves, under cold-induced stress conditions. This observation suggests that melatonin can trigger a systemic response in the plant. Researchers have successfully measured and quantified the long-distance transport of melatonin through the xylem alongside other plant hormones. Moreover, melatonin has been found to elicit remarkable changes in the expression of various genes related to plant hormones, including auxins, cytokinins, gibberellins, ethylene, and jasmonic acid. Notably, these changes predominantly involve the upregulation of biosynthesis enzymes, receptors, and elements associated with hormone signaling pathways. In watermelon plants during cold stress, the administration of melatonin led to the downregulation of the ABA receptor PYL8 gene [
36]. This finding suggests a complex interplay between melatonin and various plant hormones, wherein melatonin’s actions during cold stress may modulate the expression of genes involved in hormone biosynthesis, receptor activity, and signaling pathways.
Exogenic melatonin can control the levels of JA and SA, which are important signaling molecules involved in plant growth, development, and stress responses. Further research is needed to fully understand the underlying mechanisms of how melatonin interacts with these hormones in different plant species under adverse agroclimatic conditions.
It can be concluded that melatonin interacts with multiple phytohormones to control plant growth, development, and defense responses, highlighting its importance in plant biology. Future prospects can lead to understanding the complex interactions between melatonin and other phytohormones and their implications for agriculture and horticulture.
Table 1 and
Table 2 summarize the role and crosstalk of the melatonin hormone.
In summary, we can say that, exogenous melatonin deliberately influence horticultural plant growth by moulding (circumstances wise by upregulating and downregulating multiple hormones and related genes). From
Table 1 and
Table 2, concluded that melatonin influence on other hormones majorly protect the photosynthetic apparatus in plants. Recent research confirms that plant tolerance to various stimuli is significantly related to foliage longevity [
38]. The involvement of melatonin in photosynthetic apparatus protection via increased ROS detoxification in tomato plants cultivated under the combination of salinity and heat [
46], two of the most frequent abiotic stimuli known to act simultaneously. Melatonin aided in the prevention of protein and membrane damage caused by the combination of salinity and heat by preventing oxidation and by modulating the expression of several antioxidant-related genes and their corresponding enzymes, especially APX, GR, and SOD, GPX and Ph-GPX, which demonstrated antagonistic regulation in plants that did not receive melatonin [
46]. Exogenous MT treatment increased endogenous MT and GA content while decreasing ABA content in high-temperature-exposed plants. However, paclobutrazol (PCB, a GA biosynthesis inhibitor) and sodium tungstate (ST, an ABA biosynthesis inhibitor) treatment decreased the GA and ABA contents. In both inhibitor-treated plants, MT-induced heat tolerance was compromised [
38].
3. Melatonin Receptors in Plants
Melatonin receptors were first discovered in the mammalian brain in the early 1980s [
42]. Later, melatonin receptors were identified in other tissues of mammals and other organisms such as birds, fish, amphibians, and invertebrates [
42,
47,
48,
49]. In plants, melatonin receptors were first discovered [
50]. In
Arabidopsis thaliana, specifically in a gene known as CAND2/PMTR1.This gene regulates stomatal closure via an H
2O
2 and Ca
2+ signaling transduction cascade. More recently, studied CAND2 and proposed that the integrity of CAND2 as a melatonin receptor needs further analysis because their confocal microscopy analysis showed that CAND2 protein is localized in the cytoplasm rather than the plasma membrane in two
Arabidopsis thaliana CAND2 knockout mutant lines, SALK 071, 302 (CAND2-1) and SALK 068848 [
50,
51].
Biosynthesis of Melatonin in Crops
Melatonin biosynthesis in plants occurs via the shikimate pathway, which is also related to the biosynthesis of several other important secondary metabolites in plants. The biosynthesis pathway converts the amino acid tryptophan into melatonin through several enzymatic reactions. The biosynthesis of melatonin starts with the conversion of tryptophan to serotonin by the enzyme tryptophan decarboxylase (TDC). After that, serotonin converted into N-acetyl serotonin (NAS) by the enzyme serotonin N-acetyltransferase (SNAT). Finally, NAS is converted into melatonin by the enzyme N-acetyl serotonin O-methyltransferase (ASMT) [
7,
31]. Abiotic stresses can influence the activity of these enzymes. For instance, research has shown that drought stress can enhance the expression of TDC, SNAT, and ASMT genes in rice, subsequently increasing melatonin biosynthesis [
52]. In addition, plant hormones such as auxins and cytokinin have been shown to regulate melatonin biosynthesis in crops. Experiments have suggested that exogenous treatment of these phytohormones may increase plant melatonin biosynthesis [
16,
22].
Recent research has also uncovered the roles of other enzymes, such as caffeic acid O-methyltransferase (COMT) and acetyl serotonin deacetylase (ASDD), in the melatonin biosynthesis pathway. COMT is concerned with synthesizing the intermediate compound 5-methoxy tryptamine (5-MT), which is a precursor of NAS. At the same time, ASDD is involved in the deacetylation of NAS to form serotonin [
1]. The melatonin biosynthesis pathway in crops is complex and regulated by various factors, including environmental stresses, plant hormones, and genetic factors. Further experiments are necessary to fully understand the regulatory mechanisms involved in melatonin biosynthesis in plants and investigate the capability of manipulating melatonin biosynthesis to enhance stress tolerance and growth in horticultural crops (
Figure 2). The concept for drawing pathways has been taken [
1]. Chemical structures were retrieved from PubChem.
4. Melatonin in Horticultural Plants: Mechanism of Action
There are several proposed mechanisms through which melatonin operates in horticultural crops. One of these involves melatonin functioning as an antioxidant in horticultural crops. Melatonin is found to scavenge reactive oxygen species (ROS) and protect from oxidative damage in plants. For instance, in apple plants, melatonin application enhanced the activity of antioxidant enzymes, like superoxide dismutase (SOD) and peroxidase (POD), and reduced the buildup of ROS during alkaline stress conditions [
53]. Similarly, in litchi fruit, melatonin application has been found to enhance the activity of antioxidant enzymes and decrease the pile-up of ROS under post-harvest storage conditions [
54] and in kiwifruits under chilling stress [
35]. Another mechanism proposes that melatonin controls the expression of genes linked with various physiological processes in horticultural plants. For instance, in tomato plants, melatonin treatment has been shown to be involved in the upregulation of genes involved in abscisic acid (ABA) biosynthesis and signaling, such as NCED1, NCED2, and AAO3, and in enhancing heat tolerance [
38]. In tomato plant, SlcAPX, SIGR 1, SIGBT, and SIPh-GPX genes get upregulated and SIDHAR1 gets downregulated for enhancing the melatonin action in the plants [
46].
Melatonin’s role in regulating ion uptake and transport is another suggested mechanism of action in horticultural crops. It appears to promote the uptake of essential nutrients, such as K
+, Ca
2+, and Mg
2+ while mitigating the pile-up of toxic ions, such as Na
+ and Cl
− [
36,
40]. This has been illustrated in rice plants, in which melatonin treatment enhanced salt tolerance by regulating ion uptake and transport [
55]. Melatonin also upregulates the expression of genes associated with ion transport, such as OsSOS1 in roots and of OsCLC1 and OsCLC2 in roots and leaves, and reduces the pile-up of Na
+ and Cl
− in tomato plants under salt stress conditions. It has also been found to manage ion homeostasis in apples under salt stress conditions [
53]. Melatonin has also been proposed to control root architecture in horticultural crops, which can enhance stress tolerance by augmenting the surface area of roots and improving nutrient and water absorption. In cucumber, melatonin application enhances the length and density of lateral roots and genes related to cell wall formation and carbohydrate metabolic processes and enhances salt tolerance [
21].
Melatonin increases the expression of antioxidant genes (Cu-ZnSOD, Fe-ZnSOD, CAT, POD, etc.) and key genes involved in gibberellin (GA) biosynthesis (such as GA20ox and GA3ox) but decreases the expression of key genes involved in ABA biosynthesis (such as NECD2). Melatonin treatment after harvest increases lycopene levels, accelerates fruit softening, and improves ethylene release in tomatoes by up-regulating the expression of SlACS4, SlNR, SlETR4, SlEIL1, SlEIL3, and SlERF2 [
12]. Melatonin is a multifunctional bio-stimulant that serves as a “defense molecule” to protect plants from the harmful effects of temperature stress. Plant growth and temperature tolerance are improved by melatonin treatment by increasing numerous defense mechanisms [
56]. The HSFs MeHsf20, MeWRKY79, and MeRAV1/2 bind to the promoters of melatonin biosynthesis genes in cassava (
Manihot esculenta) to stimulate melatonin production and confer disease resistance [
50,
57]. Melatonin affects many enzymes and other components involved in amino acid biosynthesis in Krebs cycles, nitrogen-related processes, an ascorbate-glutathione (ASC–GSH) cycle, and osmoregulatory compound biosynthesis [
57].
Melatonin acts through various mechanisms to regulate physiological processes in horticultural plants, including antioxidant defense, gene expression, hormone signaling pathways, ion uptake and transport, and root architecture. However, the exact mechanisms of melatonin action in horticultural crops are yet to be fully elucidated and require further research.
4.1. The Physiological Functions of Endogenous Melatonin in Horticulture Crops
4.1.1. Function of Melatonin in Seed Germination
Melatonin performs a vital function in the seed germination of horticultural crops. Experiments have shown that the exogenous melatonin treatment can improve seed germination and seedling growth under normal and stressful conditions [
1]. In some horticultural plants, like tomato, red cabbage, carrot, and cucumber, melatonin application has been found to improve the percentage of germinated seeds and the rate of seedling emergence and growth [
58,
59,
60,
61]. Besides this, melatonin has been found to improve seedling tolerance to various abiotic stress, such as drought and salt stress, by boosting antioxidant defense systems and regulating stress-responsive genes in watermelon and cucumber [
36,
62].
Melatonin has also been reported to improve seed germination and seedling growth under suboptimal temperature conditions. In cucumber, for instance, melatonin treatment increased germination rate and seedling vigour under low-temperature stress [
58]. Similarly, melatonin treatment improved seedling growth under high-temperature stress by enhancing the activity of antioxidant enzymes and regulating the expression of stress-responsive genes in soybean and rice [
40,
63]. These shreds of evidence suggest that melatonin plays a vital role in the seed germination and seedling growth of horticultural plants, particularly under stress conditions.
4.1.2. Function of Melatonin in Root Organogenesis and Lateral Root Development
Melatonin is known to play a pivotal role in root organogenesis and lateral root development in horticultural plants. Studies have demonstrated that melatonin promotes root growth and improves the quality of the root system by regulating various physiological processes [
30]. Melatonin has been found to promote root organogenesis in various horticultural crops. For example, in grapevines, exogenous application of melatonin has been found to increase the number of adventitious roots, improve root morphology, and enhance root growth under salt stress [
64]. Likewise, in cucumber, melatonin treatment has been found to stimulate the formation of adventitious roots and improve root system architecture by enhancing the expression of genes involved in root development [
21]. Sarropoulou et al. [
65] reported that the application of 1 μM melatonin improved the adventitious root formation from shoot tip explants of cherry rootstock PHL-C (
Prunus avium L.
× Prunus cerasus L.). Melatonin has also been found to promote lateral root development in horticultural crops. In tomato, for instance, melatonin treatment has been observed to accelerate lateral root formation and increase the length of lateral roots and expressed auxin biosynthesis genes and cell cycle genes [
66].
4.1.3. Function of Melatonin in Shoot Growth and Differentiation in Horticultural Plants
Melatonin has been proven to play an important function in shoot growth and differentiation in horticultural plants. Studies have demonstrated that melatonin can boost shoot growth and regulate shoot development by influencing numerous physiological processes [
67]. Exogenous application of melatonin, for example, has been shown to improve shoot growth and amplify the expression of genes involved in shoot development in vitro in blueberry [
68]. Increased production of shoots has been observed for exogenous melatonin application in mimosa, pomegranate, and blueberry [
68,
69,
70]. Melatonin has also been associated with differential patterning of branching and leaf production [
24,
71]. Melatonin application has also been demonstrated to enhance shoot growth and improve shoot morphology. In in vitro cultured tissue of coffee (
Coffea canephora P. ex Fr.) and mimosa, melatonin led to an increase in the generation of somatic embryos from callus tissue [
69,
72].
4.1.4. Function of Melatonin in the Regulation of Vegetative and Reproductive Growth Stages and Floral Transition in Horticultural Plants
Melatonin has been discovered to be crucial in regulating vegetative and reproductive growth stages in horticultural plants. Research has demonstrated that melatonin aids in the transition from vegetative to reproductive growth and improves reproductive organ development by influencing several physiological processes [
73]. Melatonin has been found to promote the transition from vegetative to reproductive growth in various horticultural crops. Murch and Saxena [
74] observed the appearance of melatonin peak in the intermediate stages of flower development while studying the floral development of the plant St. John’s wort (
Hypericum perforatum). They observed melatonin accumulation in specific tissues and stages that acted as a signal in the development of gametophytes, transforming them into viable microspores. Murch et al. [
75] probed a narcotic plant used in natural medicine, devil’s trumpet (
Datura metel), and noted that melatonin concentration peaked at the time of flower bud maturation indicating a protective role in the plant’s reproductive parts.
Zhao et al. [
76] analyzed melatonin content during the different flower developmental stages of herbaceous peony (
Paeonia lactifora). They observed that melatonin content gradually increased from the flower bud to the initial bloom stage, which later started declining until withering. Exposure to sunlight and blue light stimulated melatonin production, whereas lower values were recorded in shade, and under white and green lights [
76].
4.1.5. Function of Melatonin in Biomass Accumulation and Differentiation in Horticultural Crops
Melatonin has been found to perform a crucial function in boosting biomass growth and differentiation in horticultural plants. Studies have shown that melatonin stimulates biomass accumulation and differentiation of various plant organs, such as leaves, stems, and roots [
77]. Exogenous treatment of melatonin, for example, has been shown to boost biomass accumulation and promote plant development in tomatoes under a variety of abiotic conditions [
37,
78]. Similarly, melatonin application has been reported in kiwi seedlings to restore and enhance biomass accumulation and plant development under drought stress [
79]. Melatonin has also been found to enhance the differentiation of various plant organs in horticultural crops. Sarropoulou et al. [
65] reported that the application of 1μM melatonin improved biomass accumulation by increasing root regeneration, photosynthetic pigments, and total carbohydrate concentration in cherry rootstock PHL-C. In apples, melatonin has been found to enhance root differentiation and improve root morphology by regulating the expression of genes related to root development [
80].
The effect of exogenous melatonin (foliar spray, 100 μmol L
−1) on the pear fruit tree [
81]. They observed an increase in melatonin in pear fruit. It was found that melatonin enhanced the size of pear fruit by enhancing the net photosynthetic rate and maximal quantum efficiency of photosystem II photochemistry during the advanced stage of pear fruit development. This led to an increase in fruit weight by 47.85% over the untreated plants. They also evaluated the biochemical parameters affected due to the exogenous use of melatonin at the ripening stage and found an increase in the contents of soluble sugar, especially sucrose, and sorbitol, possibly due to improved deposition of starch and expansion of pear fruit. These factors collectively contributed to a higher yield. They recommended that the use of melatonin in pear trees can be utilized to produce bigger and sweeter fruit of higher economic value [
81]. The application of 100 µmol L
−1 of melatonin solution through spray on the fruits of young grapes might help in promoting the build-up of endogenous melatonin contents in berries of grapes and have a substantial influence on enhancing the yield of the berries and their weight [
82].
4.1.6. Function of Melatonin in Leaf Senescence in Horticultural Plants
Melatonin has been shown to play a significant role in leaf senescence in horticultural plants. Studies have demonstrated that melatonin delays leaf senescence by regulating various physiological processes, including antioxidant activity and gene expression [
83]. Melatonin has been shown to postpone leaf senescence in a variety of horticulture crops. Exogenous melatonin, for example, has been shown to postpone leaf senescence in apples by enhancing antioxidant activity and decreasing oxidative stress [
84]. In apple melatonin also decreased chlorophyll degradation and downregulation of senescence-associated gene 12 (SAG12) [
84]. Melatonin application has also been found to postpone leaf senescence in rice and
Arabidopsis by modulating the expression of genes involved in chlorophyll breakdown and protein degradation.
Melatonin has also been found to delay leaf senescence by regulating the expression of genes involved in stress responses. In Chinese flowering cabbage, for instance, melatonin treatment has been shown to delay leaf senescence by upregulating the expression of genes involved in the ABA signaling pathway and chlorophyll degradation [
83]. Additionally, in tomato, exogenous melatonin has been found to delay leaf senescence by activation of endogenous melatonin and GA biosynthesis and inhibition of ABA biosynthesis pathways [
38].
4.1.7. Function of Melatonin in Physiological Maturity and Harvest of Crops in Horticultural Plants
Melatonin has been demonstrated to play a significant role in the physiological maturity and harvest of crops in horticultural plants. Studies have demonstrated that melatonin promotes fruit ripening and enhances fruit quality by regulating various physiological processes, including sugar metabolism and gene expression in tomato [
30]. Melatonin has been observed to promote fruit ripening in various horticultural crops. For example, in tomato, exogenous application of melatonin has been found to promote fruit ripening and improve fruit quality by increasing sugar metabolism and enhancing antioxidant activity [
30]. Similarly, in pear, melatonin treatment promoted fruit ripening and improved fruit quality by enhancing the expression of genes involved in sugar metabolism [
81].
Melatonin has been reported to enhance the shelf life of horticultural crops. In banana, for instance, melatonin treatment has been reported to delay fruit ripening and enhance fruit quality by reducing ethylene production and enhancing antioxidant activity [
85]. Additionally, in strawberry, melatonin has been shown to prolong the shelf life of fruits by reducing postharvest decay and improving fruit quality [
44]. In addition, melatonin has been found to promote seed maturity in several horticultural crops. In wine grapes (
Vitis vinifera L.), melatonin accumulation signals the transition through veraison, making the onset of seed maturation [
86].
4.1.8. Role of Melatonin in Stress Tolerance in Horticultural Plants
Melatonin plays a critical role in enhancing stress tolerance in horticultural plants. Melatonin works as a signaling molecule in response to numerous abiotic and biotic stressors, influencing many physiological functions and improving stress tolerance in horticulture crops. Melatonin has been shown to enhance stress tolerance in a variety of horticulture crops. Exogenous melatonin application has been found to improve salt stress tolerance in apple and cucumber by modulating the antioxidant enzyme activity and the expression of genes involved in osmotic adjustment and antioxidant activity [
21,
53,
87]. Melatonin has also been reported to enhance stress tolerance by regulating the expression of stress-responsive genes and hormone signaling pathways. In
Arabidopsis for instance melatonin treatment has been shown to enhance tolerance to heat stress by upregulating the expression of genes (HSP 90 and 101) involved in heat shock response [
88]. Additionally, in cucumber, melatonin has been found to enhance tolerance to salt stress by regulating the expression of genes involved in the GA and ABA signaling pathways related to their biosynthesis and catabolism [
29].
Melatonin has also been reported to enhance stress tolerance by regulating the accumulation of compatible solutes and detoxifying reactive oxygen species. This has been observed in cucumber and apple, where melatonin treatment was found to increase salt stress tolerance by facilitating the buildup of soluble sugars and boosting antioxidant enzyme activity [
29,
53]. Jahan et al. [
38] and Altaf et al. [
60] reported that exogenous melatonin helped to detoxify the over-accumulated ROS, thereby reducing the damage caused by heat stress in tomato plants. Similarly, in post-harvest peach fruits, melatonin has been found to enhance tolerance to cold and oxidative stresses by promoting the accumulation of compatible solutes and regulating the expression of genes involved in osmotic adjustment [
89]. This beneficial effect of melatonin was also observed in watermelon and kiwifruits under cold and chilling stress, respectively [
1,
41].
Melatonin has been reported to regulate water stress tolerance through changes in both physiological and anatomical characteristics. In tomato, exogenous melatonin treatment increased drought tolerance by controlling leaf cuticle synthesis and non-stomatal water loss in leaves [
90]. Furthermore, melatonin has been found to enhance stress tolerance by regulating physiological systems associated with photosynthesis. Melatonin has also been shown to improve drought tolerance in apple by modulating photosynthetic efficiency, transpiration, and stomatal conductance as well as minimizing chlorophyll degradation and oxidative damage [
14].
Furthermore, melatonin has been reported to enhance stress tolerance by regulating root architecture and nutrient uptake. In tomato plants, melatonin has been found to enhance tolerance to salt stress by regulating root morphology and ion uptake [
60] as well as ion homeostasis in apple [
53]. Specifically, melatonin treatment has been reported to increase the root length, surface area, and volume of tomato seedlings under salt stress conditions [
60]. Additionally, melatonin has been found to increase the accumulation of essential nutrients, such as K
+, Ca
2+, and Mg
2+ while reducing the accumulation of toxic ions, such as Na
+ and Cl
− [
40]. These results suggest that melatonin enhances salt stress tolerance in horticultural crops by regulating root morphology and ion uptake. Similarly, in tomato, melatonin has been found to enhance tolerance to salt stress by regulating the expression of genes involved in the ABA signaling pathway [
91].
4.1.9. Role of Melatonin in the Regulation of Autophagy in Horticultural Plants
Melatonin has been linked to the regulation of autophagy in horticultural plants. Autophagy is a cellular mechanism that degrades and recycles cellular components in order to preserve cellular homeostasis under stress conditions. Autophagy is a critical stress-response process in plants that helps them to deal with a variety of stresses like food deprivation, oxidative damage, and pathogen attack. Melatonin has been proven in studies to modulate autophagy activity and improve stress tolerance in horticulture plants.
In tomato plants, melatonin treatment has been reported to enhance tolerance to heat stress by regulating the activity of autophagy [
26]. Specifically, melatonin treatment has been found to increase the expression of genes involved in autophagy, such as TG5, ATG6, ATG8a, ATG8f, ATG12, and ATG18c, and to enhance the formation of autophagosomes. These results suggest that melatonin improves heat stress tolerance in tomato by promoting autophagy. Melatonin treatment has been shown to increase the expression of genes involved in autophagy, such as AtAPX1 and AtCATs, and enhance the formation of autophagosomes in
Arabidopsis [
92]. Additionally, melatonin treatment has been found to increase the accumulation of ROS and activate antioxidant enzymes, suggesting that melatonin enhances drought stress tolerance by promoting autophagy and regulating oxidative stress. The expression of several autophagy-related genes isolated from apple was significantly delayed in melatonin-induced leaf senescence. These pieces of evidence suggest that melatonin plays a vital role in regulating autophagy in horticultural plants, enhancing stress tolerance by fostering autophagy under stressful conditions [
84].
4.1.10. Role of Melatonin in Post-Harvest Fruit Ripening
In kiwi and pear fruits melatonin treatment has been shown to delay post-harvest ripening by inhibiting respiration and the biosynthesis and signaling of ethylene [
78,
93]. Specifically, melatonin treatment has been found to decrease the expression of genes involved in ethylene biosynthesis, such as PcACO, PcACS, MaACS1, MaACO1, and MaETR1, and to reduce the production of ethylene [
78,
94]. Additionally, melatonin treatment has been shown to enhance the activity of antioxidant enzymes and related genes and reduce the accumulation of reactive oxygen species, suggesting that melatonin delays post-harvest ripening in litchi fruit by regulating oxidative stress [
54,
95].
Melatonin treatment has also been shown to delay post-harvest ripening in apples by regulating ethylene production and signaling [
96]. It has been demonstrated that melatonin inhibits the expression of genes involved in ethylene biosynthesis, such as ACS1, ACS2, ACO1, and ERF1. Furthermore, melatonin treatment has been shown to increase antioxidant enzyme activity and decrease the buildup of reactive oxygen species, implying that melatonin delays post-harvest ripening in kiwifruit via controlling oxidative stress. However, contrasting results were found in tomatoes [
30]. The study reported an increase in the lycopene and carotenoids contents and expression of ethylene signal transduction-related genes, such as NR, SlETR4, SlEIL1, SlEIL3, and SlERF2, along with aquaporin-related genes, like SlPIP12Q, SlPIPQ, SlPIP21Q, and SlPIP22, after applying 50 µM melatonin. These all factors simultaneously promoted ripening and improved the postharvest quality of tomatoes.
4.1.11. Role of Melatonin in Circadian Cycle Regulation in Horticultural Plants
Melatonin is important for the regulation of circadian rhythms in horticultural plants. Circadian rhythms regulate several physiological activities in plants, including photosynthesis, growth, and stress responses [
97]. Melatonin has been proven in studies to control the production of clock genes and govern circadian rhythms in horticultural plants. Melatonin therapy, for example, has been demonstrated to modulate clock gene expression and increase the amplitude of circadian rhythms in
Arabidopsis plants [
98]. Melatonin has been shown to boost the expression of genes involved in the circadian clock, such as TOC1, GI, and PRR7, as well as to increase the amplitude of oscillations in clock gene expression. Furthermore, melatonin application has been demonstrated to increase the expression of photosynthesis genes and improve photosynthetic efficiency, implying that melatonin modulates circadian rhythms and photosynthesis in tomato plants.
Melatonin has also been reported to affect clock gene expression and increase the amplitude of circadian rhythms in grapevines [
99]. Melatonin application has been demonstrated to boost the expression of circadian clock genes such as LHY, CCA1, and TOC1 as well as the amplitude of oscillations in clock gene expression. Furthermore, melatonin administration has been shown to increase anthocyanin accumulation and boost secondary metabolite synthesis, implying that melatonin affects circadian rhythms and secondary metabolism in the grapevine. Based on these findings, we may conclude that melatonin performs a vital function in the regulation of circadian rhythms in horticultural plants. It can influence clock gene expression and increase the amplitude of circadian rhythms.
4.1.12. Role of Melatonin as Antioxidant
Melatonin has been reported to behave as a broad-spectrum antioxidant in plants. It can scavenge a variety of reactive oxygen species (ROS), such as hydroxyl radicals, hydrogen peroxide, and superoxide anions [
36]. Melatonin’s antioxidant potential in plants is due to its ability to neutralize ROS and boost the activity of antioxidant enzymes [
100]. Melatonin application has been found in studies to increase the activity of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) as well as to minimize the formation of ROS in a plethora of horticultural crops. Melatonin administration, for example, has been shown to boost SOD and CAT activity while decreasing ROS accumulation under salt stress conditions in tomato [
38] and apple plants [
53]. Similarly, in strawberry, melatonin treatment has been found to enhance the activity of antioxidant enzymes and reduce the accumulation of ROS under post-harvest storage conditions [
44]. In watermelon, melatonin treatment led to increased endogenous melatonin accumulation, which triggered antioxidant enzyme activity in distant untreated tissues, aiding in the mitigation of oxidative stress caused by cold stress [
41].
In addition to its direct antioxidant activity, melatonin has been reported to regulate the expression of genes involved in antioxidant defense pathways in plants. For instance, in watermelon, melatonin application has been found to enhance the expression of genes involved in the ascorbate–glutathione cycle and to reduce oxidative damage under vanadium stress conditions [
100]. Similarly, in tomato plants, melatonin treatment has been shown to enhance the expression of genes involved in the glutathione-ascorbate cycle and to reduce oxidative damage under salinity and heat stress conditions [
46] as well as in post-harvest peaches under cold stress [
89]. Melatonin improves the chilling tolerance of chloroplast by regulating photosynthetic electron flux and the ascorbate–glutathione cycle in cucumber seedlings [
101]. In apple, the application of melatonin helped to increase the expression of MdNHX1 and MdAKT1 genes in apple leaves and helped in improving ion homeostasis under salt stress. Therefore, melatonin acts as a broad-spectrum antioxidant in horticultural crops by directly scavenging ROS and regulating the expression of genes involved in antioxidant defense pathways.
4.1.13. Role of Melatonin in Enhancing Mycorrhization in Horticultural Crops
Mycorrhization is an important aspect of horticultural crop growth as it enhances nutrient uptake and can improve plant tolerance to various biotic and abiotic stresses. Melatonin has been found to play a role in promoting mycorrhization in horticultural crops. Several studies have reported that exogenous application of melatonin can promote the formation of arbuscular mycorrhizal (AM) symbiosis in plants. For example, in cucumber plants, melatonin application increased the colonization rate of AM fungi, resulting in enhanced plant growth and nutrient uptake in the fight against fusarium wilt [
102]. Similarly, in sheep grass (
Leymus chinensis) plants, melatonin application increased the number of AM spores and improved plant growth and yield under saline–alkaline conditions [
103].
The mechanism by which melatonin promotes mycorrhization in horticultural crops is not fully understood, but it is believed to be associated with the regulation of plant defense responses and root morphology. Melatonin has been shown to activate the antioxidant defense system and reduce oxidative stress in plants, which can improve their ability to establish symbiotic relationships with AM fungi [
102,
104]. Additionally, melatonin can modulate root architecture and increase the density and length of root hairs, which can facilitate AM fungal colonization [
104]. Therefore, the application of melatonin can enhance mycorrhization in horticultural crops, which can improve their growth, and yield, and impart stress tolerance.
4.1.14. Role of Melatonin in Defense in Horticultural Crops
Melatonin plays a crucial role in enhancing defense mechanisms in horticultural crops. One notable example is its involvement in plant–pathogen interactions. Studies have shown that exogenous application of melatonin can enhance the plant’s resistance to various pathogens. For instance, in grape plants infected with fungal pathogens, such as
Botrytis cinerea, melatonin treatment has been found to reduce disease severity and inhibit the pathogen growth cycle [
105]. Instead of inhibiting
Botrytis cinerea growth directly, exogenous melatonin treatment could induce disease resistance by priming defense mechanisms. Melatonin treatment inhibits grey mould and induces disease resistance in cherry tomatoes during postharvest [
106]. In another study, melatonin application in tomatoes increased the expression of defense-related genes and the production of antimicrobial compounds, leading to enhanced resistance against fungal pathogens like
Botrytis cinerea [
107]. Melatonin helped to increase the expression of the
PR1,
NPR1,
PI II, and
LoxD genes, associated with disease resistance, and elevated the contents of vitamin C, titratable acid, soluble sugar, and soluble proteins in tomatoes [
107]. These examples demonstrate the role of melatonin in triggering the plant’s defense responses and improving its ability to combat pathogenic attacks. In apple trees, melatonin treatment reduced leaf lesions and increased the production of defensive metabolites and enzymes against
Botrytis cinerea infection [
108].
Melatonin has been found to have effective antibacterial properties against phytobacterial pathogens in plant–bacteria interaction studies [
109]. In plant–virus interaction models, it has been observed that melatonin can eliminate apple stem grooving virus from apple shoots in vitro, thereby making it useful in producing virus-free plants. Additionally, it has been shown to reduce the concentration of tobacco mosaic virus, viral RNA, and virus levels in infected
Nicotiana glutinosa and
Solanum lycopersicum seedlings [
76,
109]. Melatonin has also been shown to enhance the plant’s defense against herbivorous pests [
67,
110].
To summarize, melatonin serves as a key player in enhancing defense mechanisms in horticultural crops. Its application has been shown to activate defense pathways, induce the production of antimicrobial compounds, and strengthen the plant’s antioxidant defense system. Moreover, melatonin enhances the plant’s resistance against pathogens and herbivorous pests, improving overall defense and resilience. Understanding the role of melatonin in enhancing defense in horticultural crops provides valuable insights for developing sustainable strategies to protect crops and enhance their productivity and quality.
5. Methods of Analysis of Melatonin Level in Plants
Immersion, spray, soaking, and irrigation are the methods of applying melatonin in plants. Leaves, fruits, and above-ground plant parts can be suitable portions for applying exogenous melatonin hormone [
12,
111].
Melatonin analysis in plants can be conducted utilizing a variety of analytical techniques. Some of the most common ways are:
HPLC (high-performance liquid chromatography): This is a common technique for analyzing melatonin in plants. Melatonin is separated from other components of the plant extract depending on their chemical properties and detected using a UV detector. In this scenario, several types of detectors have been utilized, including an electrochemical detector, a fluorescence detector, and a UV detector. However, when compared to the MS detector, these detectors have revealed their limitations, specifically in terms of limited sensitivity and specificity.HPLC has been employed in measuring melatonin in a variety of plant species, including rice, grapevine, and tomato [
112].
Gas chromatography–mass spectrometry (GC-MS): This technique uses gas chromatography to separate melatonin from other plant components and mass spectrometry to detect it. Although the sensitivity and specificity of melatonin quantification have improved, the need for sample volatile derivatization presents a disadvantage for its application to melatonin determination, as noted by [
113,
114]. Melatonin levels in plants such as wheat and barley have been measured using this method [
7,
112,
115].
Enzyme-linked immunosorbent assay (ELISA): This is a recent technology that detects the presence of melatonin in plant extracts by using antibodies specific to melatonin. Melatonin quantification has been a long-standing practice using immunoassays such as radioimmunoassay (RIA) and enzyme-linked immunosorbent assay (ELISA). These methods have been studied by experts [
116,
117,
118]. The ELISA kit is widely available and is a commonly used tool for melatonin analysis due to its ease of use. However, antibody cross-reactivity is a concern that cannot be avoided, resulting in a decrease in specificity and accuracy [
118,
119]. Melatonin levels in plants such as soybean and grapevine have been measured using this method [
116,
120].
LC-MS/MS (liquid chromatography–tandem mass spectrometry): Melatonin is separated from other plant components using liquid chromatography and uses tandem mass spectrometry for detection, known for its sensitivity and selectivity. The combination of LC and MS has been established as a reliable and effective approach for conducting melatonin analysis due to its exceptional precision, reproducibility, and sensitivity [
118]. Melatonin levels in plants such as tomatoes and kiwifruit have been measured using this method [
112,
115].
The method used to analyze plant melatonin is determined by sensitivity, selectivity, and sample complexity. To confirm the presence of melatonin and correctly quantify its levels, researchers frequently employ a combination of procedures.
6. Use of Exogenous Melatonin as a Potential Tool to Improve the Physiology of Horticultural Crops
When administered to horticultural crops, exogenous melatonin has been demonstrated to perform many physiological effects. It improves stress tolerance by lowering oxidative damage, regulating ion absorption and transport, and boosting root growth [
24,
36,
40]. Melatonin therapy strategy, for example, has been shown to improve salt tolerance in tomato and cucumber plants by lowering oxidative damage and encouraging root growth [
29,
60]. Exogenous melatonin has also been shown to affect numerous aspects of horticultural crop growth and development, such as seed germination, root and shoot growth, and blooming [
7,
121]. It has been proven in enhancing seed development thereby promoting seed germination, facilitating root and shoot growth, and delaying senescence in various horticultural crops [
60].
Exogenous melatonin has been reported to influence hormone signaling pathways in horticulture crops, thus regulating numerous physiological processes. Melatonin has been shown to interact with other plant hormones, such as auxins, cytokinins, and abscisic acid, in horticultural crops to increase root growth, postpone senescence, and improve stress tolerance [
15,
18,
45]. Melatonin has also been found to serve as a broad-spectrum antioxidant in horticulture crops, scavenging ROS and boosting antioxidant enzyme activity [
21,
29,
89].
In this way, exogenous melatonin has emerged as a potential tool for improving stress tolerance, growth, and development in horticultural crops. Its potential applications in crop production and stress management are increasingly being explored, and further research is necessary to fully understand the mechanisms of action and optimize its practical use in horticultural practices. The use of exogenous melatonin in alleviating abiotic stressors has been extensively studied by Ahmad et al. [
6]. Melatonin has been found to be present in both roots and seeds, and its application has shown promising results in improving plant health. In general, when plants are subjected to sodium chloride (NaCl), plants typically suffer from decreased enzyme activity, resulting in salt sensitivity. However, exogenous melatonin application has been shown to increase the level of antioxidants and enzyme activity, thereby improving plant health. Additionally, melatonin has been found to increase chlorophyll content, chloroplast gene expression, and protein content, leading to increased photosynthetic activity and chlorophyll accumulation, which is critical to plant growth. Lastly, melatonin treatment during drought stress reduces ROS and O
2− concentration while increasing chlorophyll content and physiological functions tied to plant development (
Figure 3).
It can be inferred that exogenous melatonin application has diverse effects on horticultural crops depending on the concentration and the stage of growth at which it is applied. Melatonin has been shown to promote seed germination, enhance plant growth and development, improve photosynthesis and antioxidant systems, increase fruit quality and storability, and mitigate the effects of abiotic stress factors such as drought, heat, salt, and cold. However, the optimal concentration of melatonin for each crop and growth stage needs to be further determined. Furthermore, the underlying molecular mechanisms of melatonin’s effects on horticultural crops require further research. Overall, exogenous melatonin application has enormous potential to improve the productivity and quality of horticultural crops, providing a sustainable and eco-friendly approach to crop management.
7. Economics for Using Melatonin in Horticultural Crops
The use of melatonin in horticultural crops has been shown to have potential economic benefits. Melatonin application can enhance crop growth and yield as well as improve quality attributes such as nutritional value, flavour, and shelf life. Melatonin application can increase profits for farmers and provide superior product value for consumers. One study evaluated the economic benefits of using melatonin in tomato production in China [
37]. The study found that the application of melatonin at a concentration of 100μM resulted in a 3.9% increase in tomato yield and an 18.7% increase in net photosynthesis compared to control treatments. Additionally, melatonin-treated tomatoes had higher levels of vitamin C, citric acid, lycopene, and soluble solids content, which could further enhance their market value. Similarly, a study on strawberry production found that melatonin application at a concentration of 100μM resulted in a significant increase in yield and fruit quality, including a decline in fruit decay, extended shelf life, and enhanced accumulation of ATP and antioxidants [
44]. The economic benefits of melatonin treatment were not directly evaluated in this study, but the improvements in yield and quality could translate into increased profitability for growers and improved product value for consumers.
Another study conducted on grapevines found that the application of melatonin at a concentration of 100μM increased the accumulation of secondary metabolites such as anthocyanins and flavonoids, which contribute to the colour and flavour of grapes [
122]. This could enhance the market value of the grapes and increase profitability for grape growers. Furthermore, the use of melatonin in the post-harvest storage of horticultural crops can also have economic benefits by lengthening their shelf life and minimizing post-harvest losses. For example, a study on post-harvest storage of strawberry and peach found that the application of melatonin at a concentration of 100μM delayed fruit softening and reduced decay, resulting in longer shelf life and reduced post-harvest losses [
44].
In addition to the direct economic benefits of melatonin application, there may also be indirect benefits such as reduced environmental impact and increased sustainability of horticultural production systems. Melatonin can enhance plant stress tolerance and reduce the need for chemical inputs such as fertilizers and pesticides, which can result in cost savings for growers and reduce the environmental impact of horticultural production. The use of melatonin in horticultural crops has the potential to provide economic benefits by improving crop growth, yield, and quality as well as reductions in post-harvest losses. However, the economic feasibility of melatonin application may depend on factors such as crop type, market demand, and the expense associated with melatonin treatment.
8. Future Perspectives
The future exploration of melatonin’s impact on horticultural plants opens myriad research avenues, including understanding its mechanisms of action, real-world applications, genetic manipulation, and field studies. Despite considerable progress in understanding the role of melatonin in horticulture plants, much remains to be understood about the underlying mechanisms of action. Future studies could concentrate on discovering the specific signaling pathways and molecular mechanisms involved in melatonin-regulated physiological functions. The potential applications of melatonin in horticultural practices, such as crop production and stress management, need to be explored further. Researchers can investigate the most effective ways to deliver melatonin to plants, fine-tune the optimal concentrations for different crops, and determine the optimal timing of its application. Plants’ melatonin production and signaling pathways could be modified through genetic engineering to improve stress tolerance and yield potential. Identifying genes involved in melatonin biosynthesis and signaling pathways and producing crops with increased melatonin production could be the focus of future research. Field studies could be done to assess the efficacy of melatonin in improving crop development, stress tolerance, and yield under real-world conditions. Such research would aid in determining the practical utility of melatonin in horticultural techniques. Continued research on melatonin and its role in horticultural plants are essential for improving crop productivity, stress tolerance, and food security under pressing environmental challenges.
9. Conclusions
Recent research has uncovered the diverse functions of melatonin in horticultural plants, including its role in growth and development, stress tolerance, and antioxidant defense. Melatonin’s positive impact on seed germination, root and shoot growth, and biomass accumulation in horticultural crops highlight its potential as a valuable tool in crop production. Moreover, melatonin exerts crucial regulatory control over vegetative and reproductive growth stages, floral transition, and leaf senescence in plants. By modulating root architecture, nutrient uptake, and ion transport, melatonin enhances stress tolerance, allowing plants to thrive under adverse conditions. Melatonin acts as a broad-spectrum antioxidant, effectively scavenging reactive oxygen species and boosting antioxidant activity. Its intricate mechanisms of action involve gene expressions, hormone signaling pathways, and antioxidant defense pathways. Additionally, melatonin’s interaction with other plant growth regulators, such as auxins, cytokinins, and abscisic acid, orchestrates various physiological processes in plants. The comprehensive insight provided by this review paper paves the way for further exploration and application of melatonin in horticulture. By bridging the gap between knowledge and practical implementation, researchers can uncover the full extent of melatonin’s mechanisms of action and devise effective strategies for its utilization in horticultural practices.
Undoubtedly, melatonin has emerged as a versatile chemical molecule with immense potential for enhancing crop production and managing stress in horticultural plants. As the research progresses, continued investigation into melatonin’s multifaceted functions will unlock new avenues for its utilization, benefiting both growers and the agricultural and horticultural industries. By unraveling the intricacies of melatonin’s actions and harnessing its potential, we can shape a future in which melatonin becomes an invaluable asset in optimizing horticultural practices and fostering sustainable crop production. In addition to this research, additional research is needed to completely comprehend the activities of melatonin receptors in various plant species and how they interact with other signaling pathways to control plant physiology.
Author Contributions
Conceptualization, A.K., S.K.S. and K.K.V.; methodology, A.K., S.K.S., B.M., V.K.G. and M.B.; software, A.K., S.K.S., V.K.G., M.B. and R.B.; supervision, K.K.V.; writing—review and editing, A.K., S.K.S., B.M., V.K.G., M.B., K.K.V. and R.B.; data curation, A.K. and S.K.S.; writing—original draft, A.K., and S.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Dean, College of Agriculture, Jawaharlal Nehru Krishi Vishwa Vidyalaya, GanjBasoda, Vidisha, Madhya Pradesh, India, and the Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China for providing the necessary facilities for this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| IAA | Indole-3-acetic acid |
| ROS | Reactive oxygen species |
| ARF | Adventitious root formation |
| NO | Nitric oxide |
| ABA | Abscisic acid |
| GA | Gibberellins |
| JA | Jasmonic acid |
| SA | Salicylic acid |
| BR | Brassinolides |
| TDC | Tryptophan decarboxylase |
| NAS | N-acetyl serotonin |
| SNAT | Serotonin N-acetyltransferase |
| ASMT | N-acetyl serotonin O-methyltransferase |
| COMT | Caffeic acid O-methyltransferase |
| ASDD | Acetyl serotonin deacetylase |
| 5-MT | 5-methoxy tryptamine |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| AM | Arbuscular mycorrhiza |
References
- Gao, T.; Liu, X.; Tan, K.; Zhang, D.; Zhu, B.; Ma, F.; Li, C. Introducing Melatonin to the Horticultural Industry: Physiological Roles, Potential Applications, and Challenges. Hortic. Res. 2022, 9, uhac094. [Google Scholar] [CrossRef]
- Orsini, F.; Kahane, R.; Nono-Womdim, R.; Gianquinto, G. Urban Agriculture in the Developing World: A Review. Agron. Sustain. Dev. 2013, 33, 695–720. [Google Scholar] [CrossRef]
- Umamaheswari, R.; Prasannakumar, N.R.; Sriram, S.; Sharma, S.K.; Rao, M.S.; Chaya, M.K. Biotic Stress Management in Horticultural Crops Using Microbial Intervention. In Rhizosphere Microbes; Sharma, S.K., Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Eds.; Springer: Singapore, 2020; Volume 23, pp. 619–654. ISBN 9789811591532. [Google Scholar]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, S.K.; Rai, G.K. Physiological and Biochemical Response of Plants under Drought Stress. In Plant Abiotic Stress Tolerance: Physiochemical and Molecular Avenues; Deepika Book Agency: Delhi, India, 2021; pp. 77–92. [Google Scholar]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The Role of Melatonin in Plant Growth and Metabolism, and Its Interplay with Nitric Oxide and Auxin in Plants under Different Types of Abiotic Stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Qi, Z.-Y.; Wang, K.-X.; Yan, M.-Y.; Kanwar, M.; Li, D.-Y.; Wijaya, L.; Alyemeni, M.; Ahmad, P.; Zhou, J. Melatonin Alleviates High Temperature-Induced Pollen Abortion in Solanum lycopersicum. Molecules 2018, 23, 386. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Ding, Z.; Xie, J. Prolong the Postharvest Shelf Life of Spinach through the Antioxidative Ability of Melatonin. FoodChem. X 2023, 19, 100769. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The Role of Melatonin in Salt Stress Responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Hu, L.; Zhang, J.; Yue, H.; Yang, S.; Liu, Y.; Gong, X.; Ma, F. Overexpression of MdASMT9, an N-acetylserotonin methyltransferase gene, increases melatonin biosynthesis and improves water-use efficiency in transgenic apple. Tree Physiol. 2021, 42, 1114–1126. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and Prospects of Melatonin in Plant Growth, Yield, and Quality. J. Exp. Bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef]
- Kong, X.; Ge, W.; Wei, B.; Zhou, Q.; Zhou, X.; Zhao, Y.; Ji, S. Melatonin Ameliorates Chilling Injury in Green Bell Peppers during Storage by Regulating Membrane Lipid Metabolism and Antioxidant Capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin Mediates the Regulation of ABA Metabolism, Free-Radical Scavenging, and Stomatal Behaviour in Two Malus Species under Drought Stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, S.K.; Mikhina, M.S.; Patni, B.; Krishna, G.K. Melatonin as a key regulator of plant growth and development. In Advancement of Melatonin Research in Plants: Multi-Faceted Role in Regulating Development and Stress Protection; Taylor & Francis Group: New York, NY, USA, 2023. [Google Scholar]
- Chen, Q.; Qi, W.; Reiter, R.J.; Wei, W.; Wang, B. Exogenously Applied Melatonin Stimulates Root Growth and Raises Endogenous Indoleacetic Acid in Roots of Etiolated Seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; López-Bucio, J. Melatonin Regulates Arabidopsis Root System Architecture Likely Acting Independently of Auxin Signaling: Role of Melatonin in Root Architecture and Auxin Responses. J. Pineal Res. 2012, 53, 279–288. [Google Scholar] [CrossRef]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting Roles of Melatonin in Adventitious Root Development of Solanum lycopersicum L. by Regulating Auxin and Nitric Oxide Signaling. Front. Plant Sci. 2016, 7, 718. [Google Scholar] [CrossRef]
- Yadav, S.; David, A.; Bhatla, S.C. Nitric Oxide Modulates Specific Steps of Auxin-Induced Adventitious Rooting in Sunflower. Plant Signal. Behav. 2010, 5, 1163–1166. [Google Scholar] [CrossRef]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT Improves Melatonin Production and Enhances Drought Tolerance in Transgenic Arabidopsis Thaliana Plants. J. Pineal Res. 2014, 57, 408–417. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-Seq Approach to Discriminate Gene Expression Profiles in Response to Melatonin on Cucumber Lateral Root Formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef]
- Bychkov, I.A.; Andreeva, A.A.; Kudryakova, N.V.; Kusnetsov, V.V. Cytokinin Modulates Responses to Phytomelatonin in Arabidopsis thaliana under High Light Stress. Int. J. Mol. Sci. 2023, 24, 738. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive Effects of Melatonin and Cytokinin on Alleviating Drought-Induced Leaf Senescence in Creeping Bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-Term Exogenous Application of Melatonin Delays Drought-Induced Leaf Senescence in Apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin Suppression of Heat-Induced Leaf Senescence Involves Changes in Abscisic Acid and Cytokinin Biosynthesis and Signaling Pathways in Perennial Ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Xiang, G.; Bian, F.; Yao, Y. Melatonin Promotes Ripening of Grape Berry via Increasing the Levels of ABA, H2O2, and Particularly Ethylene. Hortic. Res. 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory Metabolic Networks in Drought Stress Responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Riga, P.; Medina, S.; García-Flores, L.A.; Gil-Izquierdo, Á. Melatonin Content of Pepper and Tomato Fruits: Effects of Cultivar and Solar Radiation. Food Chem. 2014, 156, 347–352. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin Promotes Seed Germination under High Salinity by Regulating Antioxidant Systems, ABA and GA4 Interaction in Cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin Promotes Ripening and Improves Quality of Tomato Fruit during Postharvest Life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin Induces Disease Resistance to Botrytis Cinerea in Tomato Fruit by Activating Jasmonic Acid Signaling Pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef]
- Qu, G.; Wu, W.; Ba, L.; Ma, C.; Ji, N.; Cao, S. Melatonin Enhances the Postharvest Disease Resistance of Blueberries Fruit by Modulating the Jasmonic Acid Signaling Pathway and Phenylpropanoid Metabolites. Front. Chem. 2022, 10, 957581. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl Jasmonate Mediates Melatonin-Induced Cold Tolerance of Grafted Watermelon Plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, C.; Yang, R.; Zhao, S.; Han, X.; Wang, Z.; Li, S.; Gao, H. Endogenous Salicylic Acid Mediates Melatonin-Induced Chilling-and Oxidative-Stress Tolerance in Harvested Kiwifruit. Postharvest Biol. Technol. 2023, 201, 112341. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous Melatonin Confers Salt Stress Tolerance to Watermelon by Improving Photosynthesis and Redox Homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. The beneficial effects of exogenous melatonin on tomato fruit properties. Sci. Hortic. 2016, 207, 14–20. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.; Guo, S. Melatonin Pretreatment Confers Heat Tolerance and Repression of Heat-Induced Senescence in Tomato through the Modulation of ABA- and GA-Mediated Pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef]
- Sadak, M.S.; Bakry, B.A. Alleviation of Drought Stress by Melatonin Foliar Treatment on Two Flax Varieties under Sandy Soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Cui, Y.; Yin, Y. Melatonin Application Confers Enhanced Salt Tolerance by Regulating Na+ and Cl− Accumulation in Rice. Plant Growth Regul. 2017, 83, 441–454. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Zheng, J.; Dong, Y.; Liu, Q.; Yang, X.; Wei, C.; Zhang, Y.; Ma, J.; Zhang, X. Local Melatonin Application Induces Cold Tolerance in Distant Organs of Citrullus lanatus L. via Long Distance Transport. Sci. Rep. 2017, 7, 40858. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Pharmacology and Function of Melatonin Receptors. FASEB J. 1988, 2, 2765–2773. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin Treatment Delays Postharvest Senescence and Regulates Reactive Oxygen Species Metabolism in Peach Fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin Treatment Attenuates Postharvest Decay and Maintains Nutritional Quality of Strawberry Fruits (Fragaria × anannasa Cv. Selva) by Enhancing GABA Shunt Activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin Regulates the Functional Components of Photosynthesis, Antioxidant System, Gene Expression, and Metabolic Pathways to Induce Drought Resistance in Grafted Carya cathayensis Plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.; Mittler, R.; Rivero, R. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.; Rivkees, S.; Reppert, S. Localization and Characterization of Melatonin Receptors in Rodent Brain by In Vitro Autoradiography. J. Neurosci. 1989, 9, 2581–2590. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1177–1185. [Google Scholar] [CrossRef]
- Sugden, D.; Chong, N.W.S.; Lewis, D.F.V. Structural Requirements at the Melatonin Receptor. Br. J. Pharmacol. 1995, 114, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin Receptor PMTR1-Mediated Signaling Regulates Stomatal Closure in Arabidopsis Thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. The Phytomelatonin Receptor (PMRT1) Arabidopsis Cand2 is Not a Bonafide G Protein–Coupled Melatonin Receptor. Melatonin Res. 2020, 3, 177–186. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. Low Melatonin Production by Suppression of Either Serotonin N-Acetyltransferase or N-Acetylserotonin Methyltransferase in Rice Causes Seedling Growth Retardation with Yield Penalty, Abiotic Stress Susceptibility, and Enhanced Coleoptile Growth under Anoxic conditions. J. Pineal Res. 2016, 60, 348–359. [Google Scholar] [CrossRef]
- Gong, X.; Shi, S.; Dou, F.; Song, Y.; Ma, F. Exogenous Melatonin Alleviates Alkaline Stress in Malus hupehensis Rehd. by Regulating the Biosynthesis of Polyamines. Molecules 2017, 22, 1542. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Z.; Pan, J.; Li, J.; Li, X.; Khoo, H.E.; Dong, X. Melatonin Treatment Improves Postharvest Quality and Regulates Reactive Oxygen Species Metabolism in “Feizixiao” Litchi Based on Principal Component Analysis. Front. Plant Sci. 2022, 13, 965345. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; García-Caparrós, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food 2022, 13, 196–217. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Against Environmental Plant Stressors: A Review. Curr. Protein Pept. Sci. 2022, 22, 413–429. [Google Scholar]
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin Applied to Cucumber (Cucumis sativus L.) Seeds Improves Germination during Chilling Stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, B.; Zhang, H.-J.; Weeda, S.; Yang, C.; Yang, Z.-C.; Ren, S.; Guo, Y.-D. Melatonin Promotes Water-Stress Tolerance, Lateral Root Formation, and Seed Germination in Cucumber (Cucumis sativus L.): Melatonin Alleviates Water Stress in Cucumber. J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Qadir, A.; Anwar, M.; Shakoor, A.; Hayat, F. Exogenous Melatonin Enhances Salt Stress Tolerance in Tomato Seedlings. Biol. Plant. 2020, 64, 604–615. [Google Scholar] [CrossRef]
- Rosińska, A.; Andrzejak, R.; Kakkerla, V. Effect of Osmopriming with Melatonin on Germination, Vigor and Health of Daucus carota L. Seeds. Agric. 2023, 13, 749. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Liu, J.-L.; Wang, W.-X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Imran, M.; Aaqil Khan, M.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.-W.; Khan, A.L.; Lee, I.-J. Melatonin Ameliorates Thermotolerance in Soybean Seedling through Balancing Redox Homeostasis and Modulating Antioxidant Defense, Phytohormones and Polyamines Biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef] [PubMed]
- Yandi, W. Chinese Changes in Melatonin Content in Grape and Prokaryotic Expression Analysis of its Synthetic Gene SNAT; Academy of Agricultural Sciences: Beijing, China, 2018. [Google Scholar]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin Enhances Root Regeneration, Photosynthetic Pigments, Biomass, Total Carbohydrates and Proline Content in the Cherry Rootstock PHL-C (Prunus Avium × Prunus Cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, Q.; Yu, X.; Huang, L.; Xu, S.; Wang, R.; Shen, W.; Shen, W. Hydrogen Peroxide Acts Downstream of Melatonin to Induce Lateral Root Formation. Ann. Bot. 2018, 121, 1127–1136. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Saxena, P.K.; Murch, S.J. Melatonin in Plant Signalling and Behaviour. Funct. Plant Biol. 2018, 45, 58. [Google Scholar] [CrossRef] [PubMed]
- Litwinczuk, W.; Wadas-Boron, M. Development of highbush blueberry (Vacciniumcorymbosum hort. Non L.) in vitro shoot cultures under the influence of melatonin. Acta Sci. Polonorum. Hortorum Cultus 2009, 8, 3–12. [Google Scholar]
- Ramakrishna, A.; Giridhar, P.; Ravishankar, G.A. Indoleamines and calcium channels influence morphogenesis in in vitro cultures of Mimosa pudica L. Plant Signaling & Behavior 2009, 4, 1136–1141. [Google Scholar] [CrossRef]
- Sarrou, E.; Therios, I.; Dimassi-Theriou, K. Melatonin and Other Factors That Promote Rooting and Sprouting of Shoot Cuttings in Punica granatum Cv. Wonderful. Turk. J. Bot. 2014, 38, 293–301. [Google Scholar] [CrossRef]
- Okazaki, M.; Higuchi, K.; Aouini, A.; Ezura, H. Lowering Intercellular Melatonin Levels by Transgenic Analysis of Indoleamine 2,3-Dioxygenase from Rice in Tomato Plants: Lowering Intercellular Melatonin Levels by OsIDO. J. Pineal Res. 2010, 49, 239–247. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Giridhar, P.; Jobin, M.; Paulose, C.S.; Ravishankar, G.A. Indoleamines and Calcium Enhance Somatic Embryogenesis in Coffea Canephora P Ex Fr. Plant Cell Tiss. Organ. Cult. 2012, 108, 267–278. [Google Scholar] [CrossRef]
- Kolář, J.; Johnson, C.H.; Macháčková, I. Exogenously Applied Melatonin (N-Acetyl-5-Methoxytryptamine) Affects Flowering of the Short-Day Plant Chenopodium rubrum. Physiol. Plant. 2003, 118, 605–612. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. Mammalian neurohormones: Potential significance in reproductive physiology of St. John’s wort (Hypericum perforatum L.)? Naturwissenschaften 2002, 89, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Alan, A.R.; Cao, J.; Saxena, P.K. Melatonin and Serotonin in Flowers and Fruits of Datura metel L. J. Pineal Res. 2009, 47, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, R.; Liu, D.; Wu, Y.; Sun, J.; Tao, J. Melatonin andexpression of tryptophan decarboxylase gene (TDC) in herbaceous peony (Paeonia lactifora Pall.) flowers. Molecules 2018, 23, 1164. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The Physiological Function of Melatonin in Plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Zhang, H.; Cong, L.; Zhai, R.; Yang, C.; Xu, L. Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. J. Agric. Food Chem. 2019, 67, 2279–2288. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous Melatonin Promotes Biomass Accumulation and Photosynthesis of Kiwifruit Seedlings under Drought Stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Mao, J.; Niu, C.; Li, K.; Chen, S.; Tahir, M.M.; Han, M.; Zhang, D. Melatonin Promotes Adventitious Root Formation in Apple by Promoting the Function of MdWOX11. BMC Plant Biol. 2020, 20, 536. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rongrong, Y.; Min, S.; Meng, W.; Liu, C.; Rui, Z.; Chengquan, Y.; Zhigang, W.; Fengwang, M.; Lingfei, X. Effects of exogenous application of melatonin on quality and sugar metabolism in ‘Zaosu’ pear fruit. J. Plant Growth Regul. 2019, 38, 1161–1169. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Song, C.Z.; Yu, Y.; Hu, F.; Zhang, L.; Zhang, Z.W.; Xi, Z.M. Melatonin Treatment of Pre-Veraison Grape Berries to Increase Size and Synchronicity of Berries and Modify Wine Aroma Components. Food Chem. 2015, 185, 127–134. [Google Scholar] [CrossRef]
- Tan, X.; Fan, Z.; Kuang, J.; Lu, W.; Reiter, R.J.; Lakshmanan, P.; Su, X.; Zhou, J.; Chen, J.; Shan, W. Melatonin Delays Leaf Senescence of Chinese Flowering Cabbage by Suppressing ABFs-mediated Abscisic Acid Biosynthesis and Chlorophyll Degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Chang, C.; Feng, F.; Liang, D.; Cheng, L.; Ma, F. Delay in Leaf Senescence of Malus Hupehensis by Long-Term Melatonin Application Is Associated with Its Regulation of Metabolic Status and Protein Degradation. J. Pineal Res. 2013, 55, 424–434. [Google Scholar] [CrossRef]
- Wei, J.; Liang, J.; Liu, D.; Liu, Y.; Liu, G.; Wei, S. Melatonin-Induced Physiology and Transcriptome Changes in Banana Seedlings under Salt Stress Conditions. Front. Plant Sci. 2022, 13, 938262. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Hall, B.A.; Le, C.H.; Saxena, P.K. Changes in the Levels of Indoleamine Phytochemicals during Véraison and Ripening of Wine Grapes: Melatonin in Developing Wine Grapes. J. Pineal Res. 2010, 49, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating Effects of Exogenous Melatonin on Salt Stress in Cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Shi, H.; Tan, D.-X.; Reiter, R.J.; Ye, T.; Yang, F.; Chan, Z. Melatonin Induces Class A1 Heat-Shock Factors (HSFA1s) and Their Possible Involvement of Thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Shi, L.; Xu, L.; Shen, Z.; Chen, W.; Yang, Z. Melatonin Increases Chilling Tolerance in Postharvest Peach Fruit by Alleviating Oxidative Damage. Sci. Rep. 2018, 8, 806. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Wang, M.; Zhang, S. Exogenous Melatonin Improves Tolerance to Water Deficit by Promoting Cuticle Formation in Tomato Plants. Molecules 2018, 23, 1605. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Liu, M.; Zhou, R.; Jiang, F.; Sun, M.; Wen, J.; Zhu, Z.; Wu, Z. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci. Hortic. 2021, 285, 110176. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Wang, N.; Tan, D.-X.; Ma, F. Melatonin Enhances the Occurrence of Autophagy Induced by Oxidative Stress in Arabidopsis Seedlings. J. Pineal Res. 2015, 58, 479–489. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, A.; Li, H.; Huan, C.; Jiang, T.; Shen, S.; Zheng, X. Effects of Melatonin Treatment on Ethanol Fermenation and ERF Expression in Kiwifruit Cv. Bruno Dur. Postharvest. Sci. Hortic. 2022, 293, 110696. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.-X.; et al. Natural Variation in Banana Varieties Highlights the Role of Melatonin in Postharvest Ripening and Quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valverde, J.M.; Valero, D.; Serrano, M. Melatonin Treatment to Pomegranate Trees Enhances Fruit Bioactive Compounds and Quality Traits at Harvest and during Postharvest Storage. Antioxidants 2021, 10, 820. [Google Scholar] [CrossRef]
- Verde, A.; Míguez, J.M.; Gallardo, M. Melatonin Stimulates Postharvest Ripening of Apples by Up-Regulating Gene Expression of Ethylene Synthesis Enzymes. Postharvest Biol. Technol. 2023, 195, 112133. [Google Scholar] [CrossRef]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A.R. Plant Circadian Clocks Increase Photosynthesis, Growth, Survival, and Competitive Advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Pérez-García, P.; Pokhilko, A.; Millar, A.J.; Antoshechkin, I.; Riechmann, J.L.; Mas, P. Mapping the Core of the Arabidopsis Circadian Clock Defines the Network Structure of the Oscillator. Science 2012, 336, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Boccalandro, H.E.; González, C.V.; Wunderlin, D.A.; Silva, M.F. Melatonin Levels, Determined by LC-ESI-MS/MS, Fluctuate during the Day/Night Cycle in Vitis vinifera Cv Malbec: Evidence of Its Antioxidant Role in Fruits: Melatonin Day/Night Cycle Fluctuation in Vitis vinifera. J. Pineal Res. 2011, 51, 226–232. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin Pretreatment Improves Vanadium Stress Tolerance of Watermelon Seedlings by Reducing Vanadium Concentration in the Leaves and Regulating Melatonin Biosynthesis and Antioxidant-Related Gene Expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin Increases the Chilling Tolerance of Chloroplast in Cucumber Seedlings by Regulating Photosynthetic Electron Flux and the Ascorbate-Glutathione Cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Mao, Q.; Yan, Y.; Wu, M.; Wang, Y.; Ren, J.; Guo, P.; Liu, A.; Chen, S. Role of Melatonin in Arbuscular Mycorrhizal Fungi-Induced Resistance to Fusarium Wilt in Cucumber. Phytopathology 2020, 110, 999–1009. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y.; Li, Z.; Zhukova, A.; Yang, S.; Wang, J.; Tang, Z.; Cao, Y.; Zhang, Y.; Wang, D. Interactive Effects of Exogenous Melatonin and Rhizophagusintraradices on Saline-Alkaline Stress Tolerance in Leymus Chinensis. Mycorrhiza 2020, 30, 357–371. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined Application of Arbuscular Mycorrhizal Fungi and Exogenous Melatonin Alleviates Drought Stress and Improves Plant Growth in Tobacco Seedlings. J. Plant Growth Regul. 2020, 40, 1074–1087. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous Melatonin Treatment Induces Disease Resistance against Botrytis Cinerea on Post-Harvest Grapes by Activating Defence Responses. Foods 2022, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin Treatment Inhibits Gray Mold and Induces Disease Resistance in Cherry Tomato Fruit during Postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Sheng, J.P.; Zhao, R.R.; Chen, L.L.; Shen, L. Effect of pre-harvest melatonin spraying on the post-harvest disease resistance and storage quality of tomato fruit. Food Sci. 2020, 141, 188–193. [Google Scholar] [CrossRef]
- Cao, J.J.; Yu, Z.C.; Zhang, Y.; Li, B.H.; Liang, W.X.; Wang, C.X. Control efficiency of exogenous melatonin against postharvest apple grey mould and its influence on the activity of defensive enzymes. Plant Physiol. 2017, 53, 1753–1760. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, L.; Gu, P.; Zhan, X.; Zhang, Y.; Hou, C.; Wu, Z.; Wu, Y.-F.; Wang, Q.-C. Exogenous Application of Melatonin Improves Plant Resistance to Virus Infection. Plant Pathol. 2019, 68, 1287–1295. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.M.A.; Kozai, T. Melatonin in Glycyrrhiza uralensis: Response of Plant Roots to Spectral Quality of Light and UV-B Radiation. J. Pineal Res. 2006, 41, 108–115. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.; Li, M.; Wei, W. Melatonin in plants: Content, method and function. Chin. J. Appl. Environ. Biol. 2008, 14, 126–131. [Google Scholar]
- Badria, F.A. Melatonin, Serotonin, and Tryptamine in Some Egyptian Food and Medicinal Plants. J. Med. Food 2002, 5, 153–157. [Google Scholar] [CrossRef]
- Ragab, A.S.; Van Fleet, J.; Jankowski, B.; Park, J.-H.; Bobzin, S.C. Detection and Quantitation of Resveratrol in Tomato Fruit (Lycopersicon esculentum Mill.). J. Agric. Food Chem. 2006, 54, 7175–7179. [Google Scholar] [CrossRef]
- Kolář, J.; Macháčková, I. Melatonin in Higher Plants: Occurrence and Possible Functions. J. Pineal Res. 2005, 39, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by High Performance Liquid Chromatography-Mass Spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Pape, C.; Luning, K. Quantification of Melatonin in Phototrophic Organisms. J. Pineal Res. 2006, 41, 157–165. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Hernández, I.G.; Martinez, L.D.; Silva, M.F.; Cerutti, S. Analytical Tools for Elucidating the Biological Role of Melatonin in Plants by LC-MS/MS: Liquid Phase Separations. Electrophoresis 2013, 34, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mazza, G. Application of LC and LC-MS to the Analysis of Melatonin and Serotonin in Edible Plants. Crit. Rev. Food Sci. Nutr. 2011, 51, 269–284. [Google Scholar] [CrossRef]
- Cui, X.; Vasylieva, N.; Wu, P.; Barnych, B.; Yang, J.; Shen, D.; He, Q.; Gee, S.J.; Zhao, S.; Hammock, B.D. Development of an Indirect Competitive Enzyme-Linked Immunosorbent Assay for Glycocholic Acid Based on Chicken Single-Chain Variable Fragment Antibodies. Anal. Chem. 2017, 89, 11091–11097. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Li, L.; Sun, Q.; Wang, Q.; Huang, H.; Tong, Z.; Zhang, J. Melatonin-Mediated Development and Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2023, 14, 1100827. [Google Scholar] [CrossRef]
- Meng, J.F.; Yu, Y.; Shi, T.C.; Fu, Y.S.; Zhao, T.; Zhang, Z.W. Melatonin Treatment of Pre-Veraison Grape Berries Modifies Phenolic Components and Antioxidant Activity of Grapes and Wine. Food Sci. Technol. 2019, 39, 35–42. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).