Nondestructive Detecting Maturity of Pineapples Based on Visible and Near-Infrared Transmittance Spectroscopy Coupled with Machine Learning Methodologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. VIS/NIR Transmittance Spectroscopy Acquisition
2.3. Maturity Assessment and SSC Determination
2.4. Spectral DataSets Preprecessing
2.5. Modeling Algorithms
2.5.1. Quantitative Models
2.5.2. Qualitative Models
2.6. Evaluation of Model Performance
3. Results and Discussion
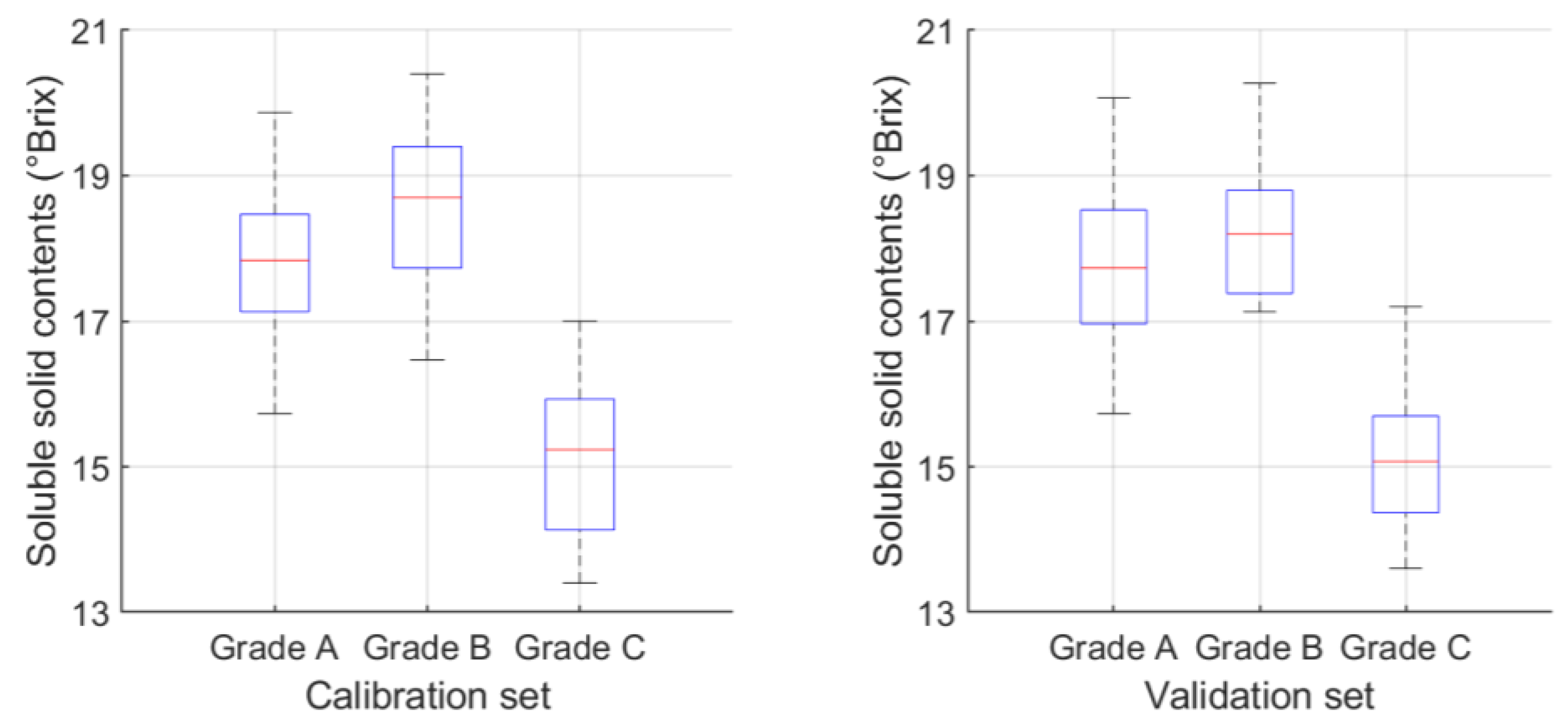
3.1. Internal Quality of Pineapple Samples
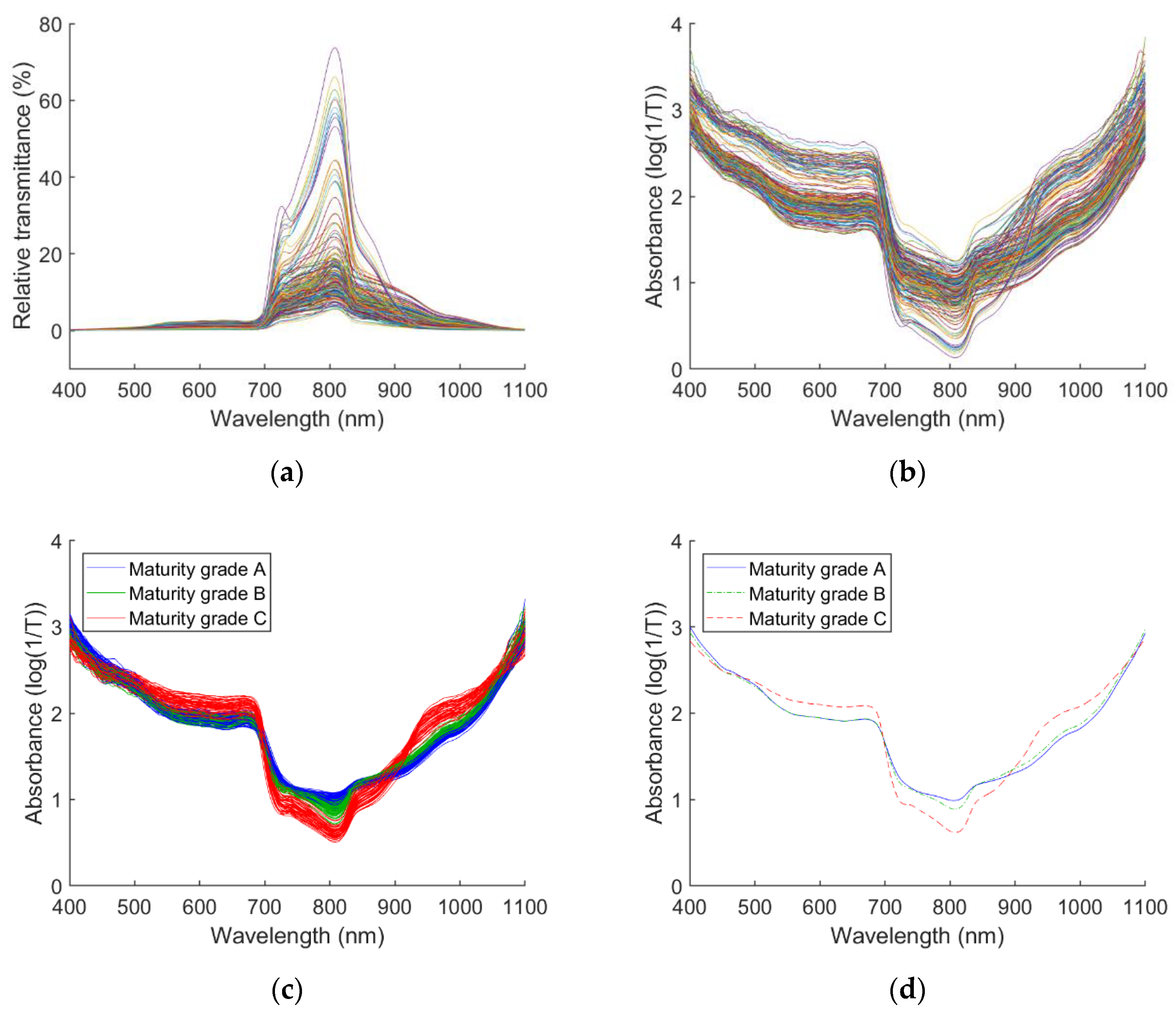
3.2. Spectrums of Pineapple Samples
3.3. Qualitative Models for Discriminating Maturity Grades
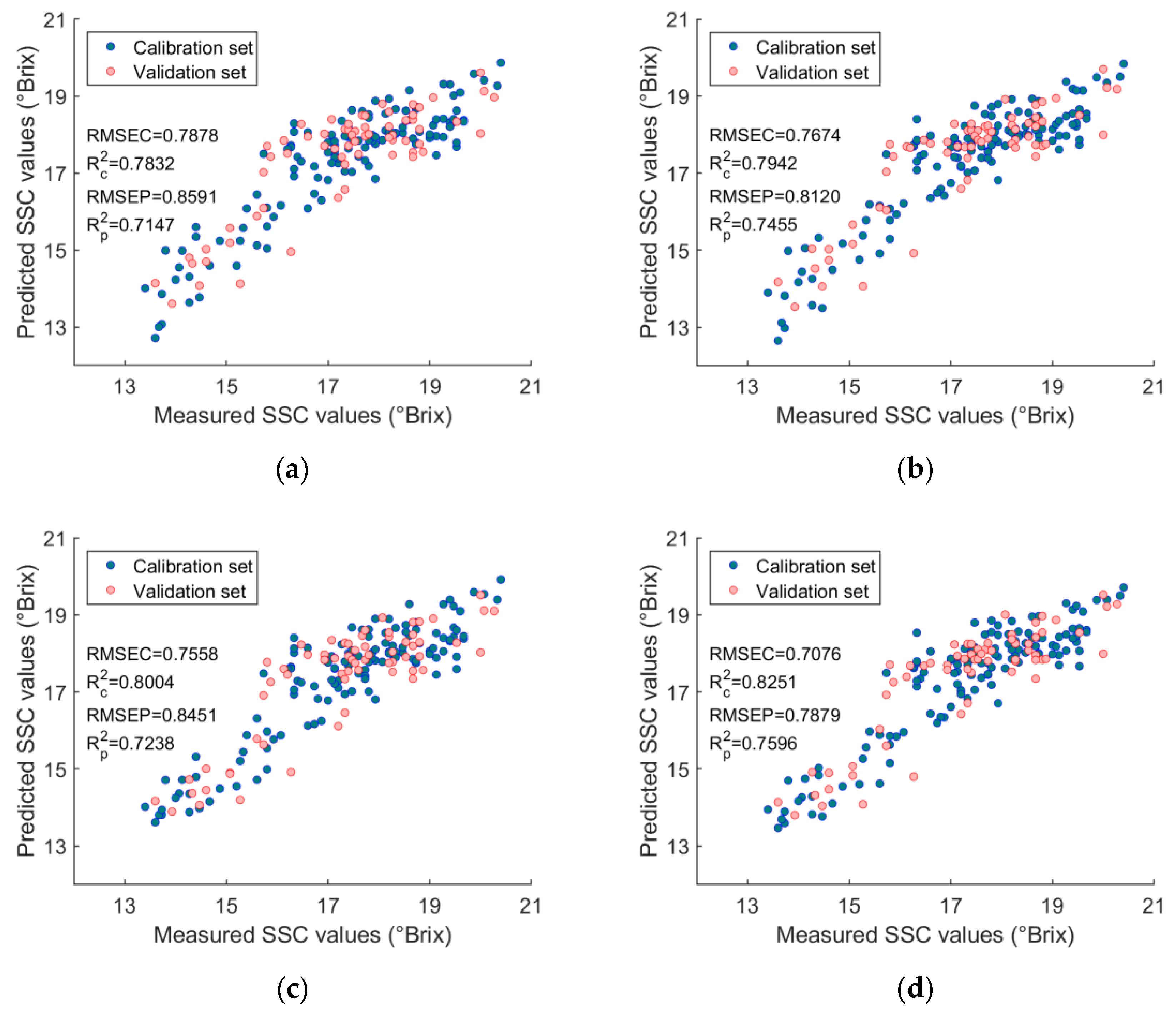
3.4. Quantification Models for Determining SSC Values
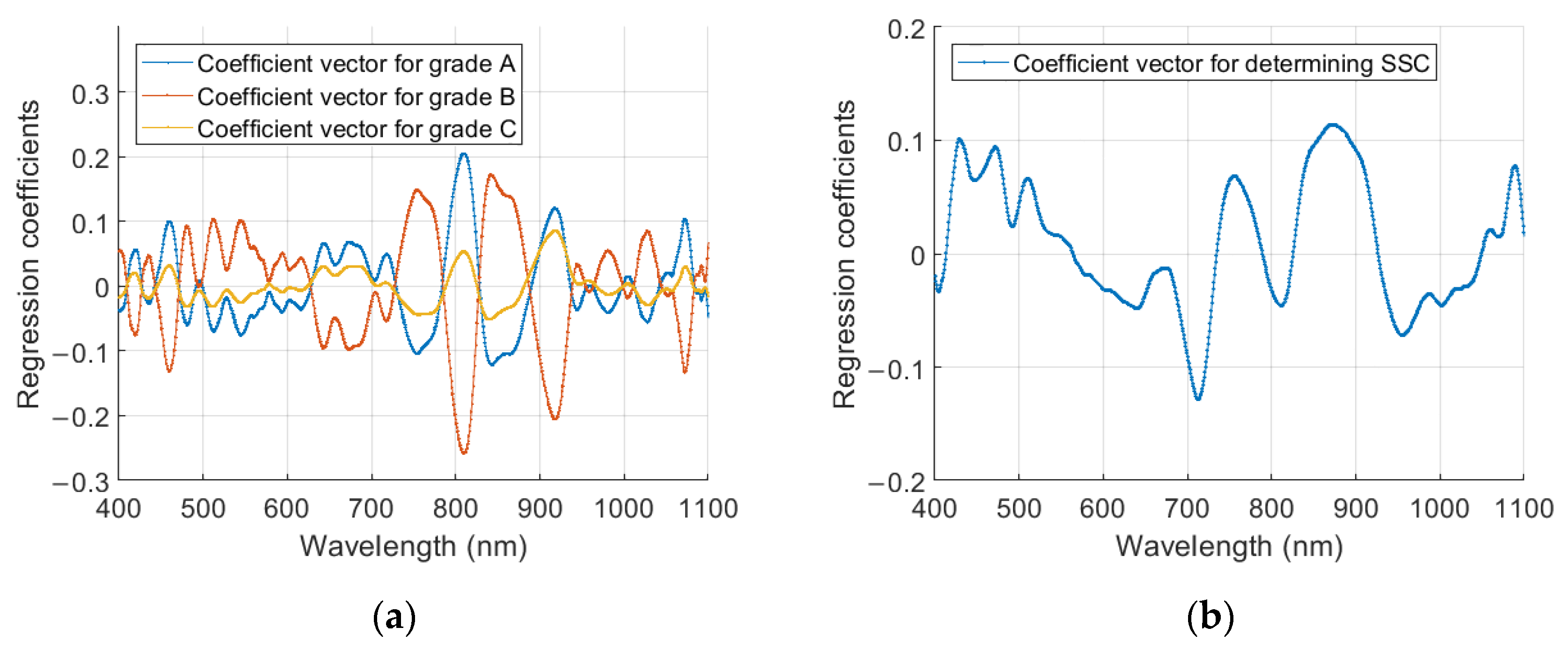
3.5. Characteristic Spectral Variables for Determining Internal Quality of Pineapple Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siti Rashima, R.; Maizura, M.; Wan Nur Hafzan, W.M.; Hazzeman, H. Physicochemical properties and sensory acceptability of pineapples of different varieties and stages of maturity. Food Res. 2019, 3, 491–500. [Google Scholar]
- Mohd, A.M.; Hashim, N.; Abd, A.S.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef]
- Tantinantrakun, A.; Sukwanit, S.; Thompson, A.K.; Teerachaichayut, S. Nondestructive evaluation of SW-NIRS and NIR-HSI for predicting the maturity index of intact pineapples. Postharvest Biol. Technol. 2023, 195, 112141. [Google Scholar] [CrossRef]
- FAO. Major Tropical Fruit Preliminary Results 2020. 2021. Available online: https://www.fao.org/3/cb6196en/cb6196en.pdf (accessed on 15 June 2023).
- FAO. Production Quantities of Pineapples in 2021. 2021. Available online: https://www.fao.org (accessed on 15 June 2023).
- Steingass, C.B.; Grauwet, T.; Carle, R. Influence of harvest maturity and fruit logistics on pineapple (Ananas comosus [L.] Merr.) volatiles assessed by headspace solid phase microextraction and gas chromatography–mass spectrometry (HS-SPME-GC/MS). Food Chem. 2014, 150, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Pu, H.; Sun, D. Innovative nondestructive imaging techniques for ripening and maturity of fruits—A review of recent applications. Trends Food Sci. Technol. 2018, 72, 144–152. [Google Scholar] [CrossRef]
- Li, B.; Lecourt, J.; Bishop, G. Advances in Non-Destructive Early Assessment of Fruit Ripeness towards Defining Optimal Time of Harvest and Yield Prediction—A Review. Plants 2018, 7, 3. [Google Scholar] [CrossRef]
- Chang, C.; Kuan, C.; Tseng, H.; Lee, P.; Tsai, S.; Chen, S. Using deep learning to identify maturity and 3D distance in pineapple fields. Sci. Rep. 2022, 12, 8749. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.M.M.; Ridwani, S.; Putri, S.P.; Fukusaki, E. GC-MS Based Metabolite Profiling to Monitor Ripening-Specific Metabolites in Pineapple (Ananas comosus). Metabolites 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Torri, L.; Sinelli, N.; Limbo, S. Shelf life evaluation of fresh-cut pineapple by using an electronic nose. Postharvest Biol. Technol. 2010, 56, 239–245. [Google Scholar] [CrossRef]
- Kaewapichai, W.; Kaewtrakulpong, P.; Prateepasen, A. A Real-Time Automatic Inspection System for Pattavia Pineapples. Key Eng. Mater. 2006, 321–323, 1186–1191. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. An overview of non-destructive approaches for quality determination in pineapples. J. Agric. Food Eng. 2020, 1, 1–7. [Google Scholar]
- Bakar, B.H.A.; Ishak, A.J.; Shamsuddin, R.; Hassan, W.Z.W. Ripeness level classification for pineapple using RGB and HSI colour maps. J. Theor. Appl. Inf. Technol. 2013, 57, 587–593. [Google Scholar]
- Cuong, N.H.H.; Trinh, T.H.; Meesad, P.; Nguyen, T.T. Improved YOLO object detection algorithm to detect ripe pineapple phase. J. Intell. Fuzzy Syst. 2022, 43, 1365–1381. [Google Scholar] [CrossRef]
- Sornsrivichai, J.; Yantarasri, T.; Kalayanamitra, K. Nondestructive techniques for quality evaluation of pineapple fruits. Acta Hortic. 2000, 529, 337–341. [Google Scholar] [CrossRef]
- Vanoli, M.; Buccheri, M. Overview of the methods for assessing harvest maturity. Stewart Postharvest Rev. 2012, 8, 1–11. [Google Scholar]
- Wang, H.; Peng, J.; Xie, C.; Bao, Y.; He, Y. Fruit Quality Evaluation Using Spectroscopy Technology: A Review. Sensors 2015, 15, 11889–11927. [Google Scholar] [CrossRef]
- Pathaveerat, S.; Terdwongworakul, A.; Phaungsombut, A. Multivariate data analysis for classification of pineapple maturity. J. Food Eng. 2008, 89, 112–118. [Google Scholar] [CrossRef]
- Chia, K.S.; Abdul Rahim, H.; Abdul Rahim, R. Prediction of soluble solids content of pineapple via non-invasive low cost visible and shortwave near infrared spectroscopy and artificial neural network. Biosyst. Eng. 2012, 113, 158–165. [Google Scholar] [CrossRef]
- Rahim, H.A.; Seng, C.K.; Rahim, R.A. Analysis for Soluble Solid Contents in Pineapples using NIR Spectroscopy. J. Teknol. 2014, 8, 7–11. [Google Scholar]
- Amuah, C.L.Y.; Teye, E.; Lamptey, F.P.; Nyandey, K.; Opoku-Ansah, J.; Adueming, P.O. Feasibility Study of the Use of Handheld NIR Spectrometer for Simultaneous Authentication and Quantification of Quality Parameters in Intact Pineapple Fruits. J. Spectrosc. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Khodabakhshian, R.; Emadi, B.; Khojastehpour, M.; Golzarian, M.R. A comparative study of reflectance and transmittance modes of Vis/NIR spectroscopy used in determining internal quality attributes in pomegranate fruits. J. Food Meas. Charact. 2019, 13, 3130–3139. [Google Scholar] [CrossRef]
- Jie, D.; Zhou, W.; Wei, X. Nondestructive detection of maturity of watermelon by spectral characteristic using NIR diffuse transmittance technique. Sci. Hortic. 2019, 257, 108718. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H.; Wang, X.; Ference, C.M.; Liang, X.; Qiu, G. Nondestructive Detection of Internal Flavor in Shatian Pomelo Fruit Based on Visible Near Infrared Spectroscopy. Hortscience 2021, 56, 1325–1330. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, W.; Wang, Q.; Wu, J.; Li, J. Detection of pears with moldy core using online full-transmittance spectroscopy combined with supervised classifier comparison and variable optimization. Comput. Electron. Agric. 2022, 200, 107231. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Zou, C.; Jiang, S.; El-Seedi, H.R.; Zou, X. General model of multi-quality detection for apple from different origins by Vis/NIR transmittance spectroscopy. J. Food Meas. Charact. 2022, 16, 2582–2595. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, H.; Zhang, H.; Zhao, Z.; Yang, Y.; Guo, S.; Wang, W. Online Detection and Classification of Moldy Core Apples by Vis-NIR Transmittance Spectroscopy. Agriculture 2022, 12, 489. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; He, C.; Zhu, S. Postharvest Exogenous Application of Abscisic Acid Reduces Internal Browning in Pineapple. J. Agric. Food Chem. 2015, 63, 5313–5320. [Google Scholar] [CrossRef]
- Xu, S.; Ren, J.; Lu, H.; Wang, X.; Sun, X.; Liang, X. Nondestructive detection and grading of flesh translucency in pineapples with visible and near-infrared spectroscopy. Postharvest Biol. Technol. 2022, 192, 112029. [Google Scholar] [CrossRef]
- Chen, C.; Paull, R.E. Sugar Metabolism and Pineapple Flesh Translucency. J. Am. Soc. Hortic. Sci. 2000, 125, 558–562. [Google Scholar] [CrossRef]
- Shamsudin, R.; Daud, W.R.W.; Takriff, M.S.; Hassan, O. Physicochemical properties of the josapine variety of pineapple fruit. Int. J. Food Eng. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Esteve Agelet, L.; Armstrong, P.R.; Romagosa Clariana, I.; Hurburgh, C.R. Measurement of Single Soybean Seed Attributes by Near-Infrared Technologies. A Comparative Study. J. Agric. Food Chem. 2012, 60, 8314–8322. [Google Scholar] [CrossRef] [PubMed]

| Data Sets | Maturity Grades | Number of Samples | SSC (°Brix) | |||
|---|---|---|---|---|---|---|
| Min | Max | Mean | d SD | |||
| Calibration set | a Grade A | 54 | 15.73 | 19.87 | 17.80 | 0.9826 |
| b Grade B | 42 | 16.47 | 20.40 | 18.51 | 1.0303 | |
| c Grade C | 34 | 13.40 | 17.00 | 15.12 | 1.1033 | |
| Total | 130 | 13.40 | 20.40 | 17.33 | 1.6983 | |
| Validation set | Grade A | 31 | 15.73 | 20.07 | 17.77 | 1.1249 |
| Grade B | 19 | 17.13 | 20.27 | 18.25 | 0.9064 | |
| Grade C | 15 | 13.60 | 17.20 | 15.05 | 0.9529 | |
| Total | 65 | 13.60 | 20.27 | 17.28 | 1.6048 | |
| Models (Parameters) | Prediction of Calibration Set | Prediction of Validation Set | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade A | Grade B | Grade C | Recall (%) | Grade A | Grade B | Grade C | Recall (%) | ||
| Actual maturity | |||||||||
| KNN (K = 5) | a Grade A | 47 | 7 | 0 | 87.0 | 27 | 4 | 0 | 87.1 |
| b Grade B | 9 | 33 | 0 | 78.6 | 3 | 15 | 1 | 78.9 | |
| c Grade C | 0 | 1 | 33 | 97.1 | 0 | 0 | 15 | 100.0 | |
| Precision (%) | 83.9 | 80.5 | 100.0 | 86.9 | 90.0 | 78.9 | 93.8 | 87.7 | |
| PLSDA (d LVs = 7) | Grade A | 47 | 7 | 0 | 87.0 | 29 | 2 | 0 | 93.5 |
| Grade B | 4 | 38 | 0 | 90.5 | 4 | 15 | 0 | 78.9 | |
| Grade C | 0 | 1 | 33 | 97.1 | 0 | 0 | 15 | 100.0 | |
| Precision (%) | 92.2 | 82.6 | 100.0 | 90.8 | 87.9 | 88.2 | 100.0 | 90.8 | |
| SVMDA (gamma = 10−6, cost = 106) | Grade A | 52 | 2 | 0 | 96.3 | 28 | 3 | 0 | 90.3 |
| Grade B | 4 | 38 | 0 | 90.5 | 3 | 15 | 1 | 78.9 | |
| Grade C | 0 | 1 | 33 | 97.1 | 0 | 1 | 14 | 93.3 | |
| Precision (%) | 92.9 | 92.7 | 100.0 | 94.6 | 90.3 | 78.9 | 93.3 | 87.7 | |
| Models | Parameters | RMSEC (°Brix) | RMSECV (°Brix) | RMSEP (°Brix) | RPD | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| PCR | a PCs = 6 | 0.7878 | 0.7832 | 0.8189 | 0.7658 | 0.8591 | 0.7147 | 1.8680 | <0.001 |
| PLSR | b LVs = 5 | 0.7674 | 0.7942 | 0.8107 | 0.7706 | 0.8120 | 0.7455 | 1.9763 | <0.001 |
| c ANN-PCA | PCs = 6, nodes = 5 | 0.7558 | 0.8004 | 0.8168 | 0.7681 | 0.8451 | 0.7238 | 1.8989 | <0.001 |
| d ANN-PLS | LVs = 5, nodes = 4 | 0.7076 | 0.8251 | 0.8093 | 0.7719 | 0.7879 | 0.7596 | 2.0369 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, G.; Lu, H.; Wang, X.; Wang, C.; Xu, S.; Liang, X.; Fan, C. Nondestructive Detecting Maturity of Pineapples Based on Visible and Near-Infrared Transmittance Spectroscopy Coupled with Machine Learning Methodologies. Horticulturae 2023, 9, 889. https://doi.org/10.3390/horticulturae9080889
Qiu G, Lu H, Wang X, Wang C, Xu S, Liang X, Fan C. Nondestructive Detecting Maturity of Pineapples Based on Visible and Near-Infrared Transmittance Spectroscopy Coupled with Machine Learning Methodologies. Horticulturae. 2023; 9(8):889. https://doi.org/10.3390/horticulturae9080889
Chicago/Turabian StyleQiu, Guangjun, Huazhong Lu, Xu Wang, Chen Wang, Sai Xu, Xin Liang, and Changxiang Fan. 2023. "Nondestructive Detecting Maturity of Pineapples Based on Visible and Near-Infrared Transmittance Spectroscopy Coupled with Machine Learning Methodologies" Horticulturae 9, no. 8: 889. https://doi.org/10.3390/horticulturae9080889
APA StyleQiu, G., Lu, H., Wang, X., Wang, C., Xu, S., Liang, X., & Fan, C. (2023). Nondestructive Detecting Maturity of Pineapples Based on Visible and Near-Infrared Transmittance Spectroscopy Coupled with Machine Learning Methodologies. Horticulturae, 9(8), 889. https://doi.org/10.3390/horticulturae9080889





