Detecting Surface Defects of Achacha Fruit (Garcinia humilis) with Hyperspectral Images
Abstract
1. Introduction
2. Materials and Methods
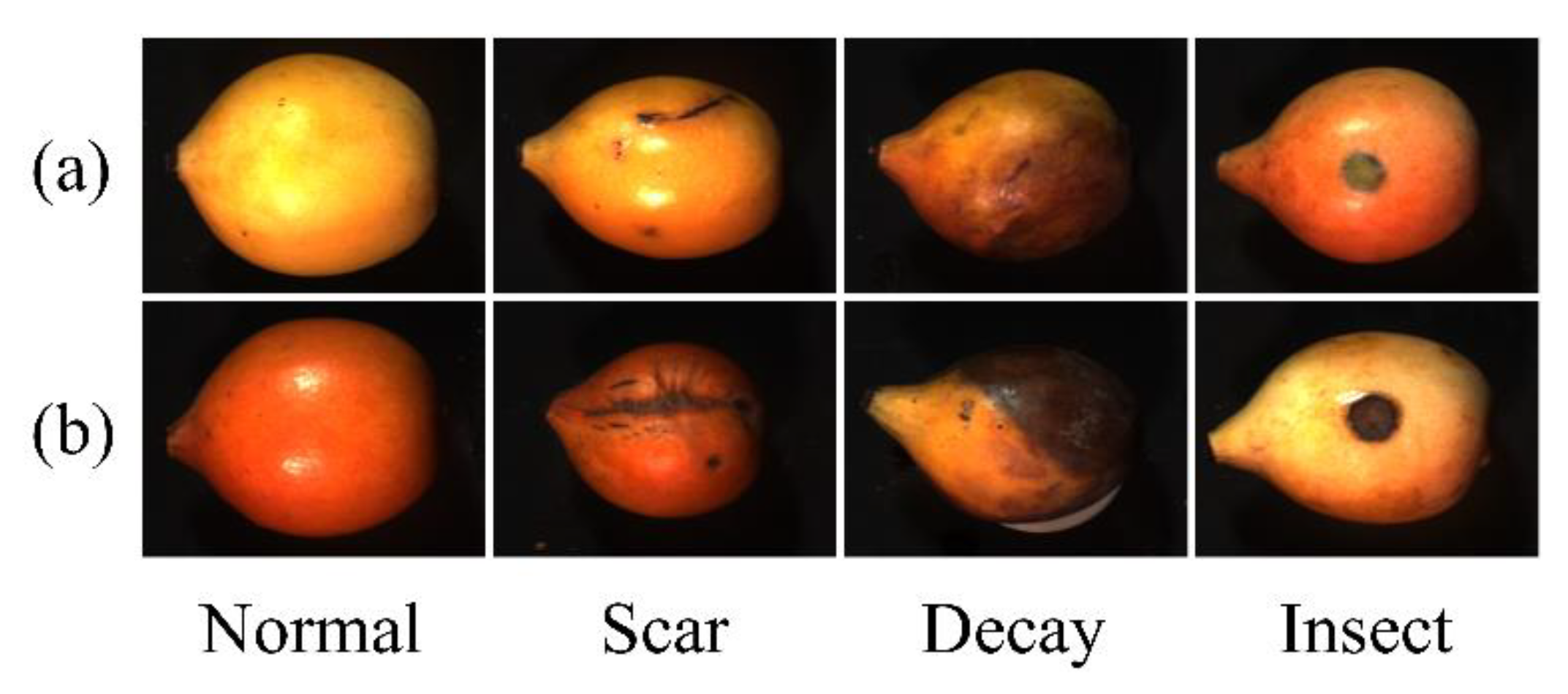
2.1. Achacha Samples and HSI-Data Acquisition
2.2. Defect Classification Methods with HSI Data
2.3. Data Reduction Using Principal Component Analysis
2.4. Stem End Detection
3. Results and Discussion
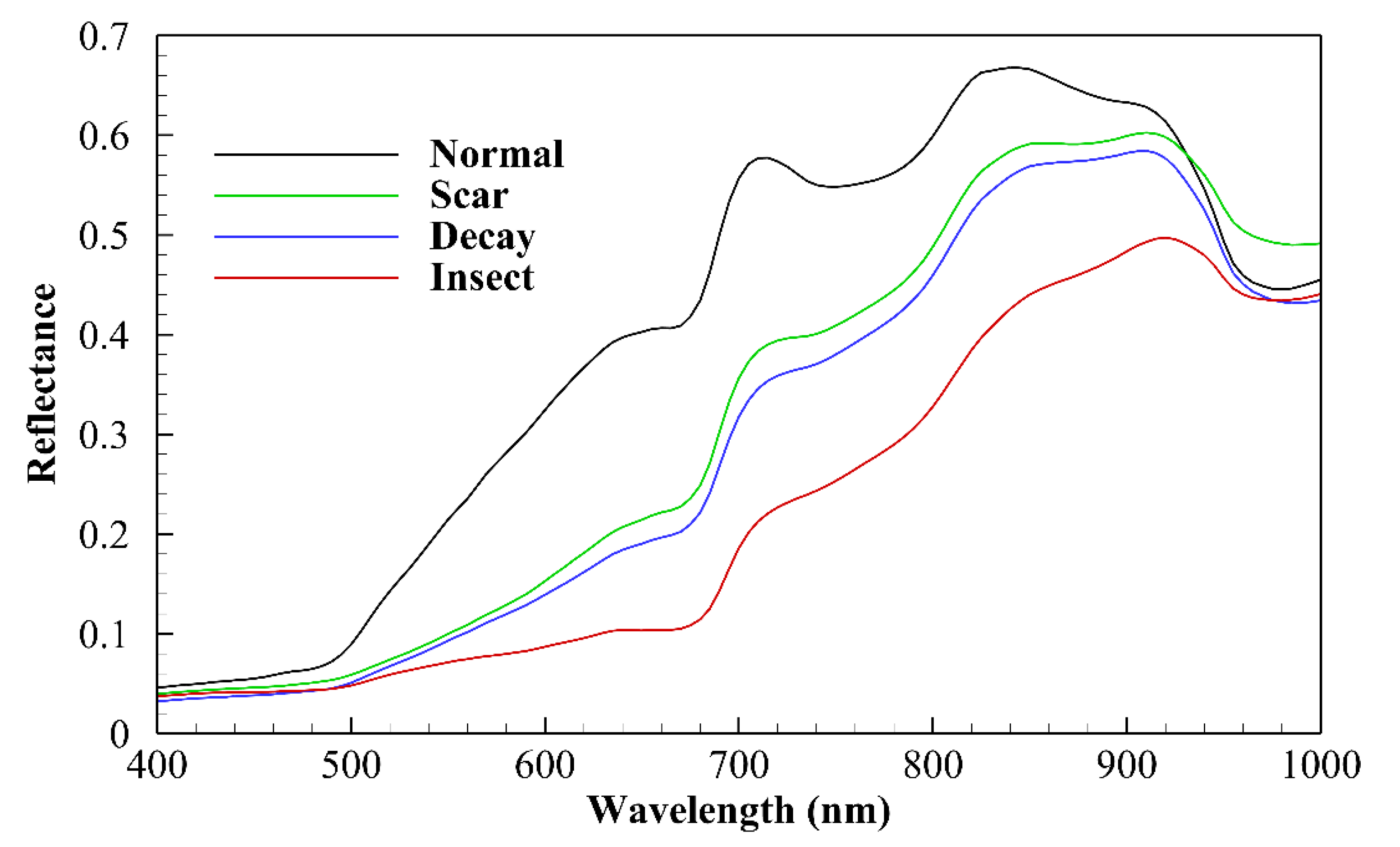
3.1. Spectral Characteristics
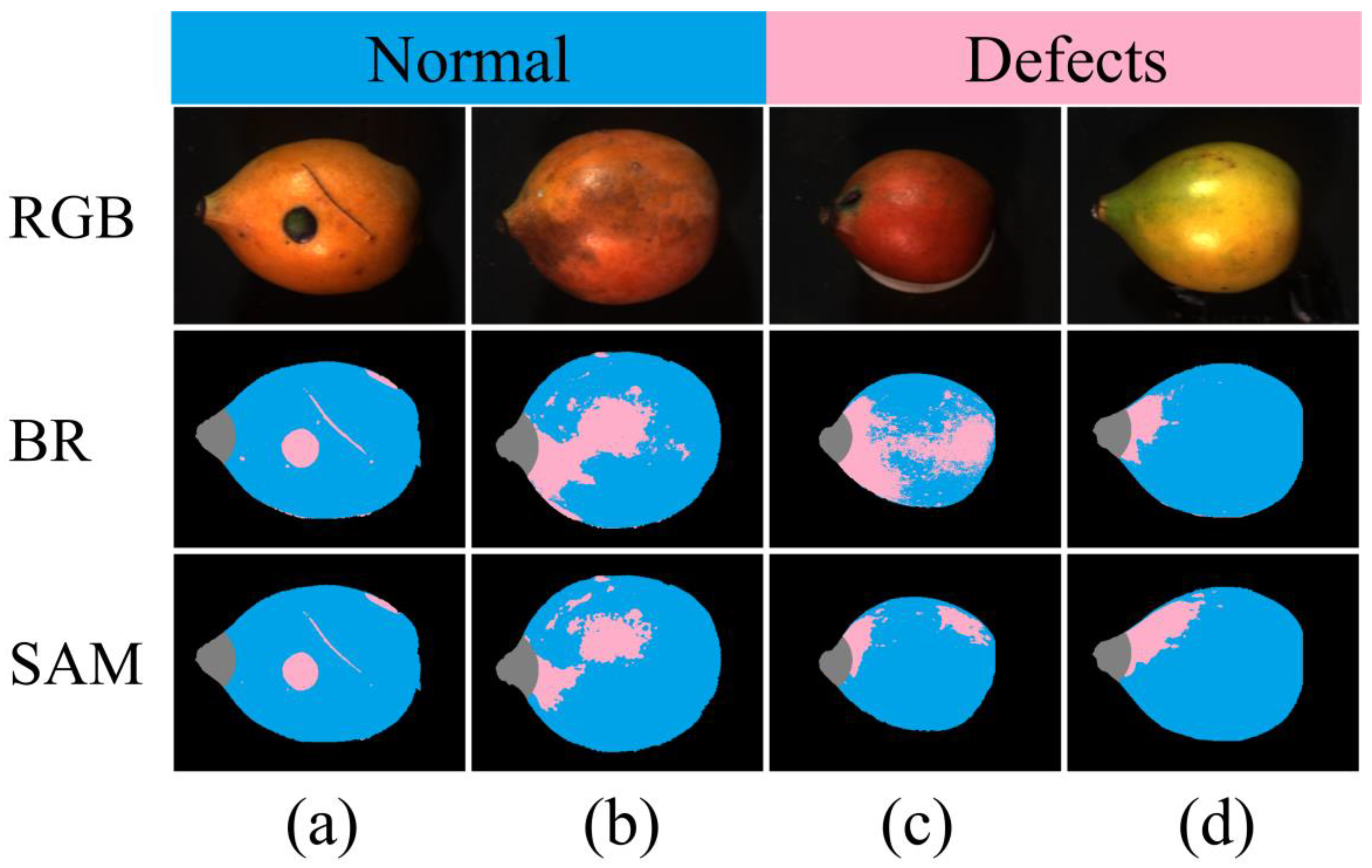
3.2. The BR and SAM Algorithms for Binary Classification
3.3. The SAM and ML Models for Multiple-Class Classification
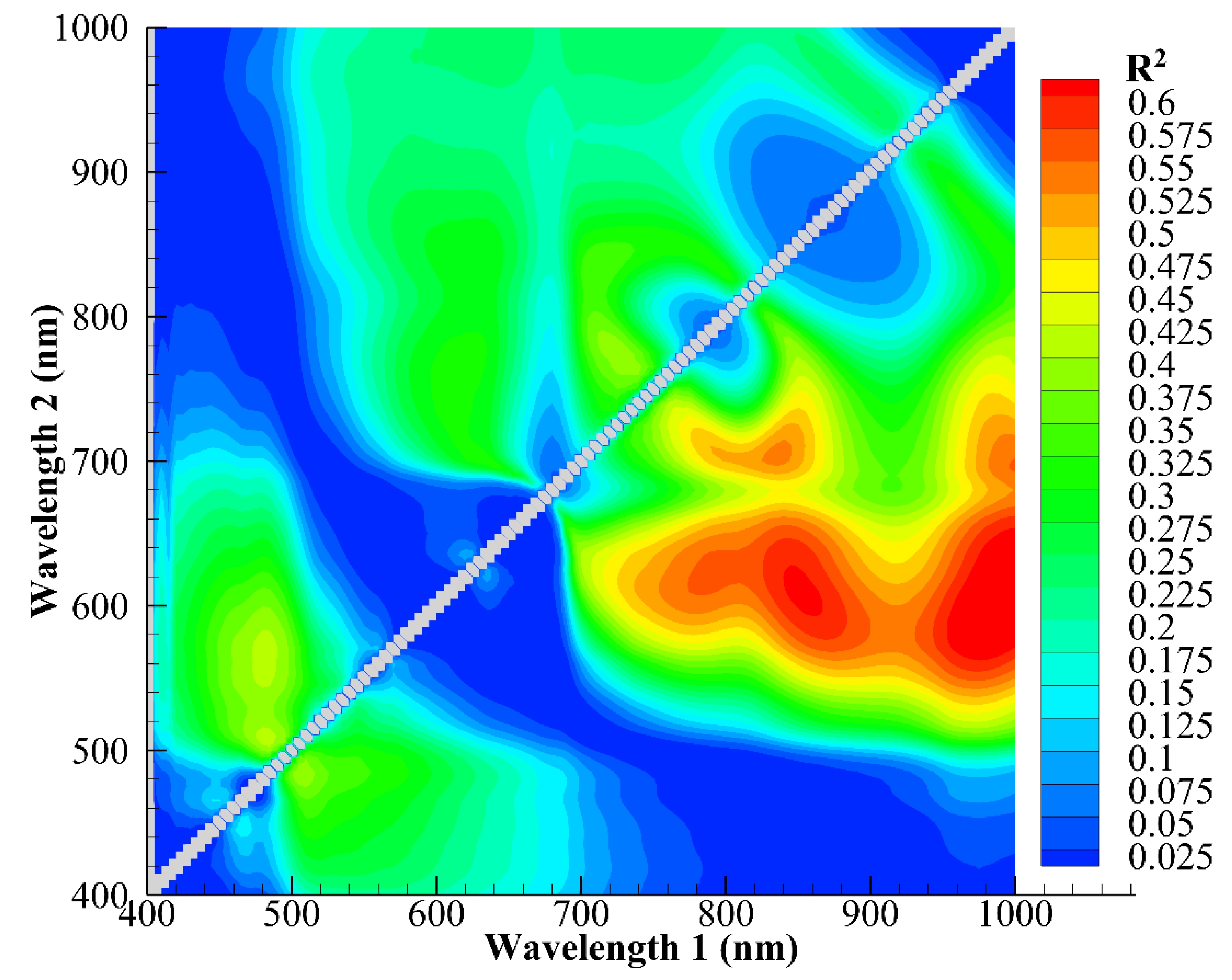
3.4. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nunes, R.; Broering, M.F.; De Faveri, R.; Goldoni, F.C.; Mariano, L.N.B.; Mafessoli, P.C.M.; Delle Monache, F.; Cechinel, V.; Niero, R.; Santin, J.R.; et al. Effect of the metanolic extract from the leaves of Garcinia humilis Vahl (Clusiaceae) on acute inflammation. Inflammopharmacology 2021, 29, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.L.J.; Santos, N.C.; Alves, I.L.; Andre, A.M.M.C.N. Evaluation of thermodynamic properties and antioxidant activities of Achachairu (Garcinia humilis) peels under drying process. Flavour. Frag. J. 2021, 36, 213–222. [Google Scholar] [CrossRef]
- Wang, N.N.; Sun, D.W.; Yang, Y.C.; Pu, H.B.; Zhu, Z.W. Recent Advances in the Application of Hyperspectral Imaging for Evaluating Fruit Quality. Food Anal. Method 2016, 9, 178–191. [Google Scholar] [CrossRef]
- Cubero, S.; Lee, W.S.; Aleixos, N.; Albert, F.; Blasco, J. Automated systems based on machine vision for inspecting citrus fruits from the field to postharvest—A review. Food Bioprocess. Technol. 2016, 9, 1623–1639. [Google Scholar] [CrossRef]
- Elmasry, G.; Kamruzzaman, M.; Sun, D.W.; Allen, P. Principles and Applications of Hyperspectral Imaging in Quality Evaluation of Agro-Food Products: A Review. Crit. Rev. Food Sci. 2012, 52, 999–1023. [Google Scholar] [CrossRef]
- Patel, K.K.; Kar, A.; Jha, S.N.; Khan, M.A. Machine vision system: A tool for quality inspection of food and agricultural products. J. Food Sci. Technol. 2012, 49, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.Z.; Figueroa, H.E.H.; Fracarolli, J.A. Computer vision based detection of external defects on tomatoes using deep learning. Biosyst. Eng. 2020, 190, 131–144. [Google Scholar] [CrossRef]
- Khoje, S.; Bodhe, S. Comparative performance evaluation of fast discrete curvelet transform and colour texture moments as texture features for fruit skin damage detection. J. Food Sci. Technol. 2015, 52, 6914–6926. [Google Scholar] [CrossRef]
- Lorente, D.; Aleixos, N.; Gomez-Sanchis, J.; Cubero, S.; Garcia-Navarrete, O.L.; Blasco, J. Recent Advances and Applications of Hyperspectral Imaging for Fruit and Vegetable Quality Assessment. Food Bioprocess. Technol. 2012, 5, 1121–1142. [Google Scholar] [CrossRef]
- Blasco, J.; Aleixos, N.; Molto, E. Computer vision detection of peel defects in citrus by means of a region oriented segmentation algorithm. J. Food Eng. 2007, 81, 535–543. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Zhuang, Z.L.; Liu, Y.; Liu, Y.; Zhang, X. Defect Classification of Green Plums Based on Deep Learning. Sensors 2020, 20, 6993. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.K.; Husin, Z.; Yasruddin, M.L.; Ismail, M.A.H. Recent technology for food and beverage quality assessment: A review. J. Food Sci. Technol. 2023, 60, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Jiang, J.; Cui, X.; Yuan, D. Identification of fungi-contaminated peanuts using hyperspectral imaging technology and joint sparse representation model. J. Food Sci. Technol. 2019, 56, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Davur, Y.J.; Kämper, W.; Khoshelham, K.; Trueman, S.J.; Bai, S.H. Estimating the ripeness of Hass avocado fruit using deep learning with hyperspectral imaging. Horticulturae 2023, 9, 599. [Google Scholar] [CrossRef]
- Hasanzadeh, B.; Abbaspour-Gilandeh, Y.; Soltani-Nazarloo, A.; Hernández-Hernández, M.; Gallardo-Bernal, I.; Hernández-Hernández, J.L. Non-Destructive Detection of Fruit Quality Parameters Using Hyperspectral Imaging, Multiple Regression Analysis and Artificial Intelligence. Horticulturae 2022, 8, 598. [Google Scholar] [CrossRef]
- Liu, G.S.; He, J.G.; Wang, S.L.; Luo, Y.; Wang, W.; Wu, L.G.; Si, Z.H.; He, X.G. Application of Near-Infrared Hyperspectral Imaging for Detection of External Insect Infestations on Jujube Fruit. Int. J. Food Prop. 2016, 19, 41–52. [Google Scholar] [CrossRef]
- Li, J.B.; Chen, L.P.; Huang, W.Q.; Wang, Q.Y.; Zhang, B.H.; Tian, X.; Fan, S.X.; Li, B. Multispectral detection of skin defects of bi-colored peaches based on vis-NIR hyperspectral imaging. Postharvest Biol. Technol. 2016, 112, 121–133. [Google Scholar] [CrossRef]
- Zhang, H.L.; Chen, Y.; Liu, X.M.; Huang, Y.F.; Zhan, B.S.; Luo, W. Identification of Common Skin Defects and Classification of Early Decayed Citrus Using Hyperspectral Imaging Technique. Food Anal. Method 2021, 14, 1176–1193. [Google Scholar] [CrossRef]
- Hernández, I.; Gutiérrez, S.; Ceballos, S.; Iñíguez, R.; Barrio, I.; Tardaguila, J. Artificial intelligence and novel sensing technologies for assessing downy mildew in grapevine. Horticulturae 2021, 7, 103. [Google Scholar] [CrossRef]
- Shurygin, B.; Smirnov, I.; Chilikin, A.; Khort, D.; Kutyrev, A.; Zhukovskaya, S.; Solovchenko, A. Mutual Augmentation of Spectral Sensing and Machine Learning for Non-Invasive Detection of Apple Fruit Damages. Horticulturae 2022, 8, 1111. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Dong, W.; Luo, W.; Huang, Y.; Zhan, B.; Liu, X. Detection of common defects on mandarins by using visible and near infrared hyperspectral imaging. Infrared Phys. Technol. 2020, 108, 103341. [Google Scholar] [CrossRef]
- Kim, D.W.; Burks, T.F.; Ritenour, M.A.; Qin, J.W. Citrus black spot detection using hyperspectral imaging. Int. J. Agric. Biol. Eng. 2014, 7, 20–27. [Google Scholar] [CrossRef]
- Pan, T.T.; Chyngyz, E.; Sun, D.W.; Paliwal, J.; Pu, H.B. Pathogenetic process monitoring and early detection of pear black spot disease caused by Alternaria alternata using hyperspectral imaging. Postharvest Biol. Technol. 2019, 154, 96–104. [Google Scholar] [CrossRef]
- ElMasry, G.; Wang, N.; Vigneault, C. Detecting chilling injury in Red Delicious apple using hyperspectral imaging and neural networks. Postharvest Biol. Technol. 2009, 52, 1–8. [Google Scholar] [CrossRef]
- Vetrekar, N.; Gad, R.S.; Fernandes, I.; Parab, J.S.; Desai, A.R.; Pawar, J.D.; Naik, G.M.; Umapathy, S. Non-invasive hyperspectral imaging approach for fruit quality control application and classification: Case study of apple, chikoo, guava fruits. J. Food Sci. Technol. 2015, 52, 6978–6989. [Google Scholar] [CrossRef]
- Wu, L.G.; He, J.G.; Liu, G.S.; Wang, S.L.; He, X.G. Detection of common defects on jujube using Vis-NIR and NIR hyperspectral imaging. Postharvest Biol. Tecnol. 2016, 112, 134–142. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.S.; Jeong, D.; Delwiche, S.R.; Chao, K.; Cho, B.K. Detection of Cracks on Tomatoes Using a Hyperspectral Near-Infrared Reflectance Imaging System. Sensors 2014, 14, 18837–18850. [Google Scholar] [CrossRef]
- Nguyen, N.M.T.; Liou, N.-S. Ripeness Evaluation of Achacha Fruit Using Hyperspectral Image Data. Agriculture 2022, 12, 2145. [Google Scholar] [CrossRef]
- Lee, K.; Kang, S.; Delwiche, S.R.; Kim, M.S.; Noh, S. Correlation analysis of hyperspectral imagery for multispectral wavelength selection for detection of defects on apples. Sens. Instrum. Food Qual. Saf. 2008, 2, 90–96. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, C.T.; Liu, F.; Kong, W.W.; He, Y.; Lou, B.G. Hyperspectral imaging analysis for ripeness evaluation of strawberry with support vector machine. J. Food Eng. 2016, 179, 11–18. [Google Scholar] [CrossRef]
- Zhang, B.H.; Huang, W.Q.; Gong, L.; Li, J.B.; Zhao, C.J.; Liu, C.L.; Huang, D.F. Computer vision detection of defective apples using automatic lightness correction and weighted RVM classifier. J. Food Eng. 2015, 146, 143–151. [Google Scholar] [CrossRef]
- Fan, S.X.; Li, J.B.; Zhang, Y.H.; Tian, X.; Wang, Q.Y.; He, X.; Zhang, C.; Huang, W.Q. On line detection of defective apples using computer vision system combined with deep learning methods. J. Food Eng. 2020, 286, 110102. [Google Scholar] [CrossRef]
- Wang, H.T.; Hu, R.; Zhang, M.Y.; Zhai, Z.Q.; Zhang, R.Y. Identification of tomatoes with early decay using visible and near infrared hyperspectral imaging and image-spectrum merging technique. J. Food Process Eng. 2021, 44, e13654. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Zubik, M.; Mazurek, W.; Sosnowska, B. Detection of fungal infections in strawberry fruit by VNIR/SWIR hyperspectral imaging. Postharvest Biol. Technol. 2018, 139, 115–126. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Zubik, M.; Mazurek, W. Detection of pits in fresh and frozen cherries using a hyperspectral system in transmittance mode. J. Food Eng. 2017, 215, 61–71. [Google Scholar] [CrossRef]
- Zhang, A.; Lipton, Z.C.; Li, M.; Smola, A.J. Dive into deep learning. arXiv 2021, arXiv:2106.11342. [Google Scholar]
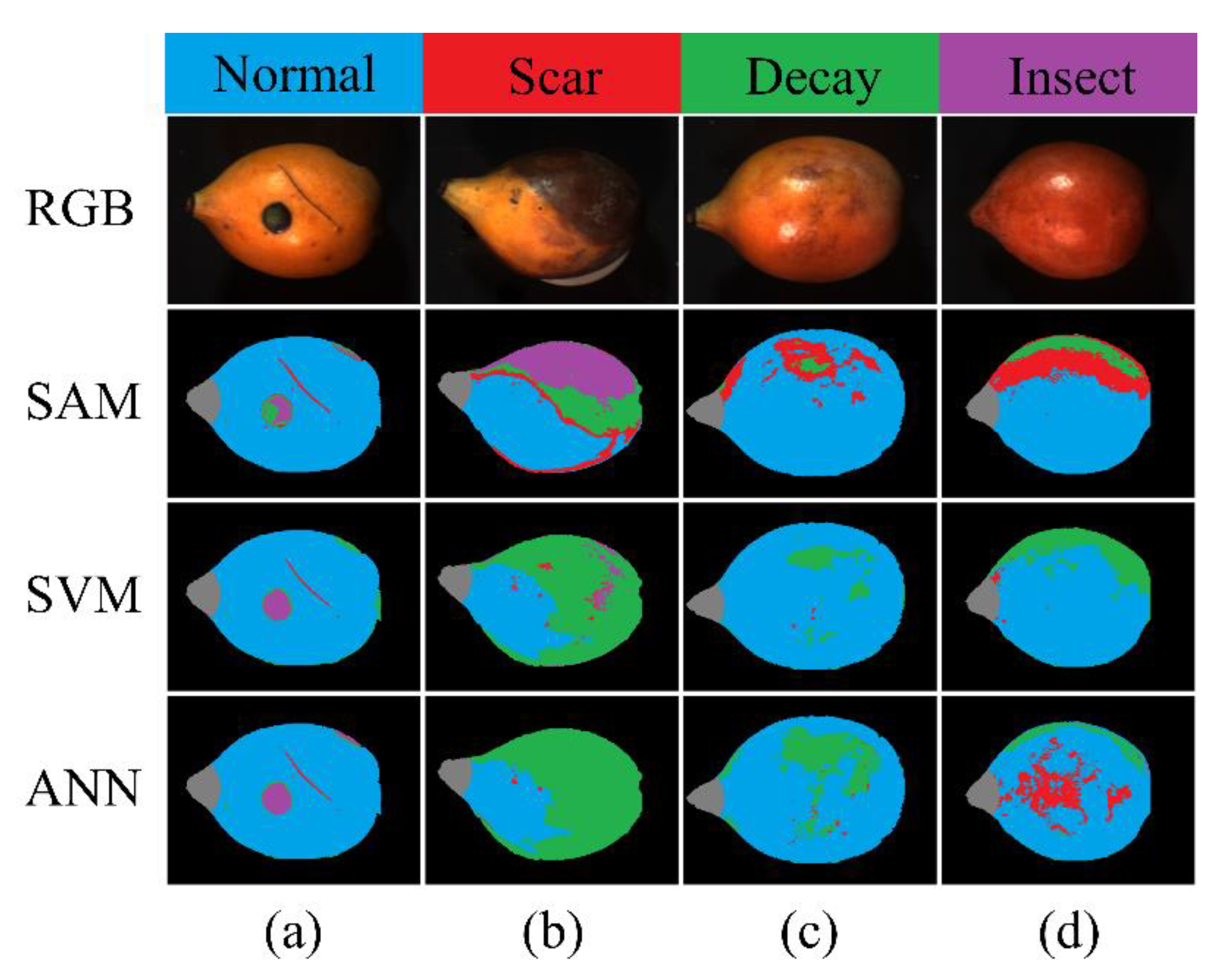
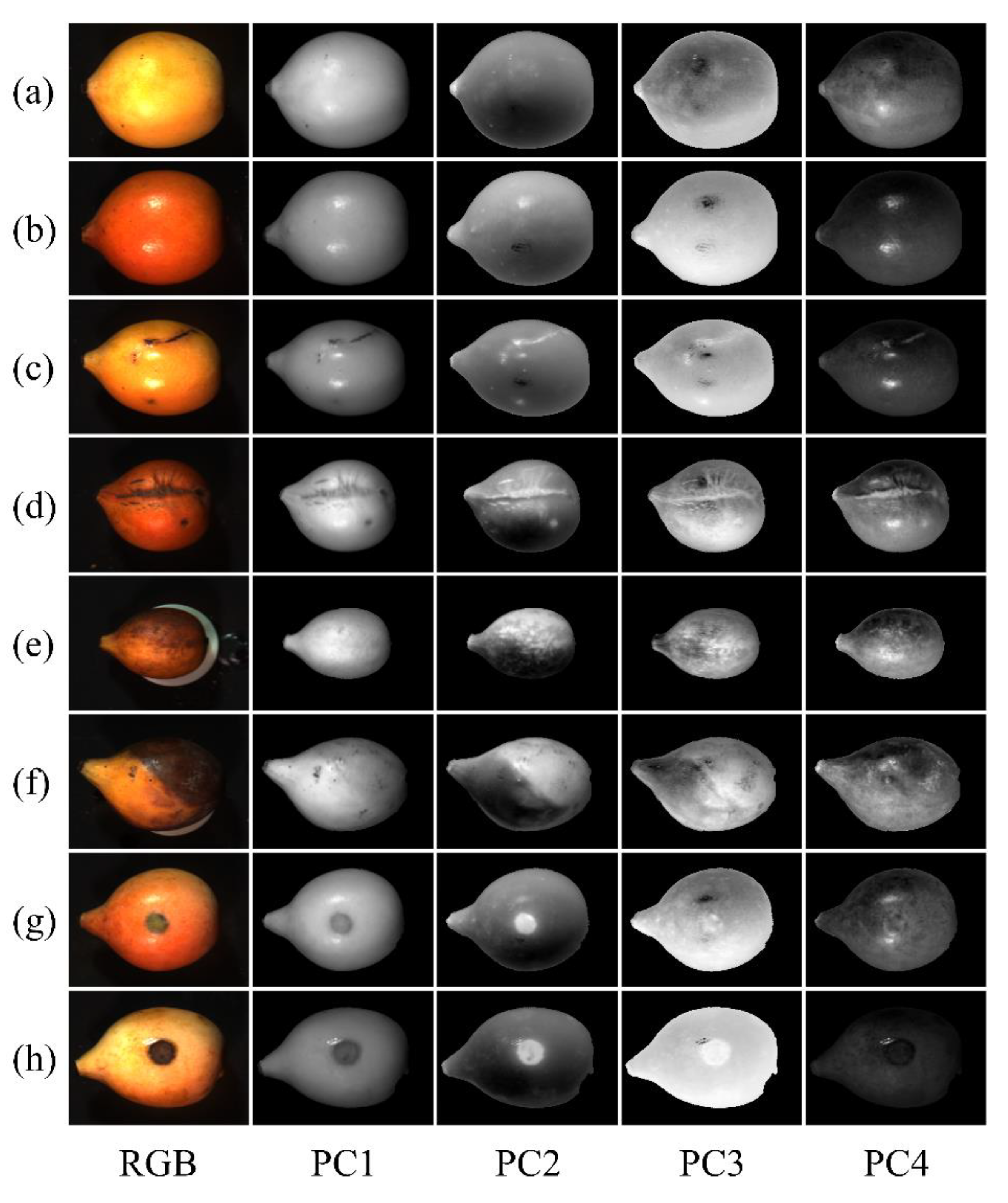
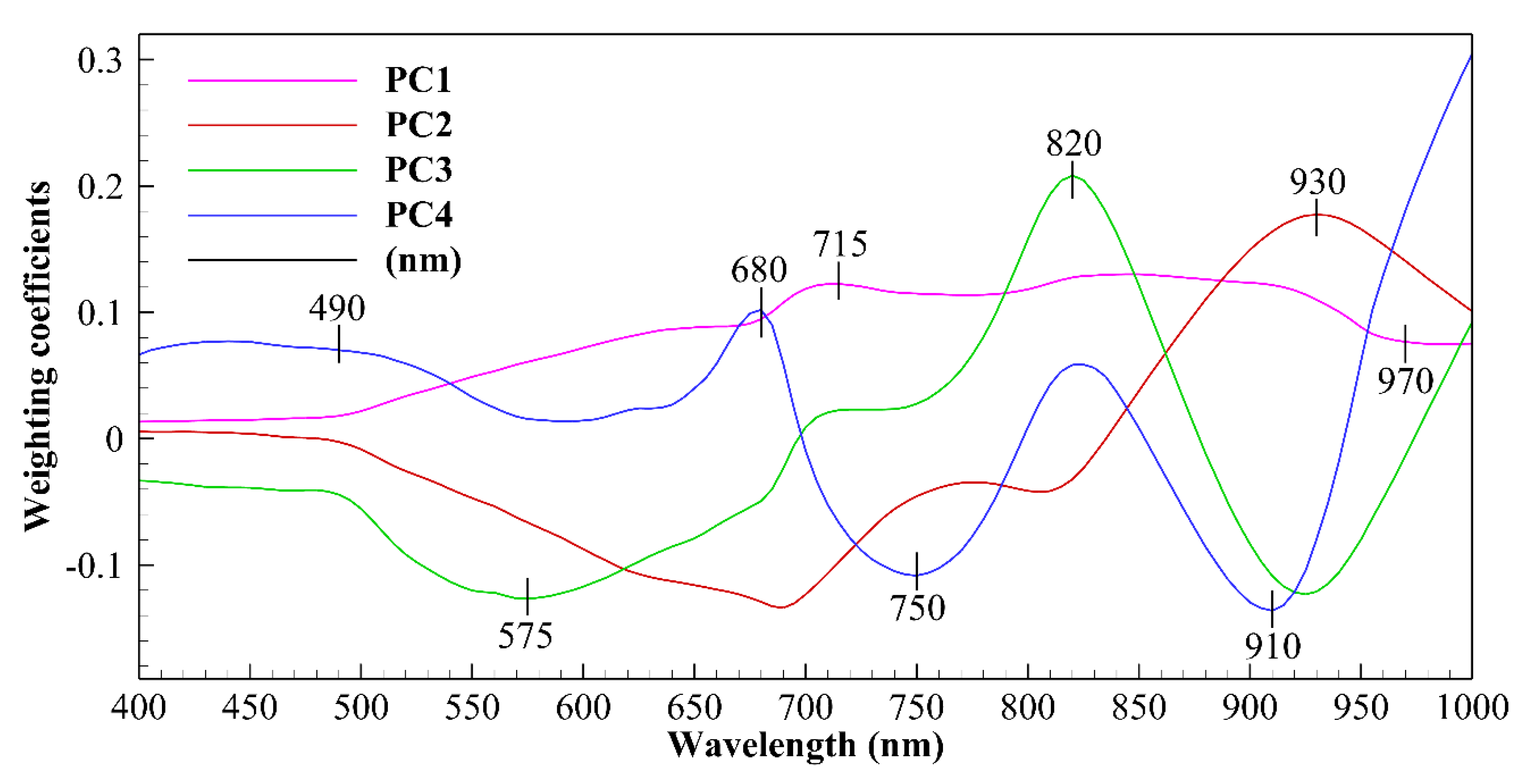
| Model | Actual | Classified as (%) | Accuracy | |||
|---|---|---|---|---|---|---|
| Normal | Scar | Decay | Insect | |||
| SAM | Normal | 85.2 | 11.3 | 3.4 | 0.1 | 58.36% |
| Scar | 6.2 | 36.1 | 17.8 | 39.9 | ||
| Decay | 13.4 | 23.9 | 39.5 | 23.2 | ||
| Insect | 2.5 | 10.4 | 14.5 | 72.6 | ||
| SVM | Normal | 92.8 | 3.2 | 4.0 | 0 | 83.59% |
| Scar | 1.6 | 78.5 | 7.8 | 12.0 | ||
| Decay | 4.8 | 11.7 | 73.7 | 9.9 | ||
| Insect | 0.2 | 4.5 | 6.0 | 89.3 | ||
| ANN | Normal | 99.9 | 0.1 | 0 | 0 | 99.88% |
| Scar | 0 | 99.9 | 0.1 | 0.1 | ||
| Decay | 0.1 | 0.1 | 99.8 | 0 | ||
| Insect | 0 | 0 | 0.1 | 100.0 | ||
| Model | Actual | Classified as (%) | Accuracy | |||
|---|---|---|---|---|---|---|
| Normal | Scar | Decay | Insect | |||
| SAM | Normal | 76.8 | 18.9 | 3.7 | 0.6 | 51.49% |
| Scar | 10.6 | 28.1 | 16.8 | 44.5 | ||
| Decay | 18.3 | 19.7 | 30.2 | 31.8 | ||
| Insect | 3.7 | 8.3 | 17.2 | 70.9 | ||
| SVM | Normal | 90.8 | 5.0 | 4.0 | 0.1 | 80.76% |
| Scar | 1.1 | 74.2 | 9.8 | 14.8 | ||
| Decay | 4.5 | 14.1 | 69.4 | 12.0 | ||
| Insect | 0.5 | 3.5 | 7.4 | 88.5 | ||
| ANN | Normal | 98.7 | 0.8 | 0.4 | 0.1 | 96.85% |
| Scar | 0.1 | 96.5 | 0.9 | 2.5 | ||
| Decay | 0.7 | 4.0 | 93.8 | 1.5 | ||
| Insect | 0 | 1.1 | 0.5 | 98.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.M.T.; Liou, N.-S. Detecting Surface Defects of Achacha Fruit (Garcinia humilis) with Hyperspectral Images. Horticulturae 2023, 9, 869. https://doi.org/10.3390/horticulturae9080869
Nguyen NMT, Liou N-S. Detecting Surface Defects of Achacha Fruit (Garcinia humilis) with Hyperspectral Images. Horticulturae. 2023; 9(8):869. https://doi.org/10.3390/horticulturae9080869
Chicago/Turabian StyleNguyen, Ngo Minh Tri, and Nai-Shang Liou. 2023. "Detecting Surface Defects of Achacha Fruit (Garcinia humilis) with Hyperspectral Images" Horticulturae 9, no. 8: 869. https://doi.org/10.3390/horticulturae9080869
APA StyleNguyen, N. M. T., & Liou, N.-S. (2023). Detecting Surface Defects of Achacha Fruit (Garcinia humilis) with Hyperspectral Images. Horticulturae, 9(8), 869. https://doi.org/10.3390/horticulturae9080869





