Distribution of Indolic Glucosinolates in Different Developmental Stages and Tissues of 13 Varieties of Cabbage (Brassica oleracea L. var. capitata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Investigation of Biological Traits
2.3. Chemicals and Reagents
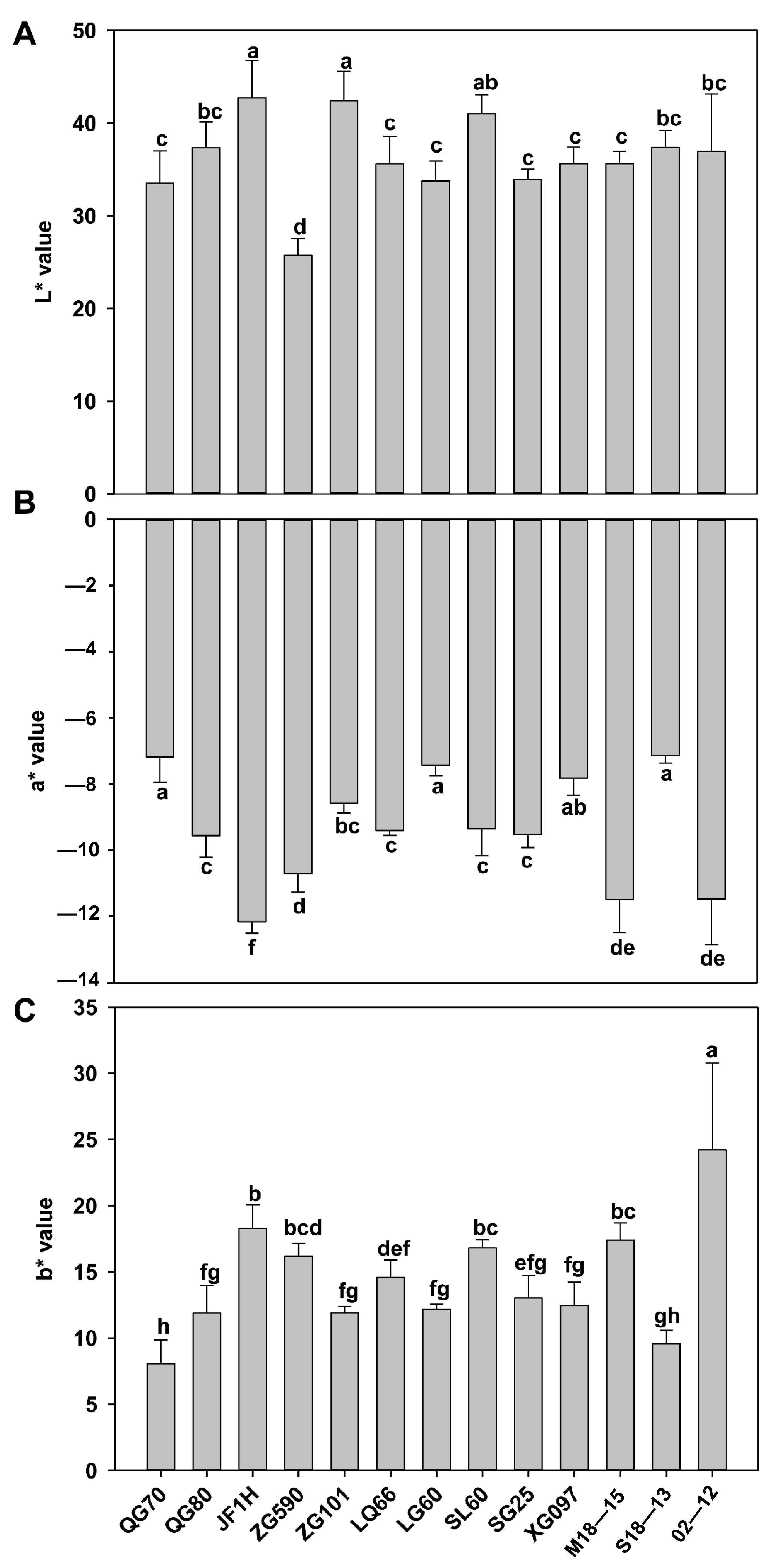
2.4. Color Difference Measurement
2.5. Determination of Chlorophyll, Carotenoid, Total Phenolic, and Flavonoids Contents
2.5.1. Determination of Chlorophyll and Carotenoid Contents
2.5.2. Determination of Total Phenolic and Flavonoid Contents
2.6. Analysis of GLS
2.7. Statistical Analysis
3. Results
3.1. Investigation on Biological Traits of 13 Cabbage Varieties
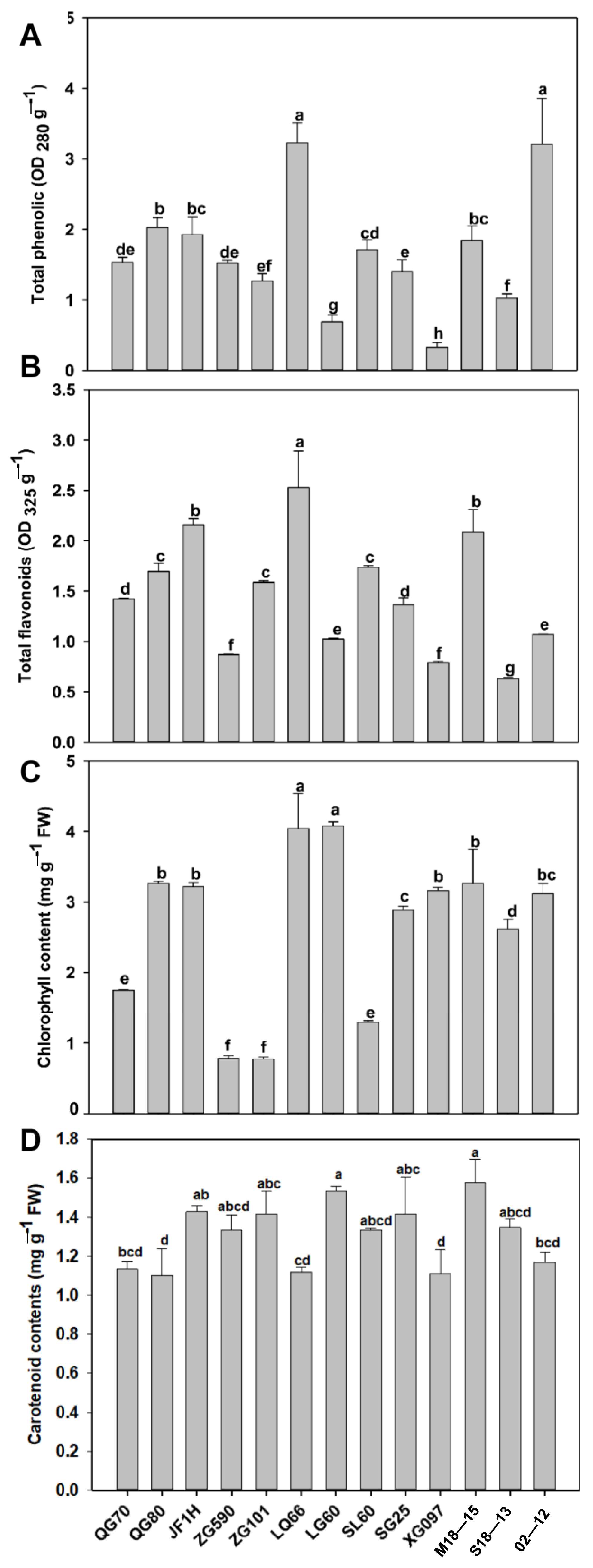
3.2. Comparison of Total Phenolic and Pigment (Flavonoid, Chlorophyll, Carotenoid) Content of 13 Cabbage Varieties
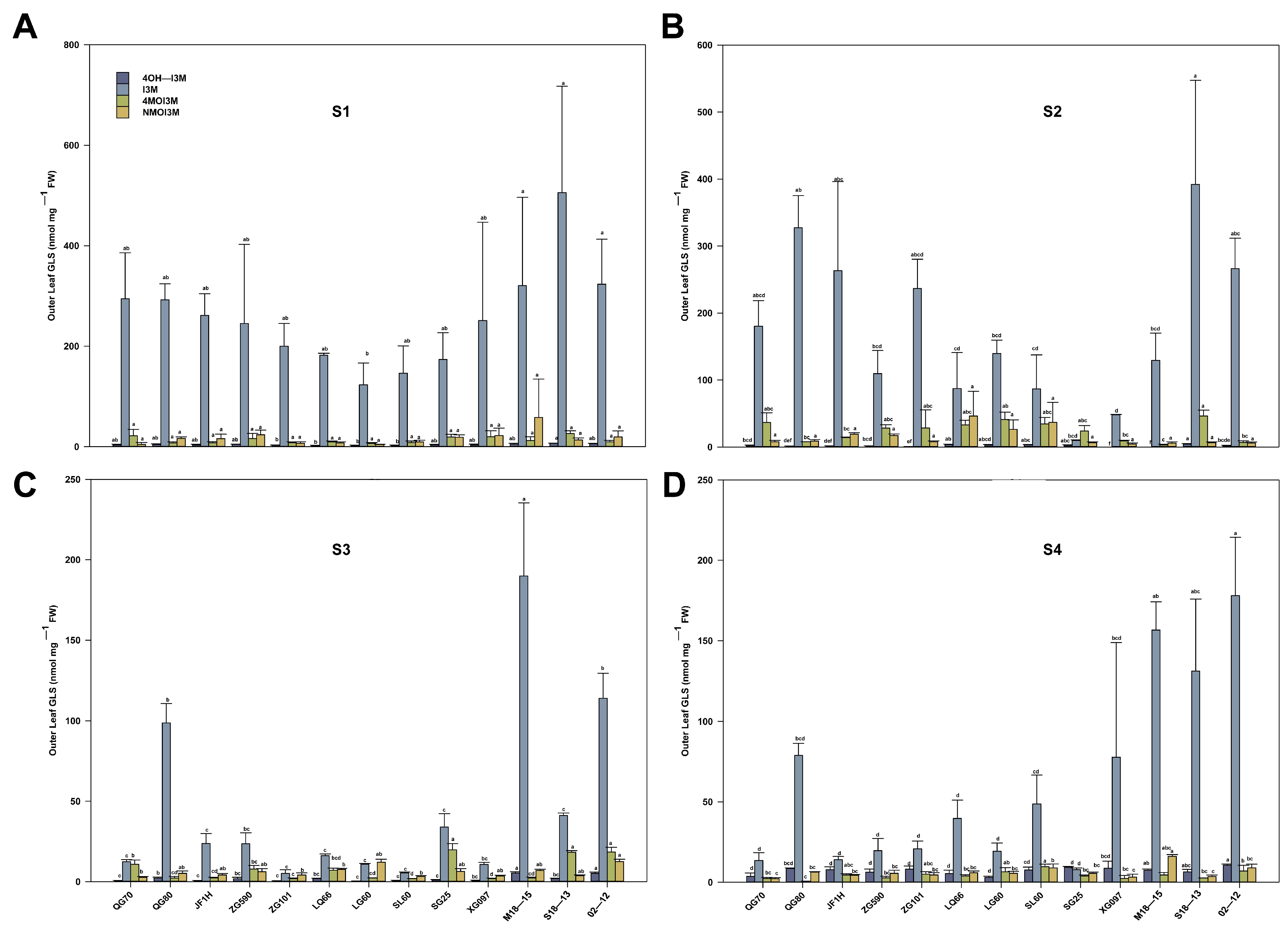
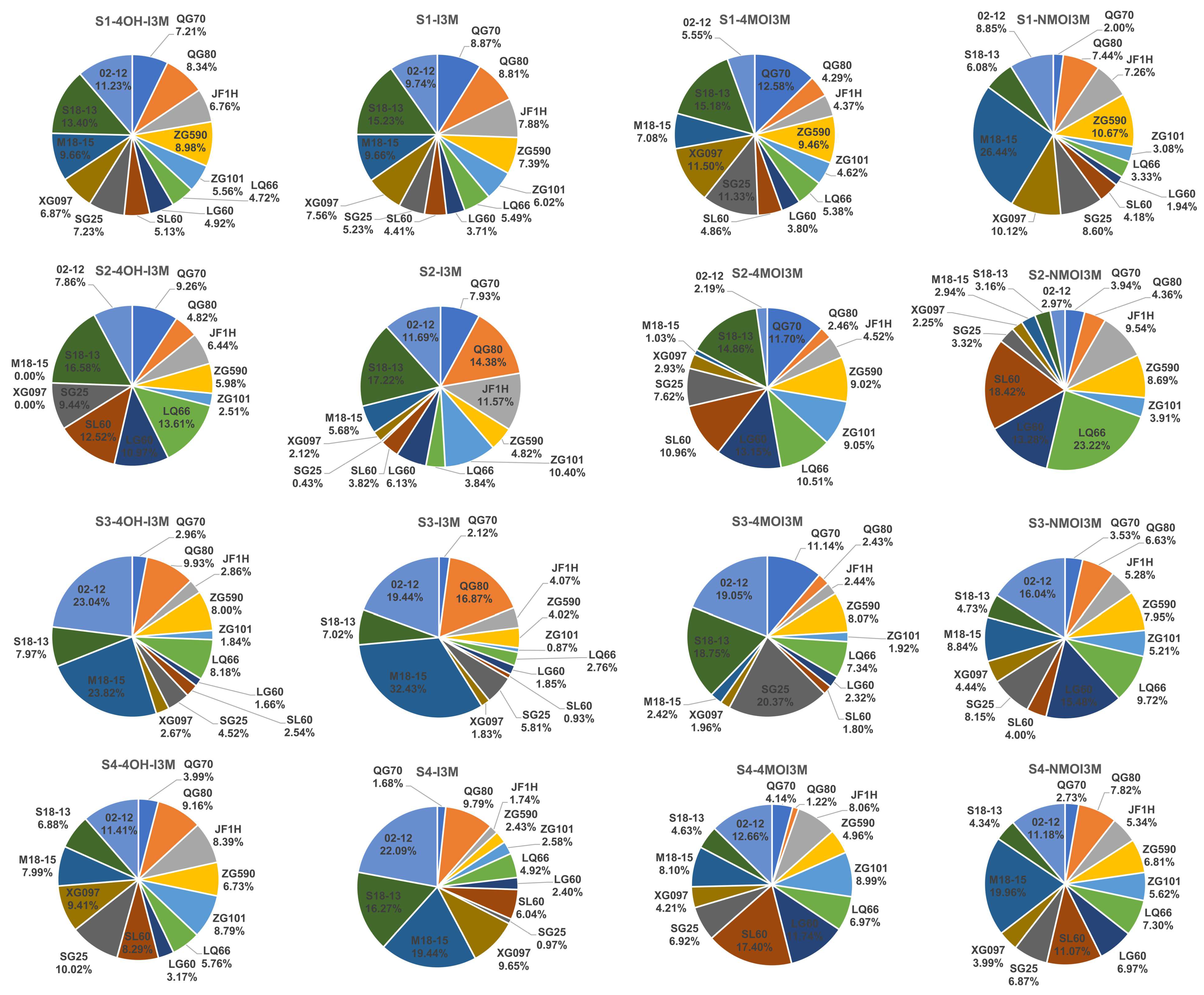
3.3. Variation of GLS Content and Components in Different Developmental Stages
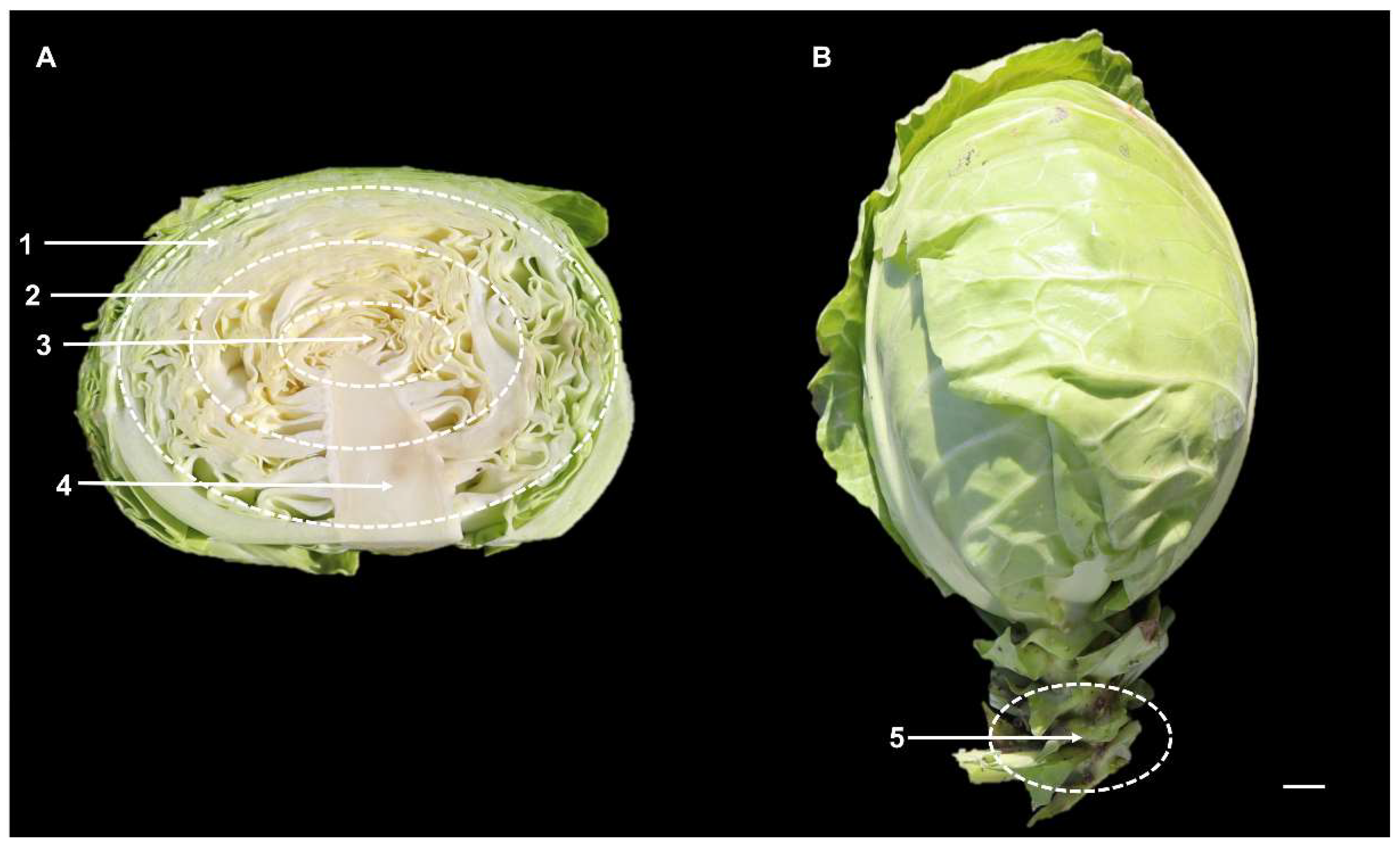
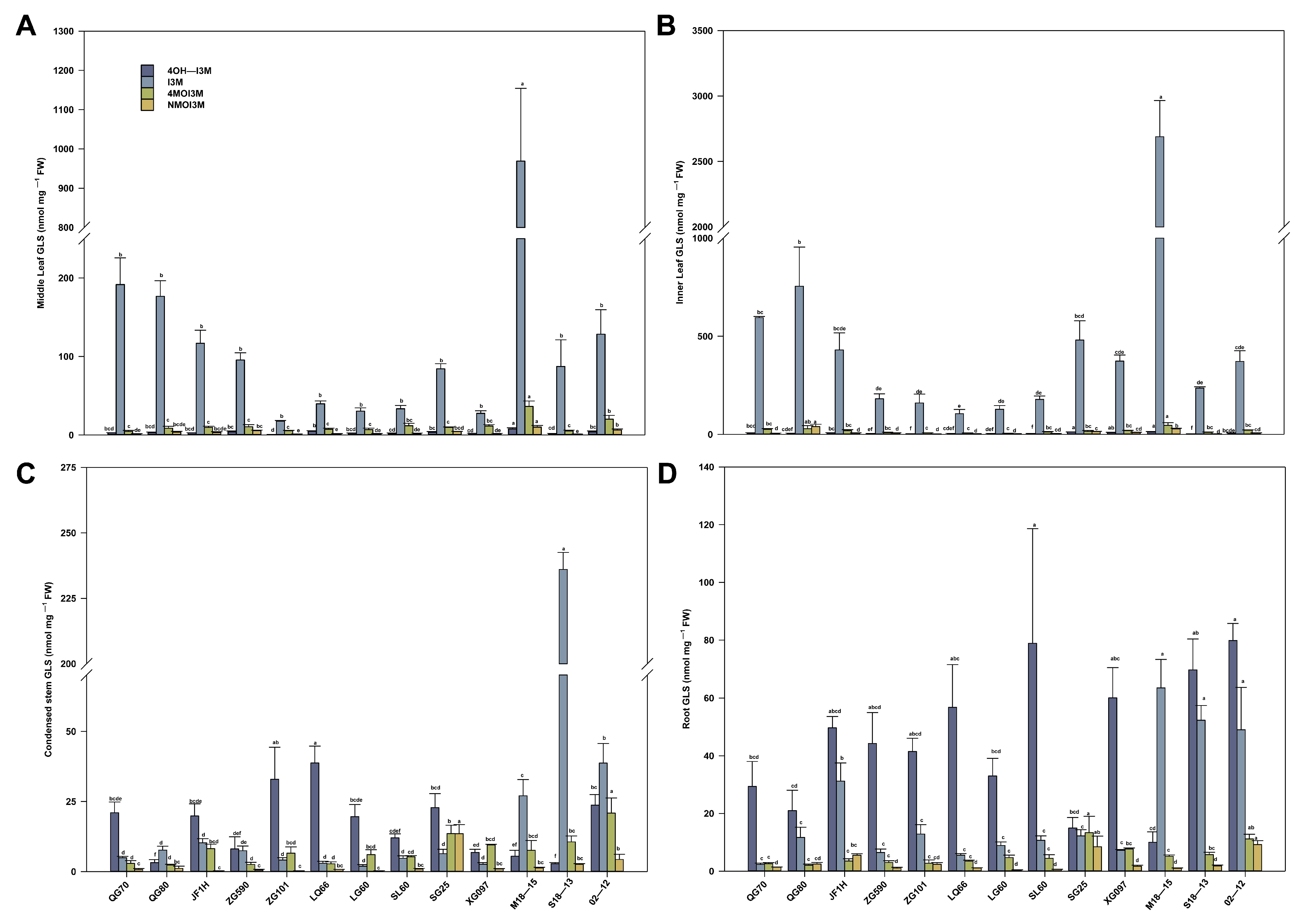
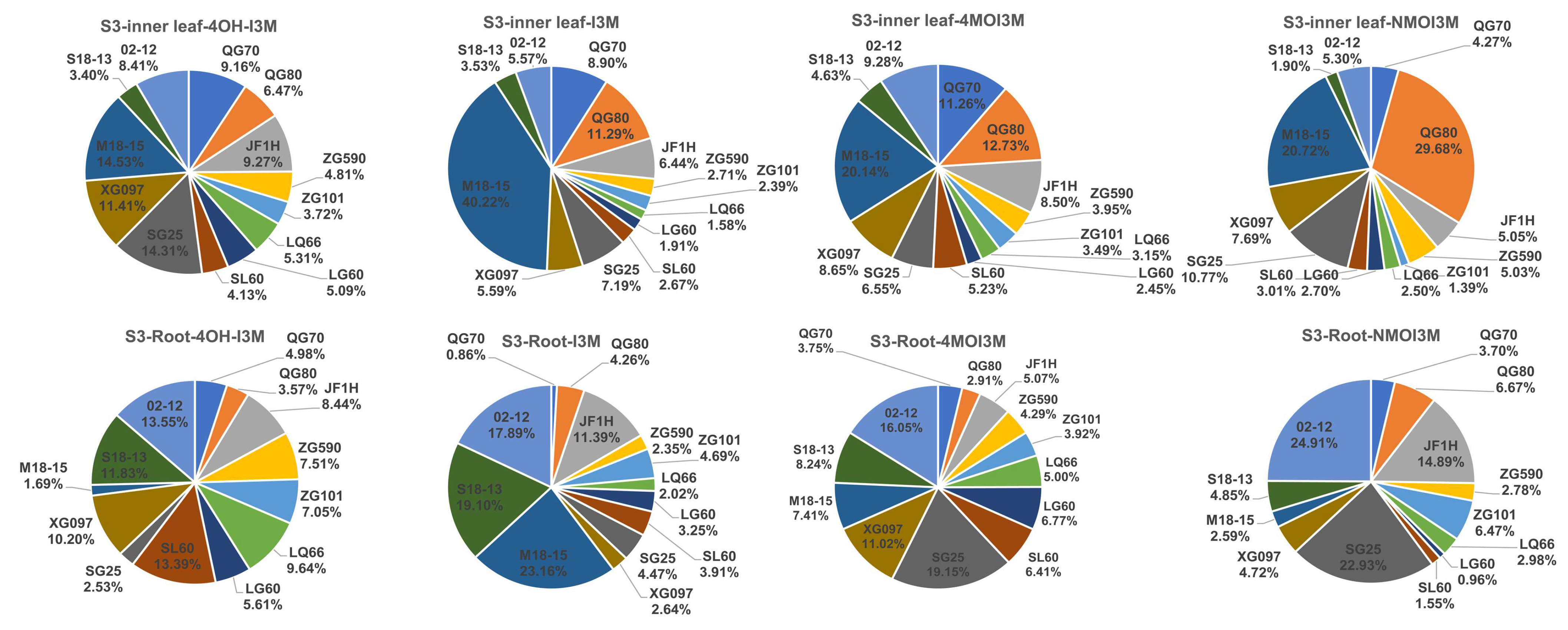
3.4. Changes of GLS Content and Components in Different Leaf Parts at S3 Stage
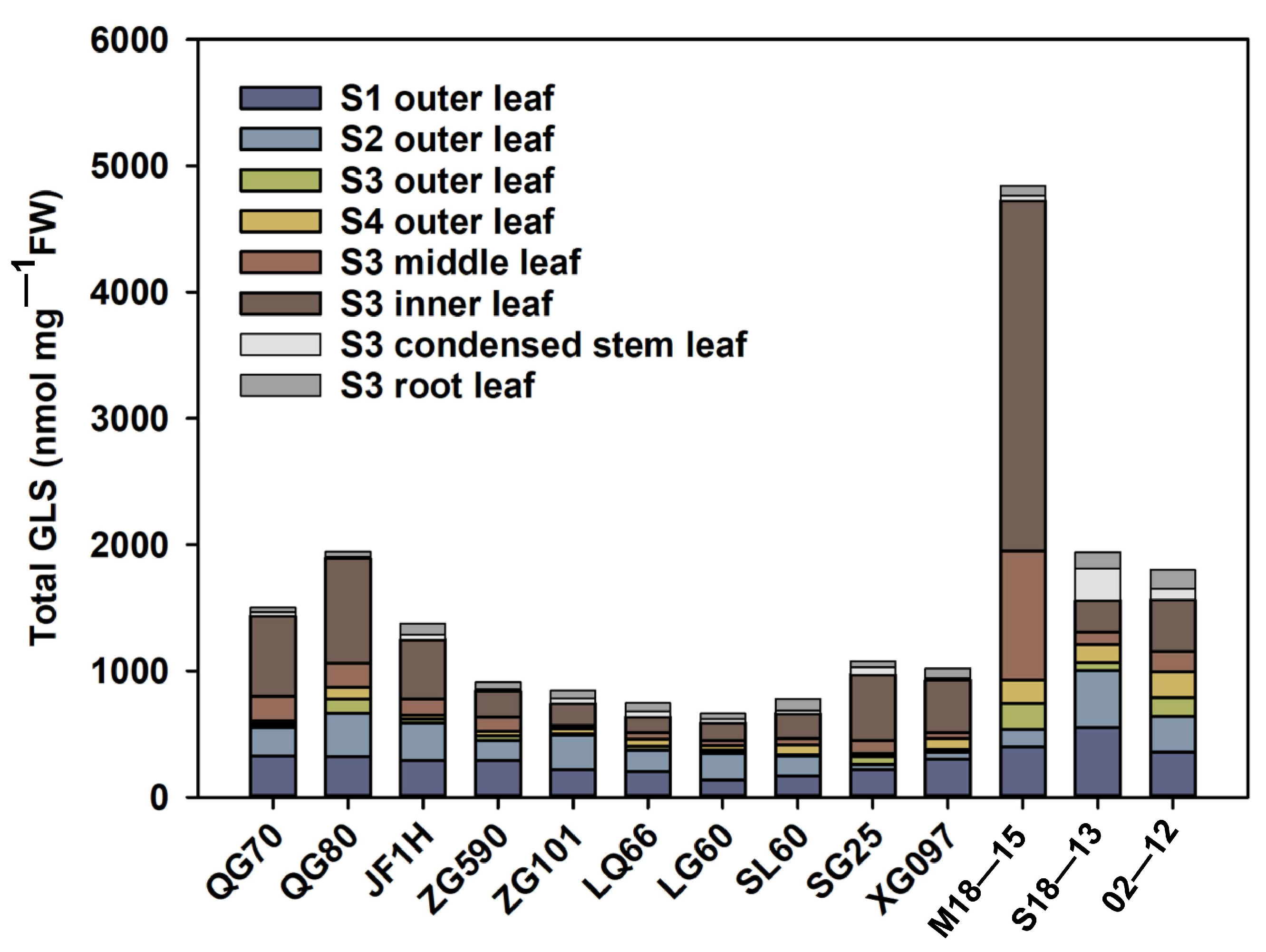
3.5. Comparison of the GLS Content in Leaves and Roots during S3 Development
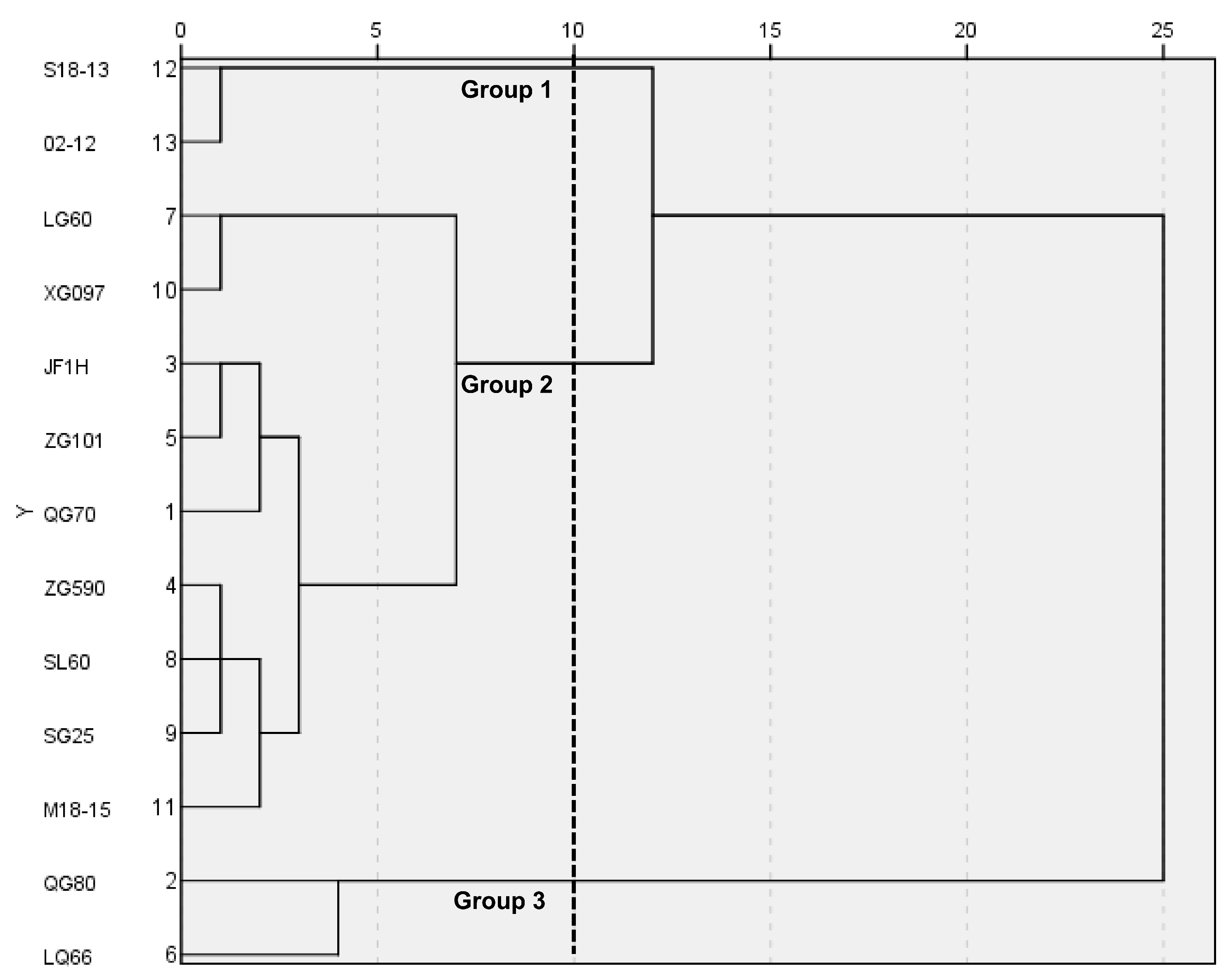
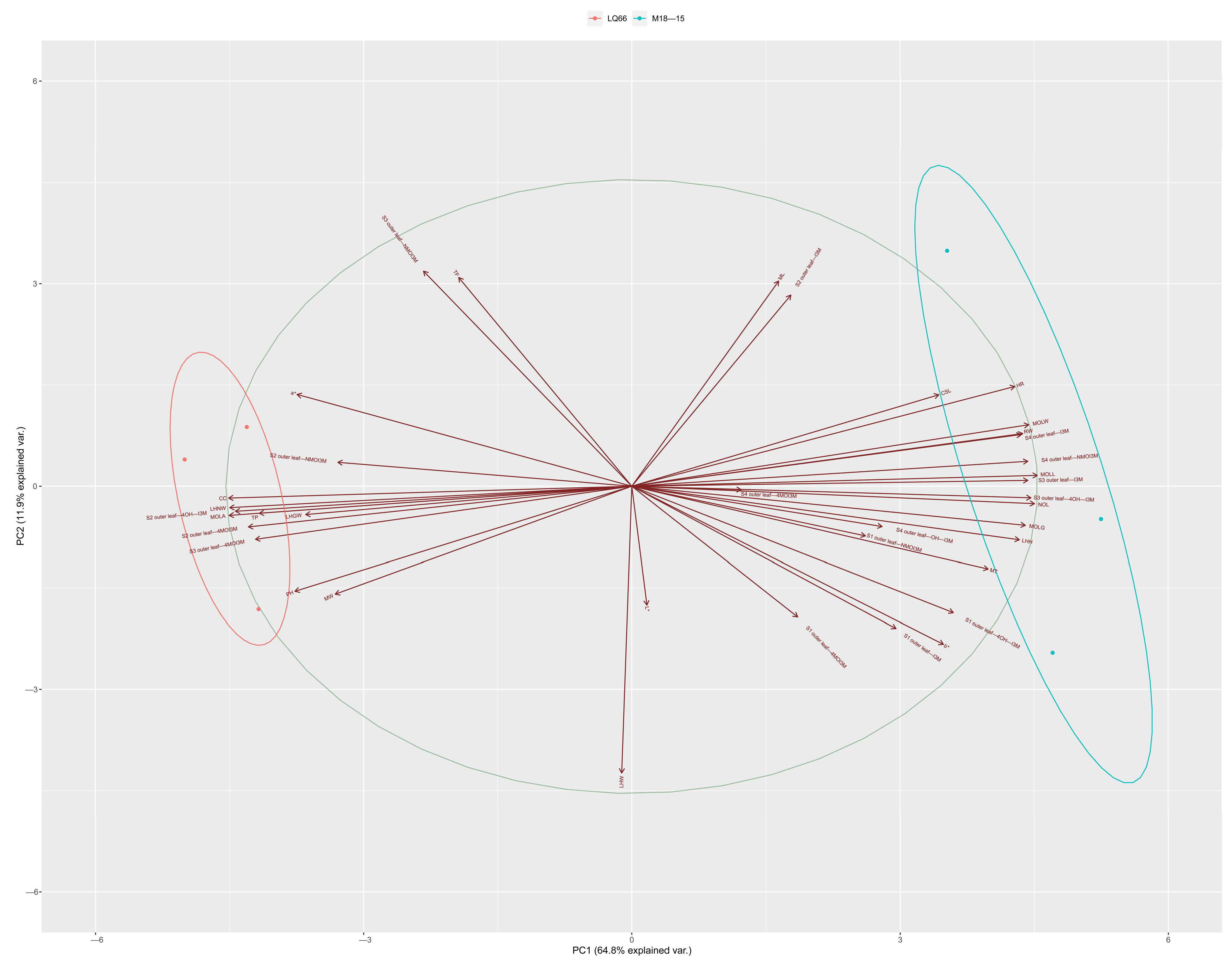
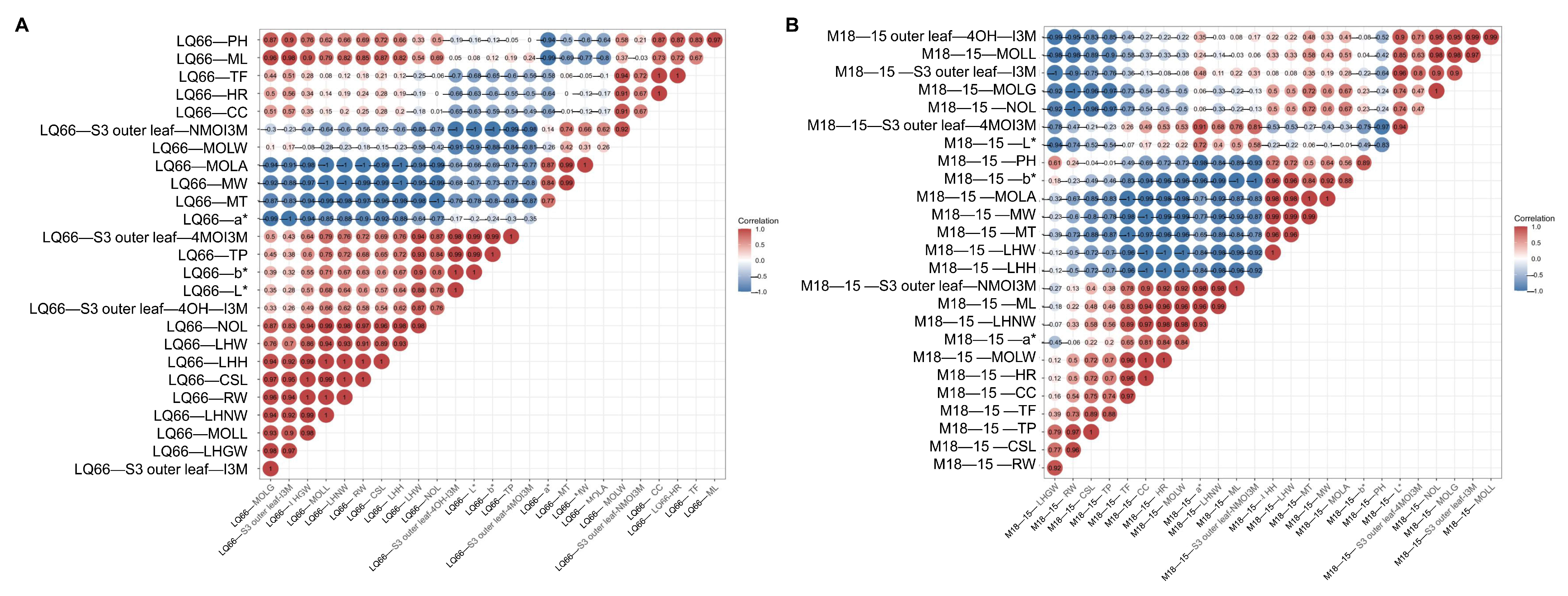
3.6. The Biological Traits, Pigment, GLS Substance Correlation, and PCA Analysis for M18-15 and LQ66
4. Discussion
4.1. Differences in Biological Traits and Pigment Accumulation of Cabbage
4.2. Comparative Analysis of GLS in Cabbage Development Period—Ecological Function
4.3. Accumulation Patterns of GLS in Various Organs of Cabbage
4.4. Comparative Analysis of above- and under-Ground GLS Substances—Transport Process
4.5. Breeders and Consumers Preferences
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.G.; Bonnema, G.; Zhang, N.; Kwak, J.H.; de Vos, R.C.; Beekwilder, J. Evaluation of glucosinolate variation in a collection of turnip (Brassica rapa) germplasm by the analysis of intact and desulfo glucosinolates. J. Agric. Food Chem. 2013, 61, 3984–3993. [Google Scholar] [CrossRef]
- Klopsch, R.; Witzel, K.; Artemyeva, A.; Ruppel, S.; Hanschen, F.S. Genotypic variation of glucosinolates and their breakdown products in leaves of Brassica rapa. J. Agric. Food Chem. 2018, 66, 5481–5490. [Google Scholar] [CrossRef]
- Andini, S.; Dekker, P.; Gruppen, H.; Araya-Cloutier, C.; Vincken, J.-P. Modulation of glucosinolate composition in Brassicaceae seeds by germination and fungal elicitation. J. Agric. Food Chem. 2019, 67, 12770–12779. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Falk, K.L.; Tokuhisa, J.G.; Gershenzon, J. The effect of sulfur nutrition on plant glucosinolate content: Physiology and molecular mechanisms. Plant Biol. 2007, 9, 573–581. [Google Scholar] [CrossRef]
- Haugen, R.; Steffes, L.; Wolf, J.; Brown, P.; Matzner, S.; Siemens, D.H. Evolution of drought tolerance and defense: Dependence of tradeoffs on mechanism, environment and defense switching. Oikos 2008, 117, 231–244. [Google Scholar] [CrossRef]
- Ratzka, A.; Vogel, H.; Kliebenstein, D.J.; Mitchell-Olds, T.; Kroymann, J. Disarming the mustard oil bomb. Proc. Natl. Acad. Sci. USA 2002, 99, 11223–11228. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The Cellular and Subcellular Organization of the Glucosinolate–Myrosinase System against Herbivores and Pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.-Y.; Kim, H.-J.; Kim, D.-K.; Jo, H.-J.; Han, B.-S.; Kim, H.-W.; Kim, J.-B. Anticancer activity and quantitative analysis of glucosinolates from green and red leaf mustard. Korean J. Food Nutr. 2011, 24, 362–366. [Google Scholar] [CrossRef]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Licznerska, B.; Baer-Dubowska, W. Indole-3-Carbinol and Its Role in Chronic Diseases. Adv. Exp. Med. Bio. 2016, 928, 131–154. [Google Scholar]
- Takada, Y.; Andreeff, M.; Aggarwal, B.B. Indole-3-carbinol suppresses NF-κB and IκBα kinase activation, causing inhibition of expression of NF-κB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood 2005, 106, 641–649. [Google Scholar] [CrossRef]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Kim, J.K.; Chu, S.M.; Kim, S.J.; Lee, D.J.; Lee, S.Y.; Lim, S.H.; Ha, S.-H.; Kweon, S.J.; Cho, H.S. Variation of glucosinolates in vegetable crops of Brassica rapa L. ssp. pekinensis. Food Chem. 2010, 119, 423–428. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef]
- Ljubej, V.; Karalija, E.; Salopek-Sondi, B.; Šamec, D. Effects of short-term exposure to low temperatures on proline, pigments, and phytochemicals level in kale (Brassica oleracea var. acephala). Horticulturae 2021, 7, 341. [Google Scholar] [CrossRef]
- Machado, M.R. Ying Chen: Trade, food security, and human rights: The rules for international trade in agricultural products and the evolving world food crisis. Agric. Hum. Values 2015, 32, 795–796. [Google Scholar] [CrossRef]
- Guo, R.; Deng, Y.; Huang, Z.; Chen, X.; XuHan, X.; Lai, Z. Identification of miRNAs affecting the establishment of Brassica Alboglabra seedling. Front. Plant Sci. 2016, 7, 1760. [Google Scholar] [CrossRef]
- Chun, J.-H.; Kim, N.-H.; Seo, M.-S.; Jin, M.; Park, S.U.; Arasu, M.V.; Kim, S.-J.; Al-Dhabi, N.A. Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Saudi J. Biol. Sci. 2018, 25, 71–82. [Google Scholar] [CrossRef]
- Gu, H.; Wang, J.; Zhao, Z.; Sheng, X.; Yu, H.; Huang, W. Characterization of the appearance, health-promoting compounds, and antioxidant capacity of the florets of the loose-curd cauliflower. Int. J. Food Prop. 2015, 18, 392–402. [Google Scholar] [CrossRef]
- Farnham, M.; Wilson, P.; Stephenson, K.; Fahey, J. Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breed. 2004, 123, 60–65. [Google Scholar] [CrossRef]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and evolutionary costs of herbivory defense: Systems biology of glucosinolate synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.D.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Jørgensen, M.E.; Nour-Eldin, H.H.; Halkier, B.A. Transport of defense compounds from source to sink: Lessons learned from glucosinolates. Trends Plant Sci. 2015, 20, 508–514. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, M.; Qiao, H.; Li, F.; Zhang, S.; Zhang, S.; Zhang, H.; Sun, R. Characterization of interspecific hybrids between flowering Chinese cabbage and broccoli. Sci. Hortic. 2018, 240, 552–557. [Google Scholar] [CrossRef]
- Teklewold, A.; Becker, H.C. Comparison of phenotypic and molecular distances to predict heterosis and F1 performance in Ethiopian mustard (Brassica carinata A Braun). Theor. Appl. Genet. 2006, 112, 752–759. [Google Scholar] [CrossRef]
- Zhou, W.; Niu, Y.; Ding, X.; Zhao, S.; Li, Y.; Fan, G.; Zhang, S.; Liao, K. Analysis of carotenoid content and diversity in apricots (Prunus armeniaca L.) grown in China. Food Chem. 2020, 330, 127223. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- Sartory, D.; Grobbelaar, J. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.-L.; Duan, F.-M.; Du, X.; Yang, Q.-C.; Zheng, Y.-J. Improvement of the growth and nutritional quality of two-leaf-color Pak Choi by supplemental alternating red and blue light. HortScience 2021, 56, 118–125. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Gershenzon, J.; Mitchell-Olds, T. Comparative quantitative trait loci mapping of aliphatic, indolic and benzylic glucosinolate production in Arabidopsis thaliana leaves and seeds. Genetics 2001, 159, 359–370. [Google Scholar] [CrossRef]
- Ashenafi, E.L.; Nyman, M.C.; Holley, J.M.; Mattson, N.S.; Rangarajan, A. Phenotypic plasticity and nutritional quality of three kale cultivars (Brassica oleracea L. var. acephala) under field, greenhouse, and growth chamber environments. Environ. Exp. Bot. 2022, 199, 104895. [Google Scholar]
- Carvalho, S.D.; Folta, K.M. Sequential light programs shape kale (Brassica napus) sprout appearance and alter metabolic and nutrient content. Hortic. Res. 2014, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-W.; Fan, Z.-Q.; Chen, Y.; Zhu, Y.-L.; Li, J.-Y.; Yin, H.-F. Functional analyses of a flavonol synthase–like gene from Camellia nitidissima reveal its roles in flavonoid metabolism during floral pigmentation. J. Biosci. 2013, 38, 593–604. [Google Scholar] [CrossRef]
- Li, P.; Su, T.; Zhang, D.; Wang, W.; Xin, X.; Yu, Y.; Zhao, X.; Yu, S.; Zhang, F. Genome-wide analysis of changes in miRNA and target gene expression reveals key roles in heterosis for Chinese cabbage biomass. Hortic. Res. 2021, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jia, W.; Wu, X.; Zhang, H.; Wang, Y.; Liu, J.; Yang, Y.; Tao, S.; Wang, X. Carbon dots can strongly promote photosynthesis in lettuce (Lactuca sativa L.). Environ. Sci. Nano 2022, 9, 1530–1540. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kusznierewicz, B. Young shoots of white and red headed cabbages like novel sources of glucosinolates as well as antioxidative substances. Antioxidants 2021, 10, 1277. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, Z.; Zhong, X.; Lei, J.; Tao, P.; Li, B. Distribution of primary and secondary metabolites among the leaf layers of headed cabbage (Brassica oleracea var. capitata). Food Chem. 2020, 312, 126028. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The glucosinolates and their bioactive derivatives in Brassica: A review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2544–2571. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef]
- Chang, J.; Wang, M.; Jian, Y.; Zhang, F.; Zhu, J.; Wang, Q.; Sun, B. Health-promoting phytochemicals and antioxidant capacity in different organs from six varieties of Chinese kale. Sci. Rep. 2019, 9, 20344. [Google Scholar] [CrossRef]
- Yeo, H.J.; Baek, S.-A.; Sathasivam, R.; Kim, J.K.; Park, S.U. Metabolomic analysis reveals the interaction of primary and secondary metabolism in white, pale green, and green pak choi (Brassica rapa subsp. chinensis). Appl. Biol. Chem. 2021, 64, 3. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Rudgers, J.A.; Lau, J.A.; Irwin, R.E. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 2002, 17, 278–285. [Google Scholar] [CrossRef]
- Shelton, A.L. Within-plant variation in glucosinolate concentrations of Raphanus sativus across multiple scales. J. Chem. Ecol. 2005, 31, 1711–1732. [Google Scholar] [CrossRef]
- Shroff, R.; Vergara, F.; Muck, A.; Svatoš, A.; Gershenzon, J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc. Natl. Acad. Sci. USA 2008, 105, 6196–6201. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.A.; Mitchell-Olds, T. Testing the optimal defense hypothesis in nature: Variation for glucosinolate profiles within plants. PLoS ONE 2017, 12, e0180971. [Google Scholar]
- Meldau, S.; Erb, M.; Baldwin, I.T. Defence on demand: Mechanisms behind optimal defence patterns. Ann. Bot. 2012, 110, 1503–1514. [Google Scholar] [CrossRef]
- McCall, A.C.; Fordyce, J.A. Can optimal defence theory be used to predict the distribution of plant chemical defences? J. Ecol. 2010, 98, 985–992. [Google Scholar] [CrossRef]
- Chen, S.; Andreasson, E. Update on glucosinolate metabolism and transport. Plant Physiol. Biochem. 2001, 39, 743–758. [Google Scholar] [CrossRef]
- Hirono, H.; Morimitsu, Y.; Kato, A.; Higashio, H. Glucosinolate profiles in cabbage (Brassica oleracea var. capitata) cultivars and their effect on the induction of a phase II detoxification enzyme. J. Jpn. Soc. Hortic. Sci. 2011, 80, 499–505. [Google Scholar]
- Pfalz, M.; Vogel, H.; Kroymann, J. The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell 2009, 21, 985–999. [Google Scholar] [CrossRef]
- Tsunoda, T.; Krosse, S.; van Dam, N.M. Root and shoot glucosinolate allocation patterns follow optimal defence allocation theory. J. Ecol. 2017, 105, 1256–1266. [Google Scholar] [CrossRef]
- De Vos, M.; Kriksunov, K.L.; Jander, G. Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiol. 2008, 146, 916–926. [Google Scholar] [CrossRef]
- Ahmad, A.; ASakr, W.; Wahidur Rahman, K. Anticancer properties of indole compounds: Mechanism of apoptosis induction and role in chemotherapy. Curr. Drug Targets 2010, 11, 652–666. [Google Scholar] [CrossRef]
- Salem, A.Z.; Medhat, D.; Fathy, S.A.; Mohamed, M.R.; El-Khayat, Z.; El-Daly, S.M. Indole glucosinolates exhibit anti-inflammatory effects on Ehrlich ascites carcinoma cells through modulation of inflammatory markers and miRNAs. Mol. Biol. Rep. 2021, 48, 6845–6855. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Fujioka, N.; Fritz, V.; Upadhyaya, P.; Kassie, F.; Hecht, S.S. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol. Nutr. Food Res. 2016, 60, 1228–1238. [Google Scholar] [CrossRef]
- Bradlow, H.L. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo 2008, 22, 441–445. [Google Scholar]
- Chen, S.; Petersen, B.L.; Olsen, C.E.; Schulz, A.; Halkier, B.A. Long-distance phloem transport of glucosinolates in Arabidopsis. Plant Physiol. 2001, 127, 194–201. [Google Scholar] [CrossRef]
- Andersen, T.G.; Liang, D.; Halkier, B.A.; White, R. Grafting arabidopsis. Bio-Protocol 2014, 4, e1164. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Savic, A.; Aradski, A.A.; Zivkovic, J.; Savikin, K.; Jaric, S.; Marin, P.D.; Duletic-Lausevic, S. Phenolic composition, and antioxidant and antineurodegenerative potential of methanolic extracts of fruit peel and flesh of pear varieties from Serbia. Pol. J. Food Nutr. Sci. 2021, 71, 225–236. [Google Scholar] [CrossRef]
- Giannino, D.; Testone, G.; Nicoladi, C.; Giorgetti, L.; Bellani, L.; Gonnella, M.; Cirdi, M.; Cappuccio, P.; Moscatello, S.; Battistelli, A. Nutritive parameters and antioxidant quality of minimally processed “cime di rapa” (Brassica rapa subsp. sylvestris) vary as influenced by genotype and storage time. Pol. J. Food Nutr. Sci. 2020, 70, 337–346. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Zhang, J.; Yan, C.; Khan, A.; Fei, S.; Lei, T.; Xu, Z.; Li, B.; Zhang, R.; Hui, M. Distribution of Indolic Glucosinolates in Different Developmental Stages and Tissues of 13 Varieties of Cabbage (Brassica oleracea L. var. capitata). Horticulturae 2023, 9, 867. https://doi.org/10.3390/horticulturae9080867
Pan Q, Zhang J, Yan C, Khan A, Fei S, Lei T, Xu Z, Li B, Zhang R, Hui M. Distribution of Indolic Glucosinolates in Different Developmental Stages and Tissues of 13 Varieties of Cabbage (Brassica oleracea L. var. capitata). Horticulturae. 2023; 9(8):867. https://doi.org/10.3390/horticulturae9080867
Chicago/Turabian StylePan, Qiming, Jiahao Zhang, Chengtai Yan, Abid Khan, Siming Fei, Ting Lei, Zhongming Xu, Baohua Li, Ruixing Zhang, and Maixia Hui. 2023. "Distribution of Indolic Glucosinolates in Different Developmental Stages and Tissues of 13 Varieties of Cabbage (Brassica oleracea L. var. capitata)" Horticulturae 9, no. 8: 867. https://doi.org/10.3390/horticulturae9080867
APA StylePan, Q., Zhang, J., Yan, C., Khan, A., Fei, S., Lei, T., Xu, Z., Li, B., Zhang, R., & Hui, M. (2023). Distribution of Indolic Glucosinolates in Different Developmental Stages and Tissues of 13 Varieties of Cabbage (Brassica oleracea L. var. capitata). Horticulturae, 9(8), 867. https://doi.org/10.3390/horticulturae9080867




